C&T













Issue 312 | September 2023
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre
Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979 cve.publications@sydney.edu.au cve.edu.au
Print Post Approval No. 10005007
DIRECTOR
Dr Simone Maher
EDITOR
Lis Churchward elisabeth.churchward@sydney.edu.au
EDITORIAL ASSISTANT
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact:
MARKETING AND SALES MANAGER
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including legal fees).
Established in 1969, this unique veterinary publication celebrates over 50 years of veterinary altruism. An ever-evolving forum gives a ‘voice’ to the profession and everyone interested in animal welfare. You don’t have to be a CVE Member to contribute an article to the C&T Series. Send your submissions to Dr Jo Krockenberger: joanne.krockenberger@sydney.edu.au
"I enjoy reading the C&T more than any other veterinary publication."
Terry King Veterinary Specialist Services, QLDThe C&T Series thrives due to your generosity. If you’re reading this and have been contemplating sending us an article, a reply or comment on a previous C&T, or would like to send us a 'What's YOUR Diagnosis?' image and question or seek feedback from colleagues, please don’t hesitate to contact us.
The C&T is not a peer reviewed journal. We are keen on publishing short pithy practical articles (a simple paragraph is fine) that our readers can immediately relate to and utilise. And the English and grammar do not have to be perfect—our editors will assist with that.
C&T authors agree that it is extremely satisfying to read their articles in print (and the digital versions) and know they are contributing to veterinary knowledge and animal welfare.
It was with some trepidation post covid that the CVE ventured back into the world of face-to-face events, not knowing how enthusiastic people would be to gather in groups. As I write, we are in our busiest period for in-person events, and it has been absolutely fantastic.
There is really nothing like gathering with a group of colleagues, catching up with those you haven’t seen for a while or making new friends, identifying with shared challenges, and connecting across a broad range of experiences. That said, we realise that not everyone has the capacity to attend in-person offerings and we remain committed to making education accessible through virtual programs, our open-access WebinarLIVE! series and of course, the C&T.
In this edition, I was fascinated to read Peter Irwin and John Beadle’s submission on canine ehrlichiosis. For a disease that remained exotic to Australia for so long to now be considered endemic is sobering. This article provides a must-read summary of what to be alert to in both presentation and clinical history.
Speaking of evolving, Imogene Ewen and Sally Coggins’ case study describing the dissolution of an atrial thrombus in a cat using antithrombotic therapy is at the cutting edge of medical treatment.
If dogs and cats aren’t your regular patients, make sure you read John Dooley’s account of treating peritonitis in a dairy cow, or Olivia Clarke’s excellent summary of dental disease in rabbits. There really is something for everyone in this edition!
Happy reading!

t. +61 8 9360 2590 |
e. p.irwin@murdoch.edu.au John Beadle Veterinarian & Practice Owner All Creatures Veterinary Hospital, Broomet. +61 8 9192 8081
C&T No. 5981
Peter has been studying tick-borne diseases of companion animals and wildlife for over 30 years.
In May 2020, in Kununurra, northern Western Australia (WA), dogs with significant fever, malaise, and haemorrhagic diatheses were diagnosed with ehrlichiosis, sparking a veterinary biosecurity alert. Despite its designation as a notifiable disease, with movement restrictions for dogs in northern Australia and the unfolding COVID-19 pandemic during 2020 (that supposedly imposed travel limitations on the dog owning public), CME was soon observed throughout most of northern WA and the Northern Territory. High morbidity and mortalities were reported, especially among dogs at indigenous communities.
Canine monocytic ehrlichiosis (CME) is a tick-transmitted disease of domestic dogs and wild canids caused by Ehrlichia canis (E. canis), a Gram-negative, intracellular bacterium belonging to the family Anaplasmataceae.¹ The organism was first described in dogs in Algeria,² and later came to prominence during the Vietnam War in the 196070’s when scores of military working dogs died of what was then referred to as ‘tropical canine pancytopenia’.³ Today, CME is recognised as a serious illness affecting dogs in tropical and subtropical regions of the world with the exception, until recently, of Australia.
The biological and clinical features of E. canis infection have been studied extensively (outside Australia). The vector is the brown dog tick Rhipicephalus linnaei (the new name for Rhipicephalus sanguineus ‘tropical strain’).⁴ This tick occurs in warm and humid climates worldwide, including northern and central Australia, and is well adapted to survival in dwellings and kennel environments.⁵
Transmission of E. canis to the dog occurs rapidly, within 3 hours of female tick attachment,⁶ and probably even faster from male ticks as they ‘graze’ on the host while
crawling around looking for females.⁷ Clinical signs develop 8-20 days after infection and intracytoplasmic inclusions (morulae) may be observed at this stage in circulating monocytes (Figure 1).⁸
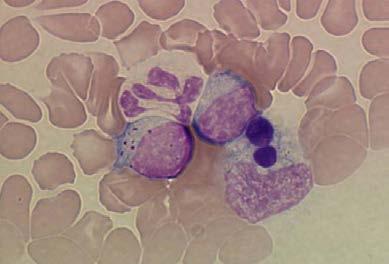
It is known from experimental studies that E. canis infection typically progresses through acute, subclinical, and chronic (terminal) phases;⁸ however, the timing of these stages is variable and unpredictable. Typical clinical signs reported globally include lethargy, weakness, decreased appetite or anorexia, fever, pale membranes, panting, bleeding diathesis, lymphadenomegaly, hepatosplenomegaly, and dehydration.8,9
The clinical picture of CME in Australia seems to have changed over the last three years. Originally, during 2020-2021, as CME established, a severe clinical syndrome was encountered in northern Australia, notably within indigenous communities. Epistaxis was often profound (Figure 2a) and life-threatening, and body temperatures as high as 41.5°C were not uncommon. Some dogs presented with dyspnoea and fever, but no bleeding, and in others cachexia was often rapid in onset. Dogs with pancytopenia rapidly progressed to sepsis and uncontrollable bleeding within a few days, and some dogs presented with blindness due to retinal haemorrhages. Another group developed generalised dependant oedema months after the acute illness, characterised by marked swelling of the face, neck and limbs (Figure 2b) and occasionally the scrotum (Figure 2c)
Now, three years on, observations by vets diagnosing CME describe a more nuanced clinical picture in endemic areas, although the severe signs persist sporadically. This is more in line with reports from overseas where CME
has achieved a level of endemic stability. Vets working in the north are reporting that some dogs which are clinically normal (therefore no suspicion of CME) develop complications during and after surgery, characterised by persistent oozing and haemorrhage. Ehrlichiosis causes mild to moderate coagulopathy through a range of mechanisms, and this is compounded by coinfection with other tick-borne diseases such as babesiosis and A. platys co-infections.

In veterinary science we refer to some diseases as ‘great pretenders’, canine hypoadrenocorticism for example, since their clinical diversity may challenge our diagnostic reasoning. Ehrlichiosis is another example—mimicking (and causing) a wide range of inflammatory and noninflammatory syndromes. CME is associated with much immune dysregulation in its own right, and can present with pyrexia of unknown origin, or signs consistent with IMPA, IMHA, ITP, and meningitis. Such presentations may not bring CME to mind immediately, especially if you work in areas outside the brown dog tick range, but the suspicion for ehrlichiosis should be increased in a patient with any of the following history:
If your practice is located within the enzootic range of the brown dog tick,
If ticks have been seen on the dog, especially in recent weeks or months. The culprit is the brown dog tick, not paralysis ticks, so further questioning and local knowledge is important here, If there is any question about the owner’s compliance with regard to tick prophylaxis, If the dog receives solely an isoxazoline product such as Nexgard, Credelio, Simparica or Bravecto. Whilst these drugs provide excellent protection against ticks generally, their mode of action is systemic. The tick needs to feed for a while in order to ingest sufficient active agent to be killed. This is too slow to protect against transmission of E. canis, which can occur in less than 3 hours (see later),
If the dog has travel history from central or northern Australia,
If the dog has been newly acquired and travel history is unknown. This is especially true for pups and dogs that have been rehomed. There are cases of CME reported in southern Australia in just this situation after it was discovered that the dogs had come from up north.
Diagnosis of CME is predicated on all-important clinical suspicion, so it’s the signs and history that should first alert the clinician. Most dogs will have one or more of these clinical signs:
Pyrexia (often >40°C in acute stage)
Lethargy and malaise
Hypo-or anorexia
Unexplained weight loss to cachexia
Ocular signs (corneal oedema, conjunctivitis)
Dry eye
Blindness
Lymph node enlargement
Petechiae
Epistaxis
Dyspnoea and coughing
Neurological signs
Diarrhoea
Persistent infections (and sepsis)
Oedema (limb, generalised or scrotal)

Abdominal distension (ascites)
Hepatosplenomegaly
Full bloodwork, including C-reactive protein (CRP), and urinalysis should be performed as screening tests. One or more abnormalities usually exist in CME, but confirmation of the diagnosis requires PCR and/or serology. The disease is notifiable (this might change) so local veterinary authorities should be advised and testing is performed in state and territory laboratories. Check their website for instructions on sample submission.
There is a pressing need for a reliable in-clinic test for ehrlichiosis in Australia. The notifiable status of CME currently mandates formal testing at regional government labs; however, we need rapid, point-of-care test(s) for suspected cases and prior to surgery in endemic areas, for example. Vets have been using a number of commercially available kits, with variable results, but none has yet been tested rigorously against a gold standard (e.g. IFAT) for the Australian strain of E. canis (see later).
A wide range of laboratory abnormalities have been reported including:
Haematology
May be normal
Thrombocytopenia
Anaemia – regenerative or non-regenerative
Inflammatory leucogram
Monocytosis
Or bi- or pancytopenia
Biochemistry
May be normal
Increased globulins (polyclonal, rarely a ‘monoclonal spike’)
Albumin normal or decreased
CRP elevation (often very high)
Liver enzyme activities increased
Pre-renal azotaemia
SDMA elevation
In addition, case reports and case series overseas associate CME with various organ pathologies and clinical syndromes, including interstitial pneumonia and pulmonary hypertension,10 hepatitis,11 meningomyelitis,12 and increased risk of developing chronic kidney disease.13
Doxycycline is the antimicrobial of choice for CME at a dose rate of 10mg/kg/day for 28 days (this can be given divided or as a single dose). There are at least 11 studies behind this ACVIM Consensus Statement recommendation from over 20 years ago.14 Evidence for its efficacy comes from in vitro studies that indicate E. canis is highly susceptible to doxycycline, requiring a low minimum inhibitory concentration (MIC) (0.03µg/mL).15
There is usually a good clinical response to doxycycline in the acute phase; however, some dogs remain bacteraemic (infectious to ticks) for unpredictably variable periods of time during the course of antimicrobial drug treatment, and some after cessation of doxycycline.16,17 Unfortunately, therefore, clinical resolution does not always equate to ‘cure’, i.e. bacterial sterilisation, and this should be remembered if dogs later develop clinical illness that could be attributable to recrudescence of ehrlichiosis.
Unfortunately not many. Different tetracycline formulations should be effective (including minocycline, oxytet) but there is less consensus about dose frequency and duration.18 Rifampicin, an ansamycin antibiotic that is used to treat mycobacterial diseases in humans, is also efficacious against E. canis in vitro, but should not be considered first line due to our responsibilities with respect to antimicrobial stewardship and because of the need to monitor liver enzyme activity during therapy. Imidocarb dipropionate (a treatment for babesiosis) was originally used to treat CME; however, recent studies have indicated this drug is not efficacious for CME.18
In practical terms there is little we can do as vets to determine the success, or otherwise, of antibiotic treatment. The sensitivity of PCR detection is low in the subclinical phase and whilst monitoring of serial antibody titres would help, there are currently no labs in Australia that provide end-point titre measurements. Furthermore, antibody levels for E. canis remain elevated for protracted periods after infection, so gaining the owners cooperation for repeated testing would most likely be challenging!
Other treatments involve managing complications and other pathological effects of E. canis infections, especially in the ‘chronic’ phase. The main considerations are:
Blood loss anaemia requiring blood transfusion(s), Sepsis as a component of bone marrow failure requiring parenteral antibiotics based on blood culture and antimicrobial sensitivity testing, Pancytopenia due to aplastic anaemia/bone marrow aplasia requiring blood transfusion(s).
Severe epistaxis, in particular, can be challenging to manage. While most cases will cease spontaneously, eventually, treatments that have been suggested including sedation, physical packing the external nares with absorbent material, whole blood transfusions (or platelet transfusions, if available), tranexamic acid, and
DDAVP, which enhances platelet function by increasing serum levels of von Willebrand factor.18
Until the arrival of CME, the most serious tick-associated disorder afflicting dogs in Australia was tick paralysis, yet in comparison with the rapid transmission of E. canis from brown dog ticks described above,⁶ the neurotoxins injected by I. holocyclus do not take effect until 3-4 days after tick attachment. Understanding this difference in timing is crucial with respect to the use of acaricides to protect against CME.
Topically-acting synthetic pyrethroids (e.g. flumethrin) are a class of acaricides that exert their protective effect through repellency (and kill), preventing tick attachment, and have been shown to protect dogs against transmission of E. canis. 19 Seresto® collars contain flumethrin in addition to imidacloprid and are now have a registration claim in Australia to reduce the risk of transmission of E. canis infection.
The other major class of acaricides currently in use in companion animal practice, the isoxazolines, act systemically, requiring ticks to feed in order to work. This prevents tick paralysis but does not protect dogs from acquiring CME,20 although isoxazoline products stop onward transmission of E. canis from an infected dog, and have proven a valuable adjunctive treatment to reduce tick burdens where dogs are housed.
Ehrlichia canis is related to several members of the Anaplasmataceae that are recognised to be zoonotic, including E. chaffeensis, E. ewingii and E. muris eauclairensis, and there is evidence for the zoonotic potential of E. canis from molecular and serological testing in Central and South America.21 However, there are no reports of infection in humans that implicate the Asian/Taiwan genogroup of E. canis that has been detected in Australia.22 It therefore seems unlikely that E. canis will be of public health concern in Australia.
It is fortunate and, indeed, somewhat surprising, that Australia remained free of CME for so long, considering the abundant population of brown dog ticks across the north. Mandatory pre-import screening (comprising IFAT) was established in September 1995, and dogs testing positive were prohibited entry into the country.
Regardless, current surveillance data indicate that infected dogs (or ticks) have been detected in most states and territories, and the disease is now (2023) regarded as endemic. There is no hope of eradication,
so veterinarians must add CME to differential diagnosis of canine illnesses in northern Australia and in travelled dogs, and start prompt treatment when cases are diagnosed. Canine ehrlichiosis is, unfortunately, here to stay.
The authors declare no conflicts of interest.
This research did not receive any specific funding.
1. Rar V and Golovljova I (2011) Anaplasma, Ehrlichia , and ‘‘ Candidatus neoehrlichia ’’ bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 11: 1842–1861. https://doi:10.1016/j.meegid.2011.09.019
2. Donatien, A., Lestoguard, F. (1935) Existence en Algerie d’une Rickettsia du chien. Bull. Soc. Pathol. Exot. 28: 418–419.
3. Nims, R.M., Ferguson, J.A., Walker, J.L. et al. (1971) Epizootiology of tropical canine pancytopenia in Southeast Asia. J. Am. Vet. Med. Assoc. 158: 53-63.
4. Slapeta, J., Chandra, S. and Halliday, B. (2021) The ‘‘tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). Int. J. Parasitol 51: 431-436. https://doi.org/10.1016/j.ijpara.2021.02.001
5. Dantas-Torres, F. (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vect. 3: 26.
6. Fourie JJ, Stanneck D, Luus HG, et al. (2013) Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Veterinary Parasitology 197: 595-603. http://dx.doi.org/10.1016/j.vetpar.2013.07.026
7. Bremer WG, Schaefer JJ, Wagner ER, et al. (2005) Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus Veterinary Parasitology 131: 95-105. doi:10.1016/j.vetpar.2005.04.030.
8. Harrus, S. (2015) Perspectives on the pathogenesis and treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet. J. 204: 239-240.
9. Harrus S, Kass PH, Klement E, et al. (1997) Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation f prognostic indicators for the disease. The Veterinary Record 141: 360-363.
10. Toom ML, Dobak TP, Broens EM et al. (2016) Interstitial pneumonia and pulmonary hypertension associated with suspected ehrlichiosis in a dog. Acta Vet Scandinavia 58:46
11. Mylonakis ME, Kritsepi-Konstantinou M, Dumler JS et al. (2010) Severe
hepatitis associated with acute Ehrlichia canis infection in a dog. J. Vet. Int. Med. 24: 633-638.
12. Frankar H, Le Boedec K, Beurlet S et al. (2023) Suppurative spinal meningomyelitis in a dog with intra-neutrophilic cerebrospinal fluid cells Ehrlichia canis morulae. Vet. Clin. Path. 52: 119-122.
13. Burton W, Drake C, Ogeer J et al. (2020) Association between exposure to Ehrlichia spp. and risk of developing chronic kidney disease in dogs. J Am Anim Hosp Assoc 56: 159-164.
14. Neer TM, Breitschwerdt EB, Greene RT, et al. (2002) Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM: American College of Veterinary Internal Medicine. Journal of Veterinary Internal Medicine 16: 309–315.
15. Branger S, Rolain JM and Raoult D (2004) Evaluation of Antibiotic Susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis , and A naplasma phagocytophilum by Real-Time PCR. Antimicrobial Agents and Chemotherapy 48: 4822-4828.
https://doi.org/10.1128/AAC.48.12.4822-4828.2004
16. Harrus S, Waner T, Aizenberg I, et al. (1998) Therapeutic effect of doxycycline in experimental subclinical canine monocytic ehrlichiosis: Evaluation of a 6-week course. Journal of Clinical Microbiology 36: 2140-2142.
17. Schaefer JJ, Needham GR, Bremer WG, et al. (2007) Tick Acquisition of Ehrlichia canis from Dogs Treated with Doxycycline Hyclate. Antimicrobial agents and Chemotherapy 51: 3394-3396.
18. Mylonakis ME, Harrus S and Breitschwerdt EB (2019) An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis) The Veterinary Journal 246: 45-53.
https://doi.org/10.1016/j.tvjl.2019.01.015
19. Stanneck D, Fourie JF (2013) Imidacloprid 10 % / Flumethrin 4.5 % Collars(Seresto®, Bayer) Successfully Prevent Long-Term Transmission of Ehrlichia canis by Infected Rhipicephalus sanguineus Ticks to Dogs. Parasitology Research 112: S21-S31. DOI 10.1007/s00436-013-3278-6.
20. Burgio F, Meyer L and Armstrong R (2016) A comparative laboratory trial evaluating the immediate efficacy of fluralaner, afoxolaner, sarolaner and imidacloprid + permethrin against adult Rhipicephalus sanguineus (sensu lato) ticks attached to dogs. Parasites & Vectors 9: 626. https://DOI 10.1186/s13071-016-1900-z
21. Unver A, Perez M, Orellana N, et al. (2001) Molecular and Antigenic Comparison of Ehrlichia canis Isolates from Dogs, Ticks, and a Human in Venezuela. Journal of Clinical Microbiology 38: 2788–2793.
22. Neave MJ, Mileto P, Joseph A, et al. (2022) Comparative genomic analysis of the first Ehrlichia canis detections in Australia. Ticks and Tick-borne Diseases 13: 101900. https://doi.org/10.1016/j.ttbdis.2022.101909
 Team Leader
Elizabeth Macarthur Agricultural Institute
Team Leader
Elizabeth Macarthur Agricultural Institute
Menangle NSW
e. mark.westman@dpi.nsw.gov.au
We have had 3 E. canis PCR-positive samples since the 2020 case we wrote up in the C&T—Billy Leso (so 4 dogs in total). Billy had recently been adopted by new owners on the South Coast after spending a few weeks with a foster carer in Sydney following relocation from the Northern Territory (NT). All 4 dogs had recently been rehomed to NSW from the NT.
Note that I am NOT including E. canis antibody-positive results here as I would not rely on solely serology for diagnosis.
If possible, ticks should also be collected from the affected dog, placed in a small container (e.g. a urine specimen jar or a plain tube) with 2 mL of 70% ethanol and submitted to the state veterinary laboratory with the collected blood samples (≥ 1 mL serum and ≥ 2 mL EDTA blood) for tick identification and E. canis testing (serology and PCR testing). If ethanol is not available, submitting ticks dry will also suffice.
Interestingly, we have had 5 A. platys positive samples since 2020! All dogs were recently rehomed from the far north QLD or SA.
C&T Download Mark Westman's article
‘Billy’ – The First Case of Ehrlichia canis Diagnosed In New South Wales, C&T No. 5897, Issue 304 Sept 2021.
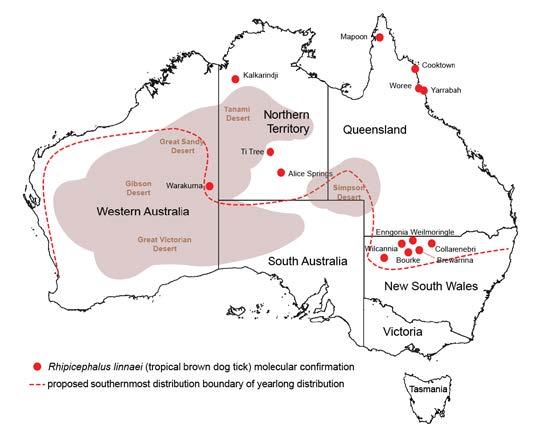
We often get asked what is the real distribution of the brown dog tick in Australia?
In Australia, only the tropical species of brown dog tick, Rhipicephalus linnaei, is found. The temperate species, Rhipicaphalus sanguineus, is not present in the country. Our current best estimate of the distribution of brown dog ticks in Australia can be seen in the figure provided (Chandra et al. 2020, 2021; Šlapeta et al. 2021). While we have observed brown dog ticks in places like Sydney, they have only been found on dogs that have been relocated from areas above the line on the figure. We have yet to confirm the sustained breeding of brown dog ticks yearround in the cooler southern regions of Australia.
The reason for the name change is that it has been discussed for over a decade, and there was a consensus that a split was necessary. This is because the tropical and temperate lineages behave differently and appear to have different tendencies for transmitting diseases. As a result, instead of using informal names, we now have two formal scientific names.
In Australia, the common name for the brown dog tick can still be used, but there is another commonly used name—the kennel tick. This name is actually very convenient because it indicates the tick’s life history. It informs us about the association of the tick with dog dwellings, such as kennels. These ticks love to breed in kennels, where they can establish their population, often in the hundreds or thousands.
References
S Chandra, GC Ma, A Burleigh, G Brown, JM Norris, MP Ward, D Emery, J Šlapeta (2020). The brown dog tick Rhipicephalus sanguineus sensu Roberts, 1965 across Australia: Morphological and molecular identification of R. sanguineus sl tropical lineage. Ticks and tick-borne diseases 11 (1), 101305
S Chandra, B Halliday, J Šlapeta (2021) Museum material of Rhipicephalus sanguineus sensu Roberts (1965) collected in 1902–1964 from Australia is identical to R. sanguineus sensu lato tropical lineage at the mitochondrial DNA 12S rRNA level. Medical and Veterinary Entomology 35 (3), 315-323
J Šlapeta, S Chandra, B Halliday (2021) The “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). I nternational Journal for Parasitology 51 (6), 431-436

