
8 minute read
Mycoplasma hyopneumoniaeUnderstanding Infected Herds
By: Dr. Jim Lowe, Production Animal Consultation
Lack of information, lack of consensus
Porcine Respiratory Disease Complex (PRDC) continues to account for a majority of the economic losses due to disease in the late finishing period. M. hyopneumoniae (Mhp) is a key contributor to PRDC in the late finishing period. While there are many things that we think we know about Mhp, under field conditions there is a paucity of peer reviewed published information to support most of the “conventional wisdom” of veterinarians in managing Mhp’s role in PRDC. This has resulted in many divergent and often directly conflicting approaches to controlling Mhp in modern production systems.
High costs, high risk
With high market values and input costs, mortality late in the growing period from PRDC is costly. Solutions that are not evidence based will result in failure, economic losses for our producers, and further marginalization of veterinarians in science driven production systems. Evidence exists to better guide our decisions on the management of Mhp in production systems.
Fix the gilts, fix mycoplasma
Control of PRDC associated with Mhp in late finishing will not be achieved until the Mhp infection status of gilts at the time of entry is managed.
•The number of pigs infected at weaning determines the disease load from Mhp in growing pigs.
•The number of pigs infected at weaning is directly related to the number of sows shedding Mhp at the time of farrowing.
•Females that are infected in the 200 days prior to farrowing are likely to shed Mhp to their piglets.
•Gilts are the animals most likely to be infected in the 200 days prior to farrowing.
Piglet infection is the root cause
The first step in understanding the potential impact of an infectious disease in a population is to understand the root mechanisms of source and transmission. In the case of Mhp, a slow growing organism with long infection to clinical impact period, infection early in life is important. Without a sufficient period for the organism to grow in the host prior to harvest, clinically important disease does not occur.
This means that for disease to occur in the population, increased transmission early in the growing period is important.
There are multiple ways to promote transmission but the simplest is to have more infected pigs in the population when the group starts, meaning that less transmission is needed to produce clinically important disease.
This hypothesis was tested by Fano et al1 where he clearly demonstrated that increasing the number of infected piglets at weaning produced earlier and more severe respiratory disease associated with Mhp. If more piglets infected at weaning are the cause of the disease in late finishing, how do piglets get infected?
Shedding sows mean infected pigs
While it is possible that piglets could get infected from lateral sources during transport or immediately after weaning, this appears to be an unlikely source of infection. The dam is the most logical source of infection in pigs at weaning for most diseases and Mhp is no exception.
There have been numerous investigations of infection status of piglets at weaning and the impact of sow infection and antibody status on piglet infections2-4
In a review of all the available evidence, Sibilia et al5 conclude that sow to pig transmission is a likely driver of clinical disease in infected herds. It is logical to assume from the available evidence that controlling this transmission could be key to improving clinical outcomes in late finishing pigs.
When, not if, is important
The available evidence would suggest that some but not all sows are shedding Mhp to their piglets as the reported infection rates at weaning are all low.2-4 This raises the question of why some sows more likely to shed than others.
There is clear evidence in the literature that animals that are within 200 days of a recent Mhp infection are capable of infecting other pigs6. This means that any female infected within the last 200 days is capable and likely to infect her offspring, but those that are infected more than 200 days ago are not likely to infect their offspring.
Gilts, not sows, are the problem
There is evidence to suggest that once an animal is infected with a single strain of Mhp, they are unlikely to be reinfected or shed the organism again7. This suggests that if the herd does not have new introductions for at least 200 days, Mhp shedding would stop. There is evidence to suggest that this does occur. In the case of Mhp elimination programs, herd closure for extended periods of time will stop sow to piglet transmission8.
In typical breeding herds with continuous or intermittent introduction and removals, the new animals are the most likely to be infected within 200 days of farrowing. There are two potential routes for this to happen. First, naïve gilts
Figure 1: The status of gilts at entry to the sow farm determines the number of infected pigs at weaning which in turn determines if there is clinically important disease associated with Mhp during the growing period. There is no other interpretation of the logic path because the alternative paths shown in red are either biologically implausible or there is sound evidence to suggest that they cannot be meaningfully changed with known interventions.
A) Farm 1: Declining S/P ratios in gilts after entry leads to less M. hyopneumoniae transmission in finishing as evidenced by lower S/P in market weight pigs.
