
11 minute read
HEARTACHE IN HIGH PLAINS FEEDYARDS
By Greta M. Krafsur, DVM, DACVP, MSc, PhD Candidate, University of Colorado Denver Anschutz School of Medicine Cardiovascular Pulmonary Research Lab
Ask anyone involved in the cattle feeding business about late day morbidity and mortality concerns in high-performing beef cattle and the subject of bovine congestive heart failure (BCHF) nearly always dominates the conversation. While most cattle feeding occurs in the High Plains, the author has observed BCHF in university feedlots and farmer-feeder operations in eastern South Dakota and southwestern Minnesota.

No matter the location or size of the feeding organization, a subset of cattle is predisposed to pulmonary hypertension culminating in right heart failure (Figure 1). The precise genesis of BCHF remains unknown, impairing our ability to develop testing strategies aimed at identifying cattle with the greatest risk of disease. Likewise, a host of bovine cardiopulmonary diseases including congenital defects, chronic respiratory disease, hardware disease, ionophore intoxication, infectious valvular endocarditis, and atypical interstitial pneumonia produce clinical signs like BCHF and frequently are misdiagnosed as such, leading to underdiagnosis of BCHF. The gross and microscopic lesions associated with BCHF are distinct from other bovine cardiopulmonary diseases, emphasizing the merit of performing a thorough postmortem and microscopic examination of heart, lung, and liver tissues to rule out these differential diagnoses. BCHF is an important cause of mortality in North American feedyards and independent of mortality, the condition negatively impacts production and carcass traits, increases antibiotic usage to address animal welfare concerns, and frustrates and causes poor morale among feedlot managers and key personnel. Regrettably, the escalating risk and incidence of the invariably terminal disease shows no signs of abating with premature culling and death the norm, not the exception.
The author herein summarizes current knowledge and potential genetic risk factors associated with feedlot BCHF, drawing upon our understanding of high mountain disease, and comparing the feedlot condition to hypoxia-induced pulmonary hypertension of high elevation.
Not-So-High Mountain Disease
While many consider BCHF a newly emerging syndrome plaguing cattle fed at low to moderate elevations (800-1600 m), the reality is the disease has been around for quite some time. In 1974, Colorado State University veterinary pathologists collaborated with feedlot veterinarians to survey causes of morbidity and mortality among 407,000 placements in 4 northern Colorado feedyards. Respiratory disease was predictably the most common cause of illness and death and tended to occur within the first 45 days of feeding. The second most common cause of death, tied with diphtheritic disease, was brisket disease (5.8%) although the cattle surveyed were fed at moderate altitude (1,600 m). Brisket disease had not previously been investigated in the feedlot setting although incidence appeared to escalate with greater days on feed. Investigators conjectured the convergence of four causative factors contributed to disease:
1) genetic predilection, 2) high elevation origin, 3) rapid growth, and 4) obesity hypoventilation. Although respiratory disease could initiate hypoxia-induced pulmonary vasoconstriction leading to brisket disease, the investigators also acknowledged that acute comorbid respiratory disease could develop in the latter stages of brisket disease, hastening clinical trajectory.
Nearly 50 years have passed since brisket disease was first described in the feedlot setting, and while several
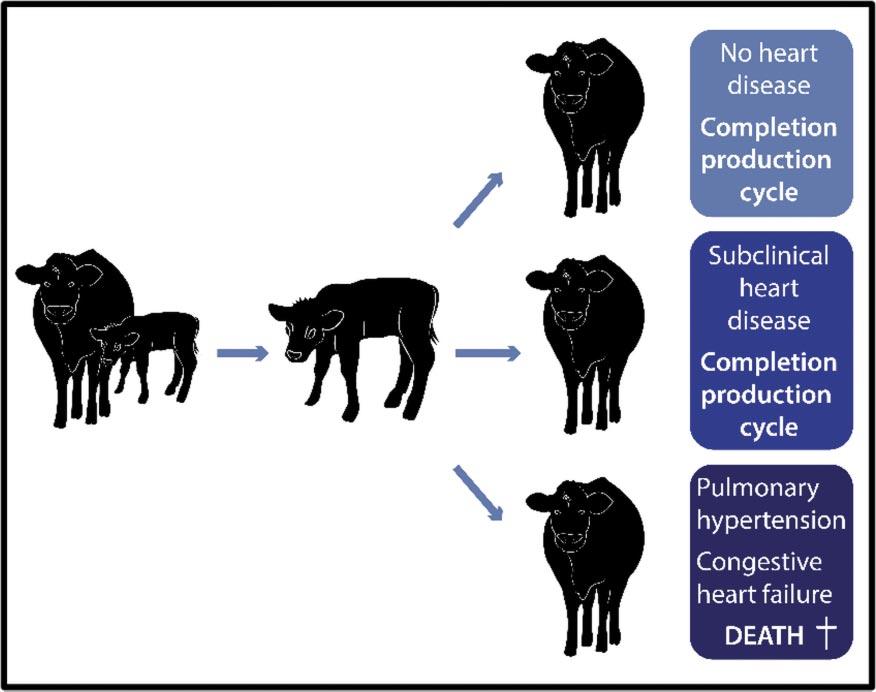
Figure 1
This cartoon depicts how a subset of cattle develop clinical manifestations of BCHF that is nearly always fatal. Another group of cattle while asymptomatic exhibit morphologic pathology in the heart and lungs indicative of subclinical disease.