If you’re reading the Control & Therapy Series for the first time, welcome. The C&T Series was established in April 1969 as a veterinary forum by the CVE’s first Director, the legendary Dr Tom Hungerford OBE BVSc FACVSc HAD. Tom wanted to get the clinicians writing.
…not the academic correctitudes, not the theoretical niceties, not the super correct platitudes that have passed the panel of review… not what he/she should have done, BUT WHAT HE/SHE DID, right or wrong, the full detail, revealing the actual “blood and dung and guts” of real practice as it happened, when tired, at night, in the rain in the paddock, poor lighting, no other vet to help
To date, 5,980 and 158 practical and relevant C&T and Perspective articles have been published in this unique forum. Authors receive no financial recompense; rather, they contribute articles to share their experiences with their peers, add to practical veterinary knowledge and improve animal welfare everywhere. (And get to enjoy reading their article in print!)
Now every one of you who has done anything practical that’s not easily obtainable in all the common textbooks, write it up.
– Tom HungerfordIt doesn’t have to be long – some of the best articles have been a mere paragraph or two. Please include figures, tables, high resolution images for print and videos for the eBook version if you have them and send to: cve.marketing@sydney.edu.au. Our veterinary editors review all submissions. Rest assured, if they have any questions or concerns, you will be contacted before publication. Join us!
…it's still the best publication by far for the practical vet out there… Every article is worth a read.
– Pete Coleshaw, Veterinarian, UKThe digital Control & Therapy (C&T ) Series is now OPEN ACCESS to the Veterinary Profession Worldwide from September 2023



North
Nowra Veterinary Hospitale. sammysingo@gmail.com

C&T No. 5982
Opal has diffuse idiopathic skeletal hyperostosis (DISH). This can cause limited spinal range of motion, nerve impingement leading to spinal pain and pain on the legs. The nerve impingement can also cause muscle weakness, paresis, and changes in spinal reflexes.
If there is lack of movement there may be muscle atrophy (plus possibly some neurogenic atrophy), plus chronic pain may lead to Opal looking and acting older than 7-years-old.
Moira van Dorsselaer BVSc
The Cat Clinic Hobart, New Town TAS
e. moira@catvethobart.com.au
t. +613 6227 8000
Opal was diagnosed with diffuse idiopathic skeletal hyperostosis (DISH) after discussion with Dr Richard Malik, as I had never actually seen a case like this before.
DISH is a systemic non-inflammatory disorder of the axial and peripheral skeleton that has also been described in humans and dogs.
DISH results in the ossification of soft tissues including spinal ventral longitudinal ligaments and sites of attachment of tendons and capsules to bone. In dogs and humans the prevalence increases with age.
In the Veterinary Quarterly 33:1, 30-42 (2013) ‘Spinal hyperostosis in humans and companion animals’ stated no studies describing DISH in cats have been published.
The Journal of Small Animal Practice (2016) 57 3335 published the article ‘Diffuse idiopathic skeletal hyperostosis of the spine in a nine-year-old cat’ which described a case of DISH in a nine-year-old intact female domestic shorthair cat and to the authors’ knowledge was the first published report of feline diffuse idiopathic skeletal hyperostosis.
Vet Record Case Reports (07 June 2021) published the article ‘Radiographic and MRI characteristics of diffuse idiopathic skeletal hyperostosis in a cat presented with a painful chronic ambulatory paraparesis’ which described a case of DISH in a nine-year-old male neutered Maine Coon and to the authors’ knowledge, this is the first case report describing the radiographic and MRI characteristics of DISH in a feline patient.
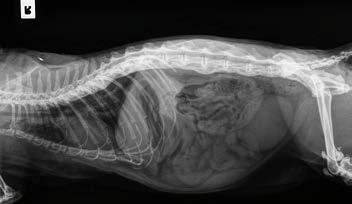
Clearly—the skeletal changes in this cat ARE SEVERE. There is every possibility that there is intervertebral disc protrusion adding to the cat’s disability by compressing the spinal cord or nerve roots exiting intervertebral foramina. The only way to determine this is by advanced imaging, CT, a CT myelogram, or an MRI scan (ideally all three!). The problem of course is we do not know what causes DISH in cats or dogs, and we cannot treat the underlying problem, so even if there is a discrete surgical lesion—can we be sure fixing it will help the cat? And the imaging would likely cost $5,000 and surgery might cost another 5-10 thousand dollars. A lot of money to pay with an uncertain prognosis.
Due to finances CT scan was not an option for Opal’s owners and we treated Opal with a single dose of Solenisa™ via subcutaneous injection to which she responded well initially. Her owners also continued with oral meloxicam daily. Sadly Opal was recently euthanised due to progressive worsening of her clinical signs, poor response to ongoing treatment and her owners not being able to cope with knowing that Opal might be suffering and in pain.
Thanks to all our readers who answered the ‘What’s Your Diagnosis?’ question in the last issue. Congratulations to Samantha who won the $100 CVE credit for the best answer!
Read
Authors’ views are not necessarily those of the CVE
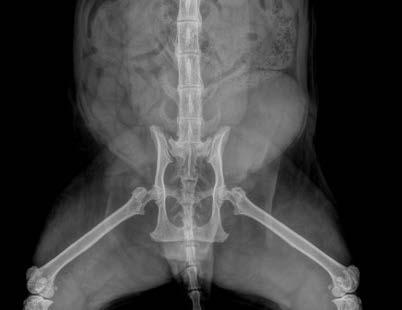
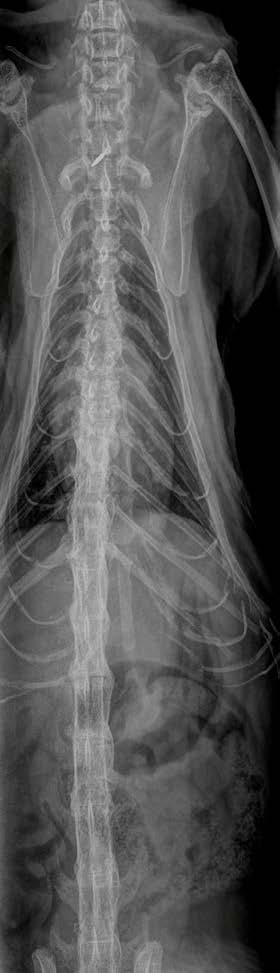
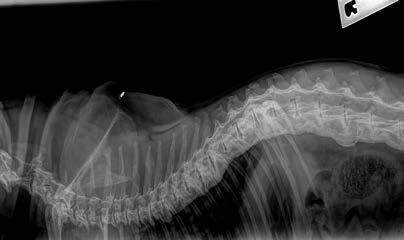
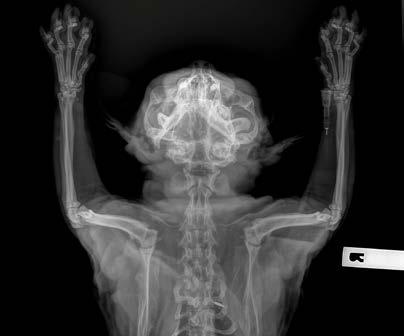 Figure 2. Dorsoventral forelimb and cervical spine
Figure 3. Lateral thoracic spine
Figure 4. Ventrodorsal hips
Figure 2. Dorsoventral forelimb and cervical spine
Figure 3. Lateral thoracic spine
Figure 4. Ventrodorsal hips
The prize is a CVE$400 voucher
Samantha Coomes BVSc
Marina Worden BVSc
4 Paws Veterinarians, Townsville

