Four randomized double-blind, placebo-controlled trials
Clairvee® relieves vaginal discharge and itching in patients without an infection in just 15 days
IN A RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRIAL OF 40 SYMPTOMATIC PATIENTS WITHOUT AN INFECTION ON CULTURE (NUGENT SCORE OF 4-6) 1
Clairvee significantly improves vaginal itching and discharge vs. placebo in just 15 days (p<0.001)
After taking Clairvee for 6 months, 83% of women maintained a balanced vaginal microbiome vs. only 21% in the placebo group2 (p<0.001) Clairvee balanced patients’ vaginal microbiome vs. placebo in just 15 days (p<0.01) and maintains a healthy vaginal pH1
Clairvee® relieves vaginal odor in just 2 weeks and provides continued relief over 6 months
The
Were no longer worried or embarrassed about their symptoms IN AN OPEN-LABEL TRIAL OF WOMEN WITH VAGINAL ODOR AND NO KNOWN VAGINAL INFECTION:1
After 6 months, 100% of moderate to severe odor patients reported a reduction in vaginal odor
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
After 6 months, the majority of women: Reported that their odor was completely eliminated* Reported improvements in vaginal irritation
In the same study, 93% of patients reported that they prefer to use Clairvee over other OTC products for vaginal symptoms
References: 1. Results from an open-label clinical study - interim data presented at the North American Menopause Society (NAMS) 2022 Annual Meeting. Full 6-month data on file. * Includes women who reported their odor as "moderate" at baseline and "absent" after 6 months using Clairvee. After 6 months, 54% said their odor was completely eliminated, 94% reported improvements in vaginal irritation, 80% were not worried about their symptoms, and 76% were not embarrassed about their symptoms. OTC = over the counter. Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™ and Bonafide® are trademarks of Bonafide Health, LLC. ©2024 Bonafide Health, LLC.
References: 1 Russo R, Karadja E, De Seta F. Beneficial Microbes. 2019;10(1): 19-26. ; 3 Russo R, Edu A, De Seta F. Arch Gynecol Obstet. 2018; 298(1):139-145. Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™, and Bonafide® are trademarks of Bonafide Health, LLC. ©2022 Bonafide Health, LLC. U.S. Patents: 10,363,222 and 11,185,545. ®
Bacterial vaginosis and vulvovaginal candidiasis are extremely common vaginal infections with high recurrence rates
30%
After intial treatment with antibiotics or antifungals: will experience a recurrence of BV within 3 months 1
Breaking the negative cycle of BV and VVC recurrence
Antibiotics or antifungals address the overgrowth of bacteria and fungi, but when treatment is stopped, BV and VVC associated pathogens quickly re-emerge in the vaginal environment, creating a vicious cycle.7,8,9
High recurrence rates after treatment are due to the survival of pathogenic bacteria and fungi without the return of lactobacilli.5,6
Antibiotic or antifungal treatment
58%
will experience a recurrence of BV within 1 year 1
with VVC will go on to have 3 or more episodes per year 2,3,4 20-34%
Reduction of lactobacilli
Recurrent Infection
In addition to treatment, restoring lactobacilli is critical to breaking the cycle of BV and VVC recurrence10
1. ACOG Practice Bulletin, Vaginitis in Nonpregnant Patients, Obstetrics & Gynecology: January 2020 - Volume 135 - Issue 1 - p e1-e17; 2. Foxman B, et al. J Low Genit Tract Dis. 2013 Jul;17(3):340-5.; 3. Blostein F, et al. Ann Epidemiol. 2017 Sep;27(9):575-582.e3; 4. Yano J, et al. BMC Womens Health. 2019 Mar 29;19(1):48.; 5. Cook R, et al. Journal of Clinical Microbiology, Apr. 1992, p. 870-877; 6. NYIRJESY, P. Am Fam Physician. 2001 Feb 15;63(4):697-703.; 7. Wilson J. Sexually Transmitted Infections 2004;80:8-11.; 8. Mayer BT, et al. J Infect Dis. 2015;212(5):793-802.; 9. Roth AC, et al. Genitourin Med. 1990 Oct;66(5):357-60.; 10. Wang, B., et al. Eur J Clin Microbiol Infect Dis 33, 1749–1756 (2014). ©2024 Bonafide Health, LLC.