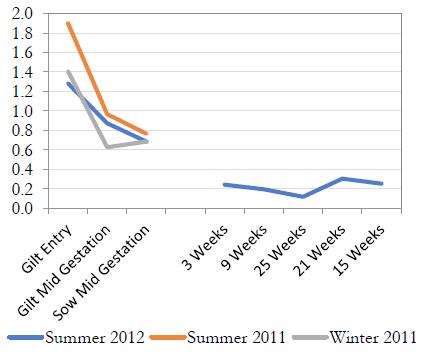
Mycoplasma could be introduced into an infected herd and become infected during the gestation period. The second scenario is gilts that have been recently infected prior to arrival (late in the development period) and have not cleared the organism prior to farrowing.
Clinically both of these scenarios have the same outcome and from the view of late finishing PRDC are not distinguishable.
It is not the vaccine
The other potential hypotheses for PRDC associated with Mhp are enhanced transmission post weaning and enhanced sow to pig transmission. In both cases there is clear evidence that these are not the likely problem. It has been demonstrated that PRRS infection does not enhance Mhp transmission in pigs9suggesting that co-factors would be likely to change the transmission rates of Mhp in pig populations. In addition there is clear evidence that conventional killed bacterins do not alter the transmission rates of Mhp in populations6,10 This means that while Mhp bacterin may improve clinical signs from Mhp in pigs11-19, it is does not alter the number of pigs that are infected. While bacterins will remain a valuable tool and serve as proven insurance for losses, they will not solve the root cause of losses or minimize the impact of Mhp on profit.
B) Farm 2: Increasing S/P ratios in gilts after entry leads to increased M. hyopneumoniae transmission in finishing as evidenced by higher S/P in market weight pigs.
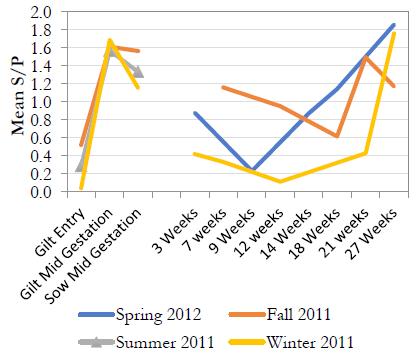
Understanding is the first step
For long term success in Mhp management, understanding infection dynamics within herds is critical. Matching infection status of replacements with the herd (+/+, -/-) is the first step. In infected herds, understanding transmission patterns of Mhp during gilt development is necessary to understand any potential solutions to the problem.
To be successful this means that we need to monitor replacement gilts during the growing period and after they enter the sow herd. There are multiple ways to monitor Mhp infection but all have challenges. Mhp only resides on the lining of the airways deep in the lung. This makes sampling with PCR testing (to look for the organism) very difficult because the samples that are likely to have high levels of Mhp are difficult to obtain.
Oral fluids can be used for PCR testing but because of the low levels and intermittent shedding of Mhp in the upper respiratory tract there are many false negative tests (infected but test negative) with this approach in chronically infected herds making its use challenging. Johnson et al20 reviewed the sensitivity and specificity of antibody testing protocols under field conditions and determined that use of the IDEXX® Mhp ELISA as a screening test produced the most repeatable results under field conditions.
We have applied the ELISA test for monitoring herds for Mhp to understand the timing of infection in gilts and how we can predict growing pig Mhp stability that results from those litters.
Figures 1 and 2 demonstrate that when gilts have increasing S/P ratios on the IDEXX Mhp ELISA test after they enter Mhp positive sow herds, the pigs are more likely to have earlier seroconversion to Mhp in the finishing phase. Seroconversion with this test occurs 3-6 weeks after exposure. This means that early seroconversion is associated with earlier transmission which means more pigs were infected at weaning.
We know that the earlier that Mhp transmission occurs in a finishing population, the greater the economic losses. Based on these facts monitoring gilt antibody levels with the IDEXX Mhp ELISA on a routine basis can be a leading indicator of future Mhp issues in finishing populations. This allows for a proactive management strategy to limit losses. Without this leading information from a routine monitoring program that provides prospective information, it is impossible to understand when the current control program is not working. While it is counterintuitive, monitoring this single population of gilts between 60 and 90 days of gestation every 4-6 months provides the most sensitive and leading indicator of future health challenges due to sow farm instability.