t. (07) 4723 1988 | e. charlesstreetvet@hotmail.com
C&T No. 5983
James Cook University, Townsville
Veterinary Pathology Resident
Bullet is a 7-year-old male Bull Arab x Border collie crossbreed. He was adopted by our client (who is one of our nurses) at 10-months-old. Castration was performed at 10-months-of-age through the rescue organisation, prior to the client adopting Bullet. Therefore, we are unsure if it was routine or cryptorchid castration. Bullet has previously been treated for heartworm infection but is currently up to date with all preventative care.
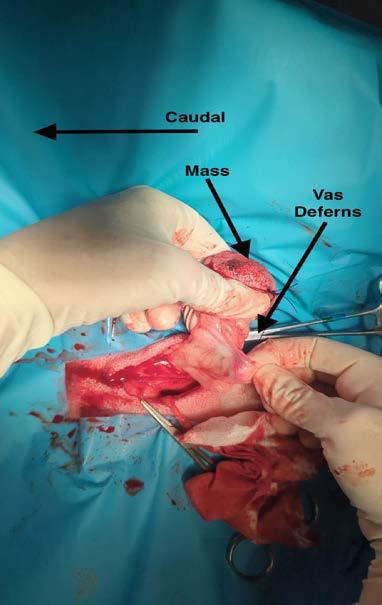
Bullet developed a mass where his scrotum would be if he were an entire male dog. The lump was noted during a bath, and was absent at vaccination two months prior. The lump remained stable for the two weeks between noticing the lump and removal. There were no other abnormalities noted at home or during physical examination.
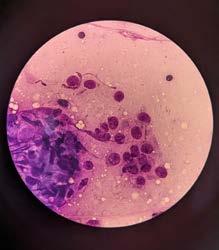
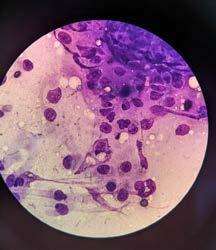
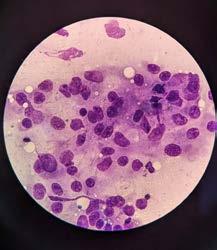
An FNA of the lump was performed during consult. After consulting with Alinta, it was determined the FNA contained germinal epithelial cells. I could be forgiven for thinking the cells were possibly spindle cells, due to the morphology of a few singular cells, or agranular round cells. Germinal epithelial cells behave like epithelial cells forming cohesive clusters of cells, whereas spindle cells routinely do not. The granular cytoplasm of germinal epithelial cells could also be mistaken for mast cells.
A lumpectomy was performed the following week. The lumpectomy was essentially a scrotal ablation, with removal of the lump through the scrotal ablation site. Care was taken not to separate the lump from the surrounding tissue. The vas deferens was located, attached to the distal edge of the lump. The vas deferens and tunica were ligated as deep as possible. The lump was submitted for histopathology.
Quoted directly from the report provided by Drs Alinta Kalns and Linda Hayes:
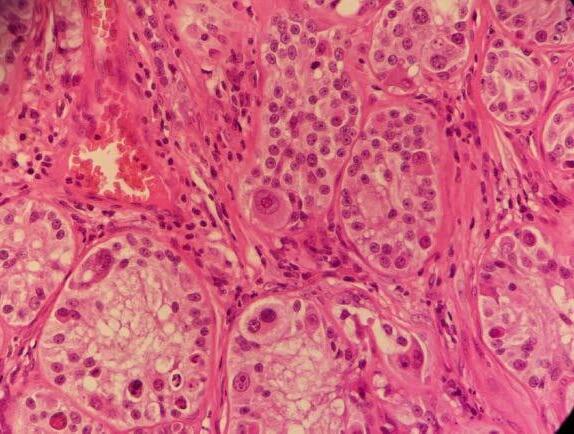
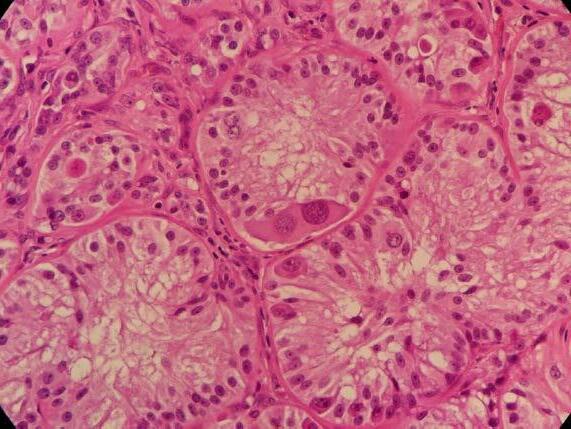
‘Scrotum: Expanding the subcutis of the scrotum, adjacent to a large vascular plexus, is an encapsulated, well demarcated, densely cellular neoplasm composed of lobules and tubules of polygonal to columnar cells arranged perpendicularly to the basement membrane and supported by a delicate to moderate fibrous
stroma. These cells exhibit moderate anisocytosis and anisokaryosis, have indistinct cell borders, and low to ample amounts of eosinophilic, granular cytoplasm that is frequently vacuolated. Nuclei are oval to round with finely stippled to vesicular chromatin and one or more prominent, eosinophilic nucleoli. Infrequent karyomegaly and binucleate cells as well as rare multinucleated cells are observed. Mitoses range from zero to two per HPF, averaging 0.7 per HPF. Frequently, cells exhibit hypereosinophilia and pyknosis.
Within one section, three separate islands of neoplastic cells expand the stroma away from the main neoplasm. Multifocally scattered throughout the neoplasm are mild to moderate perivascular infiltrates of lymphocytes and plasma cells.
In one section, the stroma between the mass and the overlying dermis, contains a poorly defined area of tortuous, variably sized clear spaces lined by flattened to mildly plump, simple squamous epithelial cells (endothelium). No mitotic figures are observed in examination of ten HPFs’.
Sertoli Cell Tumour
Secondary mass within the wall of scrotum—suspected to be an area of lymphangiomatosis.
Comments
With an absence of identifiable normal testicular tissue, the Sertoli cell tumour is likely to have arisen from a congenital or traumatic remnant of testicular tissue within the spermatic cord or scrotum. Thus, this case represents one of the rare circumstances in which this can occur and the challenges of diagnosing it as a primary clinician using in-house diagnostic techniques.
A case report of 17 castrated animals with sertoli cell tumours (Doxsee, A., Yager, J., Best, S., & Foster, R. (2006, August)) suggests ectopic testicular tissue, polyorchidism and transplantation of testicular tissue during castration as aetiologies. The median time to development of a Sertoli cell tumour was 8 years.
The second lesion, lymphangiomatosis, is a rare, benign, circulatory disturbance and/or developmental anomaly of lymphatic vessels. These are poorly circumscribed masses that can recur due to their irregular infiltrative nature. Differentials include a bloodless haemangioma or well-differentiated lymphosarcoma (however no mitotic figures were observed in ten HPFs).
Darby W. Walmsley BVSc MANZCVS (SAS) AFHEA Fcert (E&CC)
Animal Welfare League Brisbane, Queensland e. darby.walmsley@gmail.com
Adrian Wallace BVSc (Hons) MANZCVS DipECVS
Geelong Animal Referral Services, Newton Victoria e. hospital@garsvets.com.au
C&T No. 5984
An 8-year-old, entire male Jack Russell Terrier (9 kg) was referred for evaluation of a right-sided perineal swelling that had been present for approximately 3-months. The patient developed acute lethargy, pain, stranguria and tenesmus 3-days prior to presentation. Physical examination identified an extensive, right-sided perineal hernia (PH).
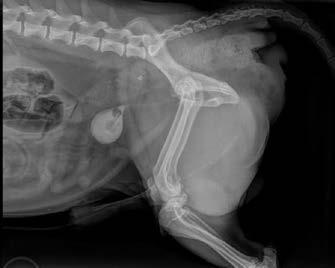
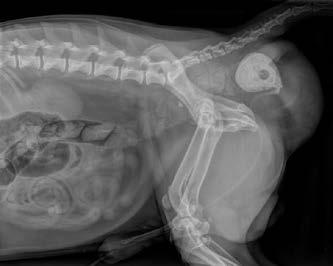
Digital rectal examination confirmed right-sided rectal sacculation with accumulation of faeces. Subjectively, the left pelvic diaphragm was intact. The remainder of the physical examination and blood testing was unremarkable.
Ultrasonography of the right perineal swelling revealed a fluid-filled object, suspected to be the urinary bladder, herniated through the pelvic diaphragm. Faecal matter was also apparent on ultrasound. Orthogonal survey abdominal radiography was performed. A urethral catheter was placed, and positive contrast cystography performed by instilling 5mL of contrast media (Urografin®; Bayer Australia Ltd, Pymble NSW, 2073, Australia) into the urinary catheter. Repeat orthogonal radiography confirmed urinary bladder retroflexion (Figure 1a). The bladder was then manually reduced into an appropriate anatomical position (Figure 1b). Due to the severity of the deficit, the bladder was unable to remain
in this position and returned into a retroflexed position despite multiple attempts at reduction.
The patient was pre-medicated with methadone (0.2 mg/ kg IV). Anaesthesia was induced with propofol (4 mg/kg IV; Lipuro 1%, Braun Australia Pty Ltd, Bella Vista NSW, Australia) and maintained with isoflurane (Isoflo®, Zoetis Australia Pty Ltd, Rhodes NSW, Australia) in oxygen. The abdomen and perineal regions were clipped and prepared with chlorhexidine and an alcohol solution, and the patient initially positioned in dorsal recumbency.
A fentanyl constant rate infusion (10 µg/kg/hr IV; Sublimaze®, Janssen-Cilag Pty Ltd, Macquarie Park NSW, 2113, Australia) was administered intra-operatively.
A midline coeliotomy with parapreputial extension was performed. The bladder was manually reduced into the
abdomen. A cystopexy was performed via seromuscular incision in the bladder apex, apposed to an incision in right body wall with 2 simple continuous suture lines (3/0 Polydioxanone). A colopexy was also performed by seromuscular incision in the colon apposed to an incision in the left body wall with 2 simple continuous suture lines. Omentum was then wrapped around the colopexy site and secured with a cruciate suture.
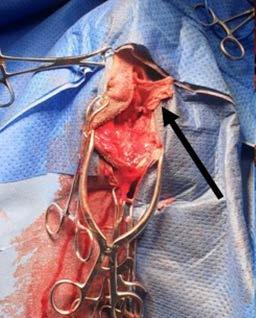
A 5 cm x 5 cm section of rectus abdominis aponeurosis was harvested from the right side of the abdomen (Figure 2) and placed within a moistened swab. The donor site was chosen to leave healthy rectus sheath immediately adjacent to midline to facilitate midline closure. No attempt was made to close the donor site.

At time of abdominal exploration, the prostate appeared enlarged, irregular, and cystic. A prostatic biopsy was taken from the left side of prostate (with histopathology later returning as benign prostatic hyperplasia). The abdomen was then copiously lavaged with sterile saline and routine midline closure was performed with 2/0 Polydioxanone in a simple continuous pattern engaging the external leaf of the rectus sheath. The subcutaneous tissue was closed with 4/0 Poliglecaprone 25 in a simple continuous suture pattern. The skin was closed with staples. Open castration was performed via a pre-scrotal incision. Vas deferens and vascular pedicle were ligated (simple ligatures) separately with 3/0 Polydioxanone and subsequently transected. The subcutaneous tissue was closed with 4/0 Poliglecaprone 25 in a simple continuous suture pattern. The skin was closed with staples.
The patient was then repositioned in sternal recumbency in a perineal stand. A paramedian approach to the right ischiorectal fossa was performed. Omental fat was immediately apparent and was bunch ligated with 3/0 Polydioxanone, and subsequently resected. Significant atrophy of both the levator ani and coccygeus muscles was apparent. Chronic bladder herniation had resulted in a large deficit between the external anal sphincter, coccygeus and levator ani muscles. An internal obturator transposition was performed with transection of the tendon of the internal obturator to facilitate increased transposition distance. The internal obturator was apposed to the external anal sphincter, coccygeus and levator ani muscles with 2/0 Polypropylene in a simple interrupted pattern (preplaced). However, a significant deficit remained dorsally following internal obturator transposition. The rectus sheath graft was apposed to the sacrotuberous ligament, the internal obturator, external anal sphincter and subcutaneous tissue dorsally with 2/0 Polypropylene in a simple interrupted pattern (Figure 3) ensuring appropriate longitudinal placement of the graft in the region of maximal tension. The subcutaneous tissue was closed with 4/0 Poliglecaprone 25 in a simple continuous pattern. The skin was closed with 4/0 Poliglecaprone 25 in an intradermal pattern.
The patient received intra-and post-operative cefazolin (22 mg/kg IV; Cefazolin-AFT, AFT Pharmaceuticals, North Ryde, NSW, Australia) and fentanyl CRI (10 µg/kg/hr IV), post-operative meloxicam (0.1 mg/kg subcutaneously; Meloxicam, Ilium, Troy Laboratories Australia Pty Ltd, Australia) and a transdermal fentanyl patch (50 µq/h; Durogesic® 50, Janssen-Cilag Pty Ltd, Macquarie Park NSW, Australia).
The patient presented at both 2- and 12- weeks postoperatively for re-evaluation. Physical examination at 2-weeks revealed some mild post-operative swelling around the right perineal region, which was no longer apparent at 12-weeks post-operatively. The remainder of the physical examination at both time points were unremarkable. Rectal examination at both time points was unremarkable. A telephone update was provided at 6 and 12 months postoperatively by the client. According to the client, the patient was clinically healthy, and displayed no signs of hernia recurrence or associated clinical signs, including tenesmus, dysuria, or perineal swelling.
PH is a common surgical condition of intact males and is likely the result of multifactorial influences.1 The proposed causes of PH include muscular atrophy,1-3 hormonal imbalances and gender variances,4 and prostatic disease.5-7 Although the understanding of the
aetiology is incomplete and ongoing, the disease process is characterised by weakening and atrophy of muscles of the pelvic diaphragm, including the levator ani and/or coccygeus muscles, resulting in the herniation of intrapelvic and/or intra-abdominal structures.1-3 The most common clinical signs ascribed to perineal herniation may include a non-painful swelling lateral to the anus, constipation, obstipation, dyschezia, tenesmus, rectal prolapse, stranguria, anuria, vomiting, and/or faecal incontinence.¹
Internal obturator muscle transposition is now considered the gold standard technique for treatment of dogs with PH.1,8 However, some surgeons advocate the use of adjunctive techniques for reinforcement of the repair if there are concerns about the severity, size, and chronicity of the hernia. A major advantage of the use of rectus abdominis aponeurosis is the low donor and recipient-site morbidity comparatively to previously described techniques when adjuvant reinforcement is required, as well as being biomechanically sound with resistance to significant tensile forces. It is in the authors’ recommendation, however, that fascia implantation should be complemented with internal obturator muscle transposition to decrease graft strain and may improve survivability. Although graft rejection was not observed clinically in the post-operative period, without further advanced imaging, the authors are unable to comment on graft survival long-term.
The use of synthetic substances, such as polypropylene mesh, has the inherent risk of suture sinuses/infection and other mechanism of failure, which require implant removal. Some surgeons therefore advocate the use of autogenous material where possible, with synthetic material reserved for salvage procedures. We were able to hypothesise that the use of an autogenous graft would likely reduce the risk of implant related complications, including infection.
1. Gill SS, Barstad RD. A Review of the Surgical Management of Perineal Hernias in Dogs. J Am Anim Hosp Assoc 2018; 54:179–187. DOI 10.5326/JAAHA-MS-6490
2. Desai R. An anatomic study of the canine male and female pelvic diaphragm and the effect of testosterone on the status of levator ani of male dogs. J Am Anim Hosp Assoc 1982;18:195–202.
3. Seim HB. Surgical management of perineal hernia. Proc North Am Vet Conference 2009;1571–3.
4. Hayes HM, Wilson GP. Hormone-dependent neoplasms of the canine perianal gland. Cancer Res 1977;37(7 Pt 1):2068–71.
5. Krahwinkel DJ. Rectal diseases and their role in perineal hernia. Vet Surg 1983;12:160–5.
6. Hosgood G, Hedlund CS, Pechman RD, et al. Perineal herniorrhaphy: per- ioperative data from 100 dogs. J Am Anim Hosp Assoc 1995;31(4):331–42.
7. Head LL, Francis DA. Mineralized paraprostatic cyst as a potential contributing factor in the development of perineal hernias in a dog. J Am Vet Med Assoc 2002;221:533–5.
8. Hardie EM, Kolota RJ, Earley TD, et al . Evaluation of internal obturator muscle transposition in treatment of perineal hernia in dogs. Vet Surg 1983;12(2):69–72.
Authors’ views are not necessarily those of the CVE
Gordon Veterinary Hospital, Pymble, NSW 2073
t. 02 9498 3000
e. info@gordonvet.com.au
C&T No. 5985
Dusty is an 11-year-old male neutered Domestic Long Hair cat (5.9 kg), who was acquired as a kitten. He is an indoor cat and is the only cat in the household. Routine F3 vaccinations and monthly parasite prophylaxis are up to date, and he is fed Hills i/d wet and dry formulations after being diagnosed with IBD in 2021. Dusty had also been diagnosed with diabetes mellitus in 2022 and has been managed with glargine insulin.
Dusty’s previous veterinarian first detected a grade III/IV systolic heart murmur and tachycardia on routine physical exam in 2013, when Dusty was 1-yearold. Subsequently, an in-house echocardiogram was performed in 2014 and he was diagnosed with hypertrophic cardiomyopathy. At this time, the left atrium (LA) and Left atrial to aortic ratio (LA:Ao) were 1.35 cm and 1.4, respectively. Systolic anterior motion of the mitral valve was observed and Dusty was started on atenolol (5mg transdermal q 12h). Annual in-house echocardiograms were recommended to monitor for disease progression.
Dusty first presented to our clinic in 2017 (now aged 6-years-old). He had a grade III/VI parasternal systolic murmur, regular heart rate, and no dyspnoea. His resting respiratory rates at home were consistently less than 30 breaths per minute. We continued to see Dusty in for annual in-house echocardiograms. Between 2019 and 2020, Dusty’s owner reported three syncopal-like episodes that appeared to be triggered by overexertion.
In 2021, during his annual health examination, an
arrhythmia was first detected (irregularly irregular). His heart murmur intensity was unchanged and he had remained well in himself at home as reported by the owner. His echocardiogram showed that the LA and LA:Ao ratio had mildly increased to 1.5 cm and 1.7, respectively. We started him on clopidogrel 75mg (1/4 tablet Plavix PO q24h), alongside the transdermal atenolol, however, Dusty would not tolerate daily tableting at this time, so was changed to Astrix Aspirin 100mg (5 granules given in food twice weekly).
In 2022, an in-house echocardiogram showed a mass effect within the LA with chamber enlargement. The arrhythmia remained present, and Dusty’s mucous membranes appeared slightly cyanotic. Despite this, Dusty had reportedly remained his normal self at home. We scheduled a referral echocardiogram and electrocardiogram (ECG) with a veterinary cardiologist, Dr Damon Leeder.
The echocardiogram confirmed that Dusty had ventricular hypertrophy with severe left and moderate right atrial enlargement (LA 2.6cm, LA:Ao 2.8) There was no evidence of left ventricular outflow tract obstruction using Doppler. The scan also confirmed the mass effect noted was in fact likely to be a large thrombus, which appeared adherent, within the left auricle/atrium (1.3 x 1.8 cm in size). This was deemed to have arisen secondary to atrial remodeling/stretch, blood flow stasis, and endothelial injury. This thrombus presented a high risk for Dusty developing severe thromboembolism or experiencing sudden cardiac death.
The ECG revealed isorhythmic dissociation although a pathologic AV block was not excluded. The arrhythmia could have been secondary to the sedation (Alfaxan was required for Dusty), however, given it had been audible prior to sedation being administered, it was considered more likely to be arising from his structural heart disease. It was not considered to be of significant hemodynamic importance.
In summary, Dusty was diagnosed with:
1. Hypertrophic cardiomyopathy - severe (stage B2)
2. Left atrial thrombus
3. Isorhythmic dissociation
Due to the presence of a large left atrial thrombus, aggressive antithrombotic therapy was recommended. The aim was to achieve slow dissolution of the thrombus without causing dislodgement or thromboembolism. However, there was a significant risk of thromboembolism with or without therapy and a guarded prognosis was provided to the owner.
After discussion with the owner and further demonstrations regarding how to medicate him