® significantly reduces the likelihood of vaginal symptoms returning up to 6 months after treating BV
For your patients treated for BV, Clairvee significantly reduces the likelihood of vaginal symptoms returning at months 1, 4, and 6 (p<0.01 vs. placebo) IN A SIX-MONTH, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED TRIAL IN 48 WOMEN WITH BACTERIAL VAGINOSIS (BV) WHO WERE TREATED WITH ANTIBIOTICS AND GIVEN EITHER CLAIRVEE OR PLACEBO 1
After 4 months
88% of patients free of itching
88% of patients free of discharge
Patents: 10,363,222 and 11,185,545.
IN A SIX-MONTH, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED TRIAL IN 48 WOMEN WITH VULVOVAGINAL CANDIDIASIS (VVC) WHO WERE TREATED WITH ANTIFUNGALS AND GIVEN EITHER CLAIRVEE OR PLACEBO 1
For your patients treated for VVC, Clairvee significantly reduces the likelihood of vaginal symptoms returning at months 3 and 6 (p<0.01 vs. placebo)
2019; 62: 328–335. Clairvee®, Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™, and Bonafide® are trademarks
Clairvee’s® patented formulation is designed to maximize probiotic colonization and support the vagina’s natural defense system
Clairvee® Vaginal Blend
4 BILLION CFU LACTOBACILLUS ACIDOPHILUS BLA-14
1 BILLION CFU LACTOBACILLUS RHAMNOSUS BHN001
Unique lactobacilli strains with benefits specific to the vaginal microbiome.1,2,3,4
In an in vitro study, the probiotic strains in Clairvee significantly inhibited the growth of a broad spectrum of vaginal pathogens compared to control, with 100% inhibition of Gardnerella vaginalis5
Lactoferrin Folate
Naturally found in the vagina, lactoferrin serves as a prebiotic and plays a key role in vaginal defense by inhibiting pathogen binding to the mucosal surface.6
CLAIRVEE® VAGINAL BLEND
CLAIRVEE® VAGINAL BLEND + LACTOFERRIN
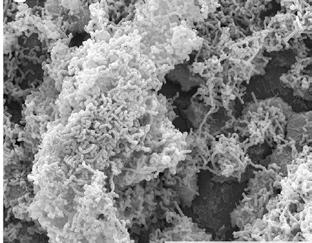
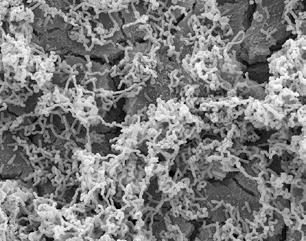
Ultrastructural analysis of the adhesion of the Lactobacillus strains in Clairvee to human cervix (HeLa) cells, with and without lactoferrin
Data suggests folate may play a role in maintaining a healthy vaginal microbiome and supporting probiotic growth7
When lactoferrin and the Clairvee® Vaginal Blend are combined they work synergistically to form a highly structured vaginal biofilm, enhancing colonization and supporting the vagina’s defense system6
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
BV = Bacterial Vaginosis, VVC = Vulvovaginal Candidiasis, GSM = Genitourinary Syndrome of Menopause. References: 1 Russo R, Karadja E, De Seta F. Beneficial Microbes. 2019;10(1): 19-26. 2 Russo R, Superti F, Karadja E, De Seta F. Mycoses. 2019; 62: 328–335. 3 Russo R, Edu A, De Seta F. Arch Gynecol Obstet. 2018; 298(1):139-145. 4 De Alberti D, Russo R, Terruzzi F, Nobile V, Ouwehand AC. Arch Gynecol Obstet. 2015; 292(4): 861-7. 5 L. Bertuccini et al. Int J Immunopathol Pharmacol. 2017 Jun;30(2):163-167.