These cattle are the “winners” as they completed the production cycle despite having silent cardiovascular and pulmonary disease. The remaining group of cattle enter the food chain with no adverse cardiopulmonary remodeling.
designations have been assigned to reflect the feedlot condition, little progress has been made in prevention because the etiologic factors and mechanistic underpinnings contributing to disease remain poorly characterized.
The Cardiopulmonary Phenotype of BCHF
Clinical manifestations in BCHF generally parallel high mountain disease stemming from exhaustion and failure of the right heart due to rigid obstructive lesions in the pulmonary circulation increasing pulmonary vascular resistance and workload on the right heart (Figures 2A, 2B). While we know that hypoxia of high elevation initiates adverse pulmonary vascular remodeling in high mountain disease, the one or more insults underlying adverse cardiopulmonary remodeling in BCHF are not yet identified. Unlike high mountain disease, however, BCHF disease and lesions are characterized by global pulmonary vascular remodeling encompassing the venous and arterial circulations, cardiac fibrosis and scarring of the left and right heart, and arteriosclerotic lesions in the coronary vasculature. Likewise, from feedlot necropsies and experimental field investigations, cattle succumbing to BCHF have excessive fatty infiltration of the heart, the so-called “well-marbled heart” described in human obesityinduced heart disease. Advanced obesity is associated with “fat in flames” and unhealthy fat “befats” inflammation and fibrosis (Figure 3). There exists widespread speculation that significant genetic improvements in performance and carcass traits emphasizing accelerated growth, frame size, muscle mass, and marbling underlie BCHF. However, it is acknowledged by the author and her contemporaries that BCHF is not necessarily a disease of advanced adiposity and frequently, the disease occurs much earlier in the feeding period before accretion of adipose. How then does one account for cardiac inflammation and fibrosis co-localized with excessive heart fat? Perhaps cardiac fat does not initiate the yet to be identified sequence of pathophysiological events directing BCHF but rather is a bystander or facilitator of cardiac inflammation and fibrosis. Unlike high mountain disease wherein hypoxia-induced pulmonary vascular remodeling initiates right heart disease, BCHF seemingly appears to involve adverse pulmonary vascular remodeling secondary to cardiac disease.