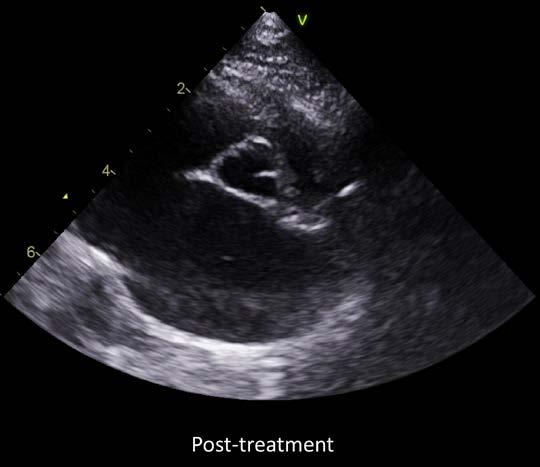
successfully orally, Dusty was re-started on clopidogrel 75mg (1/4 tablet PO q24h). Since this drug is bitter and distasteful to cats and Dusty previously did not tolerate this medication, we advised placing the 1⁄4 tablet in an empty gelatin capsule to facilitate its administration. In addition, rivaroxaban 10mg (1⁄4 tablet PO q24h) was commenced. The aspirin was discontinued and given the possible systolic dysfunction, lack of outflow obstruction, and potential inconsistency of absorption, the transdermal atenolol was also discontinued. No antiarrhythmic therapy was indicated for the ECG changes.
Aside from the thrombus, the severe left atrial enlargement indicated that Dusty was at high risk of developing congestive heart failure in the future. Consequently, the owner was instructed to continue monitoring and recording the sleeping respiratory rate, with normal rates less that 30-35 breaths/min. If the sleeping respiratory rate increased from an established baseline, or consistently exceeded 35 breaths/min, Dusty would need thoracic radiographs/TFAST ultrasound to determine if congestive heart failure had developed.
Dusty was re-evaluated by Damon 6 months later, to recheck the previously diagnosed HCM and left atrial thrombus. The echocardiographic findings were consistent with the previous diagnosis of severe hypertrophic cardiomyopathy. There was persistent markedly severe left and right atrial enlargement (LA 2.6cm, LA:Ao 2.6), and Dusty remained at high risk for CHF. However much to our surprise, following antithrombotic therapy, there was echocardiographic evidence of dissolution of the thrombus. This indicated an excellent response to therapy and the risk of thromboembolism was now much lower.
The previously prescribed antithrombotic therapy (rivaroxaban and clopidogrel) was continued unchanged. No additional cardiac medications were indicated based on this evaluation.
Cats presenting with an atrial thrombus are at high risk of developing feline arterial thromboembolism (ATE). This occurs when the thrombus embolises to a peripheral artery leading to ischemia of the affected vascular bed. Due to the extreme pain of ATE and the widely accepted poor prognosis, owners will often choose euthanasia at diagnosis of an atrial thrombus.
Therapy that includes both clopidogrel and a factor Xa inhibitor, such as rivaroxaban is currently recommended for cats that have either experienced an ATE event or at increased risk of developing ATE.¹ Unfortunately, there is very little research published on the efficacy of dual antithrombotic therapy with clopidogrel and
rivaroxaban in cats and these recommendations are largely based on human literature.² A recent study found that cats treated with clopidogrel and rivaroxaban experienced minimal side effects; had a longer medial survival time and in those cats with intracardiac thrombi, the dual therapy achieved effective thromboprophylaxis.³ Even though this study was retrospective and had a small case population, the results are promising for treatment of cats with HCM and ATE.
This case demonstrates cats diagnosed with large atrial thrombi can be successfully managed with dual antithrombotic therapy and achieve thrombus dissolution. The echocardiographic findings of a large thrombus with existing HCM and diabetes mellitus in a geriatric cat may, in some instances, prompt a discussion around euthanasia. Given Dusty had remained asymptomatic at home and quality of life appeared good, we are grateful his owner decided to treat medically, despite the risks. This case offers hope and justifies attempting dissolution treatment despite a guarded prognosis, so long as the quality of life is otherwise maintained. Dusty continues to have good quality of life and has experienced no adverse effects to the dual antithrombotic treatment with clopidogrel and rivaroxaban. His owner continues to monitor his resting respiratory rate and manage his diabetes mellitus. We plan to review his echocardiogram again in 12 months.
1. Luis Fuentes V, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med 2020; 34: 1062–1077.

2. Yuan J. Efficacy and safety of adding rivaroxaban to the anti-platelet regimen in patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol 2018; 19:1–9.
3. Lo S, Walker A, Georges C, et al. Dual therapy with clopidogrel and rivaroxaban in cats with thromboembolic disease. J Feline Med Surg 2022; 24(4): 277-283.
C&T No. 5986
Definitive diagnosis of feline infectious peritonitis (FIP) in Australia, requires consistent clinical signs and either: positive FIP immunohistochemistry on biopsies with supportive histopathology, OR positive direct immunofluorescence (DIF), typically performed on effusions. Non-effusive or ‘dry’ FIP cases typically present diagnostic challenges, as clinicians (and owners) are not prepared to put these often-debilitated patients through general anaesthesia and invasive diagnostic procedures.
An under-utilised alternative approach is to perform ultrasound guided fine needle aspirates (from lesions) into saline, typically only requiring light sedation. The sensitivity of DIF from aspirates will be lower than that from effusions (i.e., a negative result does not rule out FIP). The sensitivity on effusions is 75%, but on aspirates we estimate it to be more like 40-50%. The specificity, however, remains 100% (i.e., a positive test is definitely FIP).
The method we have had the most success with is as follows:
Perform your first couple of aspirates for routine cytology to assess for pyogranulomatous inflammation or other pathological processes. Then have 5-10 x individual 1mL syringes lined up, prefilled with 0.05-0.1mL saline. Have an equal number of needles ready for aspirates. Then have a 2mL EDTA tube (see Figure 1) Do your aspirate (with or without negative pressure), insert the needle into the EDTA vacutainer tube to utilise the negative pressure to suck cells through, then flush 0.05-0.1mL saline through the needle. Finish by drawing back 1mL air to reinstitute some negative pressure into the EDTA tube, ready for the next needle aspirate.
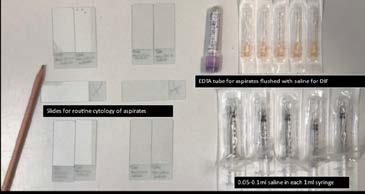
Repeat x 5-10 times.
You need a minimum of 0.5mL for DIF (but no more than 1mL or it will be too dilute).
I place the needle unattached to anything into the EDTA vacutainer first—it’s the tube’s negative
pressure that ‘sucks the cells in’. I find if I attach the saline syringe before the needle is inside the EDTA tube, the first drop of cells starts to push back out the tip of the needle and you ‘lose it’ to the outside of the rubber stopper. You want the aspirate ‘soup’ to be cloudy
You can then submit this for FIP DIF, just as you would do for an effusion. Disregard any comments from the lab technician in relation to the protein analysis, as it obviously won’t be consistent with FIP because it isn’t an effusion.
Veterinary Pathology and Diagnostic Services (VPDS, University of Sydney, B14, NSW, 2004) is the Sydney University laboratory. This is the only lab on the east coast of Australia that offers this test commercially. You can submit directly to them to save the mark-up from external labs, but you need to have an account (which is free) and arrange a courier such as TOLL or TNT (which is not free) yourself for this. Do not use Australia Post, as it will promptly be delivered to the university mail hub and then sit there for up to a week before reaching the Uni laboratory. Alternatively, you can submit via an external laboratory such as ASAP/Vetnostics or IDEXX (the former applies less mark-up).
You can also utilise this technique and submit your aspirate ‘soup’ (or part of it) for FCoV RT-Qpcr; however, you must be aware that false positive can arise, because the PCR cannot distinguish between benign enteric coronavirus (FEC) and fatal FIP. FEC can be found in up to 80% of cats without causing disease or FIP. FEC may drain from the gut in detectable levels to other abdominal sites such as the lymph node or liver and this is how false positive results can arise. IDEXX attempt to classify FIPV Biotypes, please be aware that plenty of cats that are not FIPV biotype positive are positive on definitive tests. So being FCoV positive but biotype negative does not rule out FIP. Essentially, if FCoV RNA is detected from a site unlikely to contain enteric FCoV in a cat with consistent clinical signs, it’s FIP in my book. The minimum volume required is 0.2mL. I have always used IDEXX, although ASAP/Vetnostics has also just developed this assay.
We are very comfortable starting highly suspicious cases on treatment whilst awaiting definitive test results (usually they are already getting better before you get the result back).
Figure 1. Tray made up to do U/s guided FNA of lymph nodes and kidneys in cats with ‘dry’ FIPCase Report 1
C&T No. 5987
Introduction
Canine interdigital nodular pododermatitis is a common inflammatory condition affecting the pedal skin.1–4 Also known as pedal folliculitis and furunculosis or interdigital ‘cysts’, this multifaceted and complex disorder can prove frustrating to manage given the appearance is identical irrespective of the underlying cause. Secondary infection is a common consequence of pedal inflammation, often resulting in furunculosis (rupture of hair follicles) and subsequent formation of the classic nodules and draining tracts between the digits and pads. Methicillin-resistant staphylococci (MRS) are of global concern in veterinary medicine.5,6 MRS infections frequently present to veterinarians as pyoderma, wound infections and otitis externa due to methicillin-resistant Staphylococcus pseudintermedius (MRSP) in dogs.7–10 Deep pyoderma due to MRSP affecting the paws is not uncommon in the author’s practice and can prove difficult to resolve.
The use of therapeutic light (photobiomodulation) has experienced much interest in recent years and now represents a new and appealing management option for veterinary dermatological conditions.11 A novel approach to photobiomodulation is through application of a fluorescence light energy (FLE) system consisting of blue
light which activates a topical photoconverter hydrogel containing specialised chromophores (molecules able to be excited by certain wavelengths) that generate fluorescence. FLE has been demonstrated to ameliorate and cure superficial bacterial folliculitis,12 deep pyoderma,13 canine interdigital furunculosis,14 canine perianal fistulas,15 and cutaneous calcinosis.16 In such instances, FLE was responsible for a lessening in the length of antibiotic therapy (and time needed for healing) when administered as add-on therapy.
Herein we describe the use of fluorescence biomodulation as part of a multimodal treatment regimen in resolving a case of canine interdigital nodular pododermatitis complicated by MRSP deep pyoderma.
Bruno is a 3.5-year-old, 28.3 kg, neutered male English bulldog obtained from an Australian breeder as a puppy.
Bruno initially presented to the Veterinary Dermatology Clinic in late 2021 for management of relatively generalised pruritic dermatitis with a history of recurrent otitis externa and multiple episodes of superficial bacterial pyoderma responsive to courses of cephalexin. Age of onset of pruritus was at approximately 6-months of age. Pruritus was noted to affect Bruno year-round with repeatable ‘flares’ experienced each spring. No benefit was derived from a strict novel protein diet trial using the Prime100™ kangaroo/sweet potato roll. Pruritus was reportedly both steroid- and Apoquel®responsive. Bruno was up to date with core vaccines and Bravecto® spot-on for flea/tick prevention. Bruno was an otherwise systemically well dog with no known comorbidities. A diagnosis of canine atopic dermatitis was made based on history, examination findings and by elimination. Intradermal allergy testing was performed, and allergen-specific immunotherapy subsequently commenced, based on these results, in November 2021 and administered via the subcutaneous route. Physical examination at the initial appointment revealed no complicating infections and Bruno was reportedly comfortable across the trunk on Apoquel® (16mg once daily). Despite Apoquel®, mild-moderate pruritus affecting the ears and paws persisted for which twice weekly topical steroid therapy using Elocon® lotion was prescribed.
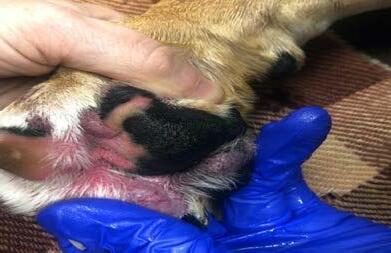
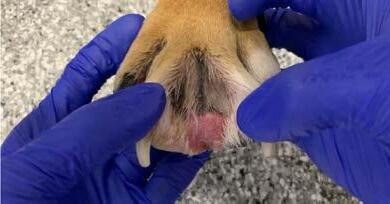
Bruno re-presented in January 2022 with a several week history of pedal pruritus and the development of ‘cysts’ between the toes on all paws despite diligent adherence to previously prescribed medications. Examination revealed diffuse furunculosis affecting the pedal skin across all paws (Figures 1-3). Cytological analysis of impression smears of haemopurulent exudate from draining tracts between digits revealed evidence of
chronic-active inflammation and intracellular diplococci consistent with interdigital nodular pododermatitis and complicating deep bacterial pyoderma. Trichography and deep skin scrapes from the paws were negative for demodectic mites. A swab of exudate was submitted to an external laboratory for aerobic bacterial culture and sensitivity. While awaiting results a tapering course of oral prednisolone (commencing at 20mg once daily) and topical Bactroban® ointment applied to all paws twice daily was commenced. Culture revealed heavy growth of Staphylococcus pseudintermedius resistant to all antibacterials tested except fusidic acid and rifampicin. Treatment options for management of extensively resistant staphylococcal deep pyoderma discussed with the client included systemic rifampicin and/or florescence biomodulation using Phovia®. The client elected the latter without the former, wanting to avoid possible rifampicin-induced hepatotoxicity. Phovia® was used per the manufacturer’s recommendations involving two, two-minute treatments of all paws once weekly. Phovia® was used in conjunction with oral prednisolone and topical mupirocin.
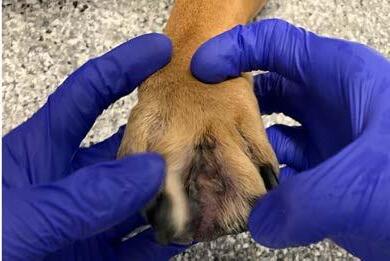
Bruno’s interdigital nodular pododermatitis with deep MRSP pyoderma resolved over a total of nine weeks of therapy without the need for systemic antibiosis (Figure 4). Comfort on the paws and pedal pruritus was greatly improved within the first two weeks of therapy. Repeat cytology was negative for diplococci at the recheck in week seven after commencing Phovia®; both Phovia® and Bactroban® ointment were continued for an additional two weeks prior to discontinuation. Atopica® 150mg once daily was instigated in week seven allowing for successful tapering and discontinuation of prednisolone over the following four weeks.
At time of writing, eight months after resolution of MRSP deep pyoderma on the paws, Bruno has remained comfortable without relapse of allergy signs including interdigital nodular pododermatitis. Atopica® has been tapered to a twice weekly frequency indicating response to allergen-specific immunotherapy with the view to discontinuing Atopica® altogether in the coming months and maintaining control of atopy with immunotherapy alone.
Resolution of deep pyoderma almost invariably requires the use of systemic antibiosis with topical therapy alone proving inadequate given insufficient penetration into the deep layers of skin to bring about microbiological cure. This case report describes the use of a novel intervention, fluorescence biomodulation (Phovia®), to resolve an extensively resistant staphylococcal deep pyoderma affecting the paws in an atopic dog without
the need for systemic antibiosis. The use of Phovia® allowed avoidance of a last-line antibacterial that can be associated with potentially fatal hepatotoxicity in dogs.
It is highly unlikely that such extensive MRSP deep pyoderma affecting the paws resolved in response to topical Bactroban®, prednisolone or allergenspecific immunotherapy indicating a pivotal role for Phovia ® in resolution of infection in this case. With the