6 L. Bertuccini et al. Lactobacilli and lactoferrin: Biotherapeutic effects for vaginal health. Journal of Functional Foods. 2018; 45: 86–94. 7 Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, Kebanoff MA. The Journal of Nutrition. 2007; 137(9): 2128–2133. Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™, and Bonafide® are trademarks of Bonafide Health, LLC. ©2024 Bonafide Health, LLC. U.S. Patents: 10,363,222 and 11,185,545.
Clairvee® provides your patients month-long protection from vaginal dysbiosis with just one capsule per day for 15 days
Just one oral capsule per day of Clairvee for 15 days is shown to produce significant vaginal colonization of the desired probiotic strains compared to placebo (p<0.001)
84% of women reported that Clairvee's dosing regimen was convenient5 ® IN A SEPARATE RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRIAL IN A TOTAL OF 40 WOMEN 3
Probiotic levels continue to increase even a week after patients stop taking Clairvee, demonstrating a prolonged dosing effect.4
L rhamnosus
L. acidophilus
L rhamnosus placebo
L. acidophilus placebo
* = p < 0.001 vs baseline
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
BV = Bacterial Vaginosis, VVC = Vulvovaginal Candidiasis, GSM = Genitourinary Syndrome of Menopause. References: 3 Russo R, Edu A, De Seta F. Arch Gynecol Obstet. 2018; 98(1):139-145. 4 De Alberti D, Russo R, Terruzzi F, Nobile V, Ouwehand AC. Arch Gynecol Obstet. 2015; 292(4): 861-7. 5. Spadt et al. Effects of Clairvee®oral probiotic supplementation on women’s vaginal odor in an open-label experience trial. Menopause. 2022. Clairvee®, Clairvee® Vaginal Blend Clairvee Capsule™, and Bonafide® are trademarks of Bonafide Health, LLC. ©2024 Bonafide Health, LLC. U.S. Patents: 10,363,222 and 11,185,545.
Clairvee Capsule™ Technology is optimized for probiotic protection, delivery and vaginal colonization

Clairvee Capsule™ Technology

Clairvee® Vaginal Blend and Lactoferrin
Outer Capsule Barrier
Liquid Folate
Inner Capsule Barrier
Double-walled, moisture-tight containers designed specifically for probiotics, providing unparalleled protection and ensuring stability and reliability.
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
BV = Bacterial Vaginosis, VVC = Vulvovaginal Candidiasis, GSM = Genitourinary Syndrome of Menopause. References: Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™, and Bonafide® are trademarks of Bonafide Health, LLC. ©2024 Bonafide Health, LLC. U.S. Patents: 10,363,222 and 11,185,545.
Clairvee® is safe, well-tolerated, and easy-to-use
GRAS FDA
Across four randomized, double-blind, placebocontrolled clinical trials in 176 women, side effects of the probiotics and lactoferrin in Clairvee were comparable to placebo. No serious adverse events reported.1,2,3,4
The probiotic strains in Clairvee are considered FDA GRAS Notified, or Generally Recognized as Safe, by the FDA, meaning that there is a consensus of expert opinion that the strains are safe for their intended use.
Lactoferrin is derived from milk, however, there are data suggesting that it does not show risk of milk allergies10
Patients with severe milk allergies or lactose intolerance should consult with their healthcare professional before use ®
CLAIRVEE IS NOT INTENDED TO REPLACE ANTIBIOTICS OR ANTIFUNGALS FOR THE TREATMENT OF BV OR VVC.