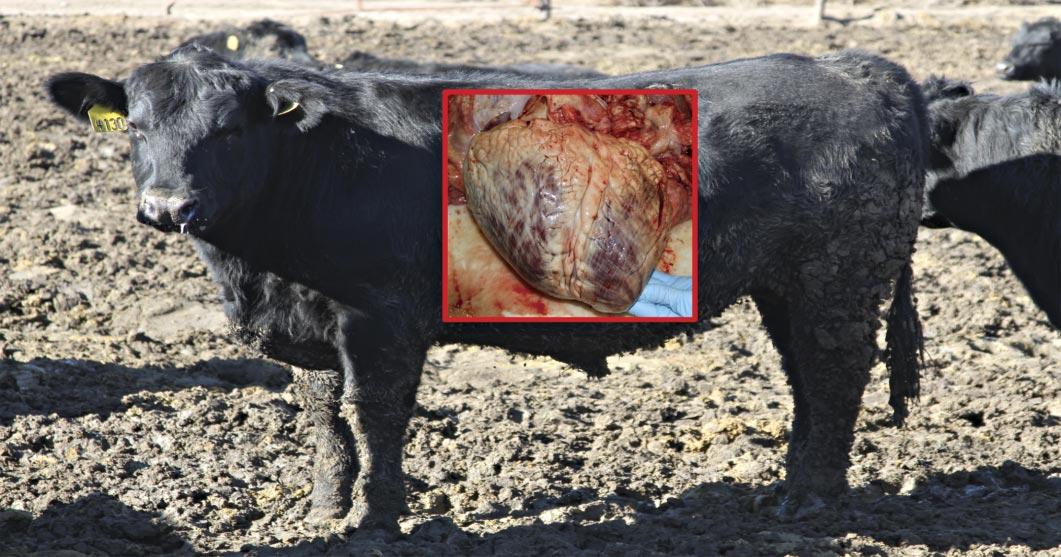
The heart is severely enlarged with an abnormal shape and enlargement of the right ventricle in particular causing rounding of the apex or the so-called “double apex”. The heart is encased by excessive fat. The steer was fed in north-central Nebraska and originated from the Dakotas. Genetic testing revealed its risk for BCHF was 28-fold compared to its pen mates.
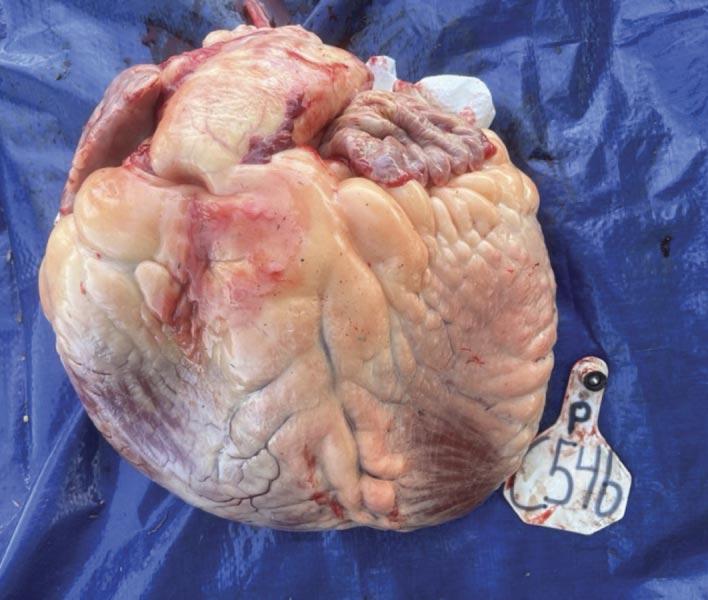

This is a heart with a normal size and shape. The apex of the heart with a sharp “point” is formed entirely by the left side of the heart.
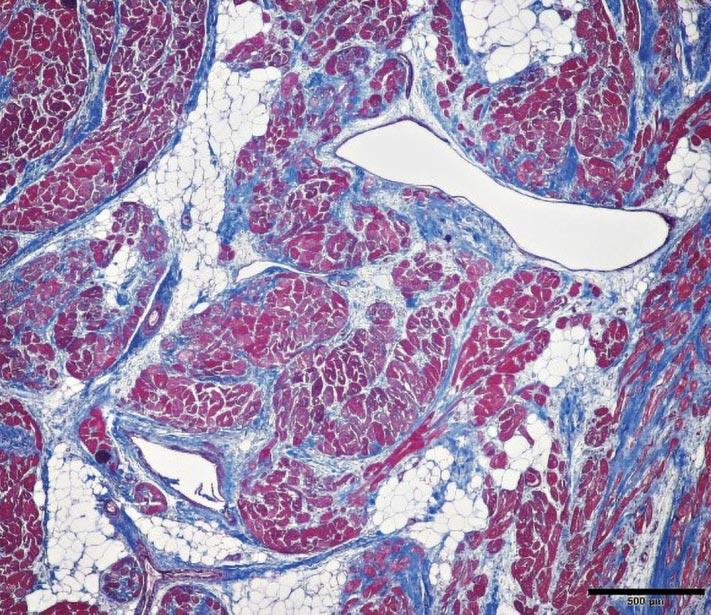
Microscopic image of the heart muscle depicting excessive fatty infiltration within the heart with concurrent fibrosis and scarring. While the animal did not exhibit clinical signs of heart failure, the steer had pulmonary hypertension and an enlarged heart encased by a massive amount of fat invading the heart muscle.
