The table shows their physical properties.
2.10
Diamond
Silicon dioxide
lustrous (shiny), transparent and colourless
white crystalline solid
very hard
very hard
very high melting point
very high melting point
insoluble in water
insoluble in water
does not conduct electricity
does not conduct electricity
3
Explain why the properties of silicon dioxide are quite similar to the properties of diamond.
4
Which two properties of diamond make it suitable for use in high temperature drilling tools?
Three-dimensional structures Diamond is not the only giant covalent structure that carbon forms. It also forms graphite. The structure of graphite is rings of carbon atoms in layers. The bonds do not act in all directions. Covalent bonding usually means that all the available electrons are shared, so they do not conduct electricity. Graphite is an exception as graphite has delocalised electrons that can move through the layers of its structure. Large structures such as quartz and diamond are hard because of a regular three-dimensional pattern in the bonding of their atoms. The melting point depends on the energy required to break all the bonds joining the atoms or molecules. As the giant covalent structure has many bonds, in many directions, the energy needed will be high so the melting point will be high. Covalent compounds are generally insoluble as the bonds are not ionic. 5
Explain why graphite is not as hard as silicon dioxide.
6
Suggest why graphite bricks are suitable for use in furnaces.
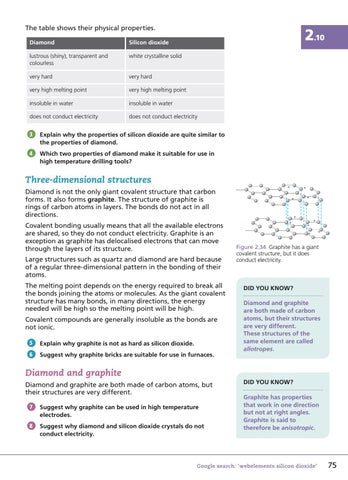
Figure 2.34 Graphite has a giant covalent structure, but it does conduct electricity.
DID YOU KNOW? Diamond and graphite are both made of carbon atoms, but their structures are very different. These structures of the same element are called allotropes.
Diamond and graphite Diamond and graphite are both made of carbon atoms, but their structures are very different. 7
Suggest why graphite can be used in high temperature electrodes.
8
Suggest why diamond and silicon dioxide crystals do not conduct electricity.
DID YOU KNOW? Graphite has properties that work in one direction but not at right angles. Graphite is said to therefore be anisotropic.
Google search: 'webelements silicon dioxide'
75054_P054_091.indd 75
75
5/24/16 8:52 PM