References
1. Fano, E., et al., Effect of Mycoplasma hyopneumoniae colonization at weaning on disease severity in growing pigs. Can J Vet Res, 2007. 71(3): p. 195-200.
2. Grosse Beilage, E., N. Rohde, and J. Krieter, Seroprevalence and risk factors associated with seropositivity in sows from 67 herds in north-west Germany infected with Mycoplasma hyopneumoniae. Prev Vet Med, 2009. 88(4): p. 255-63.
3. Sibila, M., et al., Exploratory field study on Mycoplasma hyopneumoniae infection in suckling pigs. Vet Microbiol, 2007. 121(3-4): p. 352-6.
4. Rautiainen, E. and P. Wallgren, Aspects of the transmission of protection against Mycoplasma hyopneumoniae from sow to offspring. J Vet Med B Infect Dis Vet Public Health, 2001. 48(1): p. 55-65.
5. Sibila, M., et al., Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J, 2009. 181(3): p. 221-31.
6. Pieters, M., et al., An experimental model to evaluate Mycoplasma hyopneumoniae transmission from asymptomatic carriers to unvaccinated and vaccinated sentinel pigs. Can J Vet Res, 2010. 74(2): p. 157-60.
7. Pieters, M., Duration of immunity following infection with M. hyopneumoniae J.F. Lowe, Editor. 2012: St Paul, MN.
8. Wallgren, P., et al., Control of infections with Mycoplasma hyopneumoniae in swine herds by disrupting the chain of infection, disinfection of buildings and strategic medical treatment. Zentralbl Veterinarmed B, 1993. 40(3): p. 157-69.
9. Van Alstine, W.G., G.W. Stevenson, and C.L. Kanitz, Porcine reproductive and respiratory syndrome virus does not exacerbate Mycoplasma hyopneumoniae infection in young pigs. Vet Microbiol, 1996. 49(3-4): p. 297-303.
10. Meyns, T., et al., Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine, 2006. 24(49-50): p. 7081-6.
11. Okada, M., et al., Evaluation of Mycoplasma hyopneumoniae inactivated vaccine in pigs under field conditions. J Vet Med Sci, 1999. 61(10): p. 1131-5.
12. Pallares, F.J., et al., Vaccination against swine enzootic pneumonia in field conditions: effect on clinical, pathological, zootechnical and economic parameters. Vet Res, 2000. 31(6): p. 573-82.
13. Dawson, A., et al., Studies of the field efficacy and safety of a single-dose Mycoplasma hyopneumoniae vaccine for pigs. Vet Rec, 2002. 151(18): p. 535-8.
14. Jensen, C.S., A.K. Ersboll, and J.P. Nielsen, A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev Vet Med, 2002. 54(3): p. 265-78.
15. Siugzdaite, J., K. Garlaite, and D. Urbsiene, Evaluation of antibody formation, daily weight gain and meat quality after vaccination of piglets against Mycoplasma hyopneumoniae Acta Vet Hung, 2003. 51(3): p. 273-81.
16. Moreau, I.A., G.Y. Miller, and P.B. Bahnson, Effects of Mycoplasma hyopneumoniae vaccine on pigs naturally infected with M. hyopneumoniae and porcine reproductive and respiratory syndrome virus. Vaccine, 2004. 22(17-18): p. 2328-33.
17. Baccaro, M.R., et al., Comparative efficacy of two single-dose bacterins in the control of Mycoplasma hyopneumoniae in swine raised under commercial conditions in Brazil. Vet J, 2006. 172(3): p. 526-31.
18. Tzivara, A., et al., Efficacy of an inactivated aqueous vaccine for the control of enzootic pneumonia in pigs infected with Mycoplasma hyopneumoniae. Vet Rec, 2007. 160(7): p. 225-9.
19. Maes, D., et al., Control of Mycoplasma hyopneumoniae infections in pigs. Vet Microbiol, 2008. 126(4): p. 297-309.
20. Johnson, E. et al., Screening for Mycoplasma hyopneumoniae in suspected negative herds: What do we know? Final Project, Executive Veterinary Program, U. of Illinois, 2009.