Prior Hypoxia of High Altitude and Predilection for Pulmonary Hypertension During Fattening
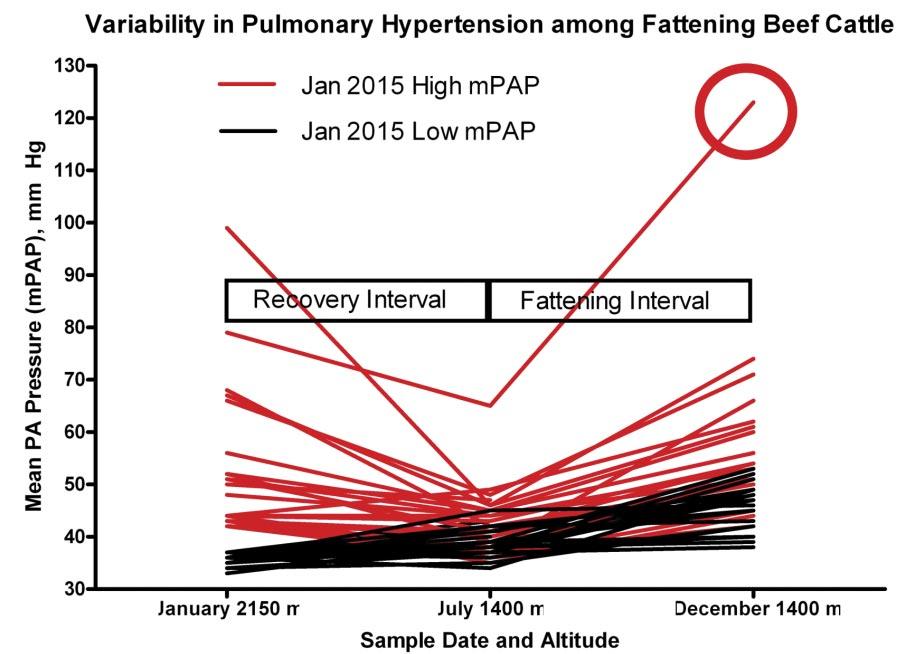
Our group has utilized cattle from the CSU Beef Improvement Center at the Rouse Ranch (Encampment, Wyoming; elevation 2150 m) to follow the natural progression of BCHF, performing sequential pulmonary arterial pressure (PAP) testing as a surrogate marker for adverse pulmonary arterial remodeling leading to pulmonary hypertension and high mountain disease. PAP testing is routinely used as a screening tool to select against bovine pulmonary hypertension in high elevation breeding programs. Even so, a subset of weanling calves remains sensitive to hypoxia of high altitude although when moved to moderate elevation (Fort Collins, Colorado; elevation 1519 m) pulmonary arterial pressures generally return to normal. However, once intense feeding and fattening commences, those same cattle most sensitive to environmental hypoxia experience the greatest increases in PAP scores (Figure 4). Similar to humans, there are individual animals that exhibit severe out of proportion pulmonary hypertension in response to hypoxic and metabolic insults. One such animal we studied had a PAP score of 123 mm Hg (normal < 41 mm Hg) approximately 3 weeks prior to scheduled harvest. Despite severe alterations in cardiac morphology at harvest, the steer did not present clinical manifestations (Figure 5). Clearly, a subset of high elevation feedyard placements enter with a measure of pulmonary hypertension exacerbated by metabolic demands incurred during accretion of muscle and adipose. Likely, hypoxia of high altitude primes the immune system, potentiating biological signaling networks underlying inflammation and metabolism during growth and fattening.
We have since eliminated the confounding factor of prior hypoxia of high altitude by utilizing a herd of moderate elevation beef cattle (northeastern Colorado) with
Figure 4
This graph depicts a subset of cattle (red lines) sensitive to environmental hypoxia of high altitude had elevated pulmonary arterial pressures that returned to normal when they were moved to lower elevation. Once feeding and fattening commenced, animals most sensitive to environmental hypoxia exhibited the greatest elevation in PAP scores although none of them developed over clinical disease.