ever-increasing prevalence of MRS infections seen by veterinarians it is crucial that we explore alternative treatments, such as Phovia ®, that can reduce reliance upon antibiotics.
Phovia ® technology includes a blue light-emitting hand-held LED lamp and a topical photoconverter gel. The gel contains chromophores (specialised molecules) that can absorb photons of directed light from the LED lamp and then re-emit them at different wavelengths in the form of fluorescence which, in turn, can manipulate the function of cells within the dermis and epidermis. Each wavelength has a discrete mechanism of action for resolving dermatitis, infection and improving skin regeneration. Phovia ® was a simple intervention that Bruno tolerated consciously on a weekly basis for the duration of therapy.
There is currently an evidence-base for use of fluorescence biomodulation for management of superficial and deep pyoderma,13 interdigital furunculosis,14 inflammatory disease of the canine interdigital skin. Lesions commonly become infected secondarily. In addition to management of the underlying cause, management of the chronic inflammatory changes in the interdigital skin created by secondary infection and by the release of keratin into deep tissues is required. Fluorescence biomodulation appears to modulate the inflammatory process in dermatological disorders and has shown promise in preliminary studies evaluating its use in superficial and deep pyoderma in dogs.
1. Bloom P. Idiopathic pododermatitis in the dog: an uncommon but frustrating disease. Vet J 2008;176:123–124.
2. Duclos D. Canine pododermatitis. Vet Clin North Am - Small Anim Pract 2013;43:57–87. Available at: http://dx.doi.org/10.1016/j. cvsm.2012.09.012.
3. Duclos DD, Hargis AM, Hanley PW. Pathogenesis of canine interdigital palmar and plantar comedones and follicular cysts, and their response to laser surgery. Vet Dermatol 2008;19:134–141.
4. Bajwa J. Diagnostic dermatology: canine pododermatitis. Can Vet J 2005;46:3. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC2834510/pdf/16454391.pdf.
5. Morris DO, Loeffler A, Davis MF, et al. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017;28.
6. van Duijkeren E, Catry B, Greko C, et al. Review on methicillinresistant Staphylococcus pseudintermedius. J Antimicrob Chemother 2011;66:2705–2714.
7. Ruscher C, Lübke-Becker A, Wleklinski C-G, et al. Prevalence of methicillin-resistant Staphylococcus pseudintermedius isolated from clinical samples of companion animals and equidaes. Vet Microbiol 2009;136:197–201.
8. Duim B, Verstappen KM, Broens EM, et al. Changes in the population of methicillin-resistant Staphylococcus pseudintermedius and dissemination of antimicrobial-resistant phenotypes in the Netherlands. J Clin Microbiol 2016;54:283–288.
9. Menandro ML, Dotto G, Mondin A, et al. Prevalence and characterization of methicillin-resistant Staphylococcus pseudintermedius from symptomatic companion animals in Northern Italy: clonal diversity and novel sequence types. Comp Immunol Microbiol Infect Dis 2019;66:101331.
10. Little S V, Bryan LK, Hillhouse AE, et al. Characterization of agr groups of Staphylococcus pseudintermedius Isolates from dogs in Texas. mSphere 2019;4:e00033-19-.
11. Godine RL. Low level laser therapy (LLLT) in veterinary medicine. Photomed Laser Surg 2014;32:1–2.
12. Marchegiani A, Spaterna A, Cerquetella M. Current applications and future perspectives of fluorescence light energy biomodulation in veterinary medicine. Vet Sci 2021;8:1–11.
13. Marchegiani A, Fruganti A, Spaterna A, et al. The effectiveness of fluorescent light energy as adjunct therapy in canine deep pyoderma: a randomized clinical trial. Vet Med Int 2021;2021.
14. Marchegiani A, Spaterna A, Cerquetella M, et al. Fluorescence biomodulation in the management of canine interdigital pyoderma cases: a prospective, single-blinded, randomized and controlled clinical study. Vet Dermatol 2019;30:371-e109.
15. Marchegiani A, Tambella AM, Fruganti A, et al. Management of canine perianal fistula with fluorescence light energy: preliminary findings. Vet Dermatol 2020;31:460-e122.
16. Apostolopoulos N, Mayer U. Use of fluorescent light energy for the management of bacterial skin infection associated with canine calcinosis cutis lesions. Vet Rec Case Reports 2020;8:1–5.
17. Marchegiani A, Fruganti A, Bazzano M, et al. Fluorescent light energy in the management of multi drug resistant canine pyoderma: a prospective exploratory study. Pathogens 2022;11.
e. mvh@mudgeevet.com.au
C&T No. 5988
Introduction
Bangers is a 4.5kg 2-year-old-male intact (cryptorchid) miniature Dachshund from the Mudgee, New South Wales.
On Friday, 20th of May 2022, Bangers’ owner noticed a large wound on his inguinal/perineal area. The owner administered 3.0mLs of Benacillin intramuscularly into Bangers’ right hind leg.
Bangers presented to Dr Liam Ryan at Mudgee Veterinary Hospital on Sunday, 22nd of May 2022 with two severe necrotizing wounds. The larger wound extended from the lateral-scrotal region to the perianal area. The second, smaller wound was on the ventral abdomen, caudal to the prepuce and cranial to the scrotum. The wounds were swollen, fluid filled, cold to touch with extensive soft tissue involvement. Bangers was able to urinate normally. His body temperature was reported normal at 38.0°C.
Two open wounds of unknown origin. A white tail spider bite or a dog bite wound was suspected (Figures 1a & 1b)
22nd May 2022
Bangers received 0.2mL Metacam® SC 0.5mL Noroclav® SC, and 0.6 ml Entrotril® SC. The large lesion on the perineal area was flushed and lavaged extensively with sterile saline. The wound was packed with manuka honey and dressed. Bangers was taken home by the owner for observation overnight and continued on oral medication.
23rd May 2022
Bangers returned to Mudgee Vet Hospital for a bandage change. The wounds were debrided with saline and sterile swabs. Observationally, the amount of necrotic
tissue appeared larger, compared to the previous visit. The wounds were redressed with Manuka honey, wound facing Jelonet™, softban and an external layer of adhesive bandage.
24th May 2022
Bangers was admitted for another bandage change and reassessment. The wounds appeared stable in size and appearance, with absence of granulation tissue. The wounds were dressed as previously described.
26th May 2022
Bangers received his first session of back-to-back (2x2 minute sessions) Phovia® FLE light therapy to both wounds. The wounds were dressed as per previously described.
2nd June 2022
Bangers returned to Mudgee Vet Hospital for a bandage change and wound assessment. There was a dramatic improvement in the lesions.
Wound 1 received another back-to-back session with the Phovia® FLE light therapy. A manuka honey dressing was applied and Bangers was sent home.
Wound 2 treatments were discontinued after rubbing of the bandage prolonged the healing process. Phovia® FLE was discontinued on wound 2 and monitoring continued
9 th June 2022
Bangers returned to Mudgee Vet Hospital for reassessment of his wounds. Phovia® FLE light therapy was applied back-to-back, for the third and final week, to Wound 1. Wound 2 continued to be monitored as an open wound.
16th June 2022
Wound 1 was nearing clinical resolution therefore treatment ceased. Wound 2 remained open closing by secondary intention and no further treatment was applied.
Veterinary staff were happy with the speed of wound closure, noting the dramatic improvement from week to week. The larger wound healed faster than expected, after receiving Phovia® FLE back- to-back for 3 weeks. FLE is a promising and safe therapy for accelerating wound healing.
Non-invasive light therapy has been utilised in human wound management and is now being adopted into veterinary medicine. Phovia®, a new product from Vetoquinol Australia and New Zealand uses a unique

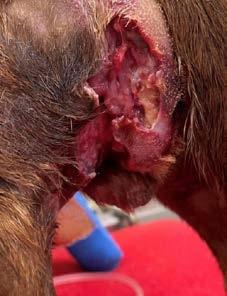
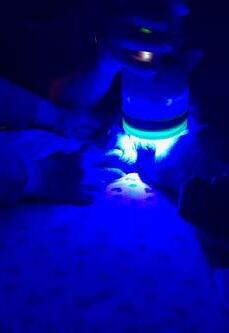
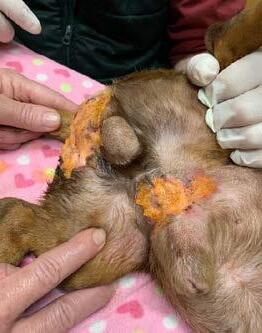
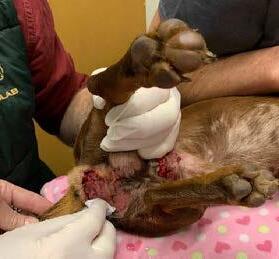

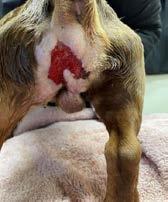
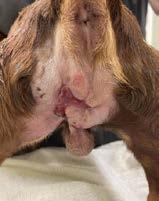
 Figure 1a & 1b. Wounds 1 and 2—22nd May 2022
Figures 2a & 2b. Bangers receiving Phovia® FLE light therapy to both wounds. The wounds were dressed as per previously described.
Figure 3 & 4. Show wounds on the 9th June 2022
4 3
2b
1b 2a
1a
Figure 1a & 1b. Wounds 1 and 2—22nd May 2022
Figures 2a & 2b. Bangers receiving Phovia® FLE light therapy to both wounds. The wounds were dressed as per previously described.
Figure 3 & 4. Show wounds on the 9th June 2022
4 3
2b
1b 2a
1a
chromophore gel and a blue light-emitting diode (LED) lamp to halve skin disorder recovery times, reducing antimicrobial use. The blue light activates the gel, generating polychromatic fluorescent light energy (FLE). The overall result is reduced inflammation, accelerating dermal fibroblast proliferation, increasing collagen synthesis and angiogenesis. Appropriate use of FLE can accelerate regeneration of damaged skin and improve wound healing time. The primary clinical protocol of FLE consists of 2 x 2-minute light therapy sessions weekly (back-to-back protocol) until clinical resolution.
Phovia® manipulates the function of cells within the dermis and epidermis, to reduce inflammation but still accelerate regeneration.

Different tissues absorb different wave lengths
Overall effect in an increase in mitochondrial cellular energy production
By stimulating natural chromophores within cells (especially within mitochondria), the efficiency of the mitochondrial electron transport chain can be increased, accelerating healing, cell repair, and cell division² Free radicals may also be reduced
Using polychromatic FLE is clinically proven to accelerate natural skin regeneration, even in situations where there is significant and deep skin damage³
The effects are anti-inflammatory, target bacteria, accelerate dermal fibroblast proliferation, increase collagen synthesis and stimulate angiogenesis
There is also solid evidence that FLE can accelerate healing of chronic inflammatory skin conditions and acute and chronic wounds in humans⁵ and dogs⁶
The Phovia® system triggers multiple cellular responses simultaneously.
Cost depends on the amount of Phovia® gels used and duration. As a guide, the lamp is ~$850 (cost price to vet, ex GST) and the lamp life lasts for 19,980 x 2-minute sessions.
The gels are ~$25 per pot (at cost price) the size of the wound will determine how many are needed.
As an example with this case study Bangers required 3 pots of gel in total over 3 weeks to apply 2 back-to-back sessions.
Most clinics so far are usually charging a 100% mark up on the gels (~$50 per pot) and a consultation fee for at least the first weeks’ session. It can then vary if they are charging for just the gel for follow up sessions or also including a fee; if so, it is usually a minimal nurse fee as the nurses are often applying the follow up treatments.
1. Scapagnini et al Management of all three phases of wound healing through the induction of fluorescence biomodulation using fluorescence light energy. Photonic Diagnosis and Treatment of Infections and Inflammatory Diseases II. Vol. 10863. International Society for Optics and Photonics.
2. Ferroni L et al. Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing. J Clin Med. Feb 18;9(2):559
3. Marchegiani, et al Fluorescence biomodulation in the management of canine interdigital pyoderma: a prospective, single-blinded, randomized and controlled clinical study. Vet Dermat ol 2019; 30: 371-e109
4. Romanelli M, et al. Evaluation of fluorescence biomodulation in the real-life management of chronic wounds: the EUREKA trial. Journal of wound care. 2018;27(11):744-753.
5. Salvaggio et al. Effect of the topical Klox fluorescence biomodulation system on the healing of canine surgical wounds. Veterinary Surgery. 2020; 49: 719– 727.
In tough times, community and purpose are even more important.
The CVE’s mission as a membership-based professional veterinary community is to provide premium, unbiased continuing education and professional development from some of the best veterinary minds to Vets, Techs + Nurses around the globe.
Join
Return On Investment (ROI): If you and/or your staff intend to enrol in CE in the next 12 months, it pays to do some quick ROI calculations. You’ll find that, as a CVE member (a range of membership types and fees available), you will be in the black. Particularly if you join as a Practice Member.
Singleton Vet Hospital, NSW
t. 02 65722077
The Lake Veterinary Hospitals, NSW
t. 02 49 459 677
e. greyhoundtarsalscreening@gmail.com
C&T No. 5989
Download C&T No. 5965
The Centre for Veterinary Education (CVE) of The University of Sydney published the report titled Control and Therapy Series, ‘The Brutal Reality of Hock Fractures in Racing Greyhounds’ C&T No. 5965 in March 2023, Issue 2010. This Report exposes an orthopaedic welfare issue that continues to plague greyhound racing worldwide. The same traumatic injury to the tarsal joint in the right rear leg, leads to the frequent outcome of surgical repair or euthanasia.
The following compelling statistics are justification that the industry needs to address this urgent welfare issue:
1. After only 12 months of racing, there is a 400% increased risk of tarsal injury (Beer, 2014)
2. The right Central Tarsal Bone (CTB) suffers a dramatic loss of bone density prior to a collapse fracture (Hercock, 2010). This may occur without triggering an obvious pain response, remaining undetected by trainers and veterinarians.
Following publication, The Report was distributed to all racing ministers in Australia, New Zealand, Republic of Ireland and the United Kingdom. It was also sent to the RSPCA (Australia and United Kingdom), Animal Welfare League (AWL) and Greyhound Welfare and Integrity Commission, NSW (GWIC).
At the time of writing this Update, the RSPCA (Australia and United Kingdom) had not acknowledged the receipt of my correspondence. The AWL responded within days and requested further information.
Lisa White is the President and Founder of the Charity Friends of the Hound Incorporated (FOTH). This rehoming charity has been funding repairs and rehabilitation of greyhounds injured on the racetrack for over 15 years.
The following is Lisa’s response to The Report:
‘…from a welfare perspective, Dr David Larratt’s article ‘The Brutal Reality of Hock Fractures in Racing Greyhounds’ has positive implications for improving Greyhound welfare. Our rescue charity continues to rescue Greyhounds suffering hock fractures and are well aware of the number of dogs euthanised due to this common racing injury. We hope this research benefits the health and well-being of Greyhounds in preventing such injury, and appreciate the opportunity this presents.’
The response from GWIC was rapid and positive with an acknowledgement that ‘…weakness in the tarsus due to a reduction in bone density may be a potential contributing factor to racing injuries that needed to be explored by the greyhound racing industry’.
Of all the state and international racing ministers contacted, only 2 have responded directly to this date.
The Hon. Kieran McAnulty (Racing Minister, New Zealand), reported that he had shared The Report to welfare groups and Massey University. He affirmed that, ‘The ongoing improvement of greyhound welfare is a priority for this government’. In response to the Robertson Review (a report into greyhound racing in New Zealand, dated July 2021), the industry was put on notice to make improvements and Minister McAnulty is consulting with key industry and animal welfare stakeholders before ultimately taking a recommendation to Cabinet. He stated that, ‘The status quo is clearly no longer acceptable, and the two options are: allowing the industry to continue under strict monitoring, or closure.’
The Hon. Madeleine Ogilvie MP (Racing Minister, Tasmania) stated the following:
1. ‘…fracture of the hock in the right limb have been universally acknowledged to be the most common site for fractures in racing greyhounds…’ , and ‘ … these fractures are believed, in most cases, to be the result of accumulation of microdamage over a period of time leading to eventual catastrophic failure.’