BV = Bacterial Vaginosis, VVC = Vulvovaginal Candidiasis, GSM = Genitourinary Syndrome of Menopause. References: 1 Russo R, Karadja E, De Seta F. Beneficial Microbes. 2019;10(1): 19-26. 2 Russo R, Superti F, Karadja E, De Seta F. Mycoses. 2019; 62: 328–335. 3 Russo R, Edu A, De Seta F. Arch Gynecol Obstet. 2018; 298(1):139-145. 4 De Alberti D, Russo R, Terruzzi F, Nobile V, Ouwehand AC. Arch Gynecol Obstet. 2015; 292(4): 861-7. 10. 10. Asthma and Allergy Foundation of America. Living with Food Allergies, 2022. Clairvee®, Clairvee® Vaginal Blend, Clairvee Capsule™, and Bonafide® are trademarks of Bonafide Health, LLC. ©2024 Bonafide Health, LLC. U.S. Patents: 10,363,222 and 11,185,545.

Patients with vaginal odor, itching, and discharge?
Recommend Clairvee® with confidence.
How to recommend Clairvee:
1. 2.
Clairvee is a once daily capsule, taken orally for 15 consecutive days each month
− Clairvee can be started anytime of the month, during or after antibiotics or antifungals and even during menses.
− For patients on antibiotics, Clairvee should be taken two hours before or after taking the antibiotics to ensure that the live probiotic strains are maximally effective.
Clairvee can provide benefits as early as 15 days, but should be taken for at least 6 months for best results
Clairvee is a dietary supplement taken as one oral capsule once daily for 15 consecutive days each month.
Clairvee is not meant to replace antibiotics or antifungals for the treatment of BV or VVC.
Four
®
1.Russo R, Karadja E, De Seta F. Beneficial Microbes. 2019;10(1): 19-26
6-month, randomized, double-blind, placebo-controlled study conducted in 48 women aged 18-50 years with symptomatic BV, Nugent score >7 and documented history of RBV who were treated with metronidazole 500 mg twice daily for 7 days and randomly assigned to receive either Clairvee or placebo (2 capsules/day for 5 days followed by further 10 consecutive days at the dosage of 1 capsule/day during the first month, and 1 capsule/day for 10 consecutive days every month after). Clairvee and placebo administration started simultaneously to the metronidazole therapy (on day 1). Vaginal dysbiosis defined as Nugent score >7 and presence of itching or discharge.
2.Russo R, Superti F, Karadja E, De Seta F. Mycoses. 2019; 62: 328–335
6-month, randomized, double-blind, placebo-controlled study conducted in 48 women aged 18-50 years with positive cultures for Candida spp., symptomatic acute episode of VVC and with documented history of recurrences confirmed by culture analysis who were treated with clotrimazole 100 mg daily for 7 days and randomly assigned to receive either Clairvee or placebo (2 capsules/day for 5 days followed by further 10 consecutive days at the dosage of 1 capsule/day during the first month, and 1 capsule/day for 10 consecutive days every month after). Clairvee and placebo administration started simultaneously to the clotrimazole therapy (on day 1). Unbalance defined as positive yeast on culture and presence of itching or discharge.
3.Russo R, Edu A, De Seta F. Arch Gynecol Obstet. 2018; 298(1):139-145
15-day, randomized, double-blind, placebo-controlled study conducted in 40 women aged 18-50 years with intermediate vaginal flora (Nugent score 4–6) who were randomly assigned to receive either Clairvee or placebo (1 capsule/day for 15 days).
4.De Alberti D, Russo R, Terruzzi F, Nobile V, Ouwehand AC. Arch Gynecol Obstet. 2015; 292(4): 861-7
21-day, randomized, double-blind, placebo-controlled study conducted in 40 healthy women aged 18-50 who were randomly assigned to receive either Clairvee or placebo (1 capsule/day for 2 weeks). Vaginal swabs were collected at 0, 1, 2 and 3 weeks and analysed for the consumed organisms by qPCR.
5.Results from an open-label clinical study - interim data presented at the North American Menopause Society (NAMS) 2022 annual meeting. Full 6-month data on file.