a history of BCHF in monitoring natural disease progression. PAP testing was performed within 3, 6, and 9 months of placement at the Eastern Colorado Research Center (Akron, Colorado; elevation 1400 m). To our astonishment, a remarkable number of 6- to 7-monthold steers weighing approximately 320 kg had elevated PAP scores within 3 months of arrival. The majority of BCHF mortalities (clinical diagnosis) occurred between 6 and 9 months of placement. Necropsy was performed on one steer euthanized for presumptive BCHF believed to be complicated by respiratory disease. The author noted the lungs were pristine. Gross alterations in cardiac morphology and chronic passive congestion in the liver were consistent with BCHF. Likewise, microscopic cardiopulmonary lesions bespoke global pulmonary vascular remodeling, biventricular cardiac fibrosis and scarring, and coronary arteriosclerotic lesions. Harvest data confirmed high PAP steers did not perform as well, having lower average daily gain and feed conversion.
Much attention has been given to the prospect of obesity hypoventilation in fattened cattle as the hypoxic insult precipitating adverse pulmonary vascular remodeling; however, our assessment of arterial-venous blood gases does not support this hypothesis.
PAP testing is fraught with logistical and technical drawbacks and while the procedure is routinely performed in high elevation breeding programs, its pitfalls limit utilization in the feedlot setting. Screening tools are needed to predict cattle at risk for BCHF; however, our ability to identify susceptible cattle mandates understanding genetic and other yet identified management and environmental factors influencing disease. Two Nebraska researchers have identified potential genetic risk factors associated with BCHF that appear to offer the promise of identifying susceptible cattle.
Figure 5
This steer prior to slaughter had a severely elevated PAP (red circle on Figure 4) and despite having a severely enlarged heart (depicted in the photo) did not exhibit any clinical signs of BCHF. Again, he was a “winner”, surviving the production cycle with no gross lesions that would have led to condemnation at harvest.
Genetic Underpinnings of BCHF
It is beyond the scope and abilities of the author to adequately address the work performed by Mike Heaton, PhD (USDA, ARS, US Meat Animal Research Center) and Brian Vander Ley, DVM, PhD, DACVPM (Great Plains Veterinary Education Center) that has led to the unveiling of 21 genetic variants associated with BCHF. Two SNP variants (NF1A-AS2, ARRDC3) are strongly associated with BCHF. The reader is advised to consult the fact sheet available at https://www.ars.usda.gov/ARSUserFiles/ 30400500/BCHF_photos/BCHF_Factsheet_03.31.2022.pdf for a more thorough description of the science behind their identification and utility of these markers to mitigate BCHF. The identification of these variants will inform and direct our efforts to appreciate the mechanistic underpinnings of BCHF and help us understand potential environmental and management effects. The author has tested for the two variants in cattle dying from BCHF and found the two SNP variants were highly correlated with disease.
In summary, although it is widely acknowledged there is a measure of risk for BCHF associated with performance and carcass traits, there is a serious lack of scientific evidence elucidating the specific mechanisms connecting these traits to disease. Until knowledge gaps concerning biological processes regulated by candidate genes in cattle are addressed, we can only speculate regarding in what manner genetics, performance traits, and environmental hypoxia converge to influence disease.
The problem of BCHF has been decades in the making, will take considerable time to alleviate, and requires a collaborative multidisciplinary approach joining scientists with caregivers. In closing, Robert F. Grover, MD said this of cattle:
“Nature sometimes determines that cattle are the professors, and we the human researchers are the students.”
As cattle caregivers, we are compelled to take note.
Selected References
Heaton, M.P., Harhay, G.P., Bassett, A.S., et al. Association of ARRDC3 and NFIA variants with bovine congestive heart failure in feedlot cattle [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research 2022, 11:385 (https://doi.org/10.12688/f1000research.109488.1).
Holt, T., and R. Callan. 2007. Pulmonary arterial pressure testing for high mountain disease in cattle. Vet. Clin. North Am. Food Anim. Pract. 23:575-596.
Jennings, K. J., Krafsur, G. M., Brown, R. D., Holt, T. N., Coleman, S. J., Speidel, S. E., Enns, R. M., Stenmark, K. R., & Thomas, M. G. (2019). Characterizing the impact of altitude and finishing system on mean pulmonary arterial pressure and carcass characteristics in Angus cattle. Translational animal science, 3(Suppl 1), 1669–1672 https://doi.org/10.1093/tas/txz052.
Jensen R, Pierson RE, Braddy PM, et al. Brisket disease in yearling feedlot cattle. JAVMA 1976; 169:515-517.