2. ‘Sensitive techniques to detect this damage and serve as an early warning are required to minimise the impact of this injury type on the greyhound industry’ .
3. ‘I am advised that your paper, including the

radiographic techniques to identify markers of such bone damage, is a valuable addition to the ‘knowledge bank’ and we encourage you to submit this information for peer-reviewed publication.’
In May 2023, in collaboration with Dr John Katakasi (Adelaide Plains Veterinary Surgery) and Dr Katrina Garrett (SASH) I commenced a series of targeted case studies to further correlate conventional tarsal radiography and CT imaging.
In October 2023, I am presenting at the annual Melbourne conference of The Australian Greyhound Working and Sporting Dog Veterinarians Group.
Topics will include:
the pathogenesis of changes in bone density which occur during running stress the introduction of guidelines to assist interpretation of radiography and CT imaging to detect adaptive load and overload in the greyhound tarsus discussion on how this information may be used by veterinarians and controlling bodies to reduce and prevent tarsal fractures
I look forward to keeping you informed of any further developments.
Beer, L.M. (2014). A study of injuries in Victorian racing greyhounds 2006-2011. Master’s Thesis, The University of Melbourne, Melbourne, Australia.
C.A.G.E.D. Nationwide (2023) www.cagednw.co.uk/greyhoundinjuries.html
Friends of The Hound (FOTH) (2023) www.friendsofthehound.org.au/
GWIC (2020), Race Injury Review Panel analysis and recommendations, 1 January 2020 – 30 June2020. https://www.gwic.nsw.gov.au/welfare/race-injuryreview-panel
GWIC (2023), Analysis of greyhound racing injuries, 1 January 2023 – 31 March 2023.
https://www.gwic.nsw.gov.au/__data/assets/pdf_file/0003/1144461/1-January31-March-2023-Injury.pdf
Hercock, C.A. (2010). Specialisation for fast locomotion: performance, cost and risk. Thesis for the degree of Doctor in Philosophy. The University of Liverpool, Liverpool, United Kingdom.
Johnson, K.A., Muir, P., Nicoll, R.G., et al. (2000) Asymmetric adaptive modeling of central tarsal bones in racing Greyhounds. Bone 2000;27:257–263.
Thompson, D.J., Cave N.J., Bridges J.P., Reuvers, K., Owen, M.C. and Firth, E.C. (2012). Bone volume and regional density of the central tarsal bone detected using computed tomography in a cross-sectional study of adult racing greyhounds, New Zealand Veterinary Journal, 60:5, 278284, DOI: 10.1080/00480169.2012.682957
Frenchies: In Vogue but Neurologically Rogue! WebinarPLUS

28 September 2023
Our FREE to members WebinarPLUS program is an economical way to keep up-to date and meet your statutory continuing education obligations. Note: Non-members enrol at $70 per course.
This flexible format allows you to engage whenever you feel like it. It’s not an arduous commitment. And the benefits are great: as well as asking your own questions you get to enjoy and learn from cases and questions posed by your peers. You can choose (or not) to challenge yourself with a short quiz and earn 1 structured CPD point.
And it’s all free. Because either you are a CVE Member or your practice has obtained CVE Membership on your behalf.
All Webinars available to you in the Webinar Library
POCUS in Cats: Rapid Cage Side Scanning WebinarPLUS with Drs Soren Boysen & Serge Chalhoub
"I found it so helpful—it was simplified in a way that makes me feel like I am actually capable of doing a POCUS scan confidentially. The presenters were exciting and engaging as well! 10/10 course to the point where I want to go do an internship at Calgary with them."
And if now you’re wondering why you didn’t enrol in the POCUS in Cats: Rapid Cage Side Scanning WebinarPLUS after the great, unsolicited feedback above, we can offer you the next best thing: FREE access to the recording in the Webinar Library which hosts all WebinarPLUS and WebinarLIVE! recordings.
FREE enrolment for Vets, Techs + Nurse Members
cve.edu.au/webinarplus cve.edu.au/webinar-library
C&T No. 5990
This enclosure was initially built in 2003.
I wanted my cats to have access to the outdoors, but in a way that would mitigate the risks associated with this exposure and avoid road trauma and the risk of cat fights.
I have a small parcel of land (approximately 225 square metres), and the enclosure covers my entire backyard (with the exception of the carport), including a sidewalkway. A much smaller space would have sufficed.
All my cats have preferred to do their toileting outside in the garden, so the cost of constructing the enclosure was rapidly offset by savings in terms of cat litter. I still have one litter tray inside, but it may only be used a couple of times per year.
I sourced the UV stabilised netting from a marine supplies business. It is attached with galvanized fencing staples. It is likely to sag a little over time and may need to be tightened. There are various galvanised fittings that are available at hardware stores that can be creatively repurposed. Originally there were tension wires spanning the width of the backyard to support the netting, but over time I found that these were not necessary.
I had existing 2.3 metre fences, and attached the netting to these. The back of the enclosure is attached to a wall constructed of trellis, with a gate in it. The walkway also has a gate, with netting spanning the distance from the side of my house to a basic wooden structure attached to treated pine posts on galvanized stirrups immediately adjacent to my neighbour’s property.

There is a tall scratching post which is made out of PVC pipe covered in marine grade carpet in the enclosure. I made a hammock out of linseed treated pine, shade cloth and UV stabilised thread. This is attached to the fence. Originally a tunnel was installed, going up to the top of the carport roof to allow views over the surrounding area. I removed this as it was rarely used and difficult to maintain.
As there are foxes in the area, I concreted below the fence line to a depth of approximately 20 cm.
I have done all of the maintenance on the cat enclosure myself.
My cats access the cat enclosure through a cat door. The backyard has a lawn, catnip, cat-grass and other plants. I removed any plants that were potentially toxic. My boys enjoy sunbaking,1 digging in the gardens, chasing leaves, eating grass, sniffing out bugs and talking to birds. On hot nights, they prefer to sleep outside and ‘supervise the possums’.
Claude loves to chase the water jet from the hose and they both like that they can hide in bushes and ambush me when I don’t see them coming.
My last cat had unrestricted access to the enclosure unless there was a need to keep her inside (e.g. fireworks, thunderstorms). As one of my current cats likes to chase insects and had an anaphylactic reaction after he tried to eat a bee, I removed many flowering plants from the
Authors’ views are not necessarily those of the CVE





backyard and try to keep him inside at times when bees were likely to be active. He actively resists this. There is no doubt that my cats’ preference is to have access to the outdoors.
An enclosure could be constructed relatively easily by a competent handyman. It would also be possible to easily enclose an existing Pergola. Council permits may be required to construct a new pergola.
Many ideas and troubleshooting advice can be found on the ‘Bunnings Cat Hacks’ Facebook page for those who wish to try to construct an enclosure themselves, otherwise a google search of ‘catio’ or ‘cat netting’ will provide links to installers and suppliers.
Inside, we have a wall with platforms and a bridge; a ‘One Fast Cat Wheel’ and the ‘Cat tree king Cat Empire’. My boys particularly like sleeping in the hammocks in their Cat Empire.
Elliot also likes to watch TV. His current favourite shows are ‘Strike Back’ and ‘Ice Age: The Meltdown’.


In some areas, there may have been regulations introduced regarding the diameter of netting which is permissible to use.
If I was going to do it again from scratch, I would consider concreting wooden posts on galvanised stirrups about 1 metre in from the fence line at intervals of about 2 metres apart and attaching netting from the fence to these so the whole yard wasn’t netted.
The corners of any enclosure are usually the most vulnerable to escape attempts so it is prudent to reinforce these with extra fencing staples or other weatherproof fasteners.


There is increasing pressure from many authorities to keep cats exclusively indoors or in enclosed or constrained outdoor domiciles. This article represents an exemplar of what can be achieved in a costeffective manner using intelligence and trial and error improvements. This should prove a fantastic resource for vets to hand out to their clients.
Download Client Handout
If Jake and Juneau were extras on the Kardashians

The ISFM & AAFP are partners with the CVE in delivering the Feline Medicine Distance Education course. Due to its comprehensive content, this course is spread over 2 years to allow the busy practitioner to fully engage with the content whilst maintaining life balance.
 Claire Woolford
RVN VTS Anesthesia & Analgesia
Claire Woolford
RVN VTS Anesthesia & Analgesia
C&T No. 5991
Claire is the lead anaesthesia nurse at Anderson Moores Veterinary Specialists, a multidisciplinary referral centre in Hampshire, where she leads the soft tissue surgical nurse team. She has worked with the Academy of Veterinary Technicians in Anesthesia and Analgesia) on the credential committee. For 3 years Claire also worked with the British Small Animal Veterinary Association (BSAVA) to develop the scientific program for the BSAVA Congress. Claire also runs a small CPD company, Synergy CPD, which provides tailored inhouse and virtual anaesthesia and analgesia training.
In 2012, the Reassessment Campaign on Veterinary Resuscitation (RECOVER) released the first clinical guidelines on cardiopulmonary resuscitation in cats and dogs. This article provides practical instruction on performing basic and advanced life support in cats, including performing effective cardiopulmonary resuscitation (CPR) following the guidelines, as well as looking at the importance of post-CPR care.
Regularly practising CPR means you can call on those skills quickly, meaning the team will be more effective and have more successful outcomes. It is recommended that refresher training is undertaken every 6 months to help maintain clinical skills.¹
Resuscitation equipment should be kept in a well organised crash cart/box located in an easily accessible central location. Regular checks (e.g. weekly) should be carried out to ensure drugs are in date, packages are sterile, and all the contents are present. The contents of the box will vary depending on the size and emergency caseload of your practice.
The key items to be included can be found in Table 1. It is also particularly helpful to have cognitive aids such as a drug dosing chart and a RECOVER CPR flowchart.¹
During a crash situation, communication is vital, so a team leader should be appointed who will allocate tasks to the team, identify problems, monitor the time and record events. Closed-loop communication between team members reduces the risk of errors through misheard directions. Instructions must be given in a clear voice and should be repeated back clearly.¹
A team leader should be appointed to oversee the crash situation and all instructions should be given in a clear voice and repeated back clearly. ¹
Some cardiac arrests can be predicted. If your patient is at high risk of arresting, prepare in advance by setting up a crash station and having equipment, drugs and additional people on standby.
Early recognition of cardiac arrest and initiation of BLS is key to improving the patient outcome with good quality procedures resulting in increased survival.² Initial patient assessment should be limited to a rapid (5–10 s) check of the airway, breathing and circulation. If a patient is unresponsive and apnoeic,³ CPR should be started immediately—do not waste time trying to find a pulse or auscultate the heart. If you suspect the patient has arrested but you are not quite sure, start CPR anyway.
Airway Endotracheal tubes
Laryngoscope with blades
Endotracheal tube ties
Cuff inflator syringe
Long dog urinary catheter (difficult airway, oxygen delivery and drug delivery)
Breathing Bag valve mask for manual intermittent positive pressure ventilation
Oxygen tubing to connect bag valve mask to oxygen source
Circulation Intravenous catheters
T-connector
Tape
Pre-drawn syringes of saline for flushing
Long dog urinary catheter
Start chest compressions immediately, without delay and do not stop.²
In cats, the cardiac pump method is the preferred technique due to high chest wall compliance.¹ To achieve this:
Place the cat in lateral recumbency (left or right).¹
Use one hand to steady the patient and compress the heart directly with the other hand using your fingers and thumb.
The heart is located just behind the patient’s elbow (Figure 1a,b).
Compress the chest/heart at a rate of 100–120 beats per minute (bpm).¹
Compress the chest at least ½–⅓ of its depth.³
Allow the chest wall to recoil in-between compressions to provide time for myocardial perfusion.³
Emergency Drugs
Selection of syringes and needles
Adrenaline
Atropine
Lidocaine
Naloxone
Flumazenil
Calcium gluconate
Sodium bicarbonate
Frusemide
Bag of 0.9% saline
Monitoring Capnography sampling line
Endotracheal tube connector for capnography attachment
ECG pads
Electrode gel
Extras Pen
Suction device
Intravenous fluids
Swabs
Syringe drug labels
Procedure pack, for example, thoracocentesis kit, chest drain kit
A 2 min uninterrupted cycle of compressions is given, after which the person giving the compressions should be switched to avoid rescuer fatigue. The new person should be ready to switch in at the end of the 2 mins to minimise interruptions to compressions. Do NOT stop chest compressions before 2 mins. Interrupting compressions early prevents maintenance of good perfusion.¹
A CPR metronome app is a helpful aid in ensuring compressions are performed at the correct rate.
Open chest CPR is not indicated in cats due to the small size of their thoracic cavity.
As veterinary patients are more likely to suffer a respiratory arrest first, securing an airway and starting intermittent positive pressure ventilation (IPPV) is vital.1 Hypoxaemia and hypercapnia will prevent return of spontaneous circulation (ROSC).3
Ideally, the patient should be connected up to an anaesthetic machine via a suitable circuit to provide 100% oxygen. If this is not available, a bag valve mask, also known as an ‘Ambu’ bag (Figure 2), can be used to deliver room air that has an oxygen content of 21%, or failing that, breathe down the tube as your expired breath contains around 16% oxygen.
It is detrimental to stop chest compressions during intubation,³ which can make placement of an endotracheal tube (ETT) difficult. Regular practice of intubation in lateral recumbency is essential to master this technique and make CPR more effective. Capnography may not confirm tracheal intubation
during CPR owing to poor cardiac output,⁴ but direct visualisation, auscultation, and assessment of chest movement during ventilation can help confirm tracheal intubation.
High tidal volumes (TVs) and high respiratory rates increase thoracic pressure opposing cerebral and coronary perfusion³ and should therefore be avoided.
Intubate your patient
A laryngoscope should always be used for good visualisation of the larynx (Figure 3).⁴
Secure the ETT in place. Inflate the cuff.
Ventilate your patient
If the patient already has an ETT in place at the time of arrest (i.e. under general anaesthesia), then check that this is patent before beginning IPPV, making sure that the anaesthetic gas is switched off first. Provide a normal-sized breath with IPPV (10ml/kg TV).
An inspiratory time of 1 s is recommended.³
Ventilate at a rate of 10 breaths per minute (one breath every 6 s).²
After 2 mins of cardiac compressions and ventilation, a very brief pause is taken to:
Assess the ECG.
Check for a pulse.
Change the person doing chest compressions.
This should take no more than 5 s,⁴ after which another 2 mins cycle of compressions and ventilation should begin and advanced life support instigated.
Specific monitoring techniques assess the effectiveness of CPR, identify ROSC and guide treatment. ECG monitoring is used to check cardiac rhythms and is assessed during the 2 min interval pause as chest compressions cause too much interference for evaluation.
Common arrhythmias seen during cardiac arrest can be divided into two groups:
Shockable rhythms: ventricular fibrillation, pulseless ventricular tachycardia;
Non-shockable rhythms: pulseless electrical activity (PEA), asystole (flatline) (Figure 4).⁴
Capnography is an invaluable tool to monitor cardiac output during CPR. It provides information on the effectiveness of compressions and potential prognosis.
In cats, the aim is to achieve an end tidal CO₂ (ETCO2) of >20 mmHg, which shows that some blood flow is delivering CO₂ to the lungs and can predict ROSC.³ An ETCO2 <15 mmHg may be a poor prognostic indicator meaning ROSC is less likely. A sudden increase in ETCO₂ indicates ROSC may have occurred, in which case CPR can be paused and the patient assessed.¹
Emergency drugs should be given intravenously where possible.⁴ Intravenous (IV) access should therefore be gained quickly in a crash situation if the patient does not already have this. If necessary, an intraosseous catheter can be placed, as all CPR drugs can safely be given via this route.
Any drugs that can be antagonised should be reversed as soon as possible. Atipamezole can be given by IV to reverse alpha-2 agonists, naloxone will antagonise opioids and flumazenil will antagonise benzodiazepines.¹
Administering emergency drugs as quickly as possible can help achieve ROSC. All drugs should be flushed with saline into the central circulation.
Adrenaline is the most commonly used CPR drug and produces profound vasoconstriction (which redistributes blood to vital organs) and increased contractility. There are concerns it may be harmful as well as helpful, as it increases myocardial oxygen demand.
Current recommendations are to administer low-dose adrenaline (0.01 mg/kg) via the IV route as early as possible, repeating the dose every 3–5 mins.⁵ A high dose (0.1 mg/kg) can be given when CPR is prolonged (i.e. more than 10 mins).
Administering emergency drugs as quickly as possible can help achieve the return of spontaneous circulation. All drugs should be flushed with saline into the central circulation.
There is limited evidence to show that atropine is beneficial but also no evidence that it is harmful. It is used where high vagal tone is suspected (eg, bradycardia prior to arrest) or PEA/asystole are identified⁵ at a rate of 0.04 mg/kg IV.
Adrenaline and atropine can also be given via the intratracheal route if IV access is not possible, although a higher dose and dilution are required for this.¹ A sterile urinary catheter is inserted down the ETT to the approximate level of the tracheal bifurcation. The drug is instilled into the lungs while holding on to the urinary catheter. Do NOT administer drugs via the intra- cardiac route.
Authors’ views are not necessarily those of the CVE
Ventricular fibrillation