This study nationally enrolled women from 3 different medical centers. To enroll, women visited their health care provider, verbally confirmed they did not have a vaginal infection, and responded yes to the following question: During the past week, have you experienced odor from your vulva or vagina? Women (age 24-70) who were enrolled in the study and reported vaginal odor at baseline were included in the present analysis (n=33). Participants were instructed to take one Clairvee capsule daily for 15 consecutive days each month. Participants reported on their experience via online questionnaires at baseline, after taking the product for two weeks, and every two weeks thereafter. The following efficacy endpoints were measured: Vulvovaginal Symptoms Questionnaire (VSQ); rating of the severity of vaginal symptoms, including itching, discharge, burning, and odor, using a four-level scale (absent; mild; moderate; severe); and product satisfaction/experience questions. VSQ is 21 yes (1) or no (0)questions with four scales: Symptoms, Emotions, Life-impact, and Sexual impact. Subjects were compensated for their participation in the study.
6.Clairvee patient-reported outcomes survey.
Results from an optional, rolling nation-wide online survey conducted over 16 months (April 2022 - August 2023) with 1,010 customers who were taking Clairvee for at least 3 months. Customers were compensated for their participation.
7. L. Bertuccini et al. Int J Immunopathol Pharmacol. 2017 Jun;30(2):163-167
In vitro study assessing the antimicrobial activities of Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus GLA-14 strains and their combination against four different pathogens; Gardenerella vaginalis, Atopobium vaginae, Staphylococcus aureus, and Escherichia coli by co-culturing assay.
8. L. Bertuccini et al. Lactobacilli and lactoferrin: Biotherapeutic effects for vaginal health. Journal of Functional Foods. 2018; 45: 86–94
In vitro study evaluating the effects of a combination of L. acidophilus GLA-14 and L. rhamnosus HN001 with lactoferrin. Results obtained using a human cervix in vitro model showed that supernatants from both probiotics exert a beneficial effect on cervix cells and that both strains were able to be grown in biofilm and exhibited aggregation and adherence properties to biotic or abiotic surfaces.
9. Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, Kebanoff MA. The Journal of Nutrition. 2007; 137(9): 2128–2133
Prospective, observational study evaluating the association between diet and the presence of BV in a subset of 1521 women. Nonpregnant women 15–45 yrs old were enrolled from August 1999 to February 2002 when presenting for routine health care at 1 of 12 clinics in the Birmingham, AL area. Women were assessed at baseline and quarterly for a year for a total of up to 5 visits. At each visit, participants completed a questionnaire and underwent a standardized pelvic examination. Vaginal flora was evaluated by Gram stain according to Nugent criteria. BV was defined as a Nugent score of ≥7 and severe BV was defined as Nugent score ≥9 and vaginal pH ≥5.
10. NAMS Position Statement. Menopause: Journal of The North American Society. 2020; 27(9):pp978-979.
The genitourinary syndrome of menopause position statement of The North American Menopause Society.
A Panel of acknowledged experts in the field of genitourinary health reviewed the literature to evaluate new evidence on vaginal hormone therapies as well as on other management options available or in development for GSM. A search of PubMed was conducted identifying medical literature on VVA and GSM published since the 2013 position statement on the role of pharmacologic and nonpharmacologic treatments for VVA in postmenopausal women. The Panel revised and added recommendations on the basis of current evidence. The Panel’s conclusions and recommendations were reviewed and approved by the NAMS Board of Trustees.
11. Gliniewicz K, Schneider G.M., et al. Front. Microbiol. 2019
A total of 45 female subjects (aged 21 - 70 years) were enrolled in the study and assigned to one of three groups: 15 pre-menopausal (pre-M), 15 post-menopausal receiving no form of hormone replacement therapy (post-M non-HRT), and 15 postmenopausal receiving HRT (post-M HRT). Self-assessed symptoms were recorded from each group (i.e. dryness, itch and difficulty with intercourse). Skin temperature and pH, and quantification of biomolecules (via tape strip samples) were obtained from the labia majora, labia minora and introitus. Vaginal pH was also collected. Vaginal swab samples were also obtained, and the vaginal microbiomes of these women were characterized by 16S rRNA gene sequencing and bacterial abundances were quantified by qPCR.