Krafsur, G. M., R. Brown, T. N. Holt, D. H. Gould, F. Garry, S. Riddle, J. M. Neary, R. Enns, M. Thomas, and K. R. Stenmark. 2017. Intense feeding and fattening regimens augment pulmonary hypertension, pulmonary venous and cardiac remodeling in beef cattle: a natural large animal model of pulmonary hypertension with left ventricular dysfunction. Amer. J. Resp. Crit. Care Med. 195:A7216.
Krafsur, Greta M., Joseph M. Neary, Franklyn Garry, Timothy Holt, Daniel H. Gould, Gary L. Mason, Milton G. Thomas, et al. “Cardiopulmonary Remodeling in Fattened Beef Cattle: A Naturally Occurring Large Animal Model of ObesityAssociated Pulmonary Hypertension with Left Heart Disease.” Pulmonary Circulation, (January 2019) doi:10.1177/2045894018796804.
Moxley RA, Smith DR, Grotelueschen DM, Edwards T, Steffen DJ. Investigation of congestive heart failure in beef cattle in a feedyard at a moderate altitude in western Nebraska. J Vet Diagn Invest. 2019 Jul;31(4):509-522. doi: 10.1177/1040638719855108. Epub 2019 Jun 6. PMID: 31170901; PMCID: PMC6857034.
Neary, J. M., F. B. Garry, T. N. Holt, A. P. Knight, D. H. Gould, and D. A. Dargatz. 2013b. Pulmonary arterial pressures, arterial blood-gas tensions, and serum biochemistry of beef calves born and raised at high altitude. Open Acc. Anim. Physiol. 5:1-8.
Neary, J. M. 2014. Epidemiological, physiological, and genetic risk factors associated with congestive heart failure and mean pulmonary arterial pressure in cattle. Ph.D. Diss., Colorado State Univ., Fort Collins.
Neary, J. M., F. B. Garry, T. N. Holt, M. G. Thomas, and R. M. Enns. 2015a. Mean pulmonary arterial pressures in Angus steers increase from cow-calf to feedlot-finishing phases. J. Anim. Sci. 93:3854-3861.
Neary, J. M., F. B. Garry, and T. N. Holt. 2015c. A case-control evaluation of cardiopulmonary physiology in feedlot steers fed the beta-2 adrenergic agonist zilpaterol hydrochloride. J. Anim. Sci. (manuscript in preparation for submission; information discussed in Neary et al., 2015b and Chapter 7 in Neary, 2014, PhD Diss. Colorado State University, Fort Collins).
Neary, J. M., F. B. Garry, T. N. Holt, R. M. Enns, and M. G. Thomas. 2015d. The altitude at which a calf is born and raised influences the rate at which mean pulmonary arterial pressure increases with age. J. Anim. Sci. 93:47144720.
Neary, J. M., B. K. Wildman, C. W. Booker, and P. S. Morley. 2016. Right-sided congestive heart failure in North American Feedlot cattle. J. Vet. Intern. Med. 30:326-334.
Rhodes, J. 2005. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J. Appl. Physiol. 98:1092-1100.
Greta M. Krafsur hails from a multigenerational diversifi ed family farm near Estelline, South Dakota, where she was the oldest of three children. Greta took the circuitous route to veterinary school, entering the professional veterinary medicine program at Colorado State University at the young age of 41 after being a stay-home mother to her three sons until they entered elementary school. She graduated with her DVM in 2013 and then two months later entered a combined anatomic pathology residency/PhD program at CSU. Through her T32 postdoc training in the UC Denver Anschutz School of Medicine Cardiovascular Pulmonary Research Lab, she has utilized a One Health approach spanning multiple disciplines and specialties to examine novel paradigms in high mountain disease and bovine congestive heart failure. Her veterinary work has spanned the Alaskan Arctic, Canadian Yukon, and Scotland where she saw practice in 2013 and was a participant in the 2017 World Angus Forum delivered by the Aberdeen Angus Cattle Society. In her spare time, she loves reading about the history of the cattle industry and Arctic exploration. She also enjoys flying with her son Joseph, a professional pilot, watching her twin son Joshua compete in Division I cross country and track for East Tennessee State University, and learning about cryptocurrency and blockchain technology from her other twin son Benjamin, the CFO for the startup company Indico Blockchain Solutions, LLC.