Pulseless electrical activity

Ventricular tachycardia

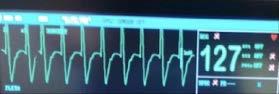

Fluid therapy should only be provided to hypovolaemic patients as shock doses of fluids in normovolaemic patients can decrease myocardial perfusion.1,4
Unfortunately, early re-arrest is common and the team should be prepared for this. Post-arrest syndromes can include multiorgan failure, cardiogenic shock and anoxic brain injuries. Goal–directed post- CPR care may help improve survival times (Table 2).1,4 oring
The current survival to discharge rate in veterinary patients undergoing CPR is between 2 and 10% despite initial ROSC in 35–45% of patients.¹ Rapid recognition of cardiac arrest and immediate commencement of CPR is therefore important as it improves the likelihood of ROSC and survival.⁶
Cardiac arrest has a high mortality rate, but being prepared as a team and starting CPR rapidly according to the RECOVER guidelines can improve the chances of success.
References
1. Fletcher DJ, Boller M, Brainard BM, et al. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 7: clinical guidelines. J Vet Emerg Crit Care 2012; 22 Suppl 1: S102–S131.
2. Brainard BM, Boller M, Fletcher DJ. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 5: monitoring. J Vet Emerg Crit Care 2012; 22 Suppl 1: S65–S84.
3. Hopper K, Epstein SE, Fletcher DJ, et al. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 3: basic life support. J Vet Emerg Crit Care 2012; 22 Suppl 1: S26–S43.
4. Ward MJ, Blong AE, Walton RA. Feline cardiopulmonary resuscitation: getting the most out of all nine lives. J Feline Med Surg 2021; 23: 447–461.
5. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 4: advanced life support. J Vet Emerg Crit Care 2012; 22 Suppl 1: S44–S64.
Blood pressure
Welcome to Research Roundup where we bring you summaries of the latest feline research. A pilot study of non-surgical contraception in female cats makes for an interesting read! And we have some great publications on foreign body removal and outcome, early identification of degenerative joint disease with the owner’s help and hypercalcaemia.
The International Society of Feline Medicine is the veterinary division of the pioneering cat welfare charity International Cat Care. Trusted by vets and nurses, it provides a worldwide resource on feline health and wellbeing, via the Journal of Feline Medicine of Surgery, by fostering an international community of veterinary professionals with a shared vision of feline welfare, and supporting professional development with practical CPD. Additionally, International Cat Care’s website provides a valuable resource of accurate information delivering what both vets and cats would want owners to know.
Capnography
Mean arterial pressure should be 70–80 mmHg. Ensures good perfusion of organs and tissues. Consider fluid therapy or vasopressor therapy if hypotensive.
End tidal CO₂ should be maintained between 32–43 mmHg. CO₂ levels influence cerebral blood flow. Hypocapnia produces cerebral vasoconstriction reducing blood flow to the brain. Hypercapnia causes cerebral vasodilation increasing blood flow to the brain risking increased intracranial pressure.
Pulse oximetry
Maintain oxygen saturation between 94 and 98% by avoiding 100% FiO₂ if possible. Hypoxaemia should obviously be avoided but hyperoxia can also be damaging through the release of reactive oxygen species, which damage cells.
Temperature
Passive warming should aim to increase temperature very slowly at a rate of 0.5°C per hour. Rapid warming can risk vasodilation and hypotension. Hypothermia is known to reduce neurological injury.
Wingham and Valley Vets, NSW 2429
e. jdooley@westnet.com.au
C&T No. 5992
A cow presented sick and inappetent 3 days post caesarian section.
Her record indicated that she was a high-producing Friesian dairy cow. Caesarean section had been necessary, after correction of the dystocia’s presentation did not permit per-vaginam* delivery of the calf. The calf had been delivered freshly dead.
Post-Sx, the cow had been treated with broad spectrum antibiotics and Oxytocin. The medical record is not available at the time of writing. The prescribed course of antibiotic was likely to have been for 5 days, and Oxytocin would have been injected at a volume of 5mLs, given twice daily for 48 hours.
What are the most likely causes of acute post-partum illness in high-producing dairy cows?
metritis
mastitis
peritonitis
acute GI disorders
metabolic
other systemic illnesses.
What other questions should we ask the farmer?
Are any other milkers or Dry cows sick?
Did this cow receive Dry Cow therapy at Drying-off?
Was this cow raised on the farm, or acquired within the last few months?
Does the cow have diarrhoea, or any other signs?
Has she suffered any known adverse event e.g. fallen while in the yard, fallen into a farm drain, wandered into udder-deep water in dam/billabong?
In this case no other cows were sick. This cow had suffered no known events other than dystocia and the corrective interventions.
She did not have diarrhoea or any other clinical signs. She was raised on this farm. This farm had previously had
cases of severe clinical Theileriosis in ‘imported’ cattle, so the fact of this patient’s being home bred made Theiliosis an unlikely diagnosis.
The cow appeared dull and lethargic, as reported. She had a normal stance.
She had no oral, nasal, or ocular discharges. She was pyrexic, but this is an unreliable indicator of disease—over several years on this farm, cows held on the yard (for AI or for vet procedures e.g. lameness examination/Rx of Cancer Eye conditions) have been found at 9am in February/March to have temperatures approaching 40⁰C, due to the combination of ambient temperature and humidity.
She had a mildly elevated HR, with cardiac auscultation findings unremarkable.
There was no indication of respiratory system involvement. Ruminal movements were present, slower and of lesser strength than normal. Palpation of her udder, and examination of milk from each quarter (by both visual and olfactory means) revealed no evidence of mastitis.
Rectal examination: rectum contained a small quantity of paint-consistency faeces, with no blood or excess mucus.
Palpation revealed a normally-contracting/involuting uterus, and no other abnormalities.
There was no evidence of crepitus in the uterine wall, urinary bladder was not palpable, and there was no evidence of developing peri-uterine or peri-vaginal adhesions.
Abdominal stethoscope auscultation revealed no pings.
Vaginal examination: No foetal membranes were present within reach, and there was a minimal volume of lochia (NB this is a quite subjective observation).
Examination ruled out systemic illness, respiratory illness, GI disorders, mastitis and metritis.
Given the history, the odds were that she did not have a primary metabolic disorder.
Peritonitis was the most likely possible diagnosis, and if this was the case, its cause was most likely to be associated with the obstetrical procedures. Possible causes would be a surgical complication from the C-section, or perforation of the uterine wall by the foetus during corrective manipulations.
The decision was taken to perform exploratory laparotomy, by re-opening the original laparotomy incision, which was on the cow’s LHS, in the caudal paralumbar fossa.
Ultrasound was not available in this case; but if it were, it may have been helpful—however, this would have involved a fee to the client, which may or may not be considered an impost (a fee is always a waivable option…). Obviously, negative scan findings or misinterpretation may have provoked a decision to not laparotomise the patient, which would then have delayed an accurate diagnosis and treatment.
The skin sutures were removed, and the edges of the skin incision were tweaked apart, to permit copious lavage of the exposed subcutis with 10% povidone solution.
Approximately 30mLs of 2% Lignocaine was then infused subcutaneously into each side of the incision, via the exposed skin margins. The incision was then re-opened. A large volume of watery fluid promptly flowed from the abdomen. This fluid was palpably slightly slimy to the touch, pale orange in colour, and contained a few small flecks of assumed fibrin clots.
The uterus was identified by both visual appraisal and by left-hand palpation, and found to be well-contracted up into the upper caudal abdomen.
The suture line in the uterine incision was identified, and painstakingly palpated along its length. A laxity in this suture line was identified, over a distance of 8-10 cms commencing at the ventral limit of the suture line, indicating that along that portion of the closure the uterine lumen was not sealed off from the abdomen—it was very easy to insert a finger through the suture line into the uterine lumen, which in a perfect closure would have been quite difficult to achieve by this time postsurgery, due to the typical very rapid fibrin deposition along the incision line, sealing the latter.
As a consequence of the imperfectly sealed closure, lochia were leaking into the abdominal cavity causing the evident peritonitis.
The appropriate corrective action was to seal the uterus effectively, and to treat the peritonitis.
The unsealed/leaking portion of the uterine closure was oversewn. This was very difficult to accomplish, having to be performed ‘blind’ and while holding the suture needle with the fingers of one hand only, due to the very caudodorsal position in the abdomen of the uterus, in its contracting state. Use of instruments e.g. needle holders was impossible.
The oversew was performed using chromic catgut* Metric 6, in a Connell pattern. The oversew was
terminated when palpation determined that the new closure extended well-caudally, beyond the limit of the previously unsealed portion. Suture knots were thrown under full visualization in the open laparotomy incision, and then one thread was held very tautly while the other thread was ‘run down’ to the uterine incision and locked tightly. The closure and knots were rechecked carefully at least once, to ensure that a complete closure/seal had been achieved.
The abdominal cavity was then lavaged thoroughly, by inserting a hose end well-sanitised with 10% Povidone, connected to a tap adjacent to the crush. This farm had the uncommon advantage of having reticulated ‘town’ water, which was therefore of potable quality. Lavage was continued until all fluid exiting the abdomen appeared to be running clean.
Of note, this case was the first of its kind which the attending clinician had encountered, so the latter had no previous experience to permit comparisons.
It was considered to be feasible, that simply closing the laparotomy and treating the cow with broad-spectrum antibiotics would have accomplished a full recovery.
However, it was considered also possible that the inflammation would persist.
The clinician had an extended-family member (one generation older) whose story of survival after having developed peritonitis consequent to abscessing/ perforated typhlitis (a burst appendix) was wellknown. The treatment had consisted of enforced dorsal recumbency, and having his abdomen lavaged several times per day via his still-open laparotomy incision. Lavage was discontinued after several days, and the laparotomy incision was permitted to close by granulation.
In this bovine case, the clinician applied the principles of this human surgical story to the bovine patient.
That the cow was unlikely to place herself into Left lateral recumbency, in consideration of her own discomfort—this would mean that wound contamination risk was thereby reduced, and that eventration of her intestines was relatively unlikely to occur, given the length of the intestinal mesentery.
That the rumen would rapidly adhere to the cranial aspect of the laparotomy wound.
The farmer was directed to gently insert the wellsanitised hose deeply and far into the abdomen, twice daily at milking time, and for a timed 2 minutes lavage the abdomen at a flow rate generating an effective efflux of water. The flow rate was demonstrated to the farmer.
The laparotomy wound margins were also to be similarly irrigated. The farmer was also reminded to monitor the patient for mastitis, even more carefully than was his habitual practice.
Biting fly control medication was applied to the wound, with this to be continued twice daily (probable brand was Flygon® spray).
Broad spectrum antibiotics were continued. The product selected was Neomycin/Penicillin 100/200 aqueous suspension for intramuscular injection (Neomycin sulphate 100mg/ml, Procaine Penicillin 200mg/ml). Label dose rate 25mLs per 500kg, dose for this patient 30mLs, injected intramuscularly, ONCE DAILY.
Although her uterus was by now likely to be not responsive to oxytocin, given the time passed since calving, it was decided to treat her with 5mLs (50iu) of injectable solution by intramuscular injection, twice daily at milking time, for a further two days more.
The patient was reported to be brighter in demeanour and with returning appetite by 24 hours post-surgery.
By 72 hours post, the farmer commented that he was ‘having trouble keeping her belly open’—the lap wound was closing rapidly, especially from its ventral extremity, and the cow’s health continued to improve.
Abdominal lavage was discontinued after a total duration of four days. The laparotomy was effectively closed along its complete length by day 8-10.
The cow survived, and re-bred successfully.
The clinician who had performed the Caesarean section was coached and counselled about ensuring proper tensioning of suture lines, with application to all surgical procedures in all species.
All clinical decisions appeared to all work in the patient’s favour—or at least, no decision appeared to be detrimental...
For abdominal lavage, homemade ‘isotonic saline’ solution made by adding an appropriate quantity of household salt
to a calculated volume of water, would be a preferred lavage option.
This was considered at the time, but was not elected to run with—for reasons of practical logistics, an effective pumping system would be required to ensure that the procedure was not too time-consuming for the farmer to carry out twice daily. Such a system was not available, and the procedure would otherwise have had to be done by tube/funnel/jug and would therefore have been quite laborious.
Warming of the lavage fluid would also have been preferable.
The clinician placed considerable faith in the wondrous healing capacity of the bovine abdomen…
*If such a case presented today, suture material routinely chosen in bovines for C sections, rumenotomies, gastropexies, lap closures etc could be monofilament such as PDS, gauge M4. That said, there is still a role for carefully handled chromic catgut suture—in which case gauge Metric 5 or 6 would be selected.
Alternative broad-spectrum antibiotics available would include TMPS (bactericidal) or Tetracyclines (bacteriostatic)…
NSAIDs would be given for pain relief...
This case may present an acceptable methodology for treating some cases of bovine peritonitis.
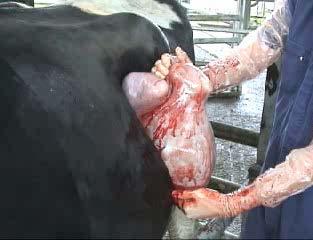
Avian and Exotics Veterinarian, Small Animal Specialist Hospital (SASH) | North Ryde NSW
e. oclarke@sashvets.com
Perspective No. 159
Introduction
Whether you are a new graduate or a seasoned veterinary practitioner, whether you work in general practice, emergency or with a high caseload of exotic pets, chances are you have, or undoubtedly will, treat at least one rabbit in your career. The popularity of rabbits as pets is continuing to grow and with dental disease being one of the most common (if not the most common) health conditions seen in this species, it is important for clinicians to understand this disease including aetiopathogenesis, clinical presentation, diagnosis and management.
The anatomy of rabbit dentition differs greatly to that of dogs and cats. Rabbits have 28 permanent teeth. The dental formula of an adult rabbit is: I 2/1, C 0/0, P 3/2, M 3/3. Typical of true herbivores, they lack canines. The powerhouse of their dentition are the premolars and molars, or collectively, the cheek teeth. These teeth, encased in alveolar bone¹ and powered by the strong masseter muscle, are responsible for shearing and grinding fibrous plant matter. Rabbit teeth are elodont (continuously growing), aradicular (lack a true root) and hypsodont (long crowned). Each tooth comprises a supragingival portion (clinical crown) and a much longer subgingival portion (reserve crown). There is a wide diastema between the incisors and cheek teeth and the occlusal pattern of the cheek teeth is anisognathic, meaning the mandible is narrow relative to the maxilla.²
Olivia is an avian and exotics veterinarian at the Small Animal Specialist Hospital (SASH) in Sydney. She has attained membership qualifications in both Avian Medicine and Surgery and Medicine and Surgery of Unusual Pets. Olivia enjoys the diversity of animals and cases that comes with being an avian and exotics veterinarian. Her favourite patients are rabbits and she has particular interests in gastrointestinal disorders, dental disease, renal disease and improving quality of life and managing comorbidities in geriatric rabbits. Aside from clinical practice, Olivia is passionate about continuing education and enjoys teaching clients and colleagues about avian and exotic pet health care. She is also regularly involved in presenting webinars, seminars and conference presentations. Olivia is continually striving to improve current practices and provide gold standard care to these species.

Tutor: Dr Olivia Clarke
CVE Members enrol for $150, Non-Members $300
Rabbit teeth grow on average approximately 2-3mm a month.³ Therefore, constant attrition is required to prevent overgrowth. Cheek teeth are brought into occlusion by lateral mandibular movement.²
Longitudinal grooves
Longitudinal grooves
Both congenital and acquired dental disease exists. Whilst acquired disease is seen most commonly, congenital dental disease is also worthy of mention as it too is seen in practice and with increasing frequency given the increased popularity of brachycephalic and dwarf rabbit breeds as pets. Tooth wear is often less effective in these breeds as their skull conformation affects mastication patterns.
Congenital disease is most common in brachycephalic breeds and breeds that are less than 1.5kg at adult body weight e.g., mini-lop and Netherland dwarf breeds. This condition is typically characterised by maxillary brachygnathism and incisor malocclusion.⁴ Primary cheek teeth abnormalities are infrequent in these cases but may occur secondarily to incisor overgrowth and malocclusion impacting occlusion and wear of the cheek teeth.² Maxillary brachygnathism has been shown to be an inherited autosomal recessive trait and as such these rabbits should not be bred from.⁵ Most cases of congenital dental disease are recognised very early in life. Complete incisor extraction is typically curative provided cheek teeth abnormalities have not developed.

