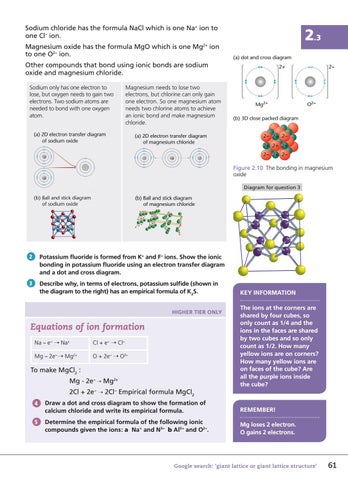
Sodium chloride has the formula NaCl which is one Na+ ion to one Cl – ion. Magnesium oxide has the formula MgO which is one Mg2+ ion to one O2− ion.
2.3 (a) dot and cross diagram
Other compounds that bond using ionic bonds are sodium oxide and magnesium chloride. Sodium only has one electron to lose, but oxygen needs to gain two electrons. Two sodium atoms are needed to bond with one oxygen atom.
Magnesium needs to lose two electrons, but chlorine can only gain one electron. So one magnesium atom needs two chlorine atoms to achieve an ionic bond and make magnesium chloride.
(a) 2D electron transfer diagram of sodium oxide Na
(a) 2D electron transfer diagram of magnesium chloride Cl
Mg
Cl
2+
Mg2+
2–
O2–
(b) 3D close packed diagram 2– 2+ 2– 2+ 2+ 2+ 2– 2+ 2–
Figure 2.10 The bonding in magnesium oxide
O Na
Diagram for question 3 ((b) b)) Ball all and a d stick ti k diag agra g am m of sodium of odi oxide ide
(b) Ball and stick diagram of magnesium chloride
2
Potassium fluoride is formed from K+ and F – ions. Show the ionic bonding in potassium fluoride using an electron transfer diagram and a dot and cross diagram.
3
Describe why, in terms of electrons, potassium sulfide (shown in the diagram to the right) has an empirical formula of K2S. HIGHER TIER ONLY
Equations of ion formation Na – e – ➝ Na+
Cl + e – ➝ Cl –
Mg – 2e – ➝ Mg2+
O + 2e – ➝ O2–
To make MgCl2 : Mg - 2e – ➝ Mg2+ 2Cl + 2e – ➝ 2Cl – Empirical formula MgCl2
KEY INFORMATION The ions at the corners are shared by four cubes, so only count as 1/4 and the ions in the faces are shared by two cubes and so only count as 1/2. How many yellow ions are on corners? How many yellow ions are on faces of the cube? Are all the purple ions inside the cube?
4
Draw a dot and cross diagram to show the formation of calcium chloride and write its empirical formula.
REMEMBER!
5
Determine the empirical formula of the following ionic compounds given the ions: a Na + and N3– b Al3+ and O2–.
Mg loses 2 electron. O gains 2 electrons.
Google search: 'giant lattice or giant lattice structure'
75054_P054_091.indd 61
61
5/24/16 8:50 PM