The most common manifestation of dental disease, this condition is often referred to as progressive acquired syndrome of dental disease (PSADD), reflecting the progressive and unfortunately incurable nature of this condition.⁶ It is of upmost importance that clinicians recognise this and are diligent in informing clients from
the very first consult that the condition is irreversible and will continue to progress despite interventions.
In the author’s experience, PSADD is usually diagnosed from two years old and onwards; however, it is sometimes diagnosed in rabbits under 12-months-of-age.

Dental wear is achieved by subjection to mechanical stress.² Anything that can affect wear or tooth position can result in malocclusion, altered attrition and eruption of the teeth.
Inadequate intake of long stem, abrasive fibre (hay and grass) is the most common cause of acquired dental disease.1,2 Lack of dental wear causes coronal elongation, stretching of masseter muscles and an increase in resting intraocclusal pressure.⁴
The theory is that this increased intraocclusal pressure places intrusive load on the teeth that results in negative growth and elongation of reserve crowns. Elongation of reserve crowns causes apical deformities and penetration of the alveolar bone.¹ Elongation of reserve crowns also impinges on sensory nerves (inferior alveolar and maxillary branches of trigeminal nerve), likely causing pain when occlusal pressure is applied during mastication. Widening of interdental spaces and periodontal ligament remodelling results in gaps that
trap debris and bacteria, often leading to periapical infections, abscesses and osteomyelitis.¹
Metabolic bone disease is also theorised to play a role in the development of dental disease in rabbits.² Rabbits have a much greater requirement for calcium due to continuous tooth eruption.⁴ Whilst rabbits are highly efficient at absorbing calcium, poor quality diets such as cereal rations are low in calcium.² Calcium deficient diets fed to laboratory rabbits resulted in 20% vertebral bony loss.⁴ Similar changes have been seen in laboratory rabbits fed vitamin D deficient diets; with a significant increase in rabbits being housed indoors, vitamin D deficiency may be prevalent in these cohorts.
In other species with continually erupting teeth such as rats (incisors), a calcium and vitamin D deficient diet results in enamel hypoplasia in only four weeks.⁴ Rats that are calcium deficient develop alveolar bone demineralisation, which can result in tooth mobility and development of malocclusion.⁴ Furthermore, many radiographic features of dental disease in rabbits are compatible with metabolic bone disease e.g., alveolar bone osteopaenia and resorption of the lamina dura.⁴
Skull trauma such as mandibular symphysial fractures can also affect tooth position hence leading to malocclusion and dental disease.²
Genetic factors also play a role in development of dental disease with an over-representation of dwarf breeds.2,4
There is a myriad of presenting clinical signs associated with dental disease in rabbits. It is important for clinicians to recognise that dental disease will not always (and frequently does not) manifest as obvious signs of dental pain such as inappetence, drooling and dysphagia. Due to the hypsodont nature of rabbit teeth with very long reserve crowns, elongation of these can impact other skull structures such as the nasolacrimal ducts, nasal cavities, maxillary sinuses, mandibular cortices and the retrobulbar space. Therefore, dental disease can present as epiphora, dacryocystitis, sneezing, chronic rhinitis, facial swellings, and exophthalmos.
Rabbits are fastidious groomers, and their dentition is employed to facilitate this process; therefore, any rabbit with an unkempt fur coat or perineal soiling should be considered as potentially having dental disease. Cutaneous myiasis or fly strike cases should likewise prompt clinicians to consider dental disease. Rabbits with dental pain and/or inadequate dietary fibre are also less likely to ingest caecotrophs directly from their anus and whilst uneaten caecotrophs may also be a sign of other conditions, this may also be a feature of dental disease. It is important that a thorough dental exam is
performed in all rabbits, especially when displaying any of the above clinical signs.
Diagnosis and staging of dental disease in rabbits involves acquiring a detailed history, including information about diet, performing a physical exam and skull imaging.
A thorough physical exam should be performed in all rabbits. As described, a myriad of clinical signs may be present in a rabbit with dental disease; therefore, all body systems need to be assessed on clinical exam. However there are specific techniques to performing a thorough dental exam in rabbits.
The first step begins before looking in the rabbit’s mouth and includes assessing for facial symmetry. Facial swellings or exophthalmos can be a sign of dental disease due to retrobulbar periapical abscessation. Furthermore, obvious changes such as incisor malocclusion may be easily detected before performing an intraoral exam.
The next step should be palpation of the skull including palpation of the hemimandibles, hemimaxillae and nasal bone for any asymmetry, swelling or irregularities. If there is reserve crown elongation of the mandibular teeth, this can usually be easily felt as bony prominences when palpating along the mandibular cortices. Gentle lateral movements of the mandible helps to identify pain and asymmetry of cheek teeth.²
Following skull palpation, a visual assessment of the eyes and nares should be performed to identify any oculonasal discharge. Tear duct patency can be assessed easily in most rabbits consciously using fluorescein via the lacrimal puncta. Topical anaesthetic can be used to facilitate this.
An intraoral exam is undoubtedly the most stressful part of the physical exam and hence it should be the last step. An otoscope or nasal speculum piece can be used depending on operator preference. First, part the lips to assess the incisors for any abnormalities such as enamel hypoplasia or malocclusion before introducing the otoscope into the mouth and assessing each dental arcade. It is important for clinicians to familiarise themselves with the normal appearance of the dentition. The mandibular cheek teeth typically have a vertical ridge creating small lingual points; these should not be confused with dental spurs.2.4 However, spurs, significant variability in crown heights and dystrophic enamel are all indicators of dental disease.
An important key to note and to emphasise to clients is that only up to 50% of dental abnormalities can be seen via conscious intraoral exam.² A complete assessment
requires a more in-depth intraoral exam under sedation or anaesthesia ideally grossly and endoscopically coupled with skull imaging.
Diagnosis: Imaging
Skull imaging should be considered a non-negotiable step when diagnosing dental disease in rabbits. Imaging is important for assessment of bony structures, reserve crowns and apices.² Skull imaging allows the clinician to stage disease, plan dental surgery, and offer more accurate prognostic information.⁶
Radiographs and computed tomography (CT) scans are most commonly used; however, intraoral dental radiography has also been described in rabbits. To obtain diagnostic images any skull imaging needs to be performed under sedation or general anaesthetic to facilitate positioning and to minimise patient stress and motion artefact.
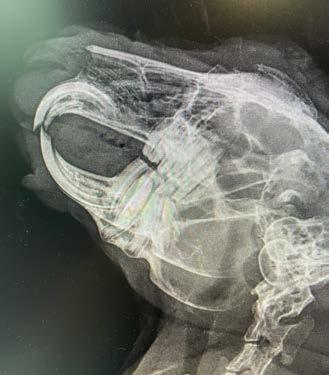
Five-view radiographs are performed due to superimposition of the skull structures and dentition. The study views include a straight lateral, a left lateral oblique, a right lateral oblique, a dorsoventral or ventrodorsal view and a rostrocaudal (skyline) view. Lateral oblique radiographs are positioned at approximately a 30-degree angle. These views are imperative to diagnosing apical abnormalities as they separate each hemimandible for individual assessment.
Imaging is used to assess the overall bone density, the occlusion of the teeth, clinical and reserve crown lengths, enamel quality, evidence of apical disease and non-dental pathology such as otitis or sinusitis.
Crossley-Boehmer lines were developed as an objective tool to diagnose dental disease radiographically in both rabbits and rodents. Anatomic landmarks are used to draw reference lines that allow identification of dental abnormalities, particularly in regard to dental elongation and malocclusion.⁶
In a rabbit unaffected by dental disease, the alveolar bone and dentition are well defined, the cheek teeth meet in a zigzag occlusal pattern and there are no apical abnormalities. There are numerous radiographic features of dental disease varying with severity of disease but include malocclusion, elongation and rounding of crowns, bony changes such as osteolysis or sclerosis and apical deformities.
For skull CT scan, the patient can be placed in sternal recumbency. CT scan may not be accessible for all practices, nor financially viable for all clients. However, CT accuracy has shown to be superior to 5-view radiography in 80% of patients for diagnosis and prognosticating.⁸ CT scan is strongly recommended for assessment of periapical abscessation, osteomyelitis and concurrent skull pathology such as otitis media or sinusitis.
Once a diagnosis of dental disease is confirmed, what happens next? One of the fundamental keys to management of dental disease in rabbits is managing client expectations. It is important that the client understands that dental disease is an irreversible, incurable, and progressive condition. Despite this, many rabbits are able to live out a normal lifespan with a good quality of life provided the owner is committed to the ongoing monitoring and palliative care required.
The mainstay of management are dental procedures, namely the coronal height reduction or commonly referred to as a ‘burr down’. This is the process of reducing the crown heights and restoring the occlusal pattern of the teeth using an electronic dental burr. A slow speed burr is used attached to a straight nose cone and specially designed mouth gags in conjunction with a light source are used to visualise and access the intraoral cavity. Magnification is always beneficial, and the author routinely utilises stomatoscopy with a rigid endoscope as well as gross visualisation of dental structures.
Individuals with more advanced dental disease may require dental extractions and surgical debridement of periapical abscesses and necrotic or infected bone. The indications for dental extractions include incisor malocclusion, highly mobile teeth, or infected teeth. Often, in cases of periapical abscesses, affected teeth can be extracted via an extraoral approach.
Dental procedures should always be performed under a general anaesthesia, ideally with airway protection via an endotracheal tube.
Prior to performing any dental procedures it is important that the patient is medically stabilised. Many patients with dental disease are metabolically compromised at time of presentation due to anorexia and pain. These patients may require fluid therapy, supportive feeding, and analgesia to ensure they are in a stable condition to undergo anaesthesia. Pre-anaesthetic blood work, urinalysis and urine protein creatinine ratio can also be considered, particularly for geriatric patients.
In addition to dental procedures, treatment should be aimed at reducing pain associated with disease and correcting diet. A detailed discussion of rabbit nutrition is beyond the scope of this article; however, a diet that comprises at least 80% fibre in the form of hay or grass is quintessential to maintaining healthy dentition and digestion in rabbits. Unfortunately, once dental disease is established, rabbits will rarely eat close to this amount of hay or grass but it is important that intake of these is encouraged. Grains, seeds and grain-based pellets need to be avoided and high sugar foods such as fruits and carrots should be fed sparingly, if at all.
Antibiotic therapy is required in cases of periapical abscessation and osteomyelitis. Antibiotics should be selected based on culture and susceptibility testing, but empirical antibiotic choices should include those that target anaerobic bacteria such as penicillin and chloramphenicol.⁶ Depending on abscess location
 Figure 6. A rabbit undergoing a dental procedure with mouth gags placed for exposure.
Figure 6. A rabbit undergoing a dental procedure with mouth gags placed for exposure.
Clinical signs
Differential diagnoses
Anorexia, hyporexia Any source of pain, illness, or stress
Weight loss
Renal insufficiency
Neoplasia
Cardiovascular disease
Rabbit megacolon syndrome (RMS)
Reluctance to eat hay or hard foods Skull or facial trauma
Sneezing, nasal discharge
Bacterial rhinitis e.g., pasteurellosis
Nasal foreign body
Sinusitis
Nasal or sinus mass
Epiphora, dacryocystitis Stenosis of nasolacrimal duct associated with conformation (e.g., brachycephalism), trauma or age.
Secondary to respiratory disease
Dysphagia Skull or facial trauma
Bruxism Any source of pain
Small, dehydrated faecal pellets, soft poorly formed faeces
Rabbit gastrointestinal stasis syndrome (RGIS)—which can be a result of dental pain.
Rabbit megacolon syndrome (RMS)
Dietary factors e.g., inadequate fibre
Perineal soiling
Urinary disease (urinary sludge, urinary tract infection, urolithiasis)
Incontinence
Diarrhoea
Obesity
Pain conditions that interfere with grooming (spondylosis, osteoarthritis)
Uneaten caecotrophs
Unkempt fur coat
Dietary factors e.g., inadequate fibre
Pain conditions that interfere with grooming (spondylosis, osteoarthritis)
Pain conditions that interfere with grooming (spondylosis, osteoarthritis)
Obesity
Nutritional deficiencies
Mites
Facial swellings
Neoplasia
Cyst (e.g., tapeworm)
Trauma
Exophthalmos
Retrobulbar mass (neoplasia, granuloma, abscess of non-dental origin)
Tapeworm cyst (retrobulbar)
Thymoma or other mediastinal mass if bilateral
and accessibility, topical antibiotics in the form of poloxamer gels or antibiotic-impregnated beads show a lot of promise when used in combination with systemic antibiotics.²
The post-operative recovery period following dental procedures varies greatly amongst individuals depending on stage of dental disease, extent of dental procedure performed, length of anaesthesia, the patient’s age and comorbidities and individual discrepancies in regard to pain tolerance and stress thresholds. Generally, recovery period can range from as little as a couple of days for basic coronal height reductions to four to six weeks for those with significant periapical abscesses and/or osteomyelitis.
An appropriate post-operative nursing plan is imperative to aiding recovery and should include supportive feeding and analgesia. Gastroprotectants, prokinetics and antibiotics may also be required in some cases. A high fibre herbivore formula should be syringe fed until the rabbit’s normal appetite and defecation resumes. Rabbits should be fed every four to six hours initially and this can be tapered as voluntary intake increases. During recovery, it is important to continue to offer all their normal foods. Opioid analgesics such as fentanyl or buprenorphine may be required for the first 24-48 hours after dental surgery.⁶ Meloxicam is a very effective anti-inflammatory analgesic used in rabbits. Adjunctive analgesics such as gabapentin or tramadol may also be required in certain cases.
Any practitioner enthusiastic about treating rabbits can play an important role in the diagnosis and long-term management of rabbit patients with dental disease.
Given the equipment, expertise and skill required to perform rabbit dental procedures and anaesthetics, it is generally recommended that dental procedures are carried out by, or under the guidance of, experienced exotics veterinarians. Advanced cases of dental disease involving periapical abscessation and osteomyelitis are particularly complex, and referral is strongly advised for management of these cases.
Dental disease is a common and complex condition seen frequently in pet rabbits. Aetiology is multifactorial and a range of clinical signs may be seen. It is important for clinicians to be informed and to understand the anatomical and physiological features of rabbits relevant to dental disease, the steps required to confirm diagnosis and the importance of both client education and developing a long-term management plan for rabbits with this condition.
1. Powers LV. 2016. Management of Dental Abscesses in Rabbits. In Pacific Veterinary Conference. San Francisco.
2. Meredith A. Rabbit Dentistry. 2007. European Journal of Companion Animal Practice . 17(1): 55-62.
3. Lord B. 2012. Management of Dental Disease in Rabbits. Veterinary Nursing Journal . 27(1): 18-20.

4. Varga M. Dental Disease. In: Varga M, ed. Textbook of Rabbit Medicine. 2. Butterworth-Heinemann; 2014: 206-227.
5. Donnelly TM, Vella D. 2016. Anatomy, Physiology and Non-Dental Disorders of the Mouth of Pet Rabbits. Veterinary Clinics of North America Exotic Animal Practice 19(3): 737-756.
6. Harcourt Brown F. 2009. Dental Disease in Pet Rabbits 3. Jaw Abscesses. In Practice 31: 496-505.
7. Capello V & Cauduro A. 2016. Comparison of Diagnostic Consistency and Diagnostic Accuracy Between Survey Radiography and Computed Tomography of the Skull in 30 Rabbits With Dental Disease. Journal of Exotic Pet Medicine . 25(2): 115-127.
8. Boehmer E, Crossley D. 2009. Objective Interpretation of Dental Disease in Rabbits, Guinea Pigs and Chinchillas. Tierärztliche Praxis Kleintiere . 37(4): 250-260.
The SSVS Annual Research Report 2023 highlights ground-breaking research and the positive impact made for animals, people, and the veterinary community. This important research has the power to transform lives and advance veterinary science.
Read in the eBook.


This course presented by Taronga will support veterinary professionals and veterinary nurses to develop knowledge and skills in native wildlife triage, including first aid, initial treatment and emergency care.


• Supported by the NSW Government Department of Planning and Environment

• AVA and VNCA certified
• Online Course (20 CPD points)
• Hands-on Workshop (12 CPD Points) –available in NSW, QLD and Vic (see website for details)
• Subsidised positions available to eligible applicants nationally
Applications open on October 9
Email: tarongprofvet@zoo.nsw.gov.au
Visit: https://taronga.org.au/vet-professional-training
Workshops available at: