








We strive to improve human health and contribute to a society enriched by smiles
Nous nous efforçons d’améliorer la santé humaine et de contribuer à une société enrichie de sourires
https://www.taihopharma.ca/en/










We strive to improve human health and contribute to a society enriched by smiles
Nous nous efforçons d’améliorer la santé humaine et de contribuer à une société enrichie de sourires
https://www.taihopharma.ca/en/

9 MESSAGE FROM THE PRESIDENT AND CEO by Andrew Casey, BIOTECanada
11 MESSAGE DU PRÉSIDENT ET CHEF DE LA DIRECTION par Andrew Casey, BIOTECanada DEPARTMENTS / SECTIONS
14 FEDERAL PERSPECTIVE / LE POINT DE VUE FÉDÉRAL
Empowering Canada’s biomanufacturing future
Préparer l’avenir de la biofabrication au Canada by the Honourable François-Philippe Champagne, P.C., M.P. Minister of Innovation, Science and Industry par l’honorable François-Philippe Champagne, C.P., député Ministre de l’innovation, des Sciences et de l’Industrie
18 INVESTMENT / INVESTISSEMENT
The 2024 BIOTECanada Investment Summit
Le sommet de l’investissement de BIOTECanada 2024
20 TALENT
Empowering commercialization to strengthen Canada’s life sciences sector
Appuyer la commercialisation pour dynamiser le secteur des sciences de la vie au Canada
24 ECOSYSTEM
Navigating the future: innovative paths to global health solutions
En exploration sur les chemins qui mèneront de l’innovation à des solutions de santé pour le monde
34 EXECUTIVE PROFILES / EXÉCUTIF
Clarissa Desjardins, Ph.D. Founder and CEO of Congruence Therapeutics
Clarissa Desjardins, Ph. D. Fondatrice et chef de la direction de Congruence Therapeutics
37 EXECUTIVE PROFILES / EXÉCUTIF
Tamer Mohamed, Founder & CEO, Aspect Biosystems
Tamer Mohamed, Fondateur et chef de la direction, Aspect Biosystems



58 LEGAL MATTERS / QUESTIONS DE DROIT
Unpacking patent boxes –federal government consultation holds promise for the life sciences industry
Régime privilégié des brevets : le gouvernement fédéral mène une consultation qui s’avère prometteuse pour l’industrie des sciences de la vie
62 KNOWLEDGE / CONNAISSANCE
British Columbia’s leap in life sciences: leading clinical trials and innovation with new research facilities
Le bond en avant de la Colombie-Britannique en sciences de la vie : elle passe à l’avant-scène des essais cliniques et de l’innovation grâce à de nouvelles installations de recherche
64 KNOWLEDGE / CONNAISSANCE
Collaborating for better access to clinical trials in Ontario
Une collaboration qui permet un meilleur accès aux essais cliniques en Ontario
66 KNOWLEDGE / CONNAISSANCE
A collaborative effort to improve access to cutting-edge treatments in Atlantic Canada
Un esprit de collaboration qui améliore l’accès aux traitements de pointe dans le Canada atlantique
84 ECOSYSTEM / ÉCOSYSTÈME
2024 New members
2024 : les nouveaux membres
90 BIONATION
Policymakers, industry leaders, and stakeholders to gather in Ottawa for BIONATION 2024
Les décideurs politiques, les dirigeants de l’industrie et les intervenants du secteur réunis à Ottawa à l’occasion de BIONATION 2024

Step into the forefront of innovation with BIOTECanada’s latest issue of insights magazine, dedicated to showcasing the best of Canadian biotechnology.
At this moment in time, with sustained momentum, Canada’s biotech industry is positioned for significant expansion. The coming years also promise to be a pivotal era for biotechnology in Canada, and together, will shape the future of this vital industry.
The spring issue of insights showcases exclusive interviews with industry leaders, federal government perspectives, and groundbreaking research highlighting the dynamic landscape and future potential of Canada’s biotech sector. From strategic biomanufacturing to solutions addressing global needs, discover the vibrant individuals, companies, and organizations propelling the ecosystem forward. Explore the next chapter of Canadian biotech, a narrative of collective momentum and innovation.
Découvrez l’avant-garde de l’innovation grâce au dernier numéro du magazine insights, consacré à la mise en valeur de ce que la biotechnologie canadienne a de mieux à offrir.
À ce point-ci dans le temps, grâce à un élan soutenu, le secteur canadien des biotechnologies pourrait bien connaître une expansion considérable. Les prochaines années promettent aussi d’être déterminantes pour les biotechs au Canada : elles façonneront l’avenir de cette industrie cruciale.
Le numéro d’insights de ce printemps contient des entrevues exclusives avec des chefs de file du secteur, et vous y découvrirez la vision du gouvernement fédéral, ainsi que les travaux de recherche de pointe qui forment le paysage dynamique du secteur canadien des biotechs et permettent d’entrevoir tout son potentiel. De la mise en place stratégique de capacités de biofabrication aux solutions destinées à répondre à des besoins mondiaux, « rencontrez » des personnes dynamiques et familiarisez-vous avec les entreprises et les organisations qui sont les moteurs de l’écosystème. Explorez le tout dernier chapitre de l’histoire canadienne des biotechnologies, fait d’innovation et d’un grand élan collectif.
Spring/Printemps 2024
Published for / Publié pour :
410 – 410 Laurier Ave West Ottawa, ON K1R 1B7 Tel: 613-230-5585 info@biotech.ca www.biotech.ca
EDITOR IN CHIEF / RÉDACTRICE EN CHEF : Nina Lewis
TRANSLATION / TRADUCTION : Sophie Campbell
Published by / Publié par :
700 – 251 Bank Street Ottawa, ON K2P 1X3 Tel: 613-317-8945 reception@taag.ca www.taag.ca
GRAPHIC DESIGNER / GRAPHISTE : Leslie Miles
PROJECT MANAGER / DIRECTEUR DE PROJET : Danielle Valois
DIRECTOR OF ADVERTISING SALES / DIRECTEUR, VENTES PUBLICITAIRES : Stephan Pigeon
©2024 BIOTECanada insights. Any errors, omissions or opinions found in this magazine should not be attributed to the publisher. The authors, the publisher and the collaborating organizations will not assume any responsibility for commercial loss due to business decisions made based on the information contained in this magazine. No part of this publication may be reproduced, reprinted, stored in a retrieval system or transmitted in part or whole, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written consent of the publisher.
©2024 BIOTECanada insights. Aucune erreur ou omission décelée dans ce magazine ou aucune opinion qui y est exprimée ne doit être imputée à l’éditeur. Les auteurs, l’éditeur et les organismes qui ont collaboré à la publication rejettent toute responsabilité à l’égard des éventuelles pertes commerciales pouvant découler de décisions d’affaires prises à la lumière des renseignements contenus dans ce magazine. Il est interdit de reproduire, de réimprimer, d’emmagasiner dans un système de recherche documentaire ou de transmettre cette publication en tout ou en partie, sous quelque forme ou par quelque moyen que ce soit (électronique, mécanique, photocopie, enregistrement ou autre), sans avoir obtenu au préalable le consentement écrit de l’éditeur.
TAAG is grateful for partial funding from the Canadian Periodical Fund, Department of Heritage. / TAAG est reconnaissante à Patrimoine canadien du financement partiel obtenu du Fonds du Canada pour les périodiques.
Funded by the Government of Canada Financé par le gouvernement du Canada
Publication Mail Agreement # / Numéro de convention de Poste-publication : #43136012 Return undeliverable Canadian addresses to: Retourner les numéros non distribuables à une adresse canadienne à : 700 – 251 Bank Street, Ottawa, ON K2P 1X3 Printed in Canada / Imprimé au Canada. Please recycle where facilities exist / Veuillez recycler là où ce service existe.





LATE 2023 AND INTO THE FIRST QUARTER OF 2024 WAS A PERIOD OF REMARKABLE SUCCESS AND GROWTH for the Canadian biotech industry. Noteworthy developments include significant investment deals and exits, major acquisitions by global pharma companies, the launch of new academic programs designed to cultivate talent in the sector, and the continued scaling up of several high potential Canadian biotech companies. This progress is a good reminder of the economic force the sector can be within the Canadian economy.
With its strong record of developing companies, entrepreneurship, and science, Canada continues to be a destination for investment and partnership. The over $4 billion in investments and partnerships Canadian biotech companies have signed with global pharma companies and other investors over recent months demonstrate the sector’s value as a generator of scientific discovery and company creation. The 2023 OECD Key biotechnology indicators rank Canada fourth globally in the number of biotech companies, a recognition of its thriving environment for discovery and development. Notably, the period from 2019 to 2023 saw biotech investment in Canada reaching $25.9 billion across 175 companies, demonstrating the confidence investors and pharma partners place in Canadian innovation.
Following the experience of solving for the pandemic and recognizing the need to prepare for future pandemic-like events, the federal government began strategically investing significantly to grow Canada’s life sciences sector and biomanufacturing capacity. The commitments and corresponding Biomanufacturing and Life Sciences Strategy have accelerated the sector’s growth. It is abundantly clear that the federal government and respective provincial governments are dedicated to growing the ecosystem and expanding the vibrant and thriving network of Canadian companies and researchers as they introduce their products into the global market. These commitments and investments demonstrate governments’ appreciation of the sector’s strategic and economic value.
Canada’s biotech industry is clearly positioned for significant expansion. The opportunity now before us is to leverage the investments and momentum to take the sector to its next level. While increasing the availability and effectiveness of investment capital continues to be essential,
often overlooked is the important role a leading domestic regulatory system can play in attracting investment and driving innovation.
With remarkable new technologies emerging, and recognized Canadian strengths in AI and regenerative medicine, it would behoove Canada to ensure its regulatory system is an equally recognized strength. An agile and leading regulatory system would greatly accelerate the attraction of new technologies while also driving the creation and growth of Canadian companies.

Investors and global pharma partners continue to view Canada as a valuable source of exciting innovation and companies in which to invest. As a result, compared to other similar sized biotech markets, Canada can be considered to be punching above its weight. But given the sector’s history of innovation and growth over recent years, the more important question becomes: is Canada punching to its true potential? Importantly, now is the time to add more stickiness to some of the high value Canadian companies.
As the Montreal, Toronto, and Vancouver biotech clusters anchor globally commercial companies, Canada is reaching its true potential as a global biotech nation.
In September, BIOTECanada will host BIONATION 2024 focussing on securing our future today with a set of scalable policies, investment tools, and regulatory measures that will build on our growing momentum.


LA FIN DE L’ANNÉE 2023 ET LE PREMIER TRIMESTRE DE 2024 ONT ÉTÉ UNE PÉRIODE DE SUCCÈS ET DE CROISSANCE EXCEPTIONNELLE pour l’industrie biotechnologique canadienne. Au nombre des réalisations notables figurent d’importantes opérations d’investissement et de sorties, des acquisitions majeures par des multinationales pharmaceutiques, la création de nouveaux programmes universitaires destinés à attirer des talents dans le secteur, et la croissance continue de plusieurs sociétés de biotechnologie canadiennes à fort potentiel. Ces avancées nous montrent bien la force économique que peut représenter le secteur au sein de l’économie canadienne. Fort de ses excellents résultats en matière de création d’entreprises, d’esprit entrepreneurial et de recherche scientifique, le Canada reste une référence en matière d’investissement et de partenariat. Les investissements et les partenariats de plus de 4 milliards $ que les entreprises canadiennes de biotechnologie ont conclus avec des multinationales pharmaceutiques et d’autres investisseurs au cours des derniers mois témoignent de la valeur du secteur en tant que vecteur de découvertes scientifiques et de création d’entreprises. Les indicateurs clés de l’OCDE en matière de biotechnologie pour l’année 2023 positionnent le Canada au quatrième rang mondial quant au nombre d’entreprises de biotechnologie, ce qui atteste de la vitalité de son environnement de recherche-développement. Plus précisément, au cours de la période de 2019 à 2023, les investissements en biotechnologie ont atteint au Canada 25,9 milliards $ répartis entre 175 entreprises, autre preuve de confiance envers l’innovation canadienne de la part des investisseurs et des partenaires pharmaceutiques. À la suite de la pandémie, le gouvernement fédéral a commencé à investir stratégiquement de manière significative pour assurer la croissance du secteur des sciences de la vie et de la capacité de biofabrication du Canada. Cela répondait à la nécessité de se préparer en vue d’éventuels événements de même nature que la pandémie. La création de la Stratégie en matière de biofabrication et de sciences de la vie s’est en ce sens avérée opportune et a permis de stimuler la croissance du secteur. Il est tout à fait clair que le gouvernement fédéral et chacun des gouvernements provinciaux sont déterminés à assurer la croissance de l’écosystème et de son réseau florissant d’entreprises et de chercheurs canadiens, alors qu’ils en sont à commercialiser leurs produits sur le marché mondial. Ces engagements et investissements témoignent de la
reconnaissance par les gouvernements de la valeur stratégique et économique du secteur.

L’industrie biotechnologique canadienne est en bonne voie de connaître une expansion significative. Il nous incombe maintenant de tirer parti des investissements et de l’élan qui en résulte pour faire passer le secteur à la vitesse supérieure. Si accroître l’accessibilité et l’efficacité des capitaux d’investissement reste essentiel, on oublie trop souvent le rôle important qu’un système réglementaire national de premier plan peut jouer dans l’obtention d’investissements et comme moteur d’innovation. Dans la foulée de l’émergence de nouvelles technologies de pointe et de la reconnaissance du Canada dans les domaines de l’IA et de la médecine régénérative, il serait souhaitable que le pays veille à ce que son système réglementaire soit également reconnu comme un atout. Un système réglementaire souple et de premier plan serait un facteur clé pour attirer de nouvelles technologies tout en entraînant la création d’entreprises canadiennes et en favorisant leur croissance. Les investisseurs et les partenaires pharmaceutiques internationaux continuent de considérer le Canada comme une formidable source d’innovations et d’entreprises prometteuses dans lesquelles investir. Par conséquent, par rapport à d’autres marchés biotechnologiques de taille similaire, le Canada peut être considéré comme jouant dans la cour des grands. Toutefois, compte tenu de l’innovation et de la croissance du secteur au cours des dernières années, la question la plus importante demeure la suivante : le Canada réalise-t-il son plein potentiel? Le moment est venu d’assurer la stabilité de certaines entreprises canadiennes à forte valeur ajoutée. À l’heure où les pôles biotechnologiques de Montréal, Toronto et Vancouver accueillent des entreprises commerciales d’envergure mondiale, il appert que le Canada est sur la bonne voie pour réaliser son plein potentiel en tant que nation biotechnologique d’envergure mondiale.
En septembre, BIOTECanada accueillera BIONATION 2024, rencontre qui sera axée sur la préparation de l’avenir dès aujourd’hui grâce à un ensemble de politiques modulables, d’outils d’investissement et de mesures réglementaires qui permettront de poursuivre sur notre lancée.
Pour obtenir de plus amples renseignements, veuillez lire ce :

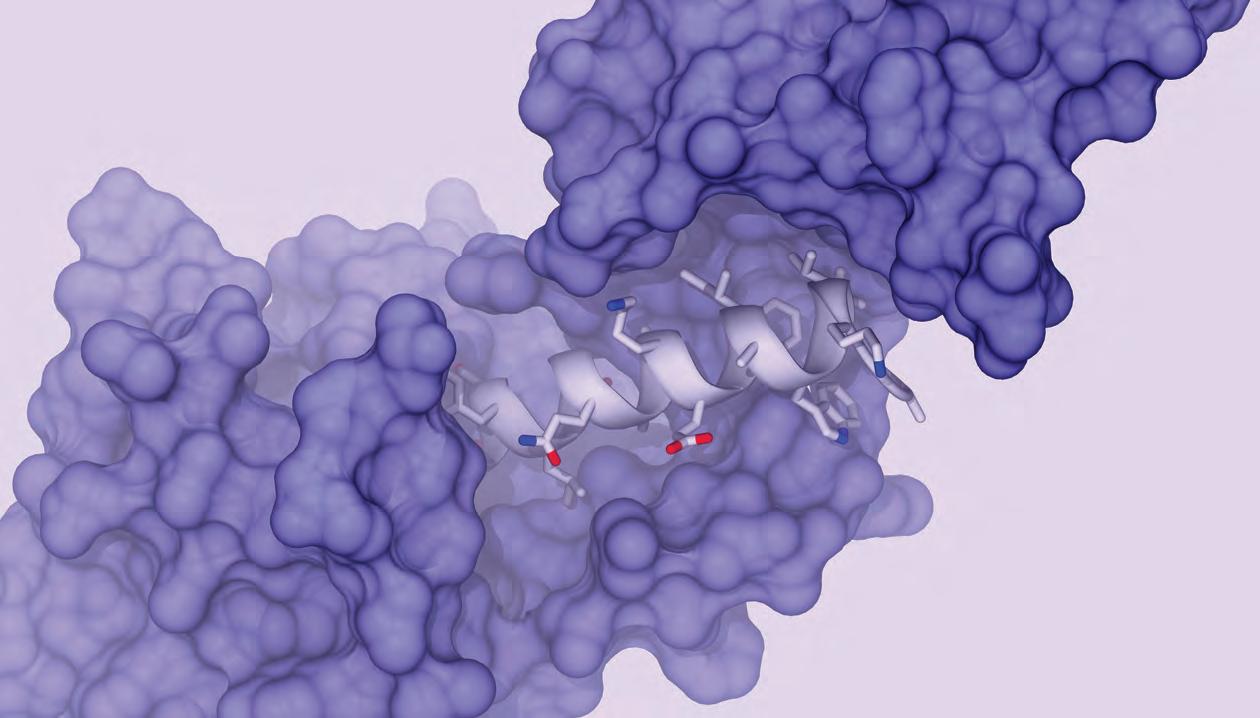

The future of precision medicine is being invented in Toronto. Again.
Toronto has been home to the biggest breakthroughs
IN 1921, BANTING AND BEST DISCOVERED THE FIRST MAJOR PEPTIDE THERAPEUTIC INSULIN.
Nearby, in 1984, Dan Drucker discovered glucagon-like peptide 1 (GLP-1), the predecessor to the blockbuster Ozempic.
In 2018, just across the street from these other discoveries, ProteinQure was founded. Since our first office in the Banting and Best building, we have been applying cutting-edge artificial intelligence (AI) to the design of novel peptide therapeutics.
WHAT ARE PEPTIDES AND WHY HAVE PEPTIDES BECOME AN IMPORTANT PART OF THE THERAPEUTICS TOOLBOX?
Peptides are small proteins. Human hormones, like insulin and GLP-1, are naturally occurring peptides and modulate a wide range of human physiology. Peptides have always had massive therapeutic potential, but their development into drugs has been limited due to a lack of traditional drug-like properties. Recent advances in chemistry and the ability to incorporate non-natural chemical building blocks into the peptides means we can now overcome those hurdles. Thus novel peptide chemistry has led to a surge of interest and blockbuster drugs (such as Mounjaro and Ozempic) over the last five years.
Peptides are a challenging class of therapeutics to design because we now have access to thousands of building blocks that just weren’t possible to incorporate a couple of decades ago. Consequently, the world lacks the large amounts of data needed to train traditional AI methods. Instead, ProteinQure has to build atomistic simulations to combine with custom deep learning architectures to model the 3D structure and predict the properties of these novel peptides.
We’ve completed over a half-dozen commercial partnerships, including three with the top 25 pharmaceutical companies in the world. Our tools have helped to discover novel therapeutics validated in the lab, spanning cancer, metabolic disease, and central nervous system (CNS) disorders. Partnerships with world-class drug discovery teams remain 50% of our work today.
The latest innovations in peptides are as delivery vehicles for important next-generation therapeutic payloads. Peptide conjugates contain three modular pieces: a targeting peptide, a linker, and a therapeutic payload (see Figure 1 below). Every tissue and cell type in the human body has a unique combination of receptors on its surface. By designing peptides to target specifically chosen receptors we can bring therapeutic payloads to the right tissue and inside the cell. Because of their ideal tolerability, size, and tissue specificity, companies are using peptides to deliver everything from gene silencers to radioisotopes. All of ProteinQure’s internal (wholly-owned) programs use patented peptides for tissue-specific delivery.
For ProteinQure’s therapeutics to impact millions of people we are going to need to develop into an integrated biotech company based in Toronto. While Toronto is well-known for AI, it has historically struggled to develop biotechs. That is why we’ve started by partnering with the best companies in the world. The validation, learning, and revenue from working with pharma companies are important; but we’ve set our ambitions on applying the technology we’ve built to construct an internally owned drug pipeline. Building a fully integrated set of R&D capabilities has historically been made challenging by the environment and reputation of life sciences in Ontario and Canada, but we are now seeing positive momentum that hopefully creates an environment where the next anchor companies can grow in Ontario.
CAN YOU TELL US HOW YOUR LEAD PROGRAM FOR BREAST CANCER FITS INTO THOSE PLANS?
ProteinQure’s lead program aims to treat the most deadly form of breast cancer (Triple Negative Breast Cancer). After completing the pre-clinical validation with Princess Margaret Cancer Centre we are moving towards Phase I clinical trials in late 2025. This program not only provides evidence that we can design novel therapeutics to address unmet clinical needs, but that a Toronto-based biotechnology company can advance wholly-owned programs from novel R&D to the clinic.
Our next internal program is focused on delivering gene therapies to the brain. This is a massive opportunity since we will potentially be able to tackle any brain-based disease with a genetic component. This gives ProteinQure a differentiated approach to treat a vast number of neurodegenerative diseases. We’ve already demonstrated a proof-of-concept in mice and are looking forward to further validating our delivery system with academics and other collaborators who are interested in the translation of our technology to save patient lives.

AT THE OUTSET OF THE PANDEMIC, CANADA HAD LIMITED BIOMANUFACTURING CAPACITY DESPITE ITS WORLD-LEADING SCIENTIFIC BASE. At that time, our government recognized the urgency of supporting anchor companies in Canada as well as the importance of collaborating to protect the health of Canadians and to respond effectively to the greatest health emergency of the century.
In response, we launched Canada’s Biomanufacturing and Life Sciences Strategy, a long-term vision to safeguard Canadians against future pandemics and other health emergencies by rebuilding our manufacturing base while fostering innovation and competitiveness in the domestic life sciences sector. The strategy consists of five pillars to support the sector, from training, to research, to testing, to the commercialization and production of life-saving vaccines and medicines.
Under the strategy, over $2.2 billion has been invested in Canada’s biomanufacturing, vaccine and therapeutics ecosystem through 38 cutting-edge projects across the country in a wide range of technology platforms, like Canada’s $415 million investment in Sanofi’s FLUZONE High-Dose Quadrivalent influenza vaccine facility in Toronto. Or our collaboration with AbCellera to develop antibody therapies during the pandemic and, based on the company’s success and growth, reinvested to help the company expand its research and manufacturing campus in Vancouver.
 P.C., M.P. Minister of Innovation, Science and Industry
P.C., M.P. Minister of Innovation, Science and Industry
And most recently, I was proud to attend the opening of Moderna’s state-of-the-art mRNA vaccine manufacturing plant in Laval, Quebec, representing a major milestone in Canada’s efforts to strengthen our domestic vaccine and therapeutics production capacity. The facility will be able to produce up to 100 million mRNA vaccine doses annually if needed. Moderna is also
D’UNE CAPACITÉ LIMITÉE EN BIOFABRICATION, MALGRÉ SA POSITION DE CHEF DE FILE MONDIAL DANS LE DOMAINE SCIENTIFIQUE . À l’époque, il a tout de suite été clair pour notre gouvernement que les entreprises phares au Canada avaient urgemment besoin d’aide et que l’on se devait d’instaurer un climat de collaboration pour protéger la santé des Canadiennes et des Canadiens et répondre efficacement à la plus grande urgence sanitaire du siècle.
En ce sens, nous avons créé la Stratégie en matière de biofabrication et de sciences de la vie, qui témoigne d’une vision à long terme en ce qui a trait à la protection des Canadiens contre les pandémies et d’autres urgences sanitaires éventuelles. L’objectif est de reconstruire notre infrastructure industrielle tout en stimulant l’innovation et la concurrence dans le secteur des sciences de la vie à l’échelle nationale. Cette stratégie repose sur cinq piliers destinés à renforcer le secteur, de la formation à la commercialisation et à la production de vaccins et de médicaments salvateurs, en passant par la recherche et les essais.
Dans le cadre de cette stratégie, plus de 2,2 milliards $ ont été investis dans l’écosystème canadien de la biofabrication, des vaccins et des produits thérapeutiques par le biais de 38 projets de pointe dans tout le pays. Parmi un large éventail de plateformes technologiques concernées par ces investissements, notons celui de 415 millions $ du Canada dans les installations de production du vaccin quadrivalent Fluzone à haute dose contre la grippe de Sanofi à Toronto. Notons aussi notre collaboration avec AbCellera pour la mise au point de traitements à base d’anticorps pendant la pandémie. À ce propos, compte tenu du succès et de la croissance de l’entreprise, nous l’avons aidée à agrandir son complexe de recherche et de production à Vancouver, grâce à un investissement supplémentaire.
Plus récemment, j’ai assisté à l’inauguration à Laval, au Québec, de l’usine ultramoderne de fabrication de vaccins à ARNm de Moderna. Nous pouvons être fiers de cette étape importante en vue de renforcer notre capacité de production nationale de vaccins et de produits thérapeutiques. L’établissement pourra produire jusqu’à 100 millions de doses de vaccins à ARNm par an si nécessaire. Moderna contribue également à la recherche-développement au Canada en établissant des partenariats avec des chercheurs et des entreprises canadiens. Elle a créé de nombreux emplois hautement qualifiés et bien rémunérés.

de la population canadienne en 2025, lorsque l’entreprise aura obtenu les certificats et autorisations réglementaires nécessaires
supporting research and development in Canada by establishing partnerships with Canadian researchers and companies, and it has created many highly skilled, wellpaying jobs.
We supported the Edmonton-based Canadian Critical Drug Initiative. The funding is being used for building a 40,000 square-foot drug manufacturing facility to produce new and critical medicines. Some of the funding will be used to upgrade an R&D facility to support early-stage companies and train technicians and scientists producing new medicines.
Those investments are a prime example of Canada seizing the moment. We had opportunities to fund the future of biomanufacturing and we took it.
In addition to these above biomanufacturing investments, we are ensuring that, in the long term, Canada has the talent, a R&D pipeline, robust SMEs, and security across the supply chain to support a dynamic and growing biologics manufacturing and innovation ecosystem. We are making important investments in building public sector biomanufacturing capacity, including the National Research Council’s new Biologics Manufacturing Centre and Clinical
Nous avons également financé l’initiative canadienne de fabrication de médicaments, dont les installations sont situées à Edmonton. Les fonds serviront à la construction d’une usine de fabrication de 40 000 pieds carrés dont la mission sera de produire de nouveaux médicaments essentiels. Une partie du financement servira à moderniser un établissement de R.-D. afin de permettre aux entreprises en phase de démarrage de se développer et de former des techniciens et des scientifiques à la production de nouveaux médicaments.
Ces investissements démontrent à quel point le Canada a su faire preuve de réactivité dans ce contexte. Nous avons saisi l’occasion qui nous avait été donnée de financer l’avenir de la biofabrication.
Au-delà de ces investissements dans la bioproduction, notre objectif est de veiller à ce qu’à long terme le Canada dispose des talents, d’un programme de R.-D., de PME solides et d’une chaîne d’approvisionnement éprouvée nécessaires au dynamisme et à la croissance de l’écosystème de l’innovation et de la fabrication de produits biologiques. Nous réalisons par conséquent d’importants investissements pour renforcer les capacités de biofabrication du secteur public,
Trial Material Facility. What’s more, the Clinical Trials Fund, led by the Canadian Institutes of Health Research, is making Canada’s largest-ever investment to support clinical trials and strengthen coordination across Canada via our universities and clinical trial networks. Additionally, to accelerate the research and development of next-generation vaccines, therapeutics and diagnostics, our government invested $127 million to support the expansion of high-containment laboratories in universities across Canada, strengthening their capacity to advance discoveries and promote training in biosciences. Over $500 million has been dedicated toward creating five research hubs led by universities in Alberta, British Columbia, Quebec and Ontario. These hubs will bring together academia, industry, and the public and not-for-profit sectors to strengthen translational and applied research,

notamment dans le nouveau Centre de production de produits biologiques (CPPB)—et ses installations de fabrication de matériel pour essais cliniques—du Conseil national de recherches du Canada. De plus, le Fonds pour les essais cliniques, dirigé par les Instituts de recherche en santé du Canada, réalise l’investissement le plus important jamais accordé par le Canada pour stimuler les essais cliniques et renforcer la coordination dans l’ensemble du pays par l’intermédiaire de nos universités et de nos réseaux d’essais cliniques. Par ailleurs, en vue d’accélérer la recherchedéveloppement en matière de vaccins, de produits thérapeutiques et de diagnostics de nouvelle génération, notre gouvernement a investi 127 millions $ dans l’agrandissement des laboratoires de haut niveau de confinement dans les universités du Canada : une aide essentielle pour leur permettre de faire avancer les connaissances et de promouvoir la formation dans le domaine des biosciences. Plus de 500 millions $ ont aussi été alloués à la création de cinq pôles de recherche dirigés par des universités de l’Alberta, de la Colombie-Britannique, du Québec et de l’Ontario. Ces pôles réuniront les universités, l’industrie, le secteur public et les organismes à but non lucratif et ont pour objectif : de renforcer la recherche translationnelle et appliquée, la formation et le perfectionnement des talents; de favoriser l’innovation, c’est-à-dire de stimuler la fabrication en aval; de contribuer à la préparation en cas de pandémie. L’objectif est de solidifier toute la chaîne de la recherche et de l’innovation en sciences de la vie, à partir des premières étapes de la recherche jusqu’aux essais cliniques et à la fabrication commerciale de médicaments et de technologies de pointe susceptibles de sauver des vies.
Le Canada accroît également ses capacités de biofabrication par le biais d’investissement dans des programmes de formation. Il s’agit par exemple de partenariats avec des organisations telles que MITACS ou d’encourager les talents qualifiés à se perfectionner dans le secteur.
Strengthening domestic capacity not only prepares the country for future health crises but also drives economic growth and establishes Canada as a leader in the biopharmaceutical industry.
training and talent development; support innovation that drives downstream manufacturing; and contribute to pandemic preparedness. The goal is to build and reinforce connections in the Canadian life sciences innovation continuum, from early-stage research through to clinical trials and commercial manufacturing of cutting-edge, life-saving medicines and technologies.
Canada is also enhancing its biomanufacturing capabilities through investments in training programs. Partnerships with organizations like Mitacs to nurturing skilled talent to grow the sector.
Because Canada is an export-oriented economy, its life sciences sector is integrated internationally on a number of fronts. Established facilities owned by Sanofi and GSK supply vaccines to domestic and international markets. Canada’s many expanding companies also have strong international client bases, such as for inputs that are manufactured here in Canada, and Canadian technology like the lipid nanoparticle contributed to the development of mRNA vaccines. Canada is also actively engaged in collaborations with like-minded partners, including through the memorandum of cooperation with the United Kingdom, which we announced last year, to advance biomanufacturing and quantum science capabilities. These partnerships demonstrate our commitment to fostering global cooperation and leveraging Canada’s unique strengths in the life sciences sector. Indeed, our efforts have not gone unnoticed: already, Canada has benefited from significant investments by leading companies such as AstraZeneca, Pfizer, J&J, Merck and Roche—all of which are contributing to our robust and growing ecosystem.
The Biomanufacturing and Life Sciences Strategy, backed by investments and partnerships, positions Canada as a competitive hub for innovation. Strengthening domestic capacity not only prepares the country for future health crises but also drives economic growth and establishes Canada as a leader in the biopharmaceutical industry.
federal perspective le point de vue fédéral
Le fait de renforcer les capacités nationales en matière de biofabrication permet non seulement de préparer le pays à d’éventuelles crises sanitaires, mais aussi de stimuler la croissance économique et de faire du Canada un leader de l’industrie biopharmaceutique.
Le Canada étant une économie orientée vers l’exportation, son secteur des sciences de la vie est présent dans plusieurs marchés sur la scène internationale. Les installations de Sanofi et de GSK permettent d’approvisionner les marchés nationaux et internationaux en vaccins. Les nombreuses entreprises canadiennes en expansion peuvent également compter sur de grands clients internationaux, notamment pour les intrants fabriqués au Canada. Une technologie canadienne comme celle des nanoparticules lipidiques a d’ailleurs contribué à la mise au point des vaccins à ARNm. Le Canada collabore aussi activement avec des partenaires aux vues similaires, notamment dans le cadre du programme de coopération avec le Royaume-Uni, annoncé l’année dernière, en vue d’augmenter ses capacités en matière de biofabrication et de science quantique. Ces partenariats témoignent de notre volonté de favoriser la coopération mondiale et de valoriser les atouts uniques du Canada dans le secteur des sciences de la vie. En effet, nos efforts ne sont pas passés inaperçus : le Canada a déjà bénéficié d’investissements importants de la part d’entreprises de premier plan telles qu’AstraZeneca, Pfizer, J&J, Merck et Roche, qui contribuent toutes à la solidité et à la croissance de notre écosystème.
La Stratégie en matière de biofabrication et de sciences de la vie, grâce à des investissements et à des partenariats, fait du Canada un pôle d’innovation concurrentiel. Le fait de renforcer les capacités nationales en matière de biofabrication permet non seulement de préparer le pays à d’éventuelles crises sanitaires, mais aussi de stimuler la croissance économique et de faire du Canada un leader de l’industrie biopharmaceutique.

The Art of Biotech Pharma Partnerships Panel: Left to right: Roberto Bellini (BSQUARED Capital), Eric Dobmeier (Chinook Therapeutics), Nancy Harrison (Amplitude Ventures), François Ravenelle (Inversago Pharma), Kristina Kitko (Eli Lilly), Avaleigh Milne (Roche)
Table ronde sur l’art des partenariats biotechpharma; de gauche à droite : Roberto Bellini (BSQUARED Capital), Eric Dobmeier (Chinook Therapeutics), Nancy Harrison (Amplitude Ventures), François Ravenelle (Inversago Pharma), Kristina Kitko (Eli Lilly), Avaleigh Milne (Roche)
CANADA HAS ESTABLISHED ITSELF AS A LEADING DESTINATION FOR BIOTECHNOLOGY INVESTMENT, research and innovation, and as a country where people want to live, and where businesses want to grow. With thousands of companies and organizations located throughout Canada feeding a national network of discovery and development, the sector is a catalyst for long-term economic growth in all regions of the country. Biotechnology is an economic success story that will continue to grow and provide opportunities for Canada’s highly skilled entrepreneurs, investors, research leaders, and medical professionals.
For the period of 2019-2023, Canada has seen record levels of investment totaling $25.9 billion into 175 Canadian companies showcasing the value of the Canadian industry. While there has been a global decline in the overall number of deals to date, the Canadian biotech sector has recorded $12 billion in 65 investment deals in 2023. This positive data for the industry set the stage for BIOTECanada’s 20 th annual Investment Summit held in March, and represented an opportune moment for both investors and companies to join the annual exclusive gathering hosted by BIOTECanada.
At the Investment Summit, BIOTECanada convened an esteemed panel of industry experts to share their personal journeys, invaluable experiences, and key lessons in forging successful global partnerships within the biotech sphere. Thank you to the distinguished panelists: Roberto Bellini of BSQUARED Capital, François Ravenelle of Inversago Pharma, Eric Dobmeier of Chinook Therapeutics, Kristina Kitko of Eli Lilly, Avaleigh Milne of Roche, and Nancy Harrison of Amplitude Ventures, for their contributions and participation, their insights have undoubtedly enriched our understanding and will inspire future collaborations in the biotech industry.

S’EST IMPOSÉ COMME UN PAYS DE PREMIER PLAN POUR LES INVESTISSEMENTS, la recherche et l’innovation dans le domaine de la biotechnologie, et il est vu comme un lieu où il fait bon vivre et qui favorise la croissance des entreprises. Grâce à des milliers de sociétés et d’établissements situés au Canada, qui alimentent un réseau national de recherche et développement, le secteur contribue, à long terme, à la croissance économique dans toutes les régions du pays. En effet, le succès économique de la biotechnologie est indéniable, et le secteur continuera à évoluer et à offrir des perspectives aux entrepreneurs, aux investisseurs, aux chercheurs et aux professionnels de la santé hautement qualifiés du Canada.
Découvrez les données sur les investissements de BIOTECanada ici :

Pour la période 2019-2023, le Canada a enregistré des niveaux record en matière d’investissements. On estime qu’au total 25,9 milliards de dollars ont été injectés dans 175 entreprises canadiennes, ce qui témoigne de la vitalité de l’industrie canadienne. Bien que le nombre d’accords conclus ait diminué à l’échelle mondiale, le secteur canadien de la biotechnologie a enregistré quant à lui 12 milliards de dollars en investissements en 2023 pour un total de 65 accords. C’est dans ce contexte positif que s’est tenu en mars le 20e sommet de l’investissement de BIOTECanada. L’occasion était parfaite pour inciter les investisseurs et les entreprises à participer à cette rencontre annuelle exclusive organisée par BIOTECanada. Le sommet de l’investissement a permis à BIOTECanada d’inviter des experts réputés à raconter, à l’occasion d’une table ronde sectorielle, leur parcours personnel, leurs expériences marquantes et les principales leçons qu’ils ont tirées des partenariats mondiaux noués dans la sphère des biotechs. Nous remercions les distingués panélistes qui ont participé à la table ronde : Roberto Bellini de BSQUARED Capital, François Ravenelle d’Inversago Pharma, Eric Dobmeier de Chinook Therapeutics, Kristina Kitko d’Eli Lilly, Avaleigh Milne de Roche et Nancy Harrison d’Amplitude Ventures. Leur participation et leurs idées ont sans aucun doute enrichi notre compréhension et préparé le terrain à d’éventuelles collaborations au sein de l’industrie de la biotechnologie.
LEADERSHIP: Boards, CEOs and management all play roles in planning for partnerships, mergers, and acquisitions.
PATIENCE: Deals can be infrequent and slow to develop. While awaiting a potential deal, leaders must protect the value of the company by staying independent and driving the companies forward on the existing plan.
PERSISTENCE: Companies are acquired, not sold. Companies must adhere to the established business plan and avoid devaluing the company by shopping it to potential buyers. Pharma partners are drawn to companies with a strong commitment to their mission.
RELATIONSHIPS: Establish and nurture relationships early (especially important following any financing round), to demonstrate progress. Leverage existing investor relationships to facilitate introductions to pharmaceutical companies.
TRANSPARENCY: Be transparent about challenges and work collaboratively with company partners to address those challenges.
ADAPTABILITY: Be open to feedback and be adaptable in strategy; incorporate insights that can refine the business plan.
PATIENT HEALTH: Prioritize patient impact in all company decisions and innovations. Global companies are attracted to a variety of technologies when they demonstrate strong patient benefit.
PREPARE EMOTIONALLY: Prepare yourself and the team for the emotions associated with a deal. In addition to the actual work of moving innovation forward, everyone will have invested significant emotional capital into the creation and building of the company. For many, the sale will be characterized by mixed emotions.

LE LEADERSHIP : Les conseils d’administration, les PDG et la direction ont tous un rôle à jouer dans la planification des partenariats, des fusions et des acquisitions.
LA PATIENCE : Il est possible que les accords soient rares et mettent du temps à se concrétiser. Dans l’attente d’un éventuel accord, les dirigeants doivent veiller à protéger la valeur de l’entreprise en préservant son indépendance et en s’appuyant sur plan d’affaires existant pour continuer à la faire évoluer.
LA PERSÉVÉRANCE : On parle d’acquisition et non de vente au sujet d’une entreprise. Ses dirigeants doivent donc respecter le plan d’affaires établi et éviter de dévaloriser l’entreprise en la proposant à des acheteurs potentiels. Les partenaires pharmaceutiques sont attirés par les entreprises qui sont profondément attachées à leur mission.
LE RÉSEAU DES RELATIONS : Il est utile d’établir et d’entretenir des relations dès le départ (et en particulier après un cycle de financement), afin de pouvoir faire état par la suite de son évolution. Tirer parti des relations existantes avec les investisseurs peut faciliter la présentation auprès de sociétés pharmaceutiques.
LA TRANSPARENCE : Faire preuve de transparence relativement aux difficultés rencontrées et travailler de concert avec les partenaires de l’entreprise pour y remédier.
LA CAPACITÉ D’ADAPTATION : Rester ouvert aux commentaires et adapter sa stratégie. Ne pas hésiter à intégrer de nouvelles données qui pourraient permettre d’affiner le plan d’affaires.
LA SANTÉ DES PATIENTS : Mettre la santé des patients au cœur de l’ensemble des décisions et des innovations de l’entreprise. Toutes sortes de technologies peuvent attirer l’intérêt des multinationales dès lors qu’elles présentent des avantages importants pour les patients. Se préparer sur le plan émotionnel : Se préparer et préparer l’équipe à faire face aux émotions que suscite une transaction. En plus du travail concret lié aux différents stades de l’innovation, il faut tenir compte de l’investissement émotionnel de chacun dans la création et l’évolution de l’entreprise. Pour beaucoup de vos collègues, l’acquisition suscitera des émotions contradictoires.
THE GLOBAL LIFE
INDUSTRY EVOLVES, THE COMPETITIVE LANDSCAPE IS TIGHTENING as many countries have invested heavily in domestic R&D and biomanufacturing capacities. These investments made in response to lessons learned from the COVID-19 pandemic included Canada injecting more than $2B into its Biomanufacturing and Life Sciences Strategy. Now, the challenge before us is: how can Canada leverage these investments as a springboard to reach and remain at the forefront of innovation and commercial development in this vital sector? The life sciences industry is not only vital for economic reasons, it should be viewed from a national security lens as well.
To achieve and sustain Canada’s role as a leader in life sciences, our policy environment, investments and initiatives should go to supporting talent acquisition and retention, training and professional development, and helping entrepreneurs and sector leaders translate their big, game-changing ideas into successful companies at commercial scale. Only through commercial development can the industry be self-sustaining and robust for the long-term.
At the heart of any successful industry lies its human capital. In the Canadian life sciences sector, attracting and retaining top talent is crucial for sustained growth and innovation. With a concerted effort to foster a supportive ecosystem for researchers, scientists, and industry professionals, Canada is becoming a magnet for skilled individuals from around the world.
Investments in research institutions, universities, and specialized training programs have nurtured a pipeline of talent equipped with the latest knowledge and skills necessary to drive breakthroughs in biotechnology, pharmaceuticals, and healthcare. Additionally, initiatives that enable Truong Ta
À MESURE QUE LE PAYSAGE CONCURRENTIEL EN SCIENCES DE LA VIE ÉVOLUE À L’ÉCHELLE MONDIALE , les perspectives se font plus rares, car de nombreux pays ont investi massivement dans leurs capacités nationales en R et D et en biofabrication. Parmi les investissements réalisés en réponse à la pandémie de COVID-19, le Canada a injecté plus de deux milliards de dollars dans la biofabrication et dans une stratégie en matière de sciences de la vie. Désormais, l’enjeu pour le Canada est le suivant : comment faire de ces investissements un tremplin pour être et rester à la pointe de l’innovation et du développement commercial dans ce secteur vital? Il ne s’agit plus aujourd’hui de considérer uniquement l’aspect économiquement essentiel des sciences de la vie : on doit aussi tenir compte de la sécurité nationale. Si l’on souhaite que le Canada devienne et reste un chef de file dans le domaine des sciences de la vie, notre environnement politique, nos investissements et nos initiatives devraient viser à assurer l’acquisition et la rétention des talents, ainsi que la formation et le développement professionnel; ils devraient également aider les entrepreneurs et les chefs de file du secteur à faire de leurs innovations qui changent la donne des entreprises prospères sur le plan commercial. Ce n’est que par le biais du développement commercial que l’industrie pourra assurer son autonomie et sa résilience à long terme.

Le capital humain est au cœur de toute industrie prospère. Il est essentiel d’attirer et de retenir les meilleurs talents dans le secteur canadien des sciences de la vie pour assurer une croissance et une capacité d’innovation durables. Le Canada est en passe de devenir, grâce à un effort concerté pour créer un écosystème favorable aux chercheurs, aux scientifiques et aux professionnels de l’industrie, un pôle d’attraction pour les personnes qualifiées des quatre coins du monde.

industrial internships such as Mitacs, mentorship programs through various incubators and accelerators, and industry-academic partnerships facilitate seamless transitions for graduates into the workforce, ensuring a steady supply of qualified professionals.
Continual learning and development are critical to this industry characterized by rapid advancements and evolving technologies. Recognizing this, stakeholders within the Canadian life sciences sector have made significant investments in training and professional development. The biopharmaceutical manufacturing training provided by CASTL is a perfect example.
From specialized courses and workshops to certification programs and conferences, professionals have access to many opportunities to enhance their skills and stay ahead of emerging trends. Cross-disciplinary collaborations and knowledge-sharing platforms also facilitate the exchange of ideas and best practices, fostering a culture of innovation and collaboration within the industry.
Les investissements dans les établissements de recherche, les universités et les programmes de formation spécialisés ont permis de constituer un vivier de talents, fort des connaissances et des compétences avancées grâce auxquelles on réalisera des percées dans les domaines de la biotechnologie, des produits pharmaceutiques et des soins de santé. Par ailleurs, les initiatives qui permettent des stages dans l’industrie, telles que celle de Mitacs, les programmes de mentorat offerts grâce à divers incubateurs et accélérateurs, et les partenariats unissant l’industrie et le milieu universitaire facilitent la transition des diplômés vers le marché du travail. Le secteur peut ainsi compter en permanence sur des cohortes de professionnels qualifiés.
La formation continue et le perfectionnement professionnel constituent des facteurs essentiels dans ce secteur aux progrès rapides et aux technologies en constante évolution. Les acteurs du secteur canadien des sciences de la vie en sont conscients et ont investi de manière considérable dans la formation et le développement professionnel. La
Entrepreneurs, visionaries, and risk-takers are essential if Canada aims to grow a dynamic life sciences industry. While government support can help to lower the risk of high-risk but high-reward endeavours, it is the entrepreneurs who will steer the industry to new heights. These visionaries may come from Canadian academia or industry, and they can access support through the aforementioned training and professional development opportunities.
Over the last several months, I had the opportunity to meet with several entrepreneurs who recently moved their startup companies here via Canada’s Visa Startup program. The Visa Startup program attracts entrepreneurs to Canada but, once here, we can improve how they are supported to access resources and navigate the different local life sciences ecosystems. The diverse background and culture of these entrepreneurs not only injects new intellectual ideas in biotechnology, pharmaceuticals, medical devices, and diagnostics into Canada, it creates an ideal situation for technologies developed in Canada to reach global markets once ready for commercialization through their global experience and network.
Despite tremendous progress made, the Canadian life sciences industry faces its share of challenges. Our startups and SMEs need an ecosystem of “navigators” to help them overcome barriers, whether scientific, regulatory, access to funding, or finding the right partners. Canadian

formation à la fabrication biopharmaceutique offerte par l’Alliance canadienne pour la formation et le développement de compétences en sciences de la vie (CASTL) en est un parfait exemple.
Qu’il s’agisse de cours et de stages spécialisés, de programmes de certification ou de conférences, de nombreuses possibilités d’améliorer leurs compétences et de rester à l’affût des nouvelles tendances s’offrent aux professionnels. Les collaborations interdisciplinaires et les plateformes de partage des connaissances facilitent également l’échange d’idées et de bonnes pratiques, ce qui favorise une culture de l’innovation et de la collaboration au sein de l’industrie.
Les entrepreneurs, les visionnaires et ceux qui prennent des risques ont un rôle à jouer au Canada si l’on souhaite voir évoluer l’industrie des sciences de la vie et lui insuffler tout le dynamisme nécessaire. Si le soutien des pouvoirs publics peut contribuer à réduire le risque des entreprises à haut risque mais à forte rentabilité, ce sont en fin de compte les entrepreneurs qui permettront à l’industrie d’atteindre de nouveaux sommets. Ces visionnaires peuvent être issus du monde universitaire ou de l’industrie canadienne, et ils peuvent bénéficier de diverses aides grâce aux possibilités de formation et de perfectionnement professionnel évoquées plus haut. Au cours des derniers mois, j’ai eu l’occasion de rencontrer plusieurs entrepreneurs qui ont récemment transféré leur entreprise en phase de démarrage au Canada dans le cadre du Programme de visa pour démarrage d’entreprise. Le programme permet d’attirer des entrepreneurs au Canada, mais, une fois qu’ils sont sur place, on pourrait faire mieux pour ce qui est de les aider à accéder aux ressources et à s’y retrouver dans les écosystèmes des sciences de la vie des différentes régions. La diversité des expériences et des cultures de ces entrepreneurs permettra de faire émerger au Canada de nouvelles perspectives intellectuelles dans les domaines de la biotechnologie, des produits pharmaceutiques, des dispositifs médicaux et du diagnostic, et leur réseau international aidera à la création d’une situation idéale pour que les technologies créées au Canada atteignent les marchés mondiaux une fois à l’étape de la commercialisation.
Malgré les progrès considérables réalisés, l’industrie canadienne des sciences de la vie se trouve face à des difficultés qui lui sont propres. Nos jeunes entreprises et nos PME doivent pouvoir compter sur un réseau de « passeurs » pour les aider à surmonter les obstacles, qu’ils soient d’ordre scientifique ou réglementaire, qu’il s’agisse de l’accès au financement ou de la recherche des bons partenaires. Les entreprises canadiennes du secteur des sciences de la vie doivent accéder à la phase de commercialisation pour être concurrentielles, ce qui nécessite une aide stratégique et des collaborations diverses.
life sciences companies need to scale commercially to be competitive, which necessitates strategic support and collaborative efforts.
Challenges bring opportunities for innovation and growth. By fostering a supportive regulatory environment, enhancing access to capital, and nurturing collaborative partnerships, we can overcome barriers and unlock the full potential of the Canadian life sciences sector.
The ripple effects of investments in research and development extend beyond job creation, driving innovation, attracting foreign investment, and bolstering Canada’s reputation as a global leader in life sciences. By leveraging our strengths in research and infrastructure, Canada is well-positioned to capitalize on emerging opportunities and solidify its position as a hub for commercial development in the life sciences industry.
The ripple effects of investments in research and development extend beyond job creation, driving innovation, attracting foreign investment, and bolstering Canada’s reputation as a global leader in life sciences.
In conclusion, empowering commercial development in the Canadian life sciences industry requires a multifaceted approach that encompasses talent acquisition, training, promoting and attracting entrepreneurs, and nurturing companies to achieve commercial status. By investing in human capital, fostering a culture of innovation, and addressing challenges proactively, Canada can continue to thrive and make significant contributions to the health and wellbeing of individuals globally.
Truong has worked in life sciences R&D, commercialization, and health policy for 20+ years at multinational and biotech firms. He has built partnerships between industry, government and stakeholders to secure transformative infrastructure and patient access investments.

Truong Ta, VP of Commercialization & Partnerships, and Julianna Weber, Director of Clinical & Regulatory Affairs, representing API at the 2023 Arab Health Conference in Dubai, bolstering Canada’s global leadership in the life sciences sector
Truong Ta, v.-p. à la commercialisation et aux partenariats, et Julianna Weber, directrice des affaires cliniques et réglementaires, représentent API à l’occasion de l’Arab Health Conference de 2023, qui s’est tenu à Dubai, et renforcent le rôle de leader du Canada dans le secteur des sciences de la vie
Il est vrai que les difficultés engendrent des possibilités d’innovation et de croissance. D’ailleurs, en favorisant un environnement réglementaire adéquat, en améliorant l’accès aux capitaux et en encourageant les partenariats et la collaboration, on pourra surmonter les obstacles et libérer tout le potentiel du secteur canadien des sciences de la vie.
Les retombées des investissements dans la recherche et le développement vont au-delà de la création d’emplois : elles stimulent l’innovation, attirent les investissements étrangers et renforcent la réputation du Canada comme l’un des leaders mondiaux du domaine des sciences de la vie. Grâce à ses atouts en matière de recherche et d’infrastructure, le Canada est bien placé pour profiter des nouvelles possibilités et consolider sa position de grand pôle de développement commercial de l’industrie des sciences de la vie.
En conclusion, pour favoriser la croissance commerciale de l’industrie canadienne des sciences de la vie, il faut adopter une approche à volets multiples qui englobe l’acquisition de talents, la formation, les efforts destinés à encourager et à attirer les entrepreneurs, ainsi que l’accompagnement des entreprises jusqu’à ce qu’elles aient franchi le cap de la commercialisation. En investissant dans le capital humain, en encourageant une culture de l’innovation et en s’attelant de manière proactive aux questions de fond, le Canada peut continuer à prospérer et à contribuer de manière importante à la santé et au bien-être des gens partout dans le monde.
Truong Ta œuvre depuis plus de 20 ans dans les domaines de la R et D en sciences de la vie, de la commercialisation et des politiques sanitaires au sein de multinationales et d’entreprises de biotechnologie. Il a œuvré à des partenariats unissant l’industrie, le gouvernement et divers intervenants en vue de l’obtention d’investissements novateurs en matière d’infrastructures et d’accès des patients aux traitements.
Aplantex, Biologics Manufacturing Centre, Noa Therapeutics, and Radiant Biotherapeutics discuss their strategic growth blueprints with BIOTECanada
Aplantex, le Centre de production de produits biologiques, Noa Therapeutics et Radiant Biotherapeutics évoquent leur plan de croissance stratégique avec BIOTECanada
WHAT IS THE FUNDAMENTAL NATURE OF APLANTEX’S TECHNOLOGY AND WHAT ARE THE
A significant biotech innovation: Aplantex uses a loop biotechnology process to leverage the DNA of select plants, our proprietary photosynthetic phytoreplicators, to efficiently clone a high yield biomass in a controlled environment and produce plant-based molecules of value to the agri-food, pharma, and cosmetics industries. At time of writing, our focus is on bioactive antioxidants, antiinflammatory, antimicrobial, and anti-aging ingredients.
Our photosynthetic phytoreplicators are grown in a controlled environment where they are fed hydroponically with a precisely metered nutritional solution under LED lighting. The biomass is automatically harvested daily, and high value molecules extracted, followed by a purification stage.
Our technology is a greener alternative to not only agriculture but also conventional chemical synthesis and contemporary fermentation-based biotech which are the current sources for these sought after molecules. Our process does not require large land surfaces, the heavy use of fertilizers and/or pesticides, nor complex industrial

QUELLE EST LA CARACTÉRISTIQUE
PRINCIPALE DE LA TECHNOLOGIE
D’APLANTEX ET QUELLES SONT LES SOLUTIONS QUE VOUS CHERCHEZ À CRÉER?
Une innovation biotechnologique majeure : Aplantex utilise un procédé biotechnologique pour extraire et réutiliser en boucle l’ADN de certaines plantes. Elle a créé des phytoréplicateurs photosynthétiques exclusifs, afin de cloner efficacement une biomasse à haut rendement dans un environnement contrôlé et de produire des molécules à base de plantes qui sont utiles aux industries agroalimentaire, pharmaceutique et cosmétique. En ce moment, nous nous intéressons aux ingrédients antioxydants, anti-inflammatoires, antimicrobiens et anti-âge de nature bioactive.
Nos phytoréplicateurs photosynthétiques sont cultivés dans un environnement contrôlé où ils sont nourris en hydroponie dans une solution nutritionnelle minutieusement dosée et où ils bénéficient d’un éclairage par diodes électroluminescentes (DEL). La biomasse est récoltée automatiquement chaque jour. Les molécules à haute valeur ajoutée sont extraites, puis sont soumises à une étape de purification.



infrastructures with their customary GHG footprint. Our production process includes the loop reuse of multiple inputs, is powered by renewable energy, generates no waste, and considerably shortens current supply chains.
WHAT ROLE HAVE/WILL ACADEMIC OR COMMERCIAL PARTNERSHIPS PLAY IN THE CREATION AND DEVELOPMENT OF THE COMPANY TO DATE OR ARE ANTICIPATED IN THE FUTURE?
The fruit of two years of R&D: The development of our proprietary photosynthetic phytoreplicators and the drafting of the comprehensive process specifications were accomplished over a two-year period in collaboration with leading universities in the province of Quebec.
The keen initial interest of major players in our various target industries underlines the strong potential for the development of commercial partnerships leading to the co-development of custom plant-based molecules.
HOW HAS APLANTEX APPROACHED SECURING INVESTMENT? WHAT STRATEGIES HAVE YOU FOUND MOST EFFECTIVE IN ATTRACTING FUNDING?
Strong investor confidence: Aplantex’s key strategic advantages, especially in the context of the unprecedented climate change upheaval, represent a viable answer to planetary concerns. By being “at the right place at the right time,” we not only enjoy the strong confidence of numerous accredited investors who have supported us to this day but are able to tap into many relevant government financial assistance programs. As our strategic plan continues to successfully unfold, we anticipate a positive response to our Q1-2025 investment drive. The funds will be used to further increase the production capacity of our Boucherville plant.
Notre technologie est une solution plus écologique que les biotechnologies fondées sur l’agriculture, sur la synthèse chimique traditionnelle et, plus récemment, sur la fermentation, qui permettent actuellement d’obtenir les molécules recherchées. Notre processus ne nécessite pas de grandes superficies, l’utilisation intensive d’engrais et de pesticides ni d’infrastructures industrielles complexes et leurs émissions de gaz à effet de serre (GES). Notre processus de production permet la réutilisation en boucle de plusieurs intrants. Il est alimenté par des énergies renouvelables, ne génère aucun déchet et permet de raccourcir considérablement les chaînes d’approvisionnement actuelles.
QUEL RÔLE ONT JOUÉ À CE JOUR OU JOUERONT À L’AVENIR LES PARTENARIATS UNIVERSITAIRES OU COMMERCIAUX DANS LA CRÉATION ET L’ÉVOLUTION DE L’ENTREPRISE?
Le résultat de deux années de R et D : La création de nos phytoréplicateurs photosynthétiques exclusifs et la rédaction des spécifications complètes du processus ont été réalisées sur une période de deux ans en collaboration avec d’importantes universités du Québec.
Le grand intérêt initial des principaux acteurs de nos diverses industries cibles montre que la conclusion de partenariats commerciaux, qui mèneraient au codéveloppement de molécules personnalisées à base de plantes, est fort possible.
COMMENT APLANTEX A-T-ELLE CHERCHÉ À OBTENIR DES INVESTISSEMENTS? QUELLES SONT LES STRATÉGIES LES PLUS EFFICACES POUR TROUVER DU FINANCEMENT?
Des investisseurs très confiants : En matière d’avantages stratégiques, en particulier dans le contexte des bouleversements sans précédent amenés par les changements climatiques, Aplantex offre une réponse durable aux enjeux planétaires. Le fait d’être « au bon endroit au bon moment » nous permet de bénéficier non seulement de la confiance de nombreux investisseurs reconnus qui nous ont soutenus jusqu’à ce jour, mais également d’accéder à de nombreux programmes d’aide financière gouvernementaux. Alors que la mise œuvre de notre plan stratégique se passe comme prévu, nous estimons que le cycle de financement du premier trimestre de 2025 sera couronné de succès. Les fonds seront utilisés pour augmenter la capacité de production de notre usine de Boucherville.
CAN YOU OUTLINE THE FUNDAMENTAL NATURE OF YOUR TECHNOLOGY AND/OR IDENTIFY THE SOLUTIONS YOU ARE AIMING TO CREATE?
The BMC is a contract manufacturing organization specialized in end-to-end bio-manufacturing. Our 58 000 Sq. Ft. GMP facility is designed to support the secure production of vaccines and other biologics within Canada and beyond. With a public good mandate, our mission extends to collaborating with industry and academia to establish a broader ecosystem allowing for an end-to-end support of the value chain in Canada, co-development projects, and addressing unmet public health needs. The BMC serves as a robust GMP manufacturing partner for mammalian and insect cellbased biologics production, specializing in vaccines (viral vector, protein subunit, virus-like particles) and therapeutic proteins (antibodies, enzymes). Our expertise extends to supporting therapeutic candidates from latestage clinical phases up to commercial manufacturing. Through fully integrated services, we, the BMC team, are the trusted partner to seamlessly support your organization in successfully completing all milestones pertaining to drug development and production through a well-defined operational strategy to ensure de-risking and speed to market with the goal to provide the right care and support to our Canadian community.
WHAT ROLE HAVE/WILL ACADEMIC OR COMMERCIAL PARTNERSHIPS PLAY IN THE CREATION AND DEVELOPMENT OF THE COMPANY TO DATE/OR ARE ANTICIPATED IN THE FUTURE?
Academic and commercial partnerships are key to our development and success as we must adhere to our 3-tiered mission;
Offer Domestic Supply Security
Playing a key role in the pandemic readiness of our country by ensuring a reliable supply of vaccines and diverse therapeutic proteins for Canadians and being actively involved in providing access to domestic production capacity in times of national and global emergencies.
Offer Collaboration Opportunities
Promoting advancements in biologics through partnerships with industry and academia. Foster the resilience and growth of the Canadian life science ecosystem offering quick turnaround times supported by our single use technology in addition to offering manufacturing services for late-stage clinical product candidates. By supporting and leading the build of a collaborative ecosystem, we and our partners will be able to allow the journey of the discovered molecules to be seamless and efficient in every aspect.
PRINCIPALE DE VOTRE TECHNOLOGIE ET/OU INDIQUER LES SOLUTIONS QUE VOUS SOUHAITEZ CRÉER?
Le Centre de production de produits biologiques (CPPB) est une organisation de sous-traitance spécialisée dans la biofabrication de bout en bout. Notre installation de 58 000 pieds carrés conforme aux BPF a été conçue pour assurer la production sécurisée de vaccins et d’autres produits biologiques destinés au Canada et à l’étranger. Notre mission d’intérêt public consiste à collaborer avec l’industrie et les universités en vue de renforcer l’écosystème et d’offrir un accompagnement de bout en bout pour la gestion de la chaîne de valeur au Canada, de participer à des projets de codéveloppement et de répondre à des besoins sanitaires non satisfaits. Le CPPB est un partenaire solide pour ce qui est de la biofabrication conforme aux BPF, en particulier en ce qui concerne la production biologique à base de cellules de mammifères et d’insectes, et il a pour spécialité le domaine vaccinal (vecteurs viraux, sous-unités protéiques, pseudoparticules virales) et les protéines thérapeutiques (anticorps, enzymes). Il a aussi pour expertise le soutien aux candidatsmédicaments, des dernières étapes du développement clinique à la fabrication commerciale.
Forte des services entièrement intégrés qu’elle offre, l’équipe du CPPB est le partenaire de confiance qui aidera en toute fluidité votre organisation à franchir avec succès toutes les étapes de mise au point et de production de médicaments grâce à une stratégie opérationnelle bien établie qui permet de réduire les risques et d’accélérer la commercialisation, et ce, dans l’optique d’offrir les soins et l’accompagnement souhaités à la communauté canadienne.
L’AVENIR LES PARTENARIATS UNIVERSITAIRES OU COMMERCIAUX DANS LA CRÉATION ET L’ÉVOLUTION DE L’ENTREPRISE?
Les partenariats universitaires et commerciaux font partie intégrante de notre développement et de notre mission en trois points :
Offrir une sécurité en matière d’approvisionnement à l’échelle nationale
Jouer un rôle majeur en vue de préparer le Canada aux éventuelles pandémies en assurant un approvisionnement fiable en vaccins et en protéines thérapeutiques diverses et participer activement à augmenter la capacité de production nationale en cas d’urgences nationales et mondiales.
Offrir des possibilités de collaboration
Encourager les avancées dans le domaine des produits biologiques grâce à des partenariats avec l’industrie et le monde universitaire. Stimuler la capacité d’adaptation et la croissance de l’écosystème canadien des sciences de la vie en offrant des délais d’exécution rapides grâce à notre technologie à usage unique et en proposant des services de


Undertaking projects that address the needs of the public, not otherwise available on the market due to high risk of failure and/or high cost/ unprofitable. Fill gaps in low volume commercial niches, less attractive to larger CMOs. Our ultimate objective being to support innovation and create value in Canada, partnerships & alliances with other players in the life science ecosystem is a must.
The BMC is a young organization which just celebrated its first anniversary. As a start-up, our most immediate focus is to create awareness through different channels to build strategic partnerships that keeps us true to our vision and missions. We pride ourselves in having an unwavering expectation of operational excellence matched by our agility through a highly talented and passionate team. Our 3-year plan is growth through diversification allowing us to expand our reach in supporting the ecosystem. What is of high importance to us is becoming a leader in building partnerships and alliances to find sustainable solutions to our biomanufacturing industry challenges. Labour shortages as well as the need for a national coordination to maximize the benefits of public and private investments in our ecosystem is a reality for all life science companies in Canada. Therefore, in the next 3 years, BMC will be an active player on panels, roundtables, advisory groups and specialized forums to advocate on the life science ecosystem needs and foster continuous improvements of the Canadian Biomanufacturing and Life Science Strategy. Simply put, we can help and we will!
fabrication pour les candidats-médicaments qui en sont aux derniers essais cliniques. En encourageant et en supervisant la mise en place d’un écosystème collaboratif, nos partenaires et nous-mêmes pourrons veiller à ce que l’élaboration des nouvelles molécules soit harmonieuse et efficace à tous égards. Apporter une aide aux initiatives d’intérêt public
Entreprendre des projets qui répondent aux besoins du public et qui autrement ne verraient pas le jour en raison d’un risque élevé d’échec et/ou d’un coût élevé ou d’un manque de rentabilité. Combler les lacunes au sein des niches commerciales à faible volume, moins attrayantes pour les grandes organisations de fabrication sous contrat. Les partenariats et les alliances avec d’autres acteurs de l’écosystème des sciences de la vie font partie intégrante de notre processus, en raison du fait que notre objectif ultime est de contribuer à l’innovation et de créer de la valeur au Canada.
SONT LES ÉTAPES PRÉVUES DANS L’ÉVOLUTION DU CPPB AU COURS DES TROIS PROCHAINES ANNÉES?
Le CPPB est une jeune organisation dont on vient tout juste de souligner le premier anniversaire. En tant que jeune entreprise, nous avons pour objectif immédiat de sensibiliser le public par le biais de différents canaux afin d’établir les partenariats stratégiques qui nous permettront de rester fidèles à notre vision et à nos missions. Notre volonté est inébranlable quant au fait d’atteindre l’excellence opérationnelle, et nous sommes fiers de notre équipe talentueuse et passionnée, qui sait démontrer de l’agilité. Notre plan triennal prévoit une croissance par la diversification, qui nous permettra de mieux assurer la stabilité de l’écosystème. Le plus important à nos yeux est de devenir un leader dans l’établissement de partenariats et d’alliances et de trouver des solutions durables aux défis de l’industrie de la biofabrication. Les pénuries de main-d’œuvre continuent d’affecter les entreprises du secteur des sciences de la vie au Canada, ce qui rend encore plus nécessaire le fait de veiller à une coordination nationale qui servira à optimiser les investissements publics et privés au sein de l’écosystème. Par conséquent, au cours des trois prochaines années, le CPPB participera activement à des groupes d’experts, des tables rondes, des groupes consultatifs et des forums spécialisés afin de faire valoir les besoins de l’écosystème des sciences de la vie et de viser l’amélioration continue dans la stratégie canadienne en matière de biofabrication et de sciences de la vie. En d’autres termes, nous pouvons nous rendre utiles et nous le ferons!
CAN YOU OUTLINE THE FUNDAMENTAL NATURE OF YOUR TECHNOLOGY AND IDENTIFY THE SOLUTIONS YOU ARE AIMING TO CREATE?
Noa Therapeutics (Noa) is a preclinical biotech company pioneering the development of homeostatic modulating small molecules, targeting barrier resolution, to address drivers of inflammatory diseases. Founded in Q3 2022 and currently residents of JLABS and MaRS, Noa is adopting a systems biology and computer assisted discovery approach, to tailor the development of novel small molecules for the treatment of immune diseases involving barrier dysfunction. Noa has established a platform of compounds to address unmet needs in over $100B of inflammatory disease markets.
WHAT ROLE HAVE/WILL ACADEMIC OR COMMERCIAL PARTNERSHIPS PLAY IN THE CREATION AND DEVELOPMENT OF THE COMPANY TO DATE/OR ARE ANTICIPATED IN THE FUTURE?
Development of novel therapeutics requires an integrated approach. Not only to de-risk assets as they advance from the laboratory to the clinic, but to optimize the potential for meaningful clinical impact. Where larger pharmaceutical companies have access to a multitude of resources, both intellectual and functional, smaller biotech companies, though nimble, can face hurdles in their development programs due to limited resources. With a keen understanding of the value of external resources, Noa is actively working to integrate academic and commercial expertise and resources. Inclusive of residency at JLABS, a Johnson & Johnson Incubator, academic collaborations, and active mentorships, Noa is leveraging a growing network of partners to accelerate and de-risk their drug development programs.

DÉCRIRE LA CARACTÉRISTIQUE PRINCIPALE DE VOTRE TECHNOLOGIE ET INDIQUER LES SOLUTIONS QUE VOUS SOUHAITEZ CRÉER?
Noa Therapeutics (Noa) est une société de biotechnologie spécialisée en recherche préclinique. Elle fait figure de pionnière en matière de petites molécules modulatrices de l’homéostasie qui visent le rétablissement d’une barrière (p. ex. intestinale) et sont destinées à lutter contre les facteurs d’apparition des maladies inflammatoires. Fondée au troisième trimestre 2022 et actuellement affiliée à JLABS et au centre MaRS, Noa a adopté une approche de la biologie des systèmes et de la recherche assistée par ordinateur en vue d’adapter la mise au point de nouvelles petites molécules destinées au traitement des maladies immunitaires liées au dysfonctionnement d’une barrière. Noa a créé une plateforme spécialisée dans les composés afin de répondre à des besoins non satisfaits en matière de maladies inflammatoires, dont on évalue le potentiel commercial entre les différents marchés à plus de 100 milliards $.
OU JOUERONT À L’AVENIR LES PARTENARIATS UNIVERSITAIRES OU COMMERCIAUX DANS LA CRÉATION ET L’ÉVOLUTION DE L’ENTREPRISE?
Le développement de nouveaux traitements nécessite une approche intégrée. L’objectif est non seulement de réduire les risques liés aux actifs lors du passage de la recherche aux essais cliniques, mais aussi d’optimiser significativement leur potentiel clinique. Alors que les grandes sociétés pharmaceutiques ont accès à une multitude de ressources, tant intellectuelles que fonctionnelles, les entreprises plus petites, bien que très agiles, peuvent se heurter à des obstacles en raison de ressources limitées. Noa est tout à fait consciente de la valeur des ressources externes et travaille activement à la mise en commun de l’expertise et des ressources universitaires et commerciales. Grâce à sa résidence à JLABS, un incubateur de Johnson & Johnson, à des collaborations universitaires et à des mentorats fructueux, Noa peut compter sur un réseau croissant de partenaires pour accélérer ses programmes de développement de médicaments et en réduire le risque.
COMMENT VOYEZ-VOUS L’ÉVOLUTION DE VOTRE TECHNOLOGIE AU COURS DES CINQ PROCHAINES ANNÉES, ET QUELLES POURRAIENT EN ÊTRE LES APPLICATIONS DANS LE DOMAINE DES SOINS DE SANTÉ À L’ÉCHELLE MONDIALE?
Noa s’est donné comme mission de bouleverser les conventions en matière de mise au point de médicaments, afin de révolutionner le traitement possible des maladies inflammatoires complexes liées à une barrière. Au cours des cinq prochaines années, Noa proposera des solutions thérapeutiques polyvalentes issues de sa plateforme de médicaments multimodaux (à trois cibles), dont une première
Noa is dedicated to creating a thriving, supportive and productive environment. Within which we will foster the development of the next generation of scientists, entrepreneurs, and innovators within the life science sector in Canada.

IN THE NEXT 5 YEARS, HOW DO YOU SEE YOUR TECHNOLOGY EVOLVING, AND WHAT IMPLICATIONS MIGHT THIS HAVE FOR GLOBAL HEALTHCARE?
At Noa, it is our mission to disrupt the convention of traditional drug development, to revolutionize treatment options for complex inflammatory barrier diseases. Within the next five years Noa will deliver convergent therapeutic solutions stemming from our trimodal drug platform, with a first indication for dermatological diseases, including atopic dermatitis, and secondary indications for gastrointestinal diseases. Noa will address unmet needs, while leveraging unprecedented reimbursement pathways to enable adoption into underserved populations. Not only expanding the absolute market potential of our first use case, but broader opportunities in greater inflammatory diseases through alignment with key strategic partners.
Integral to our journey will be the continuous development of internal skills and expertise. Noa is dedicated to creating a thriving, supportive and productive environment. Within which we will foster the development of the next generation of scientists, entrepreneurs, and innovators within the life science sector in Canada. Translating our knowledge and achievements to commercial success and to developing the talent needed to succeed.

Noa Therapeutics Team, left to right; Katrina Vizely MASc, Preclinical Strategist; Serena Mandla P.Eng. MASc, CSO & Co-Founder; Carla Spina Ph.D.., CEO & Co-Founder; Talia Fiani BSc, Research Scientist L’équipe de Noa Therapeutics, de gauche à droite : Katrina Vizely, M.Sc.A., stratège en recherche préclinique; Serena Mandla, ing., M.Sc.A., conseillère scientifique en chef et cofondatrice; Carla Spina Ph. D., présidente, chef
Noa s’efforce de créer un environnement prospère, positif et productif. Nous souhaitons ainsi contribuer à l’avènement de la prochaine génération de scientifiques, d’entrepreneurs et d’innovateurs dans le secteur des sciences de la vie au Canada.
indication liée aux maladies dermatologiques, notamment la dermatite atopique, et des indications secondaires liées aux maladies gastro-intestinales. Noa répondra à des besoins non satisfaits et tirera parti de de nouvelles voies de remboursement pour que les populations ayant des difficultés d’accès aux traitements puissent en bénéficier. Il s’agit non seulement d’élargir le potentiel de marché de notre premier cas d’utilisation, mais aussi d’offrir davantage d’options en ce qui a trait au traitement de nombreuses maladies inflammatoires grâce à la collaboration avec des partenaires stratégiques clés.
Le développement continu des compétences et de l’expertise à l’interne est un élément essentiel de notre stratégie. Noa s’efforce de créer un environnement prospère, positif et productif. Nous souhaitons ainsi contribuer à l’avènement de la prochaine génération de scientifiques, d’entrepreneurs et d’innovateurs dans le secteur des sciences de la vie au Canada. Il s’agit de transformer nos connaissances et nos réalisations en succès commerciaux et de perfectionner les talents pour paver la voie au succès.
de la direction et cofondatrice; Talia Fiani, B. Sc., chercheuse Left to right: Serena Mandla PEng. MASc, CSO & Co-Founder Noa Therapeutics; Carla Spina Ph.D., CEO & Co-Founder Noa therapeutics, Sanofi Golden Ticket Finals 2023CAN YOU OUTLINE THE FUNDAMENTAL NATURE OF YOUR TECHNOLOGY AND IDENTIFY THE SOLUTIONS YOU ARE AIMING TO CREATE?
Radiant is developing a novel multi-valent, multi-specific antibody platform called the Multabody. The Multabody platform exploits avidity—stronger binding power—coupled with multi-specificity to deliver potent multi-functional biologics with the potential to unlock cures for patients. Built on an antibody framework, Multabodies are modular, retain antibody-like developability and pharmacokinetics, creating an exciting next-generation therapeutic. By leveraging the multivalency and multi-specificity features of the Multabody, Multibodies can unlock biology that is not achieved by other antibody approaches, and in doing so, overcome limitations of existing antibody therapeutics. Multabodies have demonstrated the potential to deliver powerful biotherapeutics to tackle difficult-totreat diseases such as cancer, auto-immune diseases and infectious diseases.
POUVEZ-VOUS DÉFINIR LA NATURE FONDAMENTALE DE VOTRE
TECHNOLOGIE ET LES SOLUTIONS QUE VOUS TRAVAILLEZ À CRÉER?
Radiant œuvre à mettre au point une nouvelle plateforme d’anticorps multivalents et multispécifiques du nom de « Multabody ». La plateforme Multabody exploite « l’avidité »—une puissante force de liaison—en association avec la multispécificité; elle produit ainsi de puissants agents biologiques multifonctionnels qui ont le potentiel de mener à des remèdes pour les patients. Conçus selon une structure d’anticorps, les « Multabodies » sont modulaires, conservent le caractère évolutif et les caractéristiques pharmacocinétiques des anticorps et constituent un passionnant type de traitement de nouvelle génération.
Tirant parti de la multivalence et de la multispécificité de la plateforme, les Multabodies peuvent donner accès à des agents biologiques impossibles à découvrir au moyen des autres approches des anticorps. De cette façon, on repousse les limites que présentent les traitements actuels à base d’anticorps. On a pu constater le potentiel que recèlent les Multabodies pour ce qui est de produire de puissants biotraitements destinés à la lutte contre les maladies difficiles à traiter telles que les cancers, les maladies auto-immunes et les maladies infectieuses.

Within the next 5 years, we expect to have multiple drug candidates in clinical trials which are leveraging the features of the Multabody to exploit the biology of diseases beyond what is achieved with traditional antibody approaches.
WHAT ROLE HAVE/WILL ACADEMIC OR COMMERCIAL PARTNERSHIPS PLAY IN THE CREATION AND DEVELOPMENT OF THE COMPANY TO DATE/OR ARE ANTICIPATED IN THE FUTURE?
The discovery of the Multabody platform and foundational science supporting the formation of Radiant is based upon work from the lab of Jean-Philippe Julien, Ph.D at the Hospital for Sick Children. Radiant continues to collaborate with Dr. Julien on various projects and build upon the work being done within his lab.
The company also has collaborations with two large pharmaceutical companies as well as the Bill and Melinda Gates Foundation. These collaborations are facilitating the expansion of the Multabody platform into therapeutic areas which extend beyond the therapeutic focus of Radiant and accelerate the broad utility of the Multabody.
IN THE NEXT 5 YEARS, HOW DO YOU SEE YOUR TECHNOLOGY EVOLVING, AND WHAT IMPLICATIONS MIGHT THIS HAVE FOR GLOBAL HEALTHCARE?
Within the next 5 years, we expect to have multiple drug candidates in clinical trials which are leveraging the features of the Multabody to exploit the biology of diseases beyond what is achieved with traditional antibody approaches. The potential outcomes are best-in-class therapeutics in areas such as cancer, autoimmune disorders, and infectious diseases that are dramatically altering the course of disease and improving the lives of patients.

Au cours des cinq prochaines années, nous nous attendons à ce que plusieurs de nos candidatsmédicaments arrivent à l’étape des essais cliniques et utilisent les caractéristiques Multabody pour exploiter la biologie des maladies au-delà de ce que permettent les approches classiques des anticorps.
L’AVENIR LES PARTENARIATS UNIVERSITAIRES OU COMMERCIAUX DANS LA CRÉATION ET L’ÉVOLUTION DE L’ENTREPRISE?
Le développement de la plateforme Multabody et les travaux de recherche fondamentale qui sont à l’origine de Radiant tirent leur origine du laboratoire de Jean-Philippe Julien, Ph. D., qui fait partie du Hospital for Sick Children de Toronto. Radiant continue de collaborer avec M. Julien à divers projets et de s’appuyer sur les travaux effectués dans son laboratoire.
L’entreprise travaille aussi en collaboration avec deux grandes sociétés pharmaceutiques, ainsi qu’avec la Fondation Bill et Melinda Gates. Ces collaborations favorisent l’élargissement de la plateforme Multabody à des domaines thérapeutiques autres que ceux de Radiant et élargissent également son utilité, de plus en plus vaste.
COMMENT ENTREVOYEZ-VOUS L’ÉVOLUTION DE VOTRE TECHNOLOGIE AU COURS DES CINQ PROCHAINES ANNÉES, ET QUELLES CONSÉQUENCES CETTE ÉVOLUTION AURA-T-ELLE SUR LES SOINS DE SANTÉ À L’ÉCHELLE MONDIALE?
Au cours des cinq prochaines années, nous nous attendons à ce que plusieurs de nos candidats-médicaments arrivent à l’étape des essais cliniques et utilisent les caractéristiques Multabody pour exploiter la biologie des maladies au-delà de ce que permettent les approches classiques des anticorps. On pourrait ainsi obtenir des traitements qui seraient les meilleurs de leur catégorie dans des domaines tels que le cancer, les maladies auto-immunes et les maladies infectieuses, et qui pourraient spectaculairement modifier l’évolution des maladies et améliorer la vie des patients.






United by the same passion: A healthier future for all.
At Roche Canada, we bring together passionate people and diverse perspectives to advance science and ensure everyone has access to the healthcare they need.
We are proud to be a part of Canada’s vibrant life science ecosystem. Together, we can solve healthcare’s most complex challenges, so that we all have more time with the people we love.
For a healthier future for all.
For a stronger Canada.
Chez Roche Canada, nous réunissons des personnes passionnées et des points de vue diversifiés pour faire progresser la science et faire en sorte que chacun ait accès aux soins de santé dont il a besoin.
Nous sommes fiers de faire partie de l’écosystème dynamique des sciences de la vie au Canada. Ensemble, nous pouvons relever les défis les plus complexes de la santé, afin de passer plus de temps avec les personnes qui nous sont chères.
Pour un avenir plus sain pour tous.
Pour un Canada plus robuste.
www.rochecanada.com
If you require this information in an accessible format, please contact Roche at 1-800-561-1759. Si vous souhaitez recevoir ces renseignements dans un format accessible, veuillez communiquer avec Roche au 1-800-561-1759.
Clarissa Desjardins is the Founder and CEO of Congruence Therapeutics, a biotechnology executive and serial entrepreneur who has founded four biotechnology companies in Canada. Clarissa speaks to BIOTECanada about her journey in the biotech space.
CAN YOU RECALL ANY EARLY EXPERIENCES IN LIFE THAT FORESHADOWED YOUR PATH TOWARDS ENTREPRENEURSHIP?
There were no early signs that I would become an entrepreneur. There were no entrepreneurs in my family. The motivation to start my own business as a graduate student came from the desire to commercialize an early invention we made in the lab and share it with the rest of the world. Although commercializing inventions from the lab was not widely supported by my peers in academia at the time, I felt that it was the right thing to do. For me, the opportunity to create new jobs and generate economic

Clarissa Desjardins est la fondatrice et chef de la direction de Congruence Therapeutics, une dirigeante dans le domaine de la biotechnologie et entrepreneure en série qui a fondé quatre entreprises de biotechnologie au Canada. Clarissa raconte à BIOTECanada son parcours dans le monde des biotechs.
QUELLES ONT ÉTÉ LES EXPÉRIENCES ANTÉRIEURES QUI VOUS ONT OUVERT LA VOIE À L’ENTREPRENEURIAT?
Rien ne laissait présager que je deviendrais entrepreneure. Il n’y avait pas d’entrepreneurs dans ma famille. Lorsque j’ai obtenu mon diplôme, l’idée m’est tout simplement venue de commercialiser une invention réalisée en laboratoire et de la partager avec le reste du monde. C’est là que tout a commencé. Même si passer du banc d’essai à la commercialisation n’était pas une pratique très en vogue dans le monde universitaire à l’époque, j’ai estimé que c’était la bonne chose à faire. Je sentais pratiquement comme un devoir le fait d’avoir la possibilité de créer de nouveaux emplois et de générer de l’activité économique. J’ai compris alors que la recherche fondamentale était un privilège, dont le financement était assuré par les contribuables. Par conséquent, si vous avez la chance de faire une découverte, vous devez vous efforcer d’en assurer la commercialisation. Enfin, en tant que jeune femme en début de carrière, il me tardait de savoir si j’avais ce qu’il fallait pour diriger et faire croître une entreprise.
DANS LE CONTEXTE DE VOTRE EXPÉRIENCE AVEC CLEMENTIA PHARMACEUTICALS ET DE SON ACQUISITION, POUVEZ-VOUS NOUS DIRE DE QUELLE MANIÈRE VOUS VOUS Y ÊTES PRISE POUR SURMONTER LES DIFFICULTÉS ET TIRER VOTRE ÉPINGLE DU JEU, ET LES LEÇONS QUE VOUS AVEZ TIRÉES SUR LE RÔLE DE LEADER EN UNE PÉRIODE DE CHANGEMENT IMPORTANT?
Mon expérience en tant que fondatrice et chef de la direction de Clementia a tout simplement changé ma vie. Il se trouve que nous nous sommes attaqués à une terrible maladie osseuse de l’enfance, la fibrodysplasie ossifiante progressive, pour laquelle il n’existait aucun traitement. Nous étions la première entreprise qui se penchait sur cette
The sense of responsibility that one has when families and entire communities are praying for your clinical trial to be successful, is on a completely different level than
the sense of responsibility toward investors or even team members.
activity was practically a duty. I recognized that doing basic research is a privilege paid for by taxpayers. Therefore, if you are fortunate enough to make an invention, you should strive to ensure that it gets commercialized. Finally, as a young woman just getting started in my career, I was also motivated to see if I had what it takes to run and grow a business.
IN THE CONTEXT OF YOUR EXPERIENCE WITH CLEMENTIA PHARMACEUTICALS AND ITS ACQUISITION, CAN YOU TELL US ABOUT HOW YOU NAVIGATED THE CHALLENGES AND OPPORTUNITIES, WHAT LESSONS DID YOU LEARN ABOUT LEADERSHIP IN TIMES OF SIGNIFICANT CHANGE?
My experience as the founder and CEO of Clementia was life changing. The reason is that we worked on a terrible childhood bone disorder, fibrodysplasia ossificans progressiva, where no treatment existed. We were the first company to work on this disease and we therefore became very close with patient families. These families taught me many leadership lessons. The first is perspective. Witnessing their daily struggles, receiving their trust and confidence provided a motivation and sense of responsibility that I had never experienced before. The sense of responsibility that one has when families and entire communities are praying for your clinical trial to be successful, is on a completely different level than the sense of responsibility toward investors or even team members. We tried to moderate expectations and we worked like crazy. The entire team at Clementia felt the same way. I learned how important culture was to the success of a business. Clementia broke records of productivity and overcame quasi-insurmountable hurdles to the development and ultimate approval of our drug and we considered ourselves privileged to do so. In terms of responding to opportunities, these can be few and far between. They are a matter of luck. I was at the right place at the right time to start Clementia but I believe the team and I made the absolute best of this opportunity. Focusing on having an impact on patients’ lives was our north star and the only strategy required to guide our day-to-day decisions.
Le sentiment de responsabilité que l’on éprouve lorsque des familles et des communautés entières prient pour que l’essai clinique soit couronné de succès vous fait comprendre le sens du mot « responsabilité », et cela est d’un tout autre ordre que le sentiment de responsabilité que l’on éprouve à l’égard des investisseurs ou même des membres de l’équipe.
maladie et nous sommes donc devenus très proches des familles de patients. Ces familles m’ont beaucoup appris en matière de leadership. La première est la dimension de la perspective. Le fait d’être témoin de leur lutte de chaque instant et d’obtenir leur confiance a fait naître en moi une motivation et un sens des responsabilités que je n’avais jamais éprouvés auparavant. Le sentiment de responsabilité que l’on éprouve lorsque des familles et des communautés entières prient pour que l’essai clinique soit couronné de succès vous fait comprendre le sens du mot « responsabilité », et cela est d’un tout autre ordre que le sentiment de responsabilité que l’on éprouve à l’égard des investisseurs ou même des membres de l’équipe. Nous avons néanmoins veillé à ne pas trop nourrir d’attentes et nous avons travaillé d’arrache-pied. Toute l’équipe de Clementia était au diapason. Cette expérience m’a révélé à quel point l’état d’esprit est pour beaucoup dans les succès d’une entreprise. Clementia a battu des records de productivité et a surmonté des obstacles quasi insurmontables quant à la mise au point et à l’approbation finale de son médicament, et nous nous considérons comme privilégiés d’y être parvenus. La capacité à saisir les possibilités qui s’offrent à vous compte également, mais celles-ci sont rares. C’est une question de chance. J’étais au bon endroit au bon moment pour créer Clementia, mais il y a bien le fait que l’équipe et moi-même avons su tirer le meilleur parti de cette situation. Ce qui a guidé nos actions fut le désir de faire la différence dans la vie des patients, et ce fut même en fait la seule stratégie pour toutes les décisions à prendre au quotidien.
QU’EST-CE QUI A ORIENTÉ VOTRE PARCOURS
PROFESSIONNEL ET VOUS A MENÉ DE LA R.-D. À LA COMMERCIALISATION DANS L’INDUSTRIE
BIOTECHNOLOGIQUE? QUELS SONT LES OBSTACLES QUE VOUS AVEZ DÛ SURMONTER EN COURS DE ROUTE?
WHAT LED YOU TO CHOOSE A CAREER PATH THAT TOOK YOU FROM R&D TO COMMERCIALIZATION IN THE BIOTECH INDUSTRY? WHAT BARRIERS DID YOU OVERCOME ALONG THE WAY?
As a young person, I loved science. My father was a psychologist so I decided to study neuroscience. In my generation, during my Ph.D. studies, there were many women pursuing advanced degrees and I rarely felt that I was being treated differently than my male colleagues. The barriers to starting a business in any domain are huge and apply to everyone. I met over 100 investors to raise the first $300,000 for my first reagent business. There were likely some investors with unconscious biases that were uncomfortable funding a young person (I was 26), or a woman, as women biotech entrepreneurs were extremely rare at the time. But I did eventually attract some great investors.
GIVEN YOUR EXTENSIVE EXPERIENCE ON VARIOUS BOARDS, WHAT ADVICE WOULD YOU OFFER TO A STARTUP LOOKING TO MOVE INTO THE COMMERCIAL PHASE?
There are few start-ups in our field which can aspire to commercialize their products (therapeutic drugs) within a few rounds of financing. These would be restricted to certain reagent, diagnostic and medical device companies. For most drug development companies, the commercial phase is rarely achieved by the originator company. This is due to the inherent risks and failure rate in our business but also principally due to the length of time it takes to discover and develop a drug. A rapid progression from discovery to approval is 10 years and costs hundreds of millions to billions of dollars.
LOOKING BACK ON YOUR EARLY DAYS IN THE BIOTECH FIELD, WHAT DO YOU WISH YOU HAD KNOWN THAT YOU KNOW TODAY?
I wish I had known more about clinical development and the strategic nature of defining clinical endpoints that meet the standard of impacting “feeling, function and survival.” I wish I had known all about lead optimization and the challenge of how to take an early “hit” small molecule and turn it into a candidate drug. I wish I had learned about machine learning and protein biophysics. But I was constantly learning other things! It has been a wonderful journey with wonderful investors and colleagues and I honestly would not change a thing!
J’adore la science depuis que je suis toute petite. Mon père était psychologue, ce qui m’a incitée à aller vers les neurosciences. Durant mes études de doctorat, il y avait beaucoup de femmes qui poursuivaient des études supérieures, ce qui fait que j’ai rarement eu l’impression d’être traitée différemment de mes collègues masculins. La création d’une entreprise est toujours jalonnée de quantité d’obstacles, quel que soit le domaine, et personne ne peut y échapper. J’ai dû faire appel à plus de 100 investisseurs pour réunir les 300 000 premiers dollars nécessaires à la création de ma première entreprise spécialisée dans les réactifs. J’imagine que certains investisseurs avaient des a priori inconscients, qui faisaient qu’ils n’étaient pas vraiment disposés à financer une si jeune entrepreneure (j’avais 26 ans) ou une femme, car les femmes entrepreneures dans le domaine de la biotechnologie étaient extrêmement rares à l’époque. Mais j’ai fini par attirer des investisseurs de premier plan.
COMPTE TENU DE VOTRE VASTE EXPÉRIENCE AU SEIN DE DIVERS CONSEILS D’ADMINISTRATION, QUELS CONSEILS DONNERIEZ-VOUS À UNE JEUNE ENTREPRISE DÉSIREUSE DE FRANCHIR LE CAP DE LA COMMERCIALISATION?
Il y a peu de jeunes pousses dans notre domaine qui peuvent aspirer à commercialiser leurs produits (des médicaments) en quelques cycles de financement. Cela semble plutôt réservé à certaines entreprises qui fabriquent des réactifs, des produits de diagnostic et des instruments médicaux. Dans la plupart des cas, les sociétés qui créent des médicaments n’atteignent pas la phase commerciale. Cela est dû aux risques et au taux d’échec inhérents à notre activité, mais aussi principalement au temps nécessaire à la découverte et à la mise au point d’un médicament. Il faut compter pas moins de dix ans si l’on parle d’une progression rapide de la découverte à l’approbation, et des centaines de millions, voire des milliards de dollars d’investissements.
EN REPENSANT À VOS DÉBUTS DANS LE DOMAINE DE LA BIOTECHNOLOGIE, QU’AURIEZ-VOUS AIMÉ SAVOIR ALORS QUE VOUS SAVEZ AUJOURD’HUI?
J’aurais aimé en savoir plus sur la phase clinique et l’importance stratégique de définir des critères de résultat visant des effets sur « la souffrance, la capacité fonctionnelle et la survie ». J’aurais aimé tout savoir sur la première phase d’optimisation et sur le défi que représente la transformation d’une petite molécule « très prometteuse » en un médicament candidat. J’aurais aimé en savoir plus sur l’apprentissage machine et la biophysique des protéines. Cela dit, je n’ai cessé d’apprendre au cours des années quantité d’autres choses! Ce fut un formidable parcours aux côtés d’investisseurs et de collègues formidables et, honnêtement, je n’y changerais rien!
A globally and locally recognized bioengineer, inventor, and entrepreneur, Tamer is Founder and CEO of Aspect Biosystems—a Vancouver-based biotechnology company pioneering the development of bioprinted tissue therapeutics to transform the treatment of disease. He was named a Bloomberg New Economy Catalyst in 2022 and has received BC’s Top 40 Under 40 and Top 30 Under 30 awards. Tamer discusses his experience in the biotech industry with BIOTECanada.
CAN YOU RECALL ANY EARLY EXPERIENCES IN LIFE THAT FORESHADOWED YOUR PATH TOWARDS ENTREPRENEURSHIP?
Looking back, I think my involvement in soccer throughout childhood and into adulthood played a key role on my path towards entrepreneurship. It shaped my idea of team building, recognizing the value of multiplying what you can do as an individual by having a strong team around you. It also instilled in me the kind of tenacity and work ethic that has you pursuing a challenge and doing whatever it takes to succeed because you’re just so passionate about it.
Perhaps an even bigger step on my path towards entrepreneurship was co-founding a not-for-profit organization focused on humanitarian work in Egypt. This was a few years before co-founding Aspect, so I eventually had to make the difficult decision to stop in order to focus on the company. But it was an incredibly valuable experience. I learned about aligning people on a common mission and it made me realize how much fulfillment I got from serving others, and doing something that is intrinsically good. This is similar to the privileged opportunity we have in biotech to impact people’s lives in the way that matters most, while also combining value creation and productivity in a unique way that can deliver sustainable impact.
AS A LEADER IN REGENERATIVE MEDICINE, HOW DO YOU BALANCE THE PURSUIT OF GROUNDBREAKING INNOVATIONS WITH THE PRACTICAL CHALLENGES OF BRINGING THESE TECHNOLOGIES TO MARKET?
Bio-ingénieur, inventeur et entrepreneur reconnu à l’échelle nationale et mondiale, Tamer est le fondateur et chef de la direction d’Aspect Biosystems, une société de biotechnologie vancouvéroise pionnière de la mise au point de traitements tissulaires bio-imprimés destinés à transformer l’évolution des maladies. Bloomberg l’a inscrit à sa liste 2022 des catalyseurs de la nouvelle économie, et il a figuré parmi les 40 talents les plus prometteurs de moins de 40 ans et les 30 talents les plus prometteurs de moins de 30 ans en C.-B. Tamer fait part à BIOTECanada de son expérience au sein du secteur des biotechs.
QUELLES ONT ÉTÉ LES EXPÉRIENCES ANTÉRIEURES QUI VOUS ONT OUVERT LA VOIE À L’ENTREPRENEURIAT?
Lorsque je regarde en arrière, je me dis que mon engagement dans le soccer durant toute mon enfance et jusqu’à l’âge adulte a joué un rôle clé sur mon chemin vers l’entrepreneuriat. C’est là que s’est construite l’idée que je me fais de la constitution d’une équipe solide et que j’ai pris conscience de la richesse qu’il y a à multiplier ce qu’une personne peut faire lorsqu’il y a toute une équipe. Cette expérience m’a aussi insufflé de la ténacité et une éthique du travail qui font que je recherche les défis et que je suis prêt à faire tout ce qu’il faut pour réussir, simplement parce que cela me passionne.

Une grande étape sur mon parcours vers l’entrepreneuriat est sans doute la cofondation d’une organisation sans but lucratif qui faisait du travail humanitaire en Égypte. Cela remonte à quelques années avant la cofondation d’Aspect; à un moment donné, j’ai dû prendre la difficile décision de mettre fin à ce projet afin de me concentrer sur l’entreprise. Ce fut toutefois une expérience d’une valeur extraordinaire. J’y ai appris comment orienter tout un groupe de personnes vers une mission commune, et j’ai pris conscience du fait qu’aider les autres était pour moi très gratifiant, tout comme agir d’une façon fondamentalement bonne. Dans les biotechnologies, nous avons ce même privilège d’influer sur la vie des gens, et ce, de la manière qui compte le plus, tout en créant de la valeur et en étant productifs de façon à produire des effets uniques et durables.
Aspect is built around our proprietary full-stack tissue therapeutic platform, of which its potential applications are vast and complex. It’s tempting to seek out all the different ways that it can be applied; however, the challenge is being selective about which therapeutic areas to focus on in order to successfully address unmet medical need and bring therapies to patients. This is why we are currently laser-focused on creating implantable tissues that can replace, repair, or supplement missing biological functions in the body for metabolic and endocrine diseases.
Canada has a rich history of pioneering scientific research and discovery—particularly in the realm of regenerative medicine. Canada is home to the discovery of both stem cells and insulin. But where Canada has historically struggled is with taking it to the next level by commercializing discoveries and bringing them to patients from here in Canada. Changing this precedent and ensuring the biomanufacturing of therapies from right here in Canada is something I’m really passionate about and motivated to achieve.
WHAT LED YOU TO CHOOSE A CAREER PATH THAT TOOK YOU FROM R&D TO COMMERCIALIZATION IN THE BIOTECH INDUSTRY? WHAT BARRIERS DID YOU OVERCOME ALONG THE WAY?
I was never particularly interested in research for the sake of research. I wanted to focus on commercialization because I wanted to have a transformative impact on people’s lives. My experience with e@UBC (the entrepreneurship program at UBC) made it clear that entrepreneurship was a vehicle for delivering impact to people. So, I was motivated to get out of the lab and make it happen.
Moving from academia to business, the biggest challenges were reducing complexity to simplicity and having clarity on the strategy. In the business world, you have to break it up into bite size pieces that demonstrate value driving progress with each step. You also have to work backwards from where you need to be in terms of de-risking and creating value, and measure your progress critically along the way.
As a bioengineer, I believe an education in engineering is well suited to entrepreneurship because it’s all about defining problems and creating solutions. If you define a big enough problem to solve and apply an engineering mindset—especially if you then combine engineering with biology—you can create solutions that are arguably the most valuable because they’ll affect people’s lives.
GIVEN YOUR EXTENSIVE EXPERIENCE ON VARIOUS BOARDS, WHAT ADVICE WOULD YOU OFFER TO A STARTUP LOOKING TO MOVE INTO THE COMMERCIAL PHASE?
My main advice for a startup looking to scale is to focus on surrounding yourself with the right people. Part of this
EN TANT QUE LEADER DU DOMAINE DE LA MÉDECINE RÉGÉNÉRATIVE, COMMENT TROUVEZ-VOUS L’ÉQUILIBRE ENTRE LA RECHERCHE DE PERCÉES SCIENTIFIQUES ET LA NÉCESSITÉ DE RELEVER LES DÉFIS CONCRETS QUE REPRÉSENTE LA COMMERCIALISATION DES TECHNOLOGIES?
Aspect s’articule autour de sa plateforme complète brevetée de traitements tissulaires, dont les applications potentielles sont vastes et complexes. Il est tentant de vouloir dénicher toutes ces applications; néanmoins, le véritable défi consiste à être sélectif quant aux domaines thérapeutiques sur lesquels axer nos efforts pour réussir à répondre à des besoins médicaux non comblés et amener les traitements jusqu’aux patients. C’est pourquoi nous nous concentrons actuellement de façon très ciblée à créer des tissus implantables pouvant remplacer, réparer ou s’ajouter à des tissus biologiquement dysfonctionnels dans le contexte de maladies métaboliques et endocriniennes.
L’histoire du Canada en tant que pionnier de la recherchedéveloppement scientifique est riche, en particulier dans le domaine de la médecine régénérative. C’est au Canada qu’on a découvert les cellules souches et l’insuline. Cependant, il a été plus compliqué pour le Canada par le passé d’amener ses découvertes à l’étape de la commercialisation, afin que les patients du pays puissent en profiter. Je tiens profondément à modifier cette situation et à ce que la biofabrication des traitements ait lieu ici, au Canada; cela me motive énormément.
PROFESSIONNEL ET VOUS A MENÉ DE LA R.-D. À LA COMMERCIALISATION DANS L’INDUSTRIE
BIOTECHNOLOGIQUE? QUELS SONT LES OBSTACLES QUE VOUS AVEZ DÛ SURMONTER EN COURS DE ROUTE?
La recherche pour la recherche ne m’a jamais vraiment intéressé. Je souhaitais me concentrer sur la commercialisation, parce que je voulais avoir une influence transformatrice sur la vie des gens. Mon expérience à e@UBC (le programme d’entrepreneuriat de UBC) m’a fait clairement comprendre que l’entrepreneuriat était le bon véhicule pour influer sur la vie des gens. J’étais donc motivé à sortir du laboratoire et à agir concrètement.
Lorsque je suis passé du milieu universitaire à celui des affaires, les plus grands défis ont consisté à passer de la complexité à la simplicité, de sorte que la stratégie soit claire. Dans le monde des affaires, il faut bien segmenter les projets et démontrer la valeur des progrès réalisés à chacune des étapes. Il faut aussi visualiser le point d’arrivée, puis envisager toutes les étapes à rebours afin d’éliminer les risques, de créer de la valeur et de mesurer les progrès de façon critique tout au long du chemin.
En tant que bio-ingénieur, je suis d’avis que l’éducation en génie prépare bien à l’entrepreneuriat, car il s’agit dans les deux cas de définir les problèmes et de créer les solutions. Si l’on définit un problème à résoudre suffisamment grand et qu’on y applique le mode de réflexion d’un ingénieur—en
You
don’t need to solve everything right now. But you do need to critically assess which challenges need to be solved this week, this quarter, and this year in order to reach the next stage.
is about building the right team and appropriately transitioning from the co-founding team to a broader team of executive leaders, experts, advisors, and board members who are aligned on the mission. It also means bringing on the right investors and partners. Especially for companies with platform technologies like Aspect, working with a partner in specific fields can add a lot of value. For example, at Aspect we entered a $2.6B+ partnership with biopharma giant Novo Nordisk. We were very fortunate to be in a position where we had options on the table for who to partner with towards creating therapies for diabetes and obesity. We couldn’t be more excited to partner with Novo Nordisk because we each bring unique areas of expertise and are aligned on the common mission to bring curative therapies to patients living with these serious diseases.
LOOKING BACK ON YOUR EARLY DAYS IN THE BIOTECH FIELD, WHAT DO YOU WISH YOU HAD KNOWN THAT YOU KNOW TODAY?
In the early days, I wish I had known the importance of solving the “problem of the day.” It’s easy to fall into the trap of focusing on all the problems you need to solve in the company. But this can become overwhelming and, frankly, distracting from the main priorities. Instead, it’s crucial to identify what challenges could prevent you from unlocking the next chapter of the company, and then prioritize those. You don’t need to solve everything right now. But you do need to critically assess which challenges need to be solved this week, this quarter, and this year in order to reach the next stage. It’s all about sequencing things appropriately. For example, doing something in year 1 that is really only important for year 3 may seem like a proactive decision. But if that means you don’t actually solve the required year 1 problems, you may not even get to year 3! Identify the challenges that could block your path, understand when they need to be solved, and then create a game plan to tackle them.
particulier lorsque l’on associe génie et biologie—on peut créer les solutions qui ont le plus de valeur, au sens où elles transforment la vie des gens.
COMPTE TENU DE VOTRE VASTE EXPÉRIENCE AU SEIN DE DIVERS CONSEILS D’ADMINISTRATION, QUELS CONSEILS DONNERIEZ-VOUS À UNE JEUNE ENTREPRISE DÉSIREUSE DE FRANCHIR LE CAP DE LA COMMERCIALISATION?
Le principal conseil que je donnerais aux chefs de jeunes entreprises qui veulent les faire croître serait de veiller à s’entourer des bonnes personnes, car une partie du processus consiste à bâtir la bonne équipe et à bien faire la transition de l’équipe des cofondateurs à une équipe de dirigeants, d’experts, de conseils et d’administrateurs qui vont dans le sens de la mission établie. Cela signifie aussi d’intégrer les bons investisseurs et partenaires. Surtout pour les entreprises à technologies articulées autour d’une plateforme, comme Aspect, avoir des partenaires dans des domaines précis apporte beaucoup de valeur. Par exemple, chez Aspect, nous avons conclu un partenariat de 2,6 milliards $ avec le géant de la biopharmaceutique Novo Nordisk. Nous avons eu beaucoup de chance de nous trouver dans une position où plusieurs options étaient sur la table quant au partenariat en vue de la création de traitements contre le diabète et l’obésité. Rien n’aurait pu nous enthousiasmer davantage que ce partenariat avec Novo Nordisk, car cette entreprise et nous apportons chacun nos domaines d’expertise uniques, tout en étant en phase quant à la mission, qui consiste à offrir des traitements curatifs aux patients qui vivent avec des maladies graves.
EN REPENSANT À VOS DÉBUTS DANS LE DOMAINE DE LA BIOTECHNOLOGIE, QU’AURIEZ-VOUS AIMÉ SAVOIR ALORS QUE VOUS SAVEZ AUJOURD’HUI?
Il aurait été utile, au tout début, que je connaisse l’importance de résoudre « le problème du jour ». On peut facilement tomber dans le piège de tenter de se concentrer à la fois sur tous les problèmes à résoudre pour l’entreprise. Or, cela peut devenir décourageant et, honnêtement, constituer une distraction par rapport aux principales priorités. Il vaut mieux, et il est même crucial, de définir les difficultés susceptibles d’empêcher l’entreprise de passer à l’étape suivante, puis de les classer par priorité. On n’a pas à tout résoudre tout de suite, maintenant. Mais on doit poser un regard critique sur les difficultés à résoudre cette semaine-là, ce trimestre-là ou encore cette année-là pour passer à l’étape suivante. Il s’agit simplement de faire les choses dans l’ordre. Par exemple, faire la première année quelque chose qui ne sera important que la troisième année peut sembler proactif, mais si cela signifie ne pas résoudre les problèmes qui doivent l’être la première année, peut-être ne vous rendrez-vous pas à la troisième année! Cernez les obstacles qui se dressent sur votre chemin, déterminez à quel moment ils doivent être réglés, puis établissez un plan de match pour vous y attaquer.
AND BY
FOREFRONT
HEALTH
, consistently producing groundbreaking medical discoveries that not only save lives worldwide but also hold immense commercial potential. However, realizing this potential, and maximizing the benefits in and for Canada, is not without its challenges. It demands a concerted effort, characterized by a well-resourced, strategically coordinated innovation approach, grounded in strong collaboration among pivotal partners.
At TIAP, we have long recognized the significance of harnessing Canada’s largest academic innovation cluster, aiming to amalgamate expertise and resources to catalyze success. However, the path forward necessitates enhanced alignment and partnerships at provincial, national, and global levels. This means we all need to look beyond our individual areas of focus and beyond our own neighborhoods to partner with those across Ontario, Canada, the US and beyond who share common objectives and complementary resources. It’s crucial that we collectively pivot from competition to collaboration, particularly in early-stage programming and support, to amplify the impact on Ontario’s and Canada’s life sciences landscape, ultimately benefiting patients.

Despite notable strides in translation and commercialization through various federal and provincial initiatives over the past decade, there’s an evident need for consolidation. The life sciences sector is marred by fragmentation, with a myriad of programs that must now be streamlined for efficacy. In the post-COVID era, marked by notable shifts in funding for early-stage companies, the urgency for substantial support is palpable. Looking at our own organization, we have been able to achieve significant results via our four main pillars, LAB150, UTEST, Venture Builder and Portfolio Management, along with our Critical Technologies Program, and provide innovators and entrepreneurs access to valuable resources. However, in order to amplify that impact and fully seize the opportunity before us, greater scale and coordination is essential.
We commend the array of initiatives bolstering our sector, notably Ontario’s proactive “Taking life sciences to the next level” strategy, which aligns well with Canada’s national Biomanufacturing and Life Sciences Strategy. The establishment of the provincial Life Sciences Council (as well as federal initiatives such as the Health and Biosciences Economic Strategy Table) marks a pivotal step toward meaningful engagement and effective execution of these strategies, underscoring the necessity for alignment and coordination to thrive in the competitive life sciences arena.
TIAP envisions Ontario as a global beacon in life sciences, committed to leveraging the province’s research excellence to foster a vibrant industry and ecosystem, rich in talent, innovation, and collaboration. We extend our gratitude to our partners across government, industry, academia, the investment realm, support organizations, and patient groups. However, to ensure we can be globally competitive, retain and attract talent, and build our life sciences companies to scale, we have a call to action: coordinate, collaborate and even consolidate support and programming for our life science companies and entrepreneurs to firmly focus on the broader vision we share. Together, we stand on the cusp of realizing an extraordinary opportunity, poised to unite our strengths and achieve unparalleled success in the life sciences domain—the time is now!


For over a decade, we have dedicated ourselves to making a real difference in people’s lives, not only through the breakthroughs we achieve, but the paths we take to achieve them.
We create medicines and solutions that help patients, communities and our world.
We proudly partner with Canadian health leaders to help bring the promise of new innovation to Canadians.
Since 2013, we have launched 44 new medicines and indications in Canada to help people living with mental illness, Hepatitis C, rheumatoid arthritis, and other serious health issues.
We will continue to strive towards solving serious health challenges today and addressing the medical challenges of tomorrow.
AKEEM GARDNER RETURNED TO CANADA FROM THE UNITED KINGDOM AS A NEWLY MINTED LAW GRADUATE IN 2017 with a fresh perspective on society’s most pressing needs and determined to make a tangible impact on peoples’ lives.
With a vision that extended beyond the traditional confines of his legal training, Gardner acquired farmland near Orangeville, Ont., and began to grow industrial hemp in an effort to discover the plant’s medicinal and commercial possibilities. He quickly attracted interest from professors at the University of Guelph, who wanted to develop new natural ingredients. They were interested in novel molecules found in the industrial hemp plant that had previously gone undiscovered.
This resulted in the establishment of Canurta Therapeutics, a biotechnology company that has made tremendous strides in a short period of time. “Fast forward seven years and it is incredible what we have done with the opportunity,” says Gardner, the company’s Founder and Chief Executive Officer.
The unique aspect of Canurta’s technology is that it harnesses rare plant-derived molecules. Science shows that the company’s lead molecule, cannflavin A, found in industrial hemp at only a minute fraction of the plant’s biomass, has the potential to be an improved antiinflammatory that is safer than current market alternatives.
“Nature always had the answers and now it’s our job to use modern technology to harness its secrets to its fullest potential,” says Gardner.
Now Canurta is applying its findings to some of healthcare’s most complex and debilitating diseases, in areas such as neurodegeneration, severe chronic
inflammation and to even address the rampant opioid epidemic.
The lead asset—CNR 401—is an investigational drug looking to help those suffering from Amyotrophic lateral sclerosis, the company’s first, most urgent priority.
Cannabis has a rich history of healing, and Canurta, through Canurta’s pre-clinical program, has developed a standardized, first-in-class formulation that will help with symptom management, and provide neuroprotective potential, with the requisite IP strategy to make the asset commercially exciting with the right clinical outcomes, explains Gardner.
“Right now, ALS patients are self-medicating and using cannabis to help treat their pain, their spasticity, their appetite, and to sleep. But there is yet to be a clinical trial completed to evaluate such a formulation with our key constituents, designed for this patient population. We will be the first to show what this plant can really do, with the uniqueness of the rare cannflavins as anti-inflammatory agents that might be able to help slow neurodegeneration,” says Gardner.
“If we can stop inflammation at its source, we can help treat a variety of different complex diseases and help patients with unmet needs,” says Gardner, who explains that chronic inflammation that continues undetected can develop into many serious health problems, including joint issues, dementia, cardiovascular issues, and tumours, among other ailments.
He notes that the opioid epidemic arose because chronic pain was treated with oxycontin, which raised a whole new problem of addiction. Canurta’s suite of botanical ingredients address all of those issues, with no harsh side effects or addictive potential. These medicines are, “not going to make your stomach bleed, or give you ulcers with chronic use.”

In 3 years, Canurta has acquired the necessary data and a skilled, world-class team with the expertise and experience to execute on the company’s vision. So now they are ramping up production and getting ready to advance CNR-401 to Phase 2 clinical trials.
“We’re working hard, and we’re really excited for the impact that we think we can have in the world, and how we might be able to benefit the patients whose interest we ultimately serve.” says Gardner.


PARKINSON’S DISEASE IS A CHRONIC AND DEVASTATING NEURODEGENERATIVE DISEASE. Despite over two centuries of research, current treatments focus solely on symptom alleviation and tend to lose effectiveness over time. With over 100,000 Canadians living with Parkinson’s, and no cure to halt disease progression, novel therapies are urgently needed.
In 2022, Drs. Martin Lévesque, Claude Gravel, and Samer Hussein, from Université Laval, founded CEREBRO Therapeutics, a biotechnology company dedicated to increasing the quality of life for those living with incurable neurodegenerative disorders. Their goal is to develop a novel gene therapy for Parkinson’s disease with broader implications for other neurodegenerative conditions like Alzheimer’s disease.
“Our approach involves developing single-chain antibody fragments to target a specific protein called alphasynuclein. This protein forms clumps in the brain of Parkinson’s patients and is believed to cause the disease when it accumulates in large amounts,” said Dr. Lévesque. “The antibody fragments we are developing are five times smaller than full-size antibodies and the gene encoding them can be inserted into adeno-associated viral vectors (AAVs) to facilitate safe delivery to the brain.”
To date, the company has successfully tested two single-chain antibodies delivered using AAVs into preclinical animal models that almost completely prevent signs of disease.
“The AAVs have been engineered to target the brain, making them an ideal gene therapy candidate,” said

CEREBRO Therapeutics, an emerging Canadian biotech
Dr. Gravel. “The antibodies are then produced and confined to the brain, overcoming the challenge of crossing the blood-brain barrier, a significant obstacle in current neurological treatments.”
As CEREBRO Therapeutics and other Canadian biotech companies continue to push the boundaries of medical innovation, the prospect of transformative and personalized therapies for neurological disorders is becoming increasingly tangible.
“Ideally treatment for Parkinson’s should be individualized based on the person’s genetics, stage of disease, and specific pathology,” said Dr. Hussein. “We are hopeful that our novel approach to treating Parkinson’s will not only relieve symptoms but also serve to halt the progression of this debilitating disease.”
CEREBRO will soon begin testing the therapy in non-human primates, a pivotal step towards phase I clinical trials within the next year.
To learn more visit: cerebrotx.com



Canada’s Stem Cell Network: Powering life-saving therapies and technologies through regenerative medicine research
For over 20 years, the Stem Cell Network (SCN) has supported stem cell and regenerative medicine research in more than 26 disease areas, including heart disease, diabetes, cancer, and neurological disorders, while also providing training for the next generation of life sciences and biotechnology professionals.
Through SCN’s investment in groundbreaking research, more than 25 Canadian biotech companies have been catalyzed or enhanced, including companies like CEREBRO Therapeutics. Since 2016 SCN has invested $3.7M in neural research including research into neurogenerative diseases such as Parkinson’s, and multiple sclerosis. stemcellnetwork.ca


WHEN CYNTHIA PUSSINEN WAS APPROACHED BY AN EXECUTIVE RECRUITER IN 2023 ABOUT AN OPPORTUNITY TO LEAD SERNOVA (TSX: SVA), a Canadian life sciences company headquartered in London, Ont., making advances in cell therapeutics, she had not heard of it. But upon checking into Sernova, she was very surprised to learn about the company’s progress and accomplishments—and agreed to come on board as chief executive officer.
“When I looked into what the company was doing, and understood the data, it was simply astounding to me,” says Pussinen, a 30-year veteran of the life sciences industry.
For example, “when I reviewed the data for our Phase I/II study that is currently in the clinic for Type 1 diabetes, I was astonished by the fact that we have patients who have been insulin independent, so they have had no exogenous insulin, for anywhere between about a year and a half to almost four years and counting,” she says.
patient with Type 1 diabetes, as well as for their family members, says Pussinen.
“We have been able to demonstrate clinically meaningful benefits in patients and I want to be part of continuing the story. I want to make a difference in health outcomes in Type 1 diabetes and other chronic conditions, treating as many patients as we can around the world,” she says.
“Our vision for Sernova is a future where chronic conditions are not insurmountable obstacles.”

In addition to five of six patients in cohort 1 reaching insulin independence, all cohort 1 patients have seen their HbA1c blood sugar levels decrease into the non-diabetic range, leading to a reduction or elimination of hypoglycemic events.
Results seen thus far in Sernova’s human donor islet study are transformational and truly life changing for a
Sernova is advancing a cell therapy platform with potential applications across various chronic diseases.
“The way we are delivering therapeutic tissues or cells, hormones, etc. into the patient is through what we’re calling a Cell Pouch. The Cell Pouch is a credit card sized device. It is made of two polypropylene sheets of material and is only a couple of millimetres thick. It is very flexible with perfectly sized pores,” says Pussinen.
The drug delivery device is placed flat into the patient’s abdominal region just above the layer of muscle, so an invasive placement procedure is not involved. It is then allowed to vascularize, with pores designed in a way that vessels and tissues can grow through the Cell Pouch, which also contains small channels with tubes that create a void space.

After the Pouch has vascularized, it is ready to provide a healthy environment for the cells to be introduced to the patient. The plastic tubes are then taken out and the cells or tissues are put into the small void spaces where the tubes once were.
The cells are surrounded by vessels and tissues, ready to support the healthy oxygen glucose kinetics and islet functioning in individuals suffering from Type 1 diabetes. “It’s essentially forming a pseudo-organ of sorts,” says Pussinen.
“After a couple of weeks with the therapeutic cells in the Pouch, we begin to titrate the patient off of insulin, and then the body begins to fulfill the function of what it was not previously capable of doing. It’s quite amazing,” she adds.
Pussinen says the Cell Pouch in combination with therapeutic cells as a treatment paradigm for Type 1 diabetes patients is an improved approach compared to current treatment options. She notes that a pancreas with healthy islets has three different components—an alpha, beta and delta portion. Insulin only addresses the beta part of the islets, not the alpha and delta portions, she explains.
Sernova’s approach holistically treats the entire condition and patient, which holds the potential to minimize major health concerns and issues that Type 1 diabetes patients might have, such as cardiovascular issues, loss of limb and blindness, says Pussinen, who notes that Sernova can potentially treat insulin-dependent Type 2 diabetes patients, too.
Sernova’s next Phase I/II study will involve induced pluripotent stem cells (iPSCs), in partnership with Evotec SE in Germany which is working to engineer and produce those cells.
“There are no embryonic stem cell portions involved, and so it’s ethically derived,” explains Pussinen.
“With iPSCs, we will have a virtually endless supply of cells to treat Type 1 diabetes patients and we will not have to procure donor cells, as we do in our current clinical study. We’ll be in a position to produce/manufacture iPSC islet-like clusters whenever we need them, and ship them around the world. That will be the path to commercialization for a treatment that is broadly available to all Type 1 diabetes patients,” she predicts.
Sernova has two additional disease treatments in pre-clinical exploration, one for post-operative hypothyroidism where patients have had their thyroids removed due to benign purposes. There is also preclinical haemophilia A work under way.
“Typically when a patient has a thyroidectomy, at least a small portion of their thyroid has healthy tissue. We remove the healthy tissue and place it into the Cell Pouch; the patient would likely then not need synthetic hormones for the rest of their life. Since we’re using the patient’s own thyroid tissue, we would not require an immunosuppressant regimen for treatment, which is really quite appealing for both patients and doctors,” says Pussinen.
With respect to the treatment for haemophilia, “We plan to start with the patient’s own blood outgrowth endothelial cells and perform a gene therapy modification. Then we reintroduce the modified cells back into the patient; essentially what they’re getting is healthy cells to treat the disease,” she explains.
Pussinen notes that a majority of the treatments currently available for haemophilia either involve blood factor approaches or gene therapy approaches, both of which have limitations. With the blood factor approach, patients are still underserved, and without medication or blood factor nearby, the results could be catastrophic. Gene therapy approaches are “essentially once and done. You block yourself from getting future gene therapies,” says Pussinen.
In late 2023, Sernova received Orphan Drug and Rare Pediatric Disease Designations from the U.S. Food and Drug Administration for its third program in evaluating use of the Cell Pouch for haemophilia A patients.
The majority of Sernova’s employees are located in London, with a smaller subgroup in Toronto, and additional personnel, including Pussinen, in the United States.
“When you think about the impact of the work that we’re doing expanding to the entire world, Canadians will be able to feel really proud. It speaks to the talent—the hard working, really creative, technically savvy, highly skilled work force that has been able to bring us this far,” she says.
BIOPHARMACEUTICAL MANUFACTURERS PLANNING TO LAUNCH INNOVATIVE MEDICINES IN CANADA HAVE FACED AN INCREASINGLY COMPLEX MARKET ACCESS LANDSCAPE IN RECENT YEARS. The intersection of two issues has underpinned this evolution:
1. Many medicines launching today represent the most complex health innovations in history. They aim to cure diseases once thought to be untreatable, making these innovations simultaneously costly and priceless; and
2. T he market access pathway that ushers (but often slows) a new drug into the hands of Canadian patients is potholed with all-time high levels of uncertainty. Factors working against a smooth market access pathway in Canada include:
a) P rice regulatory ambiguity from the Patented Medicines Prices Review Board (PMPRB);
b) New pathways in health technology assessment to address evidence limitations, evaluate real-world evidence, and implement lifecycle health technology management present opportunities, and also significant and uncharted risks; and,
c) Payers’ nascent experience implementing innovative agreements and funding models for highly specialized therapies can leave manufacturers uneasy about their investments.
Combined, the unprecedented nature of new medicines launching today exacerbates the challenges of predicting how regulatory authorities, market access evaluators, and payers will value their medicines in an already highly uncertain pathway. This situation risks compromising Canada’s attractiveness for launch relative to other countries.
Notwithstanding, we appreciate global manufacturers’ critical need to precisely dissect and intensely evaluate the opportunities and risks of launching in the current climate.
In our experience, manufacturers able to
1 Connect the dots in innovative ways,
2 Ask better questions of robust datasets; and,
3 Generate alignment across market access, finance, clinical, marketing, and commercial functions, can succeed in the Canadian market, effectively addressing challenges and uncertainties while leveraging opportunities Canada offers.
PDCI Market Access, a Division of McKesson Canada (PDCI), conducts comprehensive market access assessments to provide customized solutions supporting manufacturers’ successful launches in Canada. These assessments leverage primary and secondary research methods, PDCI’s proprietary Market Access Toolkit data assets, along with our extensive market access expertise to deliver strategic solutions that achieve commercial success. In an ever-evolving Canadian landscape, we enable manufacturer preparedness to forecast various reimbursement and commercial outcomes.
It is not uncommon for systemic challenges to impede the optimal integration of new medicines into Canada’s healthcare system. This was the case for one of PDCI’s manufacturer partners. To overcome these challenges based on a foundational understanding of the product’s place in therapy, PDCI recommended investments for broader stakeholder engagement and workstreams. The result was a customized strategy for improved market access within a healthcare system that commonly lags behind the innovation seen in the pharmaceutical industry. These initiatives positioned the manufacturer to comprehensively plan and improve their positioning for market access and commercial uptake within the constraints of the Canadian healthcare system.
Figure 1: PDCI’s market access assessments connect the dots in an integrated strategy for optimized market access early in commercial planning, allowing maximum time to leverage opportunities and mitigate challenges on the horizon
Global manufacturers face questions about the Canadian reimbursement profile for their drug (e.g. public/ private payer split, patient and plan design distribution, and commercial potential) earlier in their launch planning. Manufacturers gain valuable insights from PDCI’s
proprietary data analytics tools, including the Census of Insurers, the Canadian Healthcare Reimbursement Insurance Simulation (CHRIS) and Product Reimbursement and Insurance Market Evaluation (PRIME) models. These population and epidemiology-based models forecast disease-specific therapeutic coverage profiles enabling manufacturers to visualize patient distribution across provinces, age groups and payer types to inform decisionmaking and payer negotiations. PDCI’s Market Access Ramps further support commercial forecasting, patient bridging estimates, payer negotiation strategies and break-even analyses for engagement with Canadian payers. The primary output is a visual representation of the time to listing for patient segments depending on their plan type, design, and jurisdiction.


emulated in the private payer environment. The Canadian private market has seen profound changes as Pharmacy Benefits Managers (PBMs) successfully leverage their business size to negotiate for larger market segments. This requires manufacturers’ profit and loss (P&L) forecasts to become ever more accurate to understand the financial implications of reimbursement scenarios that traditionally may have fallen within market access plans alone. PDCI’s forecasting service takes an all-encompassing approach to understanding the many payers, commercial and competitive landscape and opportunities and risks to support the development of robust and reliable P&L forecasts.
In one case, PDCI leveraged its data assets to gain a detailed understanding of reimbursement gaps based on the patient profile and distribution across plans to forecast the demand for patient support programs. In any negotiation, there are trade-offs that must be considered carefully (time to access versus price and volume). In this case, use of PDCI’s data positioned the manufacturer to avoid a poor negotiation outcome—by precisely defining the manufacturer’s trade-offs between public and private reimbursement scenarios, and its best alternative to a negotiated agreement.
Persistent uncertainties in the market access environment mean successful launch planning must be collaborative within an organization. Integration and alignment is necessary across access, marketing, commercial and finance functions. Not only are Canadian public payers negotiating from increasingly stronger positions, but consolidation of public negotiations is being
Conclusion: Canadian access evolution is constant; posing risks for manufacturers aiming to optimize their product’s success. Yet with the right partnerships, it offers immense opportunity. PDCI’s intimate knowledge and expertise across the spectrum of therapeutic areas positions us as a strategic partner from early launch planning through to loss of exclusivity. Ongoing innovation within the pharmaceutical pipeline increases complexity and requires integrated analyses that leverage PDCI’s intimate understanding of the landscape and data-driven solutions to develop strategies to optimize Canadian success.
Customized solutions that consider the critical appraisal of clinical evidence, unmet need, appreciation of budget impact and health economic value story, payer perspectives and negotiation leverage, as well as understanding of the product’s commercial potential in Canada’s uniquely challenged healthcare system—all set PDCI partners up for success despite the uncertain and ever-evolving landscape.
PDCI Market Access, a Division of McKesson Canada is Canada’s leading market access and launch consultancy. Since 1996 its senior experts have provided strategic advice to manufacturers of all sizes and needs to optimize pricing and reimbursement in Canada. For more information, please contact Info@pdci.ca
LIKE MANY COUNTRIES, CANADA HAS RECENTLY PLACED A SIGNIFICANT FOCUS ON STRENGTHENING OUR DOMESTIC LIFE SCIENCES SECTOR, enabling manufacturing and innovation to better prepare ourselves for future healthcare emergencies.
While there is obvious value in developing new technologies, such as mRNA vaccines, the traditional biologics like vaccines, antibodies and cell and gene therapies, remain critical life-saving solutions for millions of Canadians affected by illness, including rare and chronic diseases. Additionally, with antimicrobial resistance and constantly emerging new infections, the continuous development of a range of treatment options is vitally important for the future.
Canada has a rich pipeline of early-stage biologics from academia and small and medium developers, but the challenge often lies in getting these new products ready for commercial production. This involves a lengthy and complex process of pre-clinical evaluation, manufacturability assessment and small-scale production for clinical trials. In fact, for promising therapeutics, the gap between innovation and phase I clinical trials is sometimes called the “valley of death.” It’s not necessarily because a product is ineffective, but rather because navigating the development process is so difficult that many new products fail to make it through all the steps required to make it to the clinical trial stage.
The solution? Collaboration to accelerate product development and bring new innovation to Canada. One of the key takeaways from the COVID-19 pandemic was that collaboration across academia, government and the private sector is vital to developing cost-effective and timely health solutions.
The National Research Council of Canada (NRC) fosters that collaboration, working with partners from industry, hospitals, health networks, academia and government to help them navigate the complexity of the development process.
With one of the largest research and development teams dedicated to biologics development in Canada, the NRC’s Human Health Therapeutics Research Centre offers technical expertise, advisory services and leading-edge facilities across the biologics value chain, from discovery to clinical grade manufacturing.
Researchers work on solutions for some of today’s most challenging health problems, including emerging
infections, antimicrobial resistance, cancer and rare genetic disorders.
They design, develop and produce novel biologics targeting these and other health challenges, and ensure those biologics are safe, effective and ready for clinical testing, offering clients end-to-end support, including:
• filling innovation gaps in design, preclinical evaluation and bioprocessing
• addressing challenges at all stages of biologics development
• de-risking translation to commercial production.
Our mission to accelerate innovative biologics in Canada starts at the discovery stage. Our experts help design complex early-stage biologics for treating or preventing chronic diseases and emerging infections. Areas of expertise
• Target identification with proprietary databases
• Antibody (traditional & single-domain) generation and characterization
• Antibody humanization
• Viral vector design and targeting
• Cell and immunotherapy therapy platforms
• Vaccine development
• Biophysical characterization
PRE-CLINICAL RESEARCH AND DEVELOPMENT
After novel biologics have been identified as lead candidates, we help advance them through the rigorous pre-clinical evaluation required prior to entering clinical trials, including de-risking development processes and providing the required quality assurance documentation. Areas of expertise
• Small-scale manufacturing
• In vivo and in vitro pre-clinical testing
• Immune-monitoring
• Functional characterization
• Formulation and analytics
BIOPROCESS RESEARCH AND DEVELOPMENT
Our biomanufacturing experts help develop and perfect bioprocesses at small and pilot scales so the commercial scale production will be consistent, safe and cost-effective. Areas of expertise
• Cell line development
• Cell line engineering
• Upstream and downstream process development and optimization
• Manufacturability assessment and process intensification
• In-process and release assay automation
• Scale up at mammalian cell culture and microbial fermentation pilot plants
• Technology transfer to other organizations with quality assurance and control documentation
We can optimize GMP-compliant bioprocesses and produce cell-based biologics at clinical scale (up to 500 L) in our specialized GMP grade clinical trial material facility.
The NRC’s clinical trial material facility is uniquely positioned to help innovators advance novel biologics through clinical trials.
This 1,700-square-metre facility is designed for developing biopharmaceuticals such as vaccines and therapeutics produced in mammalian cell lines in collaboration with industry, hospitals, health networks, academia and other government departments. It will function as a contract manufacturing organization (CMO) for clinical trials and pharmaceutical products. It is designed to handle pre-market products in small quantity and will operate on demand.
With its state-of-the-art public infrastructure and the NRC’s established biomanufacturing R&D expertise, the clinical trial material facility is ideally suited for early process validation, production process optimization, small-scale biologics production and technology transfer for commercialization.
KEY FEATURES
• Compliant with GMP recognized by Canadian, American and European regulatory authorities
• Single-use production
• Containment levels 1 and 2
• Unidirectional materials and people-flow design
• 50 L and 500 L bioreactors
• Virus inactivation/nanofiltration capacities
• Pre- and post-virus purification train
• Tangential flow filtration (ultrafiltration)
• Cell amplification, production and clarification using depth filtration
• Chromatography skids for purification and polishing steps
• Bulk fill
• Process control capabilities
POTENTIAL
• Clinical trial material production (proteins, antibodies, vaccines, viral vectors, gene therapies)
• Technology transfer hub
• GMP manufacturing process optimization
• Early process (upstream and downstream) validation
• GMP cell banks

YOU DON’T HAVE TO NAVIGATE THE PROCESS ALONE. MAKE YOUR INNOVATION OUR NEXT PROJECT.
Through collaborative research, the NRC’s Human Health Therapeutics Research Centre helps increase success and impact for our partners from industry (startups, small and medium-sized enterprises and multinationals), hospitals, health networks, academia and government, as we work together to accelerate novel biologics through the complex development process.
We excel in bridging innovation gaps and addressing challenges across the value chain of biologics development, as well as de-risking translation to commercial production.
Contact us to learn more about how our multidisciplinary team of experts can help improve the design of your novel biologics, test their preclinical safety and efficacy, and optimize your production processes, both at lab scale and in a GMP environment.
Let’s work together to accelerate novel biologics in Canada.
Human Health Therapeutics Research Centre
National Research Council of Canada
Sue Twine Ph.D. MBA, Director General susan.twine@nrc-cnrc.gc.ca
Kelley Parato Ph.D., Director R&D Bioprocess Engineering kelley.parato@nrc-cnrc.gc.ca
Minh Tran B. Eng, Director Clinical Trial Material Facility minh.tran@nrc-cnrc.gc.ca
Paul Payette Ph.D. MBA, Director Business paul.payette@nrc-cnrc.gc.ca
AS THE NEWLY APPOINTED GENERAL MANAGER OF ALEXION CANADA, COULD YOU TELL US ABOUT YOUR CAREER PATH AND WHAT BROUGHT YOU HERE?
With this move to Alexion, I am continuing my 23-year biopharmaceutical career. Most recently I was in the United States as a Global Commercial Vice President in AstraZeneca’s Cardiovascular, Renal and Metabolism division and previously was VP in the BioPharma and Respiratory & Immunology business units in Sweden and the Nordics, respectively. Before re-locating to Europe, I was VP of Patient Access & Established Brands in Canada where I played an integral role in securing access for our portfolio of medicines and building an understanding about the value of innovative medicines and contributions of the pharmaceutical sector to the Canadian healthcare ecosystem.
Before joining AstraZeneca, I had a series of commercial leadership roles within GlaxoSmithKline Canada. I hold a BSc in Materials and Metallurgical Engineering from Queen’s University, Ontario, and a Professional Engineering Licence.
HAVING WORKED IN THE US/EU FOR A NUMBER OF YEARS, WHAT EXCITES YOU MOST ABOUT COMING BACK TO CANADA?
Coming back to Canada and stepping into this leadership role at Alexion is an amazing opportunity. While I have really enjoyed living and working abroad and learning about different cultures, there’s truly no place like home. The prospect of making
a tangible difference in the lives of Canadians affected by rare diseases is both motivating and humbling. I know that collectively as Alexion and AstraZeneca, we can help bring about positive changes to the Canadian healthcare ecosystem and I’m excited to be part of it.
My personal goal coming into Alexion is to position Canada as a best-in-class market for rare disease care and research. This hinges on the implementation of the national strategy for rare diseases. We need to transition from aspiration to action, ensuring that Canadians with rare diseases receive improved care pathways, better access to treatments, and a patient-centered care model, especially considering it takes on average 4.8 years to diagnose a rare disease1 and there are no treatments for 90% of them.2

ALEXION HAS BEEN EXPANDING ITS FOOTPRINT IN CANADA. CAN YOU TELL US MORE ABOUT WHAT SETS CANADA APART AS AN ATTRACTIVE PLACE TO INVEST AND GROW?
Canada has some of the finest researchers, academic centres and healthcare institutions in the world, making it an ideal place for investment and innovation in the rare disease space. Alexion Canada is proof of this, with more than 90 clinical trials running globally, including 14 Canadian trials in 6 provinces. We have over 200 employees in Mississauga including a dedicated research and development team focused on running clinical trials in Canada and globally. We also
While Canada has been gaining momentum when it comes to innovation in recent years, we need to ensure we have a favourable and accelerated access environment that attracts investment here, especially in supporting the national strategy for rare diseases.

have a local commercial team who ensure our treatments reach the Canadians who need them.
While Canada has been gaining momentum when it comes to innovation in recent years, we need to ensure we have a favourable and accelerated access environment that attracts investment here, especially in supporting the national strategy for rare diseases. It’s essential for fostering an environment where Canadians with rare diseases can access the necessary diagnosis, treatment, and support as quickly as possible.
IT HAS BEEN MORE THAN A YEAR SINCE CANADA ANNOUNCED ITS FIRST-EVER NATIONAL STRATEGY FOR RARE DISEASES. WHAT DO YOU HOPE THE SECTOR CAN ACCOMPLISH AS THIS WORK CONTINUES?
Reflecting on the year since the announcement of Canada’s first-ever strategy for rare diseases, it’s clear that while the government’s commitment is commendable, action is still urgently needed to bring this strategy to life. We need to do more than just catch up with other nations; we have
the opportunity to lead and set new standards in rare disease care. Alexion is already doing some of that work, but to truly become a leader on the world stage, we will need to partner with government on an approach to elevate Canada’s position in the global rare disease community.
AS ITS NEW LEADER, WHAT’S NEXT FOR ALEXION IN CANADA?
My vision for Alexion Canada is to leave a lasting impact and ensure people living with rare diseases now and in the future have more hope, more options and more access to innovative therapies that will improve their lives. The journey ahead is about pioneering change and inspiring hope and it is my ambition that we can continue to partner with stakeholders and policymakers to make this happen. I want Alexion to continue to bring innovation to the Canadian market through new therapies, more clinical trials, implementing Canada’s rare disease strategy and ultimately improving the lives of Canadians living with rare disease.
1 Global Genes. (n.d.). RARE Disease Facts. [Online] Available at: https://globalgenes.org/rare-disease-facts/.
2 National Organization for Rare Disorders (NORD). New Report Finds Medical Treatments for Rare Diseases Account for Only 11% of US Drug Spending; Nearly 80% of Orphan Products Treat Rare Diseases Exclusively. https://rarediseases.org/new-report-finds-medical-treatments-for-rare-diseases-account-for-only-11-of-us-drug-spending-nearly-80of-orphan-products-treat-rare-diseases-exclusively/. Accessed January 18, 2024.

IN THE FAST-PACED WORLD OF HEALTHCARE, WHERE EVERY DAY BRINGS NEW CHALLENGES AND OPPORTUNITIES , Daiichi Sankyo is working towards a single goal in the oncology field: to transform the way we treat cancer and improve the lives of patients. As Country Manager, I’ve had the pleasure of bringing this goal to Canada and the incredible opportunity to guide our growing talented team, fostering a culture that’s all about learning, embracing diversity and understanding our patients’ needs.
OUR PURPOSE IS TO CONTRIBUTE TO THE ENRICHMENT OF QUALITY OF LIFE
Cancer remains the leading cause of death in Canada. According to the government, every fourth Canadian is expected to die from cancer.1 These people—all of us—are at the heart of everything we do. Cancer is often a recurring disease, an unwanted, constant companion. We know that improving the lives of people living with cancer, especially those with hard-to-treat versions, is a task that cannot be accomplished overnight, but requires dedication and commitment in the long term. My aim for Daiichi Sankyo Canada is to build a future-thinking Canadian organization where our people value fulfilment at work
In spring 2023, we launched our Canadian affiliate in Toronto as a subsidiary of our Europe and Canada region. As we move forward, I’m reminded of Chinese philosopher Lao Tzu’s words, “A journey of a thousand miles begins with a single step.” Each step we take is a step closer to defeating cancer. Our strategic five-year plan for Daiichi Sankyo Canada is geared toward longevity and follows this step-by-step mantra: Beginning with “Define & Start,” progressing to “Build & Contribute,” and speeding up through “Expand & Establish,” we aim to “Succeed & Refine” by 2025.
The journey we’ve embarked upon in Canada is nothing short of extraordinary. Focusing on Medical, Commercial and Market Access functions first, I am incredibly proud to have recruited Matt Lee (Market Access), Fadie Jebrail (Medical), Niki Haley-Scott (Commercial), and Melita Sequeira (HR) to lead the Canadian Daiichi Sankyo team into the future. Over the past year, we have grown our Toronto affiliate at an impressive rate, demonstrating our long-term commitment to positively impacting the lives of people living with cancer and their families. And we’re far from done.
At Daiichi Sankyo, we know that a cancer patient may also be a mother or father who wants to see their children grow up. A partner, a friend, a daughter, or a son who longs to spend time with their loved ones. It is with this knowledge that we conduct our science and cancer research.
Our roots in Japan have taught us the value of striving for perfection, the importance of failing but never giving up, and the drive to develop groundbreaking medicines. This is why we have created a test and learn environment where curiosity is always welcome.
Equity thrives through empathy, transparency and assuming good intent in our exchanges at work.
Daiichi Sankyo prides itself on doing what’s best for people (both our employees and patients), fostering a growth mindset as well as a culture of honor and accountability. With a presence in 29 countries and a dedicated team of more than 17,000 people worldwide, we are united by our core behaviors: be inclusive & embrace diversity—develop & grow—collaborate & trust.
Every patient is unique with needs shaped by who they are, where they come from, how advanced and aggressive their cancer is, and what drives their tumor growth. That is why our approach to cancer care is to create individualized treatments for new cancer targets. What defines us is our passion for innovation. We aim to create game-changing medicines that redefine the standard of care and explore the areas of oncology where there are no solutions yet.
We seek to create value where there is a clear unmet need and untapped potential for change. With a keen focus on cutting-edge science and technology, we look forward to accelerating R&D investment in Canada. I am pleased to share that we are already increasing collaboration with research centers this year. It is our core belief that by turning scientific innovation into tangible benefits, we can have an impact on patients’ lives. One example of how this comes together is our pioneering ADC technology platform, propelling us into a new era of cancer treatment.
As I look to the future, I’m excited about the possibilities and opportunities I see for Daiichi Sankyo in Canada. I am confident in our growing team’s ability to navigate the road ahead, supported by the wider healthcare community. I’d like to invite healthcare professionals, industry partners, and future team members to join us in this mission. Together, we strive to improve standards of care for those affected by cancer and work towards a future where cancer is no longer one of the most feared diagnoses but a manageable condition.
# CA/ONP/03/24/0001
Fatih Yedikardes has been the founding Country Manager at Daiichi Sankyo Pharma Canada Limited since November 2022.
Yedikardes has been part of Daiichi Sankyo for over five years and led the Daiichi Sankyo team in Turkey as Country Manager before starting his journey in Canada. He has more than 20 years of experience in the pharmaceutical industry.

Daiichi Sankyo is an innovative global healthcare company contributing to the sustainable development of society that discovers, develops, and delivers new standards of care to enrich the quality of life around the world. With more than 120 years of experience, Daiichi Sankyo leverages its world-class science and technology to create new modalities and innovative medicines for people with cancer, cardiovascular and other diseases with high unmet medical need. For more information, please visit daiichi-sankyo.eu.


YOU WERE RECENTLY APPOINTED PRESIDENT OF ASTRAZENECA CANADA. TELL US ABOUT YOUR JOURNEY WITH THE ORGANIZATION?
I’ve been so fortunate to have spent my career with AstraZeneca—22 years and counting. I joined the company as an MBA co-op student from McMaster University; over the years, I leaned into learning everything I could about the biopharmaceutical business across therapy areas ranging from rare disease and oncology to respiratory and cardiovascular disease. I have had wonderful managers and mentors, and terrific growth opportunities that have allowed me to lead diverse and talented teams in Canada, the U.S. and globally.
I’ve spent much of the past decade in oncology, including as the VP of Oncology for AstraZeneca Canada, before transitioning to lead the Global Commercial function and development program for one of AstraZeneca’s key emerging oncology medicines in lung and breast cancer.
Most recently, I served as the General Manager in Canada of Alexion, AstraZeneca Rare Disease—a unique opportunity to lead in this highly complex and rapidly growing area of medicine. These experiences helped prepare me for the role of President of AstraZeneca Canada which I took on earlier this year—a really special “fullcircle” moment and at such an exciting time of growth and investment for the company in Canada. I’m presently also active as a Director on the Boards of Innovative Medicines Canada, as well as Safehaven and The Advanced Coronary Treatment (ACT) Foundation of Canada.

HOW WOULD YOU DESCRIBE ASTRAZENECA’S RECENT GROWTH IN CANADA?
It’s an incredibly exciting time for AstraZeneca in Canada—where we are rapidly building our research footprint in Canada and advancing our pipeline and the next generation of therapeutics leveraging promising new drug modalities and technologies, including antibody drug conjugates, epigenetics, and cell-based therapies.
Last year, our Global CEO Pascal Soriot stood alongside Prime Minister Justin Trudeau and Premier Doug Ford at our Mississauga head office as we announced a $500 million research investment, bringing more than 500 new scientific and high-tech jobs to the Greater Toronto Area in a major expansion of our Canadian research footprint. This investment includes significantly growing our existing AstraZeneca R&D Hub and creating a new Alexion Development Hub focused on rare diseases. We’ve already made great progress. We filled these new roles in just over a year and our R&D Hubs are presently leading more than 230 global clinical studies in oncology, biopharmaceuticals and rare diseases—from breast, lung and prostate cancer, to COVID-19, severe asthma and chronic kidney disease.
But we’re not stopping there. Just weeks ago, we announced an agreement to acquire Hamilton-based Fusion Pharmaceuticals, a clinical-stage biopharmaceutical company developing next-generation radioconjugates. The announcement represents one of the
It’s a very exciting time for scientific discovery, development and growth at AstraZenenca, and our decision to invest in Canada is a testament to the world-class health sciences ecosystem here—biotechnology companies, universities, hospitals, and research centres in the GTA, and the diverse scientific talent pool that exists in the region.
largest research investments made in a Canadian biotechnology company and it has the promise to redefine radiotherapy in oncology.
It’s a very exciting time for scientific discovery, development and growth at AstraZenenca, and our decision to invest in Canada is a testament to the world-class health sciences ecosystem here—biotechnology companies, universities, hospitals, and research centres in the GTA, and the diverse scientific talent pool that exists in the region.
WHAT DOES ASTRAZENECA’S AGREEMENT TO ACQUIRE FUSION PHARMACEUTICALS MEAN FOR PATIENTS? THE CANADIAN HEALTH SCIENCES INDUSTRY?
The US $2.4 billion acquisition agreement for Fusion Pharmaceuticals is a milestone investment in the Canadian life sciences sector and a testament to the breakthrough research and development work being done in our backyard.
Roughly thirty to fifty per cent of patients with cancer today receive radiotherapy at some point during treatment. Radioconjugates have emerged as a very promising modality in cancer treatment—with the potential to


redefine oncology through more precise molecular targeting of radiation to cancer cells compared to traditional radiation therapy, with the goal of improving efficacy while minimizing toxicity on normal cells.
The acquisition builds on our existing collaboration with Fusion where we have been jointly discovering and developing next-generation radioconjugates since 2020— leveraging their expertise and state-of-the-art R&D, manufacturing and supply chain capabilities in actiniumbased radioconjugates. I’m excited to work even more closely together with this dynamic, made-in-Canada organization, as well as our global teams to help deliver for patients both in Canada and around the world. This investment further strengthens AstraZeneca’s research presence in and commitment to Canada, and marks a major step forward in our ambition to transform cancer treatment and outcomes for patients by replacing traditional regimens like chemotherapy and radiotherapy with more targeted treatments.
I‘m proud to lead a science-based organization with a laser focus on delivering next-generation therapeutics that transform the lives of patients with improved outcomes and a better quality of life. I am equally proud of the positive change we are driving beyond the impact of our medicines by tackling some of the biggest sustainability challenges of our time—from climate change and biodiversity loss to health equity, health system resilience, and inclusion and diversity.
Our Global CEO Pascal Soriot was recently named by Time Magazine as a Climate Change Titan for his work taking action on climate change—bringing together business leaders from across the global healthcare system with a common goal of decarbonizing our sector. His leadership sets the tone for us and our sustainability efforts. The climate crisis is the biggest public health crisis we face, contributing to a rise in cancer, respiratory illnesses, and heart disease and undermining the capacity of health systems. Through our Ambition Zero Carbon strategy we’re accelerating our progress towards net zero and embedding sustainability into everything we do—minimizing and ultimately eliminating our environmental impact by
In Canada, we are taking important steps to help address health inequities and eliminate barriers to the delivery of healthcare. This includes supporting Canadian efforts to improve access to oncology care in underserviced rural and remote populations.
great support for their and their families’ wellbeing, and model a speak-up environment that welcomes new perspectives and approaches. It means doing whatever I and other leaders can to help people grow and reach their full potential.
It’s wonderful to be recognized externally (three prestigious milestone awards in 2023 alone—Canada’s Top 100 Employers, one of Canada’s Most Admired Corporate Cultures, and Greater Toronto’s Top Employers for the 10 th consecutive year). What drives me more is being part of a workplace where everyone is energized and feels they belong, they are learning and growing, and their work makes a such a meaningful difference. That’s magic.
reducing carbon emissions, water consumption, and waste. We also expanded our global reforestation and biodiversity initiative—AZ Forest—investing $400 million to plant and maintain 200 million trees by 2030 globally.
In Canada, we are taking important steps to help address health inequities and eliminate barriers to the delivery of healthcare. This includes supporting Canadian efforts to improve access to oncology care in underserviced rural and remote populations. Additionally, we’re supporting Canadian research that will enhance early detection, improve coordination of care, and address disparities in health equity in lung cancer, a disease that continues to be the leading cause of cancer death for Canadians, yet which receives a small fraction of research funding due to stigma. Just recently, we announced the Eureka Fellowship for Youth Changemakers in Canada, in partnership with PLAN International Canada—a national program aimed at supporting and amplifying the positive impact of inspiring youth leaders focused on tackling some of Canada’s and the planet’s biggest sustainability challenges.
At AstraZeneca, I’m truly humbled by the people that surround me every day. It’s an incredibly diverse team, rich with perspectives, talent and knowledge, all working with a powerful shared purpose to push the boundaries of science and deliver life-changing medicines to patients. That diversity of thought is foundational to our dynamic and inclusive culture—a culture that plays a hugely important role in driving innovation, solving challenges, and enabling great performance.
Our cross-functional Inclusion & Diversity Council, as well as Employee Resource Groups, lead the way by helping develop and deliver training and engagement sessions for employees—such as workshops on Neurodiversity, Gender Allyship and Supporting Psychological Safety. We also work hard to support employee growth and development, provide
In my years at AstraZeneca, I’ve experienced many exciting moments I didn’t think could be topped. Looking ahead, we’ve got such terrific momentum here in Canada— we’re rapidly building our Canadian research and footprint and are an integral part of what is a world-class health ecosystem in Canada, working with government and partners to make Canada an even more attractive environment for life sciences stakeholders.
Every day, our talented and diverse team is working to deliver life-changing science to improve the health of Canadians—partnering with healthcare practitioners to transform care and improve patient outcomes, ensuring timely access to new treatments, and supporting a more resilient healthcare system.
I couldn’t imagine a more rewarding purpose, and I’m excited about what the future looks like for AstraZeneca in Canada.

Régime
privilégié
des brevets : le gouvernement fédéral mène une consultation qui s’avère prometteuse pour l’industrie des sciences
Nancy Pei (Principal, Smart & Biggar) and Sanro Zlobec (Principal, Smart & Biggar) par Nancy Pei (directrice, Smart & Biggar) et Sanro Zlobec (directeur, Smart & Biggar)
JANUARY 2024, THE CANADIAN GOVERNMENT
RELEASED A CONSULTATION PAPER “seeking views on the suitability of adopting a patent box regime to encourage the development and retention of intellectual property stemming from R&D conducted in Canada.”1 In its paper, the government recognizes not only the value of a patent box to attract investment, but also the intrinsic value of patents to the economy, noting that “[p]atent-owning businesses grow faster and pay higher wages.” Below, we explore what a patent box may look like based on Canadian examples and its potential to further spur investment and growth in the life sciences industry in Canada.
A “patent box” or, more generally, an intellectual property (IP) box, is a tax incentive that allows companies to pay a reduced tax rate on income derived from the commercialization of certain intellectual property developed and owned by the company. Broadly speaking, an IP box is an output-driven tax incentive, which is based on the results of commercialization of innovation developed in that jurisdiction and therefore contrasts with input-driven tax incentives, such as a research and development (R&D) tax credit, which focuses on the stage of creation.
Like other countries, Canada has long provided a tax-based incentive for R&D, known here as the Scientific Research and Experimental Development (SR&ED) tax credit.2 This credit benefits companies of all sizes and levels of maturity, supporting the innovation ecosystem and spurring the development of new technologies across many industries. However, in addition to certain shortcomings regarding its operation, its scope is limited to early-stage development, without any link to commercialization efforts that take place once the fundamental research is complete.
An IP box, by contrast, is a fiscal measure intended to help encourage innovative businesses to stay in Canada, or to attract businesses from abroad, by applying a preferential tax rate to income that is derived from IP
EN JANVIER 2024, LE GOUVERNEMENT CANADIEN A PUBLIÉ UN DOCUMENT DE CONSULTATION, CAR IL « sollicite des avis sur la pertinence de créer un régime privilégié des brevets pour encourager le développement et la conservation de la propriété intellectuelle découlant de la R et D menée au Canada ». Dans son document, le gouvernement reconnaît non seulement la valeur d’un régime privilégié des brevets pour attirer les investissements, mais aussi la valeur intrinsèque des brevets pour l’économie, soulignant que les « entreprises qui détiennent des brevets connaissent une croissance plus rapide et paient des salaires supérieurs ». Dans cet article, nous présenterons, à partir d’exemples canadiens, ce à quoi pourrait ressembler un régime privilégié des brevets et son potentiel pour stimuler davantage l’investissement et la croissance dans l’industrie des sciences de la vie au Canada.
Un « régime privilégié des brevets » (ou plus généralement un régime privilégié de propriété intellectuelle ou « PI ») est un incitatif fiscal qui offre aux entreprises un taux d’imposition réduit sur les revenus tirés de la commercialisation de certains éléments de propriété intellectuelle créés et détenus par celles-ci. D’une manière générale, un régime privilégié de PI est un incitatif fiscal axé sur les extrants, qui se fonde sur les résultats de la commercialisation de l’innovation mise au point au sein du territoire concerné; les incitatifs fiscaux axés sur les intrants, tels que le crédit d’impôt pour la recherche et le développement (R et D), concernent au contraire essentiellement la phase de création.
À l’instar de plusieurs autres pays, le Canada offre depuis longtemps un incitatif fiscal en matière de R et D : le crédit d’impôt « Recherche scientifique et développement
1 Department of Finance Canada, Creating a Patent Box Regime (Consultation Paper), 31 January 2024, online: https://www.canada.ca/en/department-finance/ programs/consultations/2024/consultation-on-creating-a-patent-box-regime/ consultation-paper-on-creating-a-patent-box-regime.html.
1 Ministère des Finances Canada, Document de consultation sur la création d’un régime privilégié des brevets, 31 janvier 2024, en ligne : https://www.canada.ca/ fr/ministere-finances/programmes/consultations/2024/consultation-sur-la-creation-dun-regime-privilegie-des-brevets/document-de-consultation-sur-la-creation-dun-regime-privilegie-des-brevets.html.
created from locally generated R&D. It is from this vantage point that Canada began considering a patent box, indicating in its 2022 budget that it will consider such a regime as a way “to build innovative companies that support Canada’s competitiveness, keep intellectual property in Canada, and attract talent and investment from around the world.”3
Canada’s current consultation does not provide any details regarding a patent box regime but notes that the government would need to consider the OECD “nexus approach,” which allows “a taxpayer to benefit from an IP regime only to the extent that the taxpayer itself incurred qualifying R&D expenditures that gave rise to the IP income.”

Nancy Pei has over 25 years of life sciences patent litigation and related regulatory advice expertise. Nancy currently serves on the Board of Directors of BIOTECanada.
Nancy Pei œuvre depuis plus de 25 ans à offrir des conseils en ce qui concerne les litiges et la réglementation relatifs aux brevets dans le domaine des sciences de la vie. Elle siège actuellement au conseil d’administration de BIOTECanada.
Recognizing the close link with the SR&ED program, the federal government simultaneously launched a consultation on the SR&ED program, including to modernize and improve it in a cost-neutral way. Federal online consultations are due to be completed this spring. Additional stakeholder engagement on the policy opportunity will carry on through this year.4
While it is difficult to argue against the benefits of a new tax incentive from an individual company’s perspective, a key question that the consultations will attempt to answer is whether a Canadian IP box is indeed good for the country as a whole. Judging from global trends, the current perception is that an IP box can provide a net benefit. Today, over 20 countries, including 13 European Union members, have implemented some form of IP box, and other industrialized nations (e.g., Japan and Hong Kong) are considering doing so.
2 Canada Revenue Agency, “Scientific Research and Experimental Development (SR&ED) tax incentives” (17 January 2024), online: https://www.canada.ca/en/ revenue-agency/services/scientific-research-experimental-development-tax-incentive-program.html.
3 Department of Finance Canada, A Plan to Grow Our Economy and Make Life More Affordable (Budget 2022), 7 April 2022, online: https://budget.canada.ca/2022/ home-accueil-en.html.
4 Department of Finance Canada, Scientific Research and Experimental Development (Consultation Paper), 31 January 2024, online: https://www.canada.ca/ en/department-finance/programs/consultations/2024/consultation-on-scientific-research-and-experimental-development/consultation-paper-on-scientific-research-and-experimental-development.html.
expérimental » (RS&DE). 2 Il s’agit d’un crédit offert aux entreprises de toutes les tailles et de tous niveaux de maturité dont l’objectif est d’apporter une aide à l’écosystème de l’innovation et de stimuler le développement de nouvelles technologies dans de nombreux secteurs. Outre certaines lacunes concernant son fonctionnement, il est à noter aussi que son champ d’application est limité au développement à un stade précoce, sans aucun lien avec les initiatives en matière de commercialisation, qui ne sont mises en œuvre qu’à la suite de la recherche fondamentale.
Un régime privilégié de propriété intellectuelle, en revanche, est une mesure fiscale destinée, d’une part, à encourager les entreprises innovantes à rester au Canada et, d’autre part, à attirer des entreprises étrangères. Les entreprises bénéficient d’un taux d’imposition préférentiel pour les revenus issus de la propriété intellectuelle liée à la R et D effectuée localement. C’est dans cette optique que le Canada a commencé à envisager un régime privilégié de brevets, indiquant dans son budget de 2022 que celui-ci permettrait de « trouver d’autres moyens de bâtir des entreprises novatrices qui appuient la compétitivité du Canada, de maintenir la propriété intellectuelle au Canada et d’attirer des talents et des investissements du monde entier. » 3
La consultation menée actuellement par le Canada ne fournit aucun détail concernant un éventuel régime privilégié de brevets, mais indique que le gouvernement devrait prendre en considération les recommandations de l’OCDE en matière d’« approche du lien », celle-ci permettant « à un contribuable de bénéficier d’un régime de propriété intellectuelle uniquement dans la mesure où il a engagé des legal matters questions de droit
2 Agence du revenu du Canada, « Encouragements fiscaux pour la recherche scientifique et le développement expérimental (RS&DE) » (17 janvier 2024), en ligne : https://www.canada.ca/fr/agence-revenu/services/recherche-scientifique-developpement-experimental-programme-encouragements-fiscaux.html.
3 Ministère des Finances Canada, Un plan pour faire croître notre économie et rendre la vie plus abordable (budget de 2022), 7 avril 2022, en ligne : https://budget. canada.ca/2022/home-accueil-fr.html.
legal matters questions de droit
Today, over 20 countries, including 13 European Union members, have implemented some form of IP box, and other industrialized nations (e.g., Japan and Hong Kong) are considering doing so.
Aujourd’hui, plus de 20 pays, dont 13 membres de l’Union européenne, ont mis en place une forme ou une autre de régime privilégié de PI, et d’autres pays industrialisés (comme le Japon et Hong Kong) envisagent de le faire.
Looking abroad can provide guidance and a further incentive to implement a patent box (given that tax policies are necessarily competitive), but Canada may also find insights from experiences closer to home, with two examples of provincially sponsored IP boxes in Saskatchewan and Québec.
The Saskatchewan Commercial Innovation Incentive (SCII) was launched in 2017 and applies to a broad range of IP, even including trade secrets. However, there is no restriction on where the subject IP needs to have been developed in order to provide a tax benefit, and thus the SCII does not meet the OECD’s nexus requirement, which may partly explain why the measure will soon be phased out.
For its part, the Québec IP box, revamped from its 2016 version and in force since 2020, serves as a highly relevant example for the federal government to consider. The Québec “IDCI” measure allows eligible income to benefit from a 2% provincial tax rate compared to the regular 11.5% rate, although the actual tax rate will depend on the type of revenue (e.g., sale of goods incorporating an IP asset versus licensing of the IP asset) and the profit margin of the business. The IP asset can be a patent (or pending patent application), copyright (for software) or plant breeder’s right, as long as the IP is owned by a business established in Québec and is the result of R&D conducted in Québec. The Québec IP box is simple to apply (there is no longer a need to apportion the IP to the resulting profit), and the maximum reduction in the tax rate is among the most generous in the world (at 82.6%). The Québec IP box, importantly, provides the lower tax rate to eligible income derived from any jurisdiction, which encourages the global expansion of Québec-based businesses. However, if there is a blind spot in the Québec IP box, it is that to support a claim that an IP asset was developed from Québec-based R&D, there effectively needs to be an underlying application for a federal SR&ED credit. As commercially relevant IP can often be developed without a direct link to a SR&ED tax credit, this leaves some potentially valuable IP assets ineligible for consideration under the Québec IP box, which can make the measure less attractive than it could be, and perhaps less attractive than intended.
dépenses admissibles en R et D qui ont augmenté le revenu de la propriété intellectuelle ». Reconnaissant le lien étroit avec le programme de RS&DE, le gouvernement fédéral a simultanément lancé une consultation portant sur le programme de RS&DE et sur les façons de le moderniser et de l’améliorer sans engager de coûts . Les consultations en ligne mises en œuvre par le gouvernement fédéral devraient être achevées au printemps. D’autres échanges avec les intervenants relativement à cette possibilité stratégique auront lieu tout au long de l’année.4
S’il est vrai que pour une entreprise individuelle les avantages d’un nouvel incitatif fiscal sont indéniables, il sera intéressant que les consultations tentent de répondre à la question de savoir si un régime privilégié de PI canadien est effectivement bénéfique pour le pays dans son ensemble. Si l’on en juge par les tendances mondiales, il semble que les régimes de PI apportent des avantages nets. Aujourd’hui, plus de 20 pays, dont 13 membres de l’Union européenne, ont mis en place une forme ou une autre de régime privilégié de PI, et d’autres pays industrialisés (comme le Japon et Hong Kong) envisagent de le faire.
L’observation de ce qui se fait à l’étranger peut inspirer de nouvelles orientations et peut aussi s’avérer une forme d’incitation supplémentaire à mettre en œuvre un « régime privilégié de brevets » (étant donné que les politiques fiscales sont nécessairement concurrentielles), mais le Canada peut également tirer des enseignements d’expériences locales que sont les deux exemples de « régimes privilégiés de PI » parrainés par des provinces, soit la Saskatchewan et le Québec.
Le programme d’incitatif à l’innovation commerciale de la Saskatchewan (SCII), qui a été créé en 2017, s’applique à un large éventail de droits de propriété intellectuelle, notamment même aux secrets commerciaux. Pour offrir l’avantage fiscal, il n’impose toutefois aucune condition quant au lieu où la propriété intellectuelle en question est créée. Le programme SCII ne répond donc pas à l’exigence de l’approche du lien convenue par les pays de l’OCDE, ce qui peut expliquer en partie pourquoi la mesure sera bientôt supprimée.
4 Ministère des Finances Canada, Consultation sur la recherche scientifique et le développement expérimental (document de consultation), 31 janvier 2024, en ligne : https:// www.canada.ca/fr/ministere-finances/programmes/consultations/2024/consultation-sur-la-recherche-scientifique-et-le-developpement-experimental.html.
While the consultations for a federal IP box are at a very early stage, it is nevertheless encouraging that the Canadian government is considering further measures to attract and retain innovative companies and reward them for their success in commercializing the IP they develop here. Moreover, it is a positive sign that the value of patents as a distinct means to propel the economy is being recognized. It is our hope that the feedback received via the consultation process, coupled with a detailed study of the Québec experience and analysis of IP boxes elsewhere, will result in a system optimally tuned to benefit the life sciences industry in Canada.

Sanro Zlobec has spent the past two decades helping clients use IP to unlock value in commercial transactions and tax situations. He is one of the few IP professionals experienced with Quebec’s original “IP Box” and has been closely monitoring its evolution ever since.
Depuis une vingtaine d’années, Sanro Zlobec conseille ses clients sur l’utilisation de la PI pour optimiser la valeur des transactions commerciales et à mieux gérer leur cadre fiscal. Il est l’un des rares professionnels de la PI à posséder de l’expérience sur le terrain quant à la mouture originale de la DICI, et il en suit de près l’évolution depuis son instauration.
legal matters questions de droit
Le régime privilégié de PI du Québec, remanié par rapport à sa version de 2016 et en vigueur depuis 2020 dans sa forme actuelle, constitue quant à lui un exemple que le gouvernement fédéral aurait tout intérêt à analyser. La déduction incitative pour la commercialisation des innovations au Québec (DICI) permet de bénéficier d’un taux d’imposition provincial de 2 %, alors que le taux courant est de 11,5 %. Il est à noter que le taux d’imposition appliqué dépend du type de revenu (p. ex. la vente de biens qui incorporent un actif de PI ou l’octroi d’une licence de PI) et de la marge bénéficiaire de l’entreprise. L’actif de PI peut être un brevet (ou une demande de brevet en instance), un droit d’auteur (associé à un logiciel) ou un certificat d’obtention de variété végétale, à condition que la PI soit détenue par une entreprise établie au Québec et qu’elle soit le résultat d’activités de R et D réalisés au Québec. Le régime privilégié de PI du Québec requiert des calculs assez simples (une répartition proportionnelle PI/bénéfices n’est plus nécessaire), et la réduction maximale du taux d’imposition est l’une des plus généreuses au monde (82,6 %). Il est important de noter que le régime privilégié de PI du Québec permet de bénéficier du faible taux d’imposition sur les revenus admissibles, quel que soit le pays d’obtention des revenus, ce qui incite les entreprises québécoises à étendre leurs activités dans le monde. Il existe toutefois un angle mort dans la DICI : dans le cadre d’une demande pour un actif de PI qui a été créé à partir d’activités de R et D menées au Québec, il faut que l’entreprise ait demandé le crédit d’impôt fédéral RS&DE. Or, il arrive souvent qu’un actif de propriété intellectuelle ayant un potentiel commercial soit créé sans recours au crédit d’impôt RS&DE. Cela signifie qu’un certain éventail d’actifs de PI, pourtant précieux, sera inadmissible dans le cadre du régime privilégié de PI du Québec, ce qui fait que la mesure est peut-être moins attrayante qu’elle ne pourrait l’être ou que ce que prévoyait son objectif initial.
Si les consultations en vue de la création d’un régime fédéral de propriété intellectuelle n’en sont qu’à leurs débuts, il est néanmoins encourageant de constater que le gouvernement canadien envisage de nouvelles mesures pour attirer et retenir les entreprises innovantes et récompenser les efforts en matière de commercialisation de la propriété intellectuelle créée ici. Ces consultations constituent par ailleurs un signe indiquant que l’on reconnaît la valeur des brevets en tant que moyen particulier de stimuler l’économie. Nous espérons que les commentaires recueillis dans le cadre du processus de consultation, ainsi qu’une étude détaillée de l’expérience avec la DICI au Québec et une analyse des régimes de propriété intellectuelle dans d’autres pays, permettront de créer un système idéalement adapté aux besoins de l’industrie des sciences de la vie du Canada.
British Columbia’s leap in life sciences: leading clinical trials and innovation with new research facilities
Le bond en avant de la Colombie-Britannique en sciences de la vie : elle passe à l’avant-scène des essais cliniques et de l’innovation grâce à de nouvelles installations de rechercheby Darryl Knight, President, Providence Research par Darryl Knight, président de Providence Research
A GLOBAL LEADER IN LIFE SCIENCES, HOSTS ROUGHLY TWENTY-FIVE PER CENT OF CANADIAN CLINICAL TRIALS, with over 1,350 currently active. Vancouver stands out as a rapidly growing hub for biotechnology research, due to a collaborative community of research institutes, a vibrant and growing industry sector, and academic partners. As part of this ecosystem, Providence Health Care and its research engine, Providence Research, have long legacies of driving innovation which has impacted care and saved lives.
Of note, however, has been the lack of capacity to support Phase 1 clinical trials; a critical part of the research process where treatments are tested on healthy human volunteers for the first time. This challenge will soon be addressed by a new clinical trials unit (CTU) at Providence Health Care’s Mount Saint Joseph Hospital, in Vancouver. This CTU will enable ground-breaking life sciences innovation for years to come, leading to better patient treatments and health outcomes.
The Mount Saint Joseph Phase 1 CTU, funded with significant contributions from the Province of B.C. and Michael Smith Health Research BC, will be one of the only non-cancer Phase 1 CTU in Western Canada. The results of this collaboration will allow B.C. to host the entire life cycle of drug and therapy development within the province.
To further enhance research and discovery, and directly connected to the new St. Paul’s Hospital, the Clinical Support and Research Centre (CSRC) is being designed as a dynamic life sciences ecosystem campus that will drive research, partnerships, and collaborations between academia and Darryl Knight
LA COLOMBIE-BRITANNIQUE EST UN LEADER MONDIAL DANS LE DOMAINE DES SCIENCES DE LA VIE QUI
CONCENTRE ENVIRON VINGT-CINQ POUR CENT DES ESSAIS CLINIQUES CANADIENS, avec plus de 1 350 essais en cours. Vancouver se positionne comme un pôle de recherche en biotechnologie en pleine expansion, grâce à l’esprit de collaboration de ses instituts de recherche, à un secteur industriel dynamique et en pleine croissance, ainsi qu’à des partenaires universitaires. Providence Health Care et le moteur de ses projets de recherche, Providence Research, possèdent une longue tradition d’innovation au sein de cet écosystème, qui a fait ses preuves en matière de soins de santé et permis de sauver des vies.
Il convient toutefois de rappeler le contexte, marqué par un manque de ressources pour les essais cliniques de phase 1, une étape essentielle du processus de recherche au cours de laquelle les traitements sont testés pour la première fois sur des volontaires humains en bonne santé. Le nouveau service d’essais cliniques (SEC) à l’hôpital Mount Saint Joseph de Providence Health Care, à Vancouver, permettra bientôt de faire un pas dans la bonne direction en vue de résoudre ce problème. Ce service sera un pilier de l’innovation de pointe dans le domaine des sciences de la vie pour les années à venir, ce qui permettra d’améliorer les traitements des patients et les résultats en matière de santé.

Le SEC de phase 1 de l’hôpital Mount Saint Joseph, financé par des contributions importantes de la province de la Colombie-Britannique et de Michael Smith Health Research BC, sera l’une des seules du genre qui ne se consacre pas à la recherche sur le cancer dans l’ouest du Canada. Cette
These state-of-the-art research facilities will serve a growing demand for non-cancer Phase 1 clinical trials in B.C., and foster collaboration between researchers, clinicians, and industry experts to advance medical discovery.
Ces installations de recherche de pointe répondront à la demande croissante en matière d’essais cliniques de phase 1 pour les maladies autres que le cancer en Colombie-Britannique et favoriseront la collaboration entre les chercheurs, les cliniciens et les experts de l’industrie en vue de faire progresser la recherche médicale
industry. With a consolidated Phase 1-3 CTU, the CSRC will be one of the only facilities in Western Canada capable of conducting all phases of non-cancer clinical trials, taking life-saving treatments from concept to completion.
The Mount Saint Joseph CTU will continue to operate after the CSRC facility opens, allowing Phase 1 trials to be performed at both locations. These state-of-the-art research facilities will serve a growing demand for non-cancer Phase 1 clinical trials in B.C., and foster collaboration between researchers, clinicians, and industry experts to advance medical discovery. Industry partners will be able use the facilities to test their discoveries here in B.C., strengthening the province’s position as a world-class destination for clinical trial research, letting patients access new therapeutics sooner, and contributing to economic development by providing opportunities to attract and retain highly qualified people and keep intellectual property in Canada.

nouvelle structure collaborative permettra à la ColombieBritannique de prendre en charge l’ensemble du cycle de vie en matière de mise au point de médicaments et de traitements dans la province.
Le Centre de recherche et de services cliniques (CRSC), directement relié au nouvel hôpital St. Paul, est conçu comme un complexe en sciences de la vie qui dynamisera la recherche, les partenariats et les collaborations entre le monde universitaire et l’industrie, ce qui aura pour effet d’améliorer davantage encore la recherche-développement. Le CRSC, grâce à son SEC intégré pour les phases 1 à 3, sera l’un des seuls établissements dans l’Ouest canadien apte à mener toutes les phases des essais cliniques sur les maladies non cancéreuses et permettra de faire passer des traitements vitaux de la conception au chevet des patients.
Le SEC de l’hôpital Mount Saint Joseph poursuivra ses opérations après la mise en service du CRSC, ce qui permettra de réaliser les essais de phase 1 sur les deux sites. Ces installations de recherche de pointe répondront à la demande croissante en matière d’essais cliniques de phase 1 pour les maladies autres que le cancer en Colombie-Britannique et favoriseront la collaboration entre les chercheurs, les cliniciens et les experts de l’industrie en vue de faire progresser la recherche médicale. Les partenaires industriels pourront utiliser les installations pour valider leurs recherches, ici même en C.-B., ce qui confortera la position de la province en tant que pôle de classe mondiale pour les essais cliniques. Cette nouvelle dynamique permettra d’offrir de nouveaux traitements plus rapidement aux patients. Le rayonnement économique accru de la province permettra aussi d’attirer et de retenir des personnes hautement qualifiées et de conserver la propriété intellectuelle au Canada.
ONTARIO HAS LONG BEEN AN ATTRACTIVE JURISDICTION FOR CONDUCTING
CLINICAL TRIALS. With over 4,800 active clinical trials, Ontario leads the country in clinical trials activity and is ranked #1 in active clinical trials per capita among G7 nations. Collaboration is a key ingredient to Ontario’s success. The clinical trials ecosystem brings together patients and families, industry sponsors, government, ethics reviewers, researchers, healthcare providers and many more. These groups work together to reach a common goal of providing timely access to urgently needed therapies and offering hope to current and future patients.
At Clinical Trials Ontario (CTO), our role is to partner to find new ways of reaching that common goal quickly and effectively. CTO Stream, our flagship program that allows a single ethics review for multi-site studies, has cemented its place in Ontario’s clinical trials ecosystem with 136 participating research sites and over 200 participating pharmaceutical and medical device companies, and it continues to grow. This program, built by the clinical trials community, plays a key role in making clinical trials more efficient and cost-effective. CTO also works in lockstep with patient groups and health charities, making it easier for patients and the public to engage with clinical trials and developing resources to improve the participant experience.
But there is more we can do to strengthen our status as a competitive jurisdiction internationally. Hospital and industry leaders have come together with CTO to do just that by forming the Ontario Leadership Table for Clinical Trials. This group is focused on bringing more trial opportunities to Ontario and new therapies to patients as quickly as possible by shortening clinical trial activation times and reducing the effort required to execute the activation process.
For their top priority, members of the Ontario Leadership Table have set an ambitious target of 45 days for new study Susan Marlin
L’ONTARIO EST DEPUIS LONGTEMPS UNE PROVINCE ATTRAYANTE POUR LA RÉALISATION D’ESSAIS
CLINIQUES DE HAUTE QUALITÉ . Forte de plus de 4 800 essais cliniques en cours, l’Ontario occupe la première place au niveau national quant au nombre d’essais cliniques et se classe au premier rang des pays du G7 pour ce qui est des essais cliniques en cours par habitant. Cette situation s’explique par une volonté de collaboration qui fait partie de l’écosystème des essais cliniques. Différents groupes sont ainsi appelés à se côtoyer, parmi lesquels les patients et leurs familles, les promoteurs privés, les pouvoirs publics, les comités d’éthique de la recherche, les chercheurs, les prestataires de soins de santé et bien d’autres encore. Ceux-ci œuvrent ensemble à la réalisation d’un objectif commun : permettre l’accès à des traitements urgents et donner de l’espoir aux patients actuels et futurs.
Le rôle d’Essais cliniques Ontario (ECO) consiste à trouver des partenaires grâce auxquels on mettra en œuvre de nouvelles façons d’atteindre cet objectif commun rapidement et efficacement. CTO Stream, notre programme phare qui permet d’effectuer une seule évaluation de l’éthique en cas d’étude multisite, s’est taillé une place durable au sein de l’écosystème des essais cliniques de l’Ontario grâce à ses 136 sites de recherche et à plus de 200 sociétés pharmaceutiques et d’instruments médicaux participantes. Ce programme, en constante évolution, a été élaboré par la communauté des essais cliniques et il joue un rôle clé dans l’amélioration de l’efficacité et de la rentabilité des essais. ECO collabore aussi étroitement avec des groupes de patients et des organisations caritatives du domaine de la santé, avec pour objectif de faciliter la participation des patients et du public aux essais cliniques et de proposer des ressources qui visent à améliorer l’expérience des participants.

Nous estimons par ailleurs qu’il est possible d’en faire davantage pour renforcer notre position en tant que territoire concurrentiel à l’échelle internationale. C’est
For their top priority, members of the Ontario Leadership Table have set an ambitious target of 45 days for new study activations in sites in Ontario. Other priorities include finding collaborative solutions to improve trial recruitment, expand health data access, and improve access to therapies and trials in rare diseases.
activations in sites in Ontario. Other priorities include finding collaborative solutions to improve trial recruitment, expand health data access, and improve access to therapies and trials in rare diseases.
Through the Ontario Leadership Table, we have access to thought leaders from industry and hospital sites who understand the complexities of the evolving clinical trials landscape and what it will take to ensure Ontario remains at the top. By addressing challenges collaboratively, we can improve healthcare options, and secure Ontario’s position as one of the most important and innovative clinical trial jurisdictions in the world.
Parmi les autres priorités, citons la recherche de solutions collaboratives pour améliorer le recrutement en vue des essais, élargir l’accès aux données sur la santé et améliorer l’accès aux traitements et aux essais en ce qui concerne les maladies rares.
d’ailleurs dans cette optique que les responsables des hôpitaux et de l’industrie se sont joints à ECO pour créer l’Ontario Leadership Table for Clinical Trials (table de coordination des essais cliniques de l’Ontario). Ce groupe a pour mission de multiplier les possibilités d’essais en Ontario et d’offrir de nouveaux traitements aux patients le plus rapidement possible en raccourcissant les délais de mise en œuvre des essais cliniques et en réduisant les démarches nécessaires en ce sens.
Les membres de la table de coordination se sont fixé un objectif ambitieux de 45 jours pour la mise en place de nouvelles études en Ontario, et cela constitue leur priorité numéro un. Parmi les autres priorités, citons la recherche de solutions collaboratives pour améliorer le recrutement en vue des essais, élargir l’accès aux données sur la santé et améliorer l’accès aux traitements et aux essais en ce qui concerne les maladies rares.
Grâce à la table de coordination, nous avons accès à des personnes qui ont un rôle de prescripteur au sein du secteur et des établissements hospitaliers, sont au fait de la complexité du paysage des essais cliniques et savent ce qu’il faut faire pour que l’Ontario continue à tenir le haut du pavé. Le fait de relever des défis dans un esprit de collaboration nous permet d’améliorer les perspectives pour les soins de santé et de consolider la position de l’Ontario comme l’une des plus importantes et des plus innovantes régions du monde en matière d’essais cliniques.
de travail sur la communication du Réseau atlantique d’essais cliniques
AND CARING
IN NOVA SCOTIA, NEW BRUNSWICK, PRINCE EDWARD ISLAND, AND NEWFOUNDLAND AND LABRADOR is the primary driver of the recently formed Atlantic Clinical Trials Network (ACTN).
Announced at BIO 2022, this network is a partnership of six of the region’s health authorities: Health PEI, Horizon Health Network, IWK Health Centre, Newfoundland and Labrador Health Services, Nova Scotia Health, and Vitalité Health Network. A region of 2.5 million people may have previously been a blip on the radar, and that’s where ACTN comes in.
Since its inception, ACTN has worked closely with industry sponsors to ensure the network will improve access to cutting-edge treatments and devices. Highly trained clinical research professionals across the region are redefining operational excellence through efficient study start-ups; master agreements for confidentiality and trials; and most importantly the commitment to regular engagement and dialogue that enables two-way feedback between sites and the sponsor.
The result: seamless experience for industry sponsors wanting to undertake clinical trials in Atlantic Canada, including the launch of its multi-jurisdictional research ethics Board set for June. This means one front door for clinical trials and a “test & try” mindset that will invite new opportunities for early adoption of therapies and devices to the benefit of patients and families. The network’s successful foundation includes the support of the locally engaged sponsor representatives championing the network in their respective companies. It has led to increased awareness about what the Atlantic Region has to offer
LA RECHERCHE EST UNE FAÇON DE PRENDRE SOIN, ET LE RÉSEAU ATLANTIQUE D’ESSAIS CLINIQUES (RAEC), RÉCEMMENT CRÉÉ, EST PORTÉ PAR LE DÉSIR DE SOIGNER LES PATIENTS de la Nouvelle-Écosse, du Nouveau-Brunswick, de l’Île-du-Prince-Édouard et de Terre-Neuve-et-Labrador.
La création du RAEC, annoncé lors de BIO 2022, prend la forme d’un partenariat entre six autorités sanitaires de la région : Santé Î.-P.-É, Réseau de santé Horizon, IWK Health Centre, Newfoundland and Labrador Health Services, Santé Nouvelle-Écosse et Réseau de santé Vitalité. Cette région de 2,5 millions d’habitants—ce qui peut sembler petit—manquait jusqu’à présent d’un centre vital en matière santé, mais elle peut maintenant compter sur le RAEC.
Depuis sa création, le RAEC œuvre en étroite collaboration avec les promoteurs privés aux fins de l’amélioration de l’accès aux traitements et instruments médicaux de pointe. Des professionnels de la recherche clinique hautement qualifiés de toute la région redéfinissent l’excellence opérationnelle grâce à la mise sur pied de jeunes entreprises de recherche efficaces, à des accords-cadres pour la confidentialité et les essais et, surtout, à un engagement et à un engagement et à un dialogue constants qui favorisent l’échange d’information en continu entre les établissements et les promoteurs..

Il en résulte une expérience plus fluide pour les promoteurs du secteur qui souhaitent entreprendre des essais cliniques au Canada atlantique. Le réseau prévoit également de mettre sur pied un comité d’éthique de la recherche plurigouvernemental d’ici le mois de juin. Cela signifie que les essais cliniques passent par le guichet unique que représente le RAEC, et ce, dans un esprit d’essai et d’expérimentation qui ouvrira de nouvelles possibilités pour l’adoption
The success of ACTN’s industry partner engagement in these early days is attributed to the willingness of both sides to meet early, often and having open and honest dialogue.
Les partenaires industriels du RAEC ont dès le début pris part à l’aventure. Cet engouement est attribué à la volonté des deux parties de se réunir d’entrée de jeu, régulièrement, et d’établir ainsi un dialogue ouvert et honnête.
industry partners; and in its early days, has led to new opportunities coming to the region.
The network’s nimbleness and willingness to try novel processes and collaborations, is positioning the region as a partner in driving and finding innovative solutions to industry needs. Later this year, ACTN will launch an investigator-led, sponsor funded study aimed at improving access to care in a historically underserved community. There is also a major therapeutic focus on oncology, rare disease, and chronic illnesses based on population needs.
The success of ACTN’s industry partner engagement in these early days is attributed to the willingness of both sides to meet early, often and having open and honest dialogue. To set the network up for future success, conversations focussed on the development of meaningful relationships from the start, not just on individual study or project transactions. This has led to a foundation of trust that will benefit ACTN and its industry partners now and in the future.
rapide de traitements et d’instruments au bénéfice des patients et de leur famille. La réussite du réseau tient notamment à l’accompagnement qu’apportent les représentants des promoteurs locaux, qui s’en font les champions au sein de leur entreprise respective. Le RAEC a permis de mieux faire connaître aux partenaires industriels ce que la région atlantique peut leur offrir, tout en offrant dès le début de nouvelles perspectives dans la région.
La souplesse du réseau et sa volonté d’essayer de nouveaux processus et d’établir de nouvelles collaborations positionnent la région en tant que partenaire dans la recherche de solutions innovantes pour répondre aux besoins de l’industrie. Le RAEC lancera au cours de l’année une étude dirigée par un chercheur et financée par un promoteur, dont l’objectif est d’améliorer l’accès aux soins dans une collectivité historiquement mal desservie. Le réseau axe une grande partie de ses efforts sur l’oncologie, les maladies rares et les maladies chroniques, en fonction des besoins de la population.

Les partenaires industriels du RAEC ont dès le début pris part à l’aventure. Cet engouement est attribué à la volonté des deux parties de se réunir d’entrée de jeu, régulièrement, et d’établir ainsi un dialogue ouvert et honnête. Le succès à long terme du réseau passe par le désir qui s’est manifesté dès le départ d’établir des relations significatives plutôt que de simplement réaliser des études particulières ou des transactions liées à des projets. Une confiance mutuelle est ainsi née. Elle constitue pour le RAEC et ses partenaires industriels une assise qui porte fruit dès maintenant et permet d’envisager l’avenir.

IN THE HEART OF CANADA LIES MANITOBA, A PROVINCE KNOWN FOR ITS SPRAWLING LANDSCAPES, RICH CULTURAL HERITAGE, AND STRONG SENSE OF COMMUNITY. Behind the scenes of Manitoba lies a thriving bioscience industry driven by collaboration, partnership, and access.
While collaboration might not be the first thing that comes to mind when one thinks of Manitoba, it is a secret well-kept. One that has propelled the province forward in terms of innovation, economic growth, and global competitiveness.
In 2022, Manitoba was the second largest province for Pharmaceutical Manufacturing in Canada. Medicaments generated over $2 billion making it one of Manitoba’s number one export. With a confluence of factors including access to resources, a supportive government, and an expanding economy, Manitoba is rewriting the narrative of bioscience collaboration.
COLLABORATION: THE HIDDEN STRENGTH OF MANITOBA’S BIOSCIENCE INDUSTRY
Collaboration is the backbone of innovation. What sets Manitoba apart is its culture of collaboration—a culture deeply ingrained in the fabric of its research institutions, businesses, government, and people.
Manitoba thrives on partnerships that bring together diverse expertise, resources, and perspectives. These partnerships often grow bigger than what could have been done on their own.
ACCESS TO RESOURCES AND PEOPLE: A KEY INGREDIENT FOR SUCCESS
One of the driving forces behind Manitoba’s collaborative spirit is the accessibility of resources and people. From world-class research facilities like the National Microbiology Lab to a rich talented pool of scientists, engineers, and entrepreneurs looking for an exciting career in Manitoba. Manitoba has a rich ecosystem where ideas flourish and innovations can take flight.
The close-knit nature of the bioscience community means that individuals and organizations are readily accessible. This fosters a culture of open communication and knowledge sharing.
Through various initiatives, diverse perspectives converge to tackle complex challenges in health, agriculture, and environmental sustainability. This collaborative approach not only accelerates innovation but also cultivates a dynamic ecosystem where breakthroughs are nurtured and translated into real-world solutions.
Crucial to Manitoba’s collaborative ecosystem is the proactive involvement of the government. Manitoba’s government is known for its willingness to listen and collaborate on ideas, initiatives, and policies that support the bioscience industry.
Through strategic investments, supportive regulatory frameworks, and partnership-building initiatives, the Government of Manitoba has played a pivotal role in fostering a conducive environment for collaboration and innovation to thrive.
Additionally, the Government of Manitoba actively engages with industry stakeholders to identify and address challenges. This ensures policies are responsive to the evolving needs of the bioscience sector. Enabling proactive approaches fosters a dynamic ecosystem where ideas are nurtured, innovations are accelerated, and the bioscience industry can reach its full potential.
To illustrate the impact of collaboration in Manitoba’s bioscience industry, here are a few notable success stories:
The Fight Against the Opioid Crisis – Emergent BioSolutions Emergent BioSolutions, a company rooted in Manitoba, stands committed to combating the opioid crisis through its groundbreaking initiative with NARCAN® Nasal Spray. By launching this life-saving product over the counter, Emergent has made it accessible to everyone. They empower individuals to respond effectively to opioid overdose emergencies.
This milestone is a result of collaborative efforts between Emergent, leading retailers, and public interest groups. Collaboration has broadened access and awareness nationwide. Emergent has maintained affordability and expanded distribution channels that demonstrate unwavering dedication to public health.
As the opioid epidemic continues to affect communities, Emergent’s proactive approach underscores the power of collaboration in addressing complex health challenges. Emergent remains at the forefront of the fight against opioid overdoses globally. They are committed to protecting and enhancing life.
The Protection, Detection, and Prevention of Type 2 Diabetes – Scimar
Scimar, a biotech company based in Manitoba is changing how the world prevents, detects, and treats type 2 diabetes. Founded in 2009, Scimar commercialized Dr. W. Wayne Lautt’s groundbreaking discovery of hepatalin which pushed them to the forefront of diabetes research and innovation.
Through collaborative efforts with researchers, healthcare professionals, and investors, Scimar is driving forward with cutting-edge therapies aimed at addressing the complexities of type 2 diabetes.
Scimar’s innovative approach extends beyond scientific collaboration. By streamlining investment processes and making them accessible to all, the company is expanding access to investment opportunities. This has enabled broader participation in their mission. Through easy and affordable investment opportunities, Scimar empowers individuals to contribute to groundbreaking diabetes research. They embody Manitoba’s attitude of inclusivity and collaboration in the bioscience industry.
Cerebra, a Winnipeg-based medical technology company, is at the forefront of advancing sleep disorder diagnostics.
Cerebra emphasizes the significance of combining convenience with comprehensive analysis, underscoring the benefits for patients and sleep specialists alike. This partnership exemplifies Manitoba’s innovative ecosystem and leverages locally developed technology to improve healthcare outcomes.
While Manitoba’s bioscience industry has made significant strides in fostering collaboration, there is work ahead. Issues such as talent retention, access to capital, and regulatory barriers continue to pose hurdles to growth and innovation. However, with the collective effort of stakeholders across the public and private sectors, these challenges can be addressed. The solutions to these challenges will pave the way for continued success and prosperity in Manitoba’s bioscience industry.
Collaboration is the secret sauce that has propelled Manitoba’s bioscience industry to new heights. With a culture of openness, accessibility, and partnership, Manitoba has created a collaborative ecosystem where innovation thrives, economic growth flourishes, and lives are transformed.


Integrated Nanotherapeutics has found a way to tell the immune system not to attack our own body and has had early success in reversing type 1 diabetes in mice.

PATIENTS WHOSE AUTOIMMUNE CONDITIONS ARE MANAGED WITH IMMUNOSUPPRESSIVE DRUGS know the drugs are designed to suppress an overactive immune system. But there are risks to this method of treatment in that they may not be able to fight off actual infections due to the immune suppressant drug that’s controlling their condition, and as soon as the drugs are stopped, the symptoms usually come roaring back.
Given that 300 million people worldwide suffer from auto-immune diseases, it’s a quandary requiring more attention, one for which Integrated Nanotherapeutics (INT) has a unique value proposition. The biotech startup has found a way to teach the immune system not to attack our own cells, but instead to tolerate the antigen that’s encouraging the attack in the first place. The company is developing therapeutics to treat immune diseases with known antigens.
“We are developing a treatment method that can target the root cause of the disease,” explains INT co-founder and CEO Dr. Chris Tam. “We have found a way to tell the immune system to recognize these antigens, but not to react to them—to just ignore them.”
In its early trials, INT has found that its differentiated multi-cargo lipid nanoparticle (LNP) system can stop the progression of autoimmune-driven diabetes, in NOD mice, a model of type 1 diabetes. After a few low-dose treatments, the approach has shown compelling efficacy data, with close to 90 per cent of mice having no diabetes after that course of treatment.
“That is really encouraging,” Tam says. “Our scientific advisers who have been studying type 1 diabetes for a long time say that as far as efficacy data goes, this is as good as it gets. But we believe that we can do even better, for example, by altering the dosing method and schedule.”
Tam also sees applications in many other human ailments, including other notable autoimmune diseases, such as MS or lupus, as well as in organ transplant rejection and allergies. For example, the same therapeutic approach can be applied for celiac disease because the disease has a known antigen—gluten.
“Type 1 diabetes isn’t a simple disease because it involves multiple antigens, but we started with this condition because it affects a lot of people and our technology can handle multiple antigens,” Tam says, adding that her team uses mRNA technology in the process. “INT has a plug and play system. We are developing a pipeline of therapeutics to benefit a range of different diseases.”
INT’s differentiator is a powerful multi-cargo LNP technology that can co-deliver any type of drug modality in the same nanoparticle. The use of small molecule immunomodulators with mRNA is essential to achieve immune tolerance. Others have tried, but INT has figured out how to secure a small molecule modulator in the same LNP carrier as the mRNA and that is the key to INT’s success so far.
Tam and her team are encouraging industry partners, researchers and clinicians to get in touch if they want to collaborate in developing novel treatments for autoimmune conditions.

WEX PHARMACEUTICALS INC. IS A PRIVATE BIOPHARMACEUTICAL COMPANY HEADQUARTERED IN VANCOUVER, Canada, a subsidiary of CK Life Sciences Int’l., (Holdings) Inc. A pioneer in non-opioid analgesia, WEX’s aims are to address the complexities of pain management amidst an opioid crisis. At the core of WEX’s commitment lies Halneuron®, a breakthrough in modern pain relief.
Halneuron® leverages the therapeutic potential of Tetrodotoxin (TTX), a well-established sodium channel blocker known for its efficacy and safety in analgesia. Halneuron’s® unique mechanism of action, coupled with its preliminary signs of efficacy in clinical trials, presents a new beginning for the field of pain management. By selectively targeting sodium channel Nav 1.7, Halneuron® offers a direct, effective approach to pain relief. Non-habit forming and lacking the common side effects associated with opioid analgesics, Halneuron® is a promising alternative to traditional pain therapies, presenting a novel approach to alleviate pain without the risks commonly associated with opioid medications.
Currently, Halneuron® is in late-stage clinical development. While primarily targeting moderate-to-severe chemotherapy-induced neuropathic pain (CINP) and cancer pain, Halneuron® has also shown promising success in studies targeting burn pain and other serious pain

conditions. WEX has undertaken an extensive clinical program, conducting over 15 trials with TTX featuring more than 600 patients to date. This approach to rigorous research and development underscores WEX’s dedication to advancing innovative pain management solutions.
For WEX, the goal in developing Halneuron® is not simply filling a need in the pharmaceutical market but changing the narrative surrounding pain relief. As it currently stands, opioids are a risky but necessary cornerstone of analgesia, one that doctors and patients alike turn to for effective pain mitigation lacking many viable alternatives. While this has resulted in effective pain management, it has also contributed to an opioid crisis that has taken countless lives and affected families across the globe. Halneuron®, with its ability to successfully and safely target pain in a variety of circumstances, presents a revolution; a different reality in which opioids are not the be-all-end-all of pain relief.
WEX Pharmaceuticals Inc. is driven by a steadfast commitment to patient well-being and scientific innovation, with a vast network of connections that allow for the realization of mutual goals. Through strategic partnerships with leading academic institutions and research organizations, WEX has access to first-rate resources, allowing for further research and testing of Halneuron® in patient-focused, expertly run trials. Simultaneously, these entities are afforded the invaluable opportunity of being at the forefront of analgesia research and medical progress.

As WEX Pharmaceuticals approaches regulatory approval and commercialization of Halneuron®, it stands poised to revolutionize the field of non-opioid analgesia. With its innovative approach, unwavering dedication to patient-centricity, and commitment to scientific excellence, WEX is set to redefine the standard of care in pain management, offering hope, healing and safety to millions of individuals worldwide.


BETWEEN PAPEASY’S DEVICE THAT COULD EVENTUALLY ALLOW WOMEN TO ADMINISTER THEIR OWN PAP TESTS, Octane XO’s biomaterials that mimic bone and could change the way the world does spinal fusions, KINARM’s interactive robots that provide accurate brain function assessments to determine if a person’s behaviour is within a healthy range and Spectra Plasmonics’ device that does drug analysis either for vulnerable populations or forensics, Kingston is home to several groundbreaking devices and discoveries.
“We are a hotbed for medical devices in the health and life sciences space,” says Ben McIlquham, investment manager in health innovation at Kingston Economic Development. “Kingston is making remarkable strides.”
Kingston has an unique combination of innovative startups, long-standing institutions and centres of excellence in the life science sector and it’s making waves across Canada and internationally

The city punches above its weight by fostering innovative startups and helping multinational corporations grow. It ranked 9th in Canada on StartupBlink’s 2023 Startup Ecosystem Index, competing against cities several times its size. It is a lower-cost jurisdiction, strategically located within a two- to three-hour drive of Montreal, Ottawa and Toronto. Add the size of the U.S. market within an eighthour drive of Kingston and its neighbouring market grows to 130 million.
Wiley Chung, who relocated SMART Biomedical, his Calgary company, to Kingston, says Kingston is “special,” especially for startups. SMART Biomedical has developed Smart Drain, a corkscrewshaped medical device that facilitates chest-tube insertion to treat collapsed lungs in patients who often die because the tube goes into the wrong place.
“We knew the product worked, but we needed a patent,” Chung says. “Through the Kingston Innovation Fund, we accessed the services of a patent advisor for virtually nothing. We won a pitch competition through the Queen’s Innovation Centre in 2021 and we received close to $30,000 and used that to pay for patent lawyers.”
The moment he started making connections in Kingston, he knew he was in the right place.
“Who gives you free office space?” he asks. “We have that here. People have to know how great it is to be a startup here. They will get you to the next step, help you connect. Startups need nurturing and mentorship. I don’t think big cities can do that.”
In addition to its startup supports, Kingston is recognized for its global talent. With the most Ph.D. graduates per capita in Canada, it has been called the “smartest workforce” in the country. Between its two world-class universities—Queen’s and the Royal Military College—and St. Lawrence College, the city also has a high percentage of certificate, diploma and degree holders.

“There’s an attraction for top talent who either want out of urban centres or want the best that Kingston has to offer,” says Joe Brazda, spinal implant program manager at Octane EXO. “It’s always overwhelming when we see the quality and caliber of the applicants when we post jobs. It makes you feel fortunate to work here and want to work harder.”
Kingston also has business parks with leading-edge infrastructure at lower costs than those in larger cities and development charges are waived for industrial uses. It has low corporate taxes and access to more than $40 million in federal and provincial incentives.
The city has several centres of excellence, including the Centre for Health Innovation, Queen’s Cardio Pulmonary Unit, Canadian Cancer Trials Group, the Centre for Neuroscience Studies and the Centre for Advanced Computing.
Within the health sciences sector, genomics are part of Kingston’s advantage.
During COVID, Kingston was recognized as a leader in testing and test development with members of the Kingston Health Sciences Centre developing their own COVID assay and being selected as a centre of excellence for genomics.
“Kingston provides genomic surveillance for COVID-19 for all of Eastern Ontario,” says Prameet M. Sheth, director of molecular microbiology and infectious disease surveillance at the Kingston Health Sciences Centre. “Because Kingston developed its own assays, it wasn’t competing globally for reagents and dealing with supplychain issues.”
Today, the city continues to develop new tests for emerging pathogens, including MPOX, and more established pathogens such as RSV and influenza.
To add heft of the genomics sector, the Centre for Health Innovation and aforementioned Queen’s Cardio Pulmonary Unit (QCPU)—an $8-million 8,000-square foot research facility—have genetic testing equipment to help companies commercialize products and services. QCPU is a comprehensive research platform that performs experiments on everything from molecules to populations
and offers faculty, students and startups access to state-of-the-art research tools for confocal microscopy, flow cytometry, cell culture and transcriptomics, to name a few.
“A $15 million grant will help build a substantial expansion and the next phase of this invaluable translation research centre,” says Dr. Stephen Archer, director of the institute that oversees the QPCU.
Many of Kingston’s startups have spun out of Queen’s University, including Spectra Plasmonics, which has created a device that will allow “comprehensive drug testing anywhere, by anyone,” according to its tagline.
“We moved to Innovation Park, the commercial life sciences lab space in the city,” says CEO Malcolm Eade. “We ended up growing here during the pandemic and it’s worked out because we’ve been able to tap into all of Kingston’s resources.”
McIlquham says the strengths extend to regenerative medicine, with companies such as Octane Medical Group, which is developing the aforementioned bone-mimicking material, producing orthobiologic cells that regenerate cartilage for knee surgeries and making devices and materials that can improve the outcomes of spinal fusion surgery. The company has also partnered with Swiss multinational Lonza on the Lonza Cocoon, a revolutionary process that streamlines cell therapy manufacturing.
The medical devices and consumables sector is also strong, as is the health informatics field, with institutions such as the Centre for Advanced Computing, which are transforming health-care outcomes through advanced data analysis.
Bioprocessing and pharmaceutical are growing areas with groundbreaking work in drug development and manufacturing under way. Companies such as PnuVax and Neuractas are leading the charge.
“This dynamic growth and innovation underline Kingston’s role as a beacon of medical and life sciences advancement,” McIlquham says. “Our ecosystem not only supports the scaling and development of emerging companies, but also nurtures established firms looking to scale.”


IN THE RAPIDLY EVOLVING LANDSCAPE OF BIOPHARMACEUTICALS, PROTEIN THERAPEUTICS HAVE EMERGED AS A CORNERSTONE OF MODERN MEDICINE. These biologic drugs, derived from living organisms, offer targeted treatments for a wide array of diseases, ranging from cancer to autoimmune disorders. As our understanding of molecular biology deepens and technological advancements surge forward, the journey from DNA to Investigational New Drug (IND) approval is becoming increasingly streamlined and efficient.
The process of developing protein therapeutics begins at the genetic level, with the identification and isolation of genes that encode for specific therapeutic proteins. Modern biotechnology techniques, such as recombinant DNA technology and gene editing, have revolutionized this initial step, allowing researchers to precisely manipulate DNA sequences to produce desired proteins.
For nearly 40 years, SGS Canada has served as a trusted partner to pharmaceutical and biopharmaceutical companies around the world. With a sophisticated array of testing solutions, SGS provides comprehensive support through the entire drug development journey—from molecule to market.
SGS has GMP-certified facilities in Mississauga, Ontario, which are equipped to deliver consistent, highquality testing that meets stringent deadlines and production demands.
The world class SGS Canada team stays at the forefront of rapid advancements in protein therapeutics, ensuring they comply with all regulatory standards. They work closely with clients, becoming a seamless extension of the
team as they safeguard the quality and efficacy of medicines that will ultimately improve the lives of countless patients.
Protein therapeutics are an emerging class of biopharmaceutical drugs that harness the therapeutic potential of proteins or peptides to treat various medical conditions. These therapies hold immense potential across a variety of areas, including oncology, hematology, cardiology, dermatology, ophthalmology and nephrology. Protein therapeutics—such as monoclonal antibodies, fusion proteins and antibody drug conjugates—are designed to interact with specific targets in the body to modulate biological pathways, regulate immune responses or replace deficient proteins.
One of the key features of protein therapeutics is their specificity and selectivity in targeting particular receptors or molecules in the body. This allows for more precise treatment of diseases and often results in fewer side effects compared to traditional drugs. They have revolutionized the treatment landscape for many diseases and improved outcomes for patients worldwide.
While traditional small-molecule drugs are typically synthesized through chemical processes, protein therapeutics must be manufactured using living cells. This involves intricate processes, including cell line selection, upstream processing and downstream purification—which all affect the final product
characteristics, such as incomplete disulfide bond formation, glycosylation, N-terminal pyroglutamine cyclization, C-terminal lysine processing, deamidation, isomerization, oxidation, amidation of the C-terminal amino acid, as well as noncovalent associations with other molecules, conformational diversity, fragmentation and aggregation. These attributes all affect the characteristics of the final product and impact the overall safety, efficacy and quality of the drug.
Sahil Shah is an operations manager at the SGS laboratory in Mississauga who specializes in protein characterization. He explains that when developing high-quality protein therapeutics, it is critical to develop and use analytical methods that can monitor the critical quality attributes at each stage of drug development from cell line to fill finish.
“The right analytical strategy is extremely crucial to accelerate the submission of the CMC package for regulatory filings and approvals,” says Shah. “Most biopharmaceutical companies need help navigating these challenges effectively in compliance with regulatory requirements. They partner with SGS because we have the infrastructure and expertise to handle such complex molecules.”
The SGS Mississauga laboratory where Shah works boasts state-of-the-art infrastructure spread across a vast 24,684 square feet of lab space. Equipped with sophisticated instrumentation, including Sanger Sequencing, ddPCR and mass spectrometry, SGS has made strategic investments to cater to the growing demands of the biopharmaceutical industry. This commitment to innovation and technological advancement enables SGS to provide comprehensive analytical solutions tailored to the evolving needs of its clients.
Central to SGS Canada’s success is its team of over 260 highly skilled professionals, with more than 90% holding advanced degrees such as Ph.D.s and Masters degrees. These seasoned experts bring years of experience and expertise to every project, from method development and validation to stability testing and quality control. The dedicated project management team ensures timely delivery, which is critical for meeting CMC milestones and regulatory deadlines.
“Innovation thrives in an environment where infrastructure meets expertise,” says Dr. Ida Dossou-Yovo, general manager of the SGS Missisauga laboratory. “With our unparalleled facilities, seasoned professionals and unwavering dedication to regulatory excellence, we pave the way for transformative discoveries in pharmaceutical development, ensuring the success of every project from conception to commercialization.”
SUPPORTING CLIENTS FROM MOLECULE TO MARKET
Pharmaceutical and biopharmaceutical companies face a variety of challenges to bring safe and effective therapeutics to the market. With job cut announcements affecting the pharmaceutical workforce, many biopharmaceutical companies are facing the challenge of producing FDA-certified therapeutics with fewer human resources. Few have the capacity to manage the full scope of drug development in-house and rely on trusted partners to provide reliable testing services. Outsourcing product testing to trusted partners like SGS Canada is a costeffective way to ensure the highest level of quality and safety for patients.
Whether clients need support through every stage of the drug development cycle or just when their in-house capabilities are at full capacity, SGS can meet their needs.
“In the drug development space, we offer testing services for clients across all touchpoints. For our biopharma clients, we do the heavy lifting with respect to the analytical phase.” says Mahdi Roohnikan, Ph.D., a technical client manager at the Mississauga laboratory.
The SGS team leverages its vast experience and expertise to conduct comprehensive testing that is required for FDA approval, all within stringent timelines.
“Our clients say we put their mind at ease,” says Dr. Roohnikan. “They know we’re capable of completing testing quickly with faultless quality assurance so that the end product meets all regulatory requirements and is FDA and Health Canada compliant.”
As pioneers in biopharmaceutical testing, SGS Canada remains at the forefront of innovation and quality excellence. By anticipating and adapting to the evolving demands of the biopharmaceutical industry, SGS continues to lead as a centre of excellence and quality for the proteinbased and cell and gene therapy space. The implementation of cutting-edge tools and methodologies underscores SGS Canada’s commitment to offering comprehensive analytical solutions and delivering high-quality results.
In conclusion, the journey from DNA to IND approval for protein therapeutics is undergoing a remarkable transformation, fueled by advances in biotechnology. By leveraging cutting-edge technologies and embracing a culture of innovation, SGS can further accelerate the development of biologics and bring life-changing therapies to patients in need.
For more information on SGS Canada and their work in protein therapeutics and biopharmaceutical testing, please visit sgs.com/pharmaqc


A RECENT REPORT FROM THE ADMARE INSTITUTE FINDS THAT CANADA NEEDS AN ANCHOR COMPANY FOR THE LIFE SCIENCES ECOSYSTEM to ensure the sector continues to grow and experiences the positive competitive advantages for the entire sector. Pharmascience, along with its related companies, is building the anchor company that showcases exactly what the Canadian life sciences ecosystem can offer.
Anchor companies are companies that are uniquely positioned to support the entire chain of the innovation ecosystem, and Pharmascience is the keystone we know that supports the successful development of the sector.
As the largest Canadian-owned pharmaceutical company, with over 1,500 employees proudly headquartered in Montréal, Pharmascience manufactures a range of pharmaceutical products, including innovative, generic and over-the-counter products. It also offers a wide range of services to other companies in the life sciences space, offering contract development and manufacturing services, bioanalytical testing and commercialization services.
Pharmascience is also well-established: the company recently celebrated its 40 th anniversary of serving patients in Canada and around the world.
Pharmascience’s Executive Chairman David Goodman reflects on this milestone: “Pharmascience has come so far in 40 years, and experienced significant and stable growth. From where we started as a distributor serving the local
market, we are now a global player, serving patients in over 50 countries and producing billions of doses of medicines each year that we have developed and manufactured ourselves.
“As I look forward to the future of Pharmascience, I reflect on what our goals and ambitions should be for the coming years. I truly believe that with our diverse service offerings, our intensive focus on R&D, and our unique contract development and manufacturing concept, Pharmascience will be known as the anchor of Canada’s life sciences ecosystem.”
As Pharmascience grows and matures, the family of companies surrounding Pharmascience is growing as well, with the creation of a new division at Pharmascience exclusively focused on CMO and CDMO as well as a new standalone venture capital fund that will support the future of medicine.
Finchley Healthcare Ventures is a new fund that invests in companies that are pushing the boundaries of medical research and development, with a particular emphasis on those developing novel therapies. The Fund seeks out opportunities where breakthrough technologies and transformative ideas have the potential to address significant unmet medical needs and revolutionize patient care.

“Finchley’s unique approach allows us to support innovation by providing entrepreneur both the financial means to develop their novel therapies and leveraging the supports offered by the Pharmascience group of companies,” said Pierre Beauparlant, Managing Director of Finchley Healthcare Ventures. “We are able to offer so much more than financial support. It also includes access to quality bioanalytical and manufacturing expertise. It’s a win for us and a win for the life sciences sector.”
Pharmascience is also building on its manufacturing expertise. In the Fall edition of Insights magazine, Pharmascience shed the light on the $120-million expansion of its sterile injectable manufacturing facility in Candiac, Québec, which will triple its capacity and create new manufacturing opportunities. Construction is well underway and the project is expected to be completed on time in 2026.
The Candiac sterile injectable manufacturing facility is currently manufacturing liquid and lyophilized products in a variety of sizes and formats and is one of the very few sites that are capable of manufacturing cytotoxic products in North America.
On the cutting edge of technology, research and drug formulation expertise, the site upgrade and expansion positions Pharmascience to meeting the unmet medical needs of the future. In addition, the cytotoxic capabilities create a specialized ability to manufacture complex products like antibody-drug conjugates, including the ability to fill and finish emerging advanced therapy products.
“With a vision to continue our expansion while supporting local development and manufacturing, we see a tremendous opportunity to expand our R&D services to meet the needs of a growing pharma and biotech client base, whose complex injectable product supply requirements can be met by the extraordinary expertise of the Pharmascience team,” said Martin Arès, CEO of Pharmascience.
“Creating our new CDMO business unit represents an excellent way to promote our unique development services and maximize the value of every unit we produce at our Candiac site,” he continued.

“Our unique CDMO model is what sets us apart from other players in this space where we can provide world class services while looking beyond pure fee for service business models. We can manage commercialization and sale rights for global markets, letting you focus on the markets that are most important to your business,” said John Foy, General Manager of Pharmascience’s CDMO unit. “We also know that having these injectable service capabilities available in Canada can be a strategic advantage for our customers and gives them peace of mind and satisfaction knowing their products will be manufactured with high quality standards.”
Highlighting these two commercial opportunities of the group of companies related to Pharmascience, it is also important to mention the commercial offerings of Royalmount Laboratories, which offers bioanalytical testing for small and large molecules, based on accepted Health Canada, FDA, EMA and ICH standards.
If you’re seeking a partner for your innovative products, look no further to Pendopharm, a specialty pharmaceutical partner dedicated to developing, in-licensing and commercializing innovative products to improve the lives of Canadian patients and their families.
All in all, Pharmascience and its related companies are ready to make their mark on the global life sciences and biomanufacturing ecosystem. Dedicated to growth and expansion, while solidifying the company’s Canadian roots, Pharmascience seeks to be the key player ready to jump on exciting opportunities wherever they may be—testing, licencing, R&D, manufacturing, commercialization.
“We are proud of the role we play in supporting Canada’s healthcare system. We know that our pivotal role connects and creates opportunities for other players in the biomanufacturing sector in Canada,” added Martin Arès. “And we want to continue our impact on a global scale.”

IN THE CONTINUALLY EVOLVING FIELD OF BIOTECHNOLOGY, ONE AREA IS EMERGING AS A GAME-CHANGER, poised to redefine our pathways to health and greener living: glycomics. The study of glycomics examines sugars called glycans, which coat every cell in organisms—plants, animals, humans, even bacteria and viruses. Glycans play critical roles in almost every biological process, from determining cell structure and function to influencing the immune system.
Glycans are far more variable, complex and information-rich than genes and proteins because the “building blocks” of glycans can be linked together in many more different combinations. Understanding glycan changes and interactions not only expands our knowledge of biology, but also equips us with new tools to solve pressing health and environmental challenges. For example, subtle changes in glycans influence blood type and organ donor compatibility—small differences with a big impact on saving lives.
As with genomics and proteomics, breakthroughs in glycomics are now fundamentally revolutionizing life sciences, thanks to critical advances in technology. Glycomics encompasses glycan structure elucidation, chemical and enzymatic synthesis, glyco-proteomics and, most recently, glycoengineering. With these technological leaps forward, glycomics is expected to generate rapid growth, like that of genomics and proteomics, over the next decade in the biotechnology and pharma sectors.
Glycomics offers a fresh lens through which to view biology, medicine, and the sustainability of our planet. The applications of glycomics extend to many industrial sectors that contribute to One Health, including human and animal health, and sustainable agri-food systems.
In health, glycomics is driving the development of novel diagnostics, vaccines, and therapies. Early detection of diseases like cancer, Alzheimer’s, and infectious diseases is becoming more refined and new treatments are being pursued thanks to advances in glycan analysis. Glycomics is spurring the development of new drug alternatives and delivery strategies to address global issues such as antimicrobial resistance. Five of the top ten global protein therapeutics are glycan-linked proteins that had total sales of US $58 billion in 2020.1 A 2022 analysis by The Insight Partners estimates that the global market for glycomic therapeutics will reach US $257 billion by 2028.2
1 Sagonowsky, E. The top 20 drugs by worldwide sales in 2020. https://www.fiercepharma.com/special-report/top-20-drugs-by-2020-sales (accessed Dec 15, 2021).
2 Glycomics Therapeutics Market: Forecast to 2028, The Insight Partners, 2022
Glycomics is also at the forefront of establishing ecosustainable agricultural approaches to bolster global food security. The livestock industry faces significant challenges exacerbated by the effects of global climate change, including heightened heat stress, diminishing reproductive capabilities, and weakened immune systems among animals. Studying distinct glycan profiles in the gut bacteria of healthy versus sick animals uncovers early indicators of vulnerability to illness and disease progression. This insight is enabling glyco-scientists to develop pioneering diagnostic tools and targeted therapies, ensuring timely and precise interventions.
As we look ahead, continued research, collaboration, and investment in glycomics remain important to leveraging its full potential.
Canada has a history of over 90 years of glycomics research and is now home to one of the world’s most impactful glycomics technology clusters. Since 2015, GlycoNet has mobilized scientists across the country—becoming the largest nonprofit glycomics research network in Canada and one of the world’s leaders in glycomics research and innovation. As a recipient of funding through the Government of Canada’s Strategic Science Fund, GlycoNet continues to grow partnerships and co-fund research and innovation, de-risk technologies, and translate discoveries into made-inCanada solutions, including diagnostics, vaccines, therapeutics, biomaterials, and biomanufacturing methods.
At GlycoNet, we believe collaboration is the cornerstone of groundbreaking scientific advancement. We invite organizations, researchers, and innovators across industries to join us in this exciting journey to accelerate research, development, innovation, and commercialization of tomorrow’s glycomics-based solutions, leading to a healthier, more sustainable world.
If you share our passion for innovation, we would love to hear from you. Whether you’re in the biotech sector, pharmaceuticals, or agri-food systems, we’re open to exploring new partnerships and discussing how glycomics can advance your research and development goals. Together we can unlock new possibilities and make significant strides toward a healthier and more sustainable future.

Under the network, GlycoNet Integrated Services (GIS) operates as a national platform of glycomics services, leveraging state-of-the-art infrastructure investments in seven institutions across Canada. GlycoNet and GIS together provide one-stop access to glycomics tools, specialized expertise, consulting, and collaborative opportunities to academia and industry, both nationally and internationally.

QUARK VENTURE LP AND ITS LEAD FUND, THE GLOBAL HEALTH SCIENCE FUND (“GHS” FUND), is one of only a few biosciences focused global venture capital firms headquartered in Canada. It is dedicated to the growth of a burgeoning biotechnology and health sciences industry in Canada, as well as internationally.
Quark Venture and GHS Fund are led by a management team with deep experience and expertise in multiple scientific, technical, business and financial fields from the biotechnology, big pharma, medical technology and investment banking sectors. The GHS team is headquartered in Vancouver and based in Boston, Berkeley and Hong Kong. An impressive and diversified portfolio of some 38 companies has been financed since the GHS Fund’s inception, pursuing promising opportunities using leading-edge technology to advance medical science. A particular early focus for the fund has been on converging technologies and next generation of “tech meets biotech/ medtech” companies like Canary Medical and Cathworks.
“We want to invest in and enable the next generation of cutting-edge technologies impacting healthcare,” says Karimah Es Sabar, the Vancouver-based chief executive officer and general partner of Quark Venture. “But we don’t just cut a cheque and wait for things to happen as a passive investor. We capitalize on our experienced, globally connected team, and our KOL, academic and industry worldwide networks to enable the companies in their areas of need.”
Early global investments and exits include Keros, a company focused on novel treatments for patients suffering from hematological and musculoskeletal disorders, which went public in the spring of 2020, and Cathworks, which has a co-promotion agreement with Medtronic. As early investors in Cathworks, GHS saw the potential for their FFRangio platform, which provides diagnostic and physiologic information through artificial intelligence, to completely disrupt traditional practices and revolutionize the management of coronary artery disease.
GHS has also seeded and led investments into several
Canadian companies, including Canary Medical, founded by serial entrepreneur Bill Hunter, and ARTMS, an isotope production spin-out from TRIUMF, BCCA, CPDC and Lawson Health. “ARTMS is a prime example of our mandate to build and nurture companies,” says Dr. Kaley Wilson, Principal and Director of Business Development.
“We seeded the company as the sole investor in 2017, I joined the team as interim CEO for 18 months, we attracted Deerfield as a co-investor and recently announced an acquisition with Telix Pharmaceuticals.” Importantly, Telix plans to continue to operate and expand ARTMS’ R&D and production capabilities at the Burnaby location. Canary Medical, which has developed a proprietary implantable sensor technology, has enabled its partner Zimmer Biomet to debut the world’s first FDA approved smart knee implant, Persona IQ. GHS lead Canary Medical’s Series A, bringing in international co-investors.
Quark Venture and the GHS Fund have established unique relationships with partners and have built a syndicate of VCs and top tier investors with whom they make serial co-investments and help build exciting, high impact companies. “Amazing science, remarkable technologies, and outstanding talent and entrepreneurship are emerging exponentially, so GHS Fund looks forward to investing in the next generation of bioscience companies,” says Es Sabar.




VASOMUNE THERAPEUTICS INC., A TORONTO-BASED BIOPHARMACEUTICAL COMPANY, has successfully developed innovative basic research, invented at Sunnybrook Research Institute, into clinical stage technology.
Vasomune has grown into an independent clinical stage company financed largely through non-dilutive sources via corporate partnerships and government grants. To date, the company has received $2.8M from the National Research Council of Canada (NRC IRAP), $1.5 million through Genome Canada’s Genomic Applications Partnership Program (GAPP), and $9.5M USD in Peer Reviewed Medical Research Program (PRMRP) of the US Department Of Defense to support the development of its lead molecule, AV-001.
Vasomune’s therapeutic strategy is aimed at combating diseases driven by vascular endothelial dysfunction, and research has generated positive results across multiple indications. The host vascular response (HVR) is an important pathological process in a variety of diseases, including acute respiratory distress syndrome (ARDS), acute kidney injury, sepsis, hemorrhagic shock, stroke and vascular dementia. In response to trauma or infection, the HVR can become unconstrained, driving the loss of vascular barrier function, inflammation, and vascular leak resulting in edema and damage in critical organs.

AnGes, Inc. (Tokyo, TYO:4563), a Japan-based pharma focused on developing biotherapeutics. The co-development contributions provided by AnGes, Inc., which included upfront and clinical milestone fees, “has taken our lead molecule through development into Phase 2. AV-001 is a product of research which was performed by the late Dr. Daniel J. Dumont at Sunnybrook Research Institute in Toronto,” said Dr. Harold Kim, Vice-President of Research and Scientific Affairs at Vasomune.

The recent global pandemic led Vasomune to prioritize development of its drug as a treatment for hospitalized patients with pneumonia and ARDS. Infection of pulmonary endothelial cells frequently results in microvascular leaks, which in turn leads to respiratory distress and ARDS by altering blood vessel barrier integrity and inducing vascular inflammation. Pre-clinical models have shown that AV-001 can stabilize the vasculature by blocking vascular instability and leak precipitated by a variety of lethal pathogens. The demonstration of AV-001 as a pathogen agnostic therapeutic provides encouraging evidence for its potential effectiveness against known pathogens and new emergent infectious diseases.
Vasomune’s lead clinical candidate, AV-001, is a first-inclass synthetic Tie2 tyrosine kinase receptor agonist targeting a key regulatory protein expressed on the cell surface of endothelial cells in the vasculature. In healthy individuals, Tie2 is responsible for maintaining normal vascular function, endothelial stability, and preventing vascular leakage. In animal studies, AV-001 significantly improved survival, reduced pulmonary edema and increased lung function in a lethal model of viral infection. Importantly, AV-001 also increased survival independent of the type of pathogen, being highly effective against, for example, H3N2, PR8 (H1N1) and 2009 swine-origin pandemic H1N1 viral strains.
The development of AV-001 was further catalyzed by the initiation of a global co-development agreement with
Following the successful phase 1 study (NCT04737486), Vasomune initiated a phase 2a study of AV-001 for the treatment of hospitalized patients with pneumonia and ARDS (NCT NCT05123755).
“We are tremendously pleased at the positive response from the clinical and scientific community for our novel therapeutic approach to reversing vascular endothelial dysfunction. We are grateful for financial support from Genome Canada, the NRC, and US Department Of Defense PRMRP programs. This support enabled acceleration of the clinical development of AV-001,” said Dr. Brian Jahns, President and Chief Operating Officer of Vasomune.
“Results from the Phase 2a study will determine if our therapeutic strategy of normalizing the host vasculature can improve survival, reduce progression and shorten hospital stays for critically ill patients.”
For more information on Vasomune Therapeutics, visit: vasomune.com
FOUNDED IN 2016, SHADOW LAKE GROUP (SLG) IS A CANADIAN BOUTIQUE M&A ADVISORY COMPANY that helps companies globally with their business development needs in the Pharmaceutical/Biotech Industry.
Since that time SLG has completed over $3 USD Billion in transactions on behalf of clients all over the world including Canada
SLG’S SUCCESS?
Our big picture global focus in combination with a practical entrepreneurial approach based on significant business operational experience of both Catherine Miner and John Proffett (Managing Partners)
“We try to customize our approach to meet the various needs of our clients. Not all clients come to us with the same level of experience, skillset or bandwidth required to get transactions done, so we need to adapt to their needs, capabilities, and personalities. This also applies to the companies and individuals that we negotiate with on behalf of our clients—we try to customize our approach to them as well to get the best possible outcome. We describe ourselves as a ‘Swiss Army Knife’ being able to provide multiple types of approaches/services due to our extensive and diverse experience and creative mind-set,” says Catherine Miner, SLG Managing Partner.
IS YOUR VIEW ON THE CANADIAN PHARMA/ BIOTECH INDUSTRY?
There is incredible science and innovation going on in Canada, but it is our observation that companies do not think big enough and do not raise enough funds early on to see them through to critical business inflection points.
We have all heard the complaints that there is not enough investment capital in Canada for the biotechnology industry. BIOTECanada has reported that $25.9 Billion was invested between 2019 and 2023. Only $3.8 Billion came from venture capital, which is barely enough to cover the development of 3-5 drugs though to regulatory approval. On the positive side, there was $15.2 Billion in M&A activity. This proves that Canadian biotech is a good investment!

Hopefully, these statistics and the recent success story of the Fusion acquisition by AstraZeneca will provide inspiration for other Canadian entrepreneurs to push harder for the necessary investment levels in their companies.
WHAT ARE SOME OF THE TRANSACTIONS THAT YOU ARE MOST PROUD OF?
At Shadow Lake Group, we have been very fortunate to be able to work with some of Canada’s top pharmaceutical executives, especially early on at critical inflection points in the business to take them to the next level or launch the company.
Some examples are:
• HLS Therapeutics (Canadian Specialty Pharmaceutical Company)
▶ SLG sourced and facilitated HLS’s acquisition of both:
▷ Clozaril from Novartis for the US and Canadian markets
▷ VASCEPA from Amarin for the Canadian market
• Vasomune Therapeutics (Canadian Biotech Company):
▶ SLG sourced and brokered the pivotal transaction for their main drug candidate AV-001 with a Japanese company AnGes. The drug is in Phase 2 clinical trials.
• $1.4B Global CNS transaction (client details are not disclosed due to confidentiality reasons)
▶ SLG both sourced and advised our client to secure the global rights to a prominent CNS brand from a top ten pharmaceutical company.
The most common mistake that we see Biotech companies making is not understanding and quantifying the commercial potential and pathway of their project.
Having great scientific evidence to support your hypothesis is critically important but what we see so often is that the company has not explored the commercial potential of the opportunity prior to investing in the research/development programs.
The hard cold truth is that if the drug or technology is not financially viable from a commercial perspective, then the project is doomed to fail—regardless of how amazing the science is. You need to know who your customer is, and it is not just the patient. It is also the physician, the regulatory authority, the healthcare payer, and your potential partner.
An analogy would be creating the best land-line telephone which back in 1970’s/80’s would have been a hit—but now everyone uses mobile phones and there is no market for land-line phones—regardless of the technology, so no longer financially viable from a commercial perspective. We know that this seems obvious, but we run into examples like this unfortunately quite often.
The other side of this coin is that the financial potential could be massive and understanding this early on could be critical to securing the right pharma partner or investors to sufficiently fund the project.


1. Keep the message simple, high-level and memorable.
a. Remember that potential partners view 1,000’s of pitches—the easier you make it for them to understand and talk about internally without referring to their notes—the better.
b. Identify the problem you are addressing and describe your solution before diving into the science
2. K now the commercial potential and pathway to get there. Understand your commercial viability!
a. Quantify the problem and show how your solution is the best approach.
b. Have the data to back it up
3. Be confident and likeable
a. I n the end everyone is human, and it never ceases to amaze us how people can be influenced significantly by their emotions.
b. People want to work with someone they like and who can instill confidence.
4. Think BIG!


35 Pharma is a clinical-stage biopharmaceutical company specializing in TGF-beta superfamily therapeutics for the treatment of Pulmonary Hypertension, Heart Failure and obesity. 35Pharma leverages its scientific leadership in TGF-beta biology combined with superior protein engineering to discover innovative compounds that selectively and potently neutralize validated pathological TGF-beta ligands while sparing beneficial homeostatic ligands. 35Pharma believes in connecting rigorous science with their innate sense of urgency to rapidly generate breakthrough therapies for patients in need of a better quality of life.

As a green biotech company and circular economy leader, Aplantex’s mission is to enhance the molecular power of plants by improving access to the phytochemical ingredients that contribute to our well-being in terms of our health, beauty, and diet while also simplifying the supply chain and embracing environmental, social and governance principles. Aplantex has developed an innovative and exclusive green biotechnology process able to produce the valuable molecules coveted by the pharmaceutical, cosmetics, and food & beverage industries in accordance with ESG standards.

Radiant Biotherapeutics is a revolutionary antibody platform company leading the new frontier of multi-valent, multi-specific therapeutics to deliver transformative therapies for patients. Radiant’s proprietary Multabody™ platform leverages avidity and multi-specificity simultaneously, to generate highly efficacious Multabodies with superior potency than other antibody platforms.
These powerful Multabodies have potential to deliver a new class of biologics to tackle complex, heterogenous diseases, such as cancer, that often have challenging targets and mechanisms.
35Pharma est une société biopharmaceutique au stade des essais cliniques, spécialisée dans les facteurs de la superfamille des TGF-bêta, destinés au traitement de l’hypertension pulmonaire, de l’insuffisance cardiaque et de l’obésité. 35Pharma allie à son expertise scientifique en biologie des TGF-bêta une capacité de pointe en ingénierie des protéines, ce qui lui permet de développer des composés innovants qui neutralisent de manière sélective et efficace les ligands pathologiques connus de type TGF-bêta, tout en épargnant les ligands homéostatiques bénéfiques. Grâce à un savoir-faire scientifique associé à un sens de l’urgence depuis ses débuts, 35Pharma possède la capacité de mettre rapidement au point des traitements révolutionnaires qui permettent d’améliorer la qualité de vie des patients.
En tant qu’entreprise de biotechnologie soucieuse de l’écologie et chef de file de l’économie circulaire, Aplantex s’est donné pour mission de renforcer le pouvoir moléculaire des plantes en améliorant l’accès aux composés phytochimiques qui contribuent à notre bien-être en matière de santé, de soin de beauté et d’alimentation, tout en simplifiant la chaîne d’approvisionnement et en respectant des principes environnementaux, sociaux et de gouvernance. Aplantex a élaboré un procédé innovant et exclusif en biotechnologie verte permettant de produire les précieuses molécules convoitées par les industries pharmaceutique, cosmétique et agroalimentaire conformément aux normes environnementales, sociales et de gouvernance (ESG).
Radiant Biotherapeutics est une société dont le programme d’anticorps innovant ouvre de nouvelles perspectives en matière de traitements polyvalents et multispécifiques et de traitements transformateurs offerts aux patients. La plateforme Multabody™, exclusive à Radiant, s’appuie sur un processus appelé « avidité » et sur des cibles multispécifiques, pour générer des « multabodies », dont l’efficacité est supérieure à celle des autres programmes d’anticorps. Ces puissants anticorps « multabodies » pourront potentiellement constituer une nouvelle gamme de produits biologiques pour le traitement des maladies complexes et hétérogènes, telles que le cancer, dont les cibles et les mécanismes sont souvent difficiles à cerner.

Neurenati Therapeutics is a Québec-based biotech company dedicated to developing therapies for rare diseases. The first technology targets Hirschsprung disease (HSCR), a life-threatening gastrointestinal (GI) birth defect characterized by the lack of nerves in parts of the lower GI tract. Neurenati proposes an innovative therapy via growth factor to treat newborns with HSCR, thereby averting the need for surgery and its associated complications.

Inteligex is developing proprietary stem cellbased therapeutics to restore function in patients with spinal cord injury (SCI) and for other diseases of the Central Nervous System. Successful outcomes from the development program would position Inteligex as the first in the world with a treatment affecting hundreds of thousand patients worldwide. Inteligex is developing state-of-the-art Artificial Intelligence solutions that allow for the accurate prediction of disease progression along with biomaterial strategies that, when combined with stem cell therapy, will provide a personalized treatment approach for SCI. While Inteligex’s primary focus is on acute and chronic forms of SCI, Inteligex is confident that its stem cell technology can be applied to other diseases of the CNS including ALS, MS and stroke.

CryoStasis has developed a set of solutions to allow biological material to go unfrozen at subzero temperature. The company has developed a core platform that can be deployed using multiple mechanisms. The first is the Ambient Pressure Sub-Zero Unfrozen (APSU) solution that provides novel process and equipment to depress the freezing point of its media to subzero providing up to a week of storage with no cryoinjury and minimal mortality, permitting shipping & short-term storage. The second is High Pressure Sub-Zero Unfrozen (HPSU) which induces suspended animation & provides a means of storing biological material indefinitely permitting tissue and organ banking.
Neurenati Therapeutics est une société de biotechnologie basée au Québec qui se consacre à l’élaboration de traitements pour les maladies rares. Elle a créé une première technologie pour s’attaquer à la maladie de Hirschsprung (HSCR), ou mégacôlon congénital, une malformation congénitale gastro-intestinale potentiellement mortelle, caractérisée par l’absence de nerfs dans certaines parties du tractus gastro-intestinal inférieur. Neurenati propose un traitement innovant autour des facteurs de croissance pour le traitement des nouveau-nés atteints d’HSCR, évitant ainsi le recours à la chirurgie et les complications qui y sont associées.
Inteligex élabore des traitements à base de cellules souches exclusives en vue de restaurer les fonctions chez les patients souffrant de lésions de la moelle épinière et d’autres maladies du système nerveux central. Si le programme de développement parvient à son terme, Inteligex deviendrait la première entreprise au monde à disposer d’un traitement pour une maladie qui touche des centaines de milliers de patients partout sur la planète. Inteligex a créé des solutions d’intelligence artificielle de pointe qui permettent de déterminer la progression de la maladie. Elle utilise également des biomatériaux qui, combinés au traitement par cellules souches, offriront une approche thérapeutique personnalisée pour les lésions médullaires. Si Inteligex utilise la technologie des cellules souches principalement pour le traitement des formes aiguës et chroniques des lésions médullaires, la société est convaincue que sa technologie des cellules souches peut être appliquée à d’autres maladies du SNC, notamment la SLA, la sclérose en plaques et les accidents vasculaires cérébraux.
CryoStasis a mis au point un ensemble de solutions permettant de conserver du matériel biologique à une température inférieure à zéro, sans congélation. La plateforme conçue par l’entreprise peut être utilisée à diverses fins grâce à différentes solutions. La première est une solution qui offre une pression ambiante sous zéro sans congélation (APSU –Ambient Pressure Sub-Zero Unfrozen). Elle s’appuie sur un processus et un équipement novateurs pour abaisser le point de congélation de son milieu à un niveau inférieur à zéro, ce qui permet de conserver le biomatériau jusqu’à une semaine sans lésions cryogéniques et avec un faible taux de mort cellulaire, et de l’expédier et de le stocker à court terme. La seconde est un processus de conservation à haute pression sous zéro sans congélation (HPSU – High Pressure Sub-Zero Unfrozen), qui induit un arrêt momentané de la vitalité et autorise la conservation indéfinie du matériel biologique, ce qui permet de créer des banques de tissus et d’organes. ecosystem écosystème

Aramis Biotechnologies leverages the unique capabilities of plant cells to develop a new generation of biomanufacturing platform within plants. Benefiting from a unique technology derived from 20 years of research and development, the company is committed to shaping a future in which biotechnology sustainably meets global human health needs. The company’s technology provides the capacity to produce different vaccines and antibodies at very high speed, paving the way for novel solutions in human health and pandemic response.
ProteinQure is a biopharmaceutical company that designs novel exotic non-canonical amino acid containing peptide ligands with multiple therapeutic applications including conjugates appended with cytotoxics, oligonucleotides and radioisotopes. The platform incorporates computational and experimental approaches like biophysical modelling, molecular machine learning, and display screening technologies. The company’s capabilities have been validated both internally and externally with top-tier pharmaceutical companies as highlighted by multiple successes both in vivo and unprecedented in vitro design campaigns.

BioCanRx is a network of scientists, clinicians, cancer stakeholders, academic institutions, NGOs and industry partners working together to accelerate the development of leading-edge immune oncology therapies for the benefit of patients, and with a vision to turn all cancers into curable diseases. As a leader in the translation, manufacture and adoption of cancer immunotherapies, they invest in translating world-class technologies from the research lab into clinical trials. BioCanRx provides researchers with access to funding, expertise, training and biomanufacturing facilities. BioCanRx trains and develops the talent needed for a thriving Canadian health biotechnology sector.
Aramis Biotechnologies utilise les caractéristiques uniques des cellules végétales pour élaborer une plateforme de biofabrication de nouvelle génération à l’intérieur même des plantes. La technologie unique qu’elle a créée est le fruit de 20 ans de recherche et développement et témoigne de la mission de l’entreprise à long terme : façonner un avenir dans lequel la biotechnologie répondra de manière durable aux besoins en matière de santé humaine à l’échelle mondiale. L’entreprise peut produire différents vaccins et anticorps très rapidement, grâce à une technologie qui ouvre la voie à de nouvelles solutions en matière de santé humaine et en cas de pandémies.
ProteinQure est une société biopharmaceutique qui conçoit de nouveaux ligands peptidiques non canoniques contenant des acides aminés et ayant de multiples applications thérapeutiques, notamment des conjugués avec agents cytotoxiques, oligonucléotides et radio-isotopes. La plateforme intègre des approches informatiques et expérimentales telles que la modélisation biophysique, l’apprentissage automatique d’identification des molécules et les technologies de criblage. Les capacités de l’entreprise ont été validées à la fois à l’interne et à l’externe avec des sociétés pharmaceutiques de premier plan, comme en témoignent les nombreux succès obtenus in vivo et les campagnes de conception in vitro sans précédent.
BioCanRx est un réseau de scientifiques, de cliniciens, d’acteurs de la lutte contre le cancer, d’établissements universitaires, d’ONG et de partenaires industriels qui travaillent ensemble à accélérer le développement de traitements immunitaires oncologiques de pointe pour les patients. L’objectif du réseau est de faire en sorte que tous les cancers puissent être guéris. En tant que leader dans l’application, la fabrication et la mise en œuvre d’immunothérapies contre le cancer, BioCanRx investit en vue d’amener les technologies de classe mondiale du laboratoire aux essais cliniques. Cela permet aux chercheurs d’accéder au financement, à l’expertise, à la formation et aux installations de bioproduction. BioCanRx forme les talents nécessaires pour faire de la biotechnologie canadienne de la santé un secteur florissant.

The Biologics Manufacturing Centre (BMC) Inc. is a non-profit organization specialized in the end-to-end GMP biomanufacturing facility designed to support the secure production of vaccines and other biologics within Canada. With a public good mandate, its mission extends to collaborating with industry and academia, co-development projects, and addressing unmet public health needs. The BMC serves as a robust GMP manufacturing partner for mammalian and insect cellbased biologics production, specializing in vaccines (viral vector, protein subunit, viruslike particles) and therapeutic proteins (antibodies, enzymes). The expertise extends to supporting therapeutic candidates from late-stage clinical phases up to commercial manufacturing.

Canurta Therapeutics has brought together leading experts with decades of research experience on polyphenols including flavonoids, cannflavins and stilbenes, to discover novel mechanisms of actions and therapeutic potential with technology-driven drug development. Their proprietary technology precisely isolates, extracts and standardizes these and other valuable compounds. This enables the creation of both extracts and single molecules that adhere to strict CMC standards. Optimized by bioinformatics, Canurta’s approach streamlines drug development, maximizing innovation, efficiency and therapeutic potential.

Myeloid Enhancement (ME)
Therapeutics is an early-stage Vancouver based biotechnology company involved in the discovery and development of novel immuno-oncology therapeutics targeting immune suppression in cancer. ME Therapeutics has two myeloid cell targeted drug development programs and one drug discovery program currently underway. All three programs target distinct areas of myeloid cell biology in order to inhibit the suppressive effects of suppressive myeloid cells on the anti-cancer immune response. These drug candidates are being developed to target pathways of myeloid cell biology that are not currently being targeted effectively.
Le Centre de production de produits biologiques (CPPB) est une organisation à but non lucratif spécialisée dans les installations de biofabrication qui respectent les bonnes pratiques de fabrication (BPF) de bout en bout. Son objectif est de garantir la production de vaccins et d’autres produits biologiques au Canada. Sa mission d’intérêt public consiste à collaborer avec l’industrie et les universités, à des projets de codéveloppement et à la réponse aux besoins de santé publique non satisfaits. Le CPPB est un partenaire solide pour ce qui est de la biofabrication conforme aux BPF, en particulier en ce qui concerne la production biologique à base de cellules de mammifères et d’insectes, et il a pour spécialité le domaine vaccinal (vecteurs viraux, sous-unités protéiques, pseudo-particules virales) et les protéines thérapeutiques (anticorps, enzymes). Il a aussi pour expertise le soutien aux candidats-médicaments, des dernières étapes du développement clinique à la fabrication commerciale.
L’entreprise Canurta Therapeutics compte en son sein de grands experts et des décennies d’expérience dans le domaine des polyphénols, dont les flavonoïdes, les cannflavines et les stilbènes, et elle vise à découvrir, grâce à la mise au point de médicaments fondée sur les technologies, de nouveaux mécanismes d’action et un potentiel thérapeutique. Sa technologie brevetée permet d’isoler avec précision, d’extraire et de normaliser les types de composés susmentionnés et d’autres précieux composés. Ainsi, on peut créer des essences et des molécules uniques qui respectent des normes rigoureuses de chimie, de fabrication et de contrôle. Optimisée grâce à la bio-informatique, la démarche de Canurta constitue une simplification du développement des médicaments, et elle permet de maximiser l’innovation, l’efficacité et le potentiel thérapeutique.
L’entreprise Myeloid Enhancement (ME) Therapeutics est une société de biotechnologie vancouvéroise en phase de démarrage, dont les travaux visent la recherchedéveloppement de traitements immuno-oncologiques novateurs ciblant l’immunosuppression en contexte de cancer. Deux programmes de mise au point de médicaments ciblant les cellules myéloïdes et un programme de recherche pharmaceutique sur le même sujet sont en cours chez ME Therapeutics. Les trois programmes visent des aspects distincts de la biologie des cellules myéloïdes, mais cherchent tous à inhiber les effets des cellules myéloïdes suppressives sur la réponse immunitaire anti-cancéreuse. On œuvre à mettre au point les candidats-médicaments de façon à cibler les mécanismes biologiques des cellules myéloïdes qui ne sont actuellement pas efficacement ciblés. ecosystem écosystème

Central to iProgen’s innovative efforts is an assortment of pioneering protein delivery technologies and extensive experience and expertise in antibody payload conjugation. They have developed conditionally activated antibody delivery and internalization technologies (Smart Antibodies™) that can boost existing antibody and ADC therapies. Additionally, the company is investigating a wider range of payloads, including oligonucleotides, peptides, PROTAC, lipid and even sugar.

Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines. Moderna’s mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases.

Sustained Therapeutics is a clinical-stage company utilizing its advanced clinical and research expertise to develop non-opioid locally injected sustainedrelease therapeutics primarily for the treatment of pain. A spin-off from the University of British Columbia and the Vancouver Prostate Centre, their novel sustained-release drug delivery platform has the potential to provide effective, long-lasting solutions for managing both acute and chronic pain. The company also plans to develop the platform to enable sustained release treatments for cancer and inflammatory diseases.
Au cœur des efforts d’innovation d’iProgen, il y a un ensemble de technologies novatrices d’administration des protéines, ainsi qu’une expérience et une expertise approfondies en matière de conjugaison de charges d’anticorps. La société a mis au point des technologies d’administration et d’internalisation d’anticorps activés sous conditions (Smart Antibodies™) qui peuvent renforcer les traitements existants à base d’anticorps et de conjugués anticorps-médicaments. L’entreprise s’intéresse par ailleurs à d’autres types de « charges », notamment des oligonucléotides, des peptides, des PROTAC, des lipides et même des sucres.
Grâce à ses travaux portant sur les technologies à base d’ARNm, Moderna transforme, au bénéfice de tous, la façon dont on fabrique les médicaments, de même que la manière dont on traite et prévient les maladies. Ayant travaillé à l’intersection des sciences, des technologies et de la santé durant plus d’une décennie, cette entreprise a mis au point des médicaments, dont l’un des premiers et des plus efficaces vaccins contre la COVID-19, dans un délai et avec une efficacité inégalés. S’articulant autour de l’ARNm, la plateforme de Moderna a permis le développement de traitements et de vaccins contre des maladies infectieuses, en immuno-oncologie, ainsi que contre des maladies rares et des maladies auto-immunes.
La société Sustained Therapetics en est au stade des essais cliniques et s’appuie sur son expertise de pointe dans le domaine clinique et en recherche pour mettre au point des produits injectables non opioïdes à libération prolongée destinés principalement au traitement de la douleur. Ayant essaimé de l’Université de la Colombie-Britannique et du Vancouver Prostate Centre, elle travaille à une plateforme d’administration de médicaments à libération prolongée qui pourrait produire des solutions de gestion de la douleur aiguë et de la douleur chronique efficaces et durables. L’entreprise prévoit aussi de développer son programme afin de proposer des traitements à libération prolongée pour les cancers et les maladies inflammatoires.

Langara College offers tailored academic pathways featuring a two-year Associate of Science or Diploma, or the only Bachelor of Science in Bioinformatics in British Columbia. In today’s data-driven world, industries need experts who can handle and make sense of vast amounts of biological based data. The field of Bioinformatics is booming, with a projected value of over USD $46 billion by 2027. Langara’s BSc in Bioinformatics not only equips students with essential skills but also provides valuable hands-on experience through two co-op terms and a year-long capstone project with industry partners. These transferable skills will prepare students to thrive in the ever-expanding global data economy.

Evolved Therapeutics has two complimentary technology platforms designed to enable the discovery of ultra-rare antibodies with unique functional activity to develop as first-in-class therapeutics. The Company’s pipeline is currently focused on treating inflammatory diseases. Evo-AbGen™ is an ultra-high-throughput, function-first screening platform capable of mining an entire immune repertoire (> 1 billion antibodies) which eliminates binding-biased screening to enable the discovery of ultra-rare functional antibodies. EvoBispecific™ takes this a step further, creating millions of unique bispecific combinations and screening with a function-first approach potentially enabling the discovery of novel mechanisms of action not possible with a monospecific approach.
As the national industry association for biotechnology, BIOTECanada represents the full ecosystem and all life sciences sectors including healthcare, agriculture, biomanufacturing, investment, academia and knowledge/ service providers.
BIOTECanada delivers targeted support through key areas of focus including:
• Business development and networking;
• National advocacy and dialogue; and
• Industry and public awareness

Le Langara College propose des parcours d’études parmi lesquels un programme spécialisé de deux ans et le seul baccalauréat en bio-informatique de la Colombie-Britannique. Dans le monde actuel, très axé sur les données, les industries ont besoin d’experts pouvant gérer et comprendre de vastes quantités de données biologiques. Le domaine de la bioinformatique est en plein essor, et sa valeur devrait atteindre 46 milliards $ US d’ici 2027. Le baccalauréat en bio-informatique procure aux étudiants des compétences essentielles, mais leur offre aussi une expérience pratique grâce à deux stages coop et à la nécessité de réaliser un projet de fin d’études (sur un an) en collaboration avec des partenaires du secteur. Les compétences transférables acquises préparent les étudiants à une carrière florissante au sein de l’économie mondiale des données, qui ne cesse de croître.
Evolved Therapeutics possède deux plateformes technologiques complémentaires conçues pour la recherche d’anticorps ultra-rares dotés d’une activité fonctionnelle unique propice à leur développement en tant que premiers produits thérapeutiques de leur catégorie. Le programme de la société est actuellement axé sur le traitement des maladies inflammatoires. Evo-AbGen™ est une plateforme de criblage à très haut débit, axée avant tout sur la caractérisation fonctionnelle, apte à l’exploration de répertoires immunitaires entiers (plus de 1 milliard d’anticorps), qui élimine du criblage les biais causés par les liaisons et permet ainsi la découverte d’anticorps fonctionnels ultra-rares. Evo-Bispecific™ va encore plus loin en créant des millions de combinaisons bispécifiques uniques et en effectuant le criblage selon une approche axée avant tout sur la caractérisation fonctionnelle qui pourrait permettre la découverte de nouveaux mécanismes d’action, impossible lorsque la méthode est monospécifique.
À titre d’association sectorielle nationale des biotechnologies, BIOTECanada représente l’ensemble de l’écosystème et tous les secteurs des sciences de la vie, dont la santé, l’agriculture, la biofabrication, les investissements, le milieu universitaire et les fournisseurs de services et de savoir.
BIOTECanada offre une aide ciblée dans des domaines clés, parmi lesquels :
• le développement de l’entreprise et le réseautage;
• la défense des intérêts du secteur et le dialogue à son sujet, à l’échelle nationale;
• la sensibilisation des membres de l’industrie et du grand public
Découvrez les avantages de l’adhésion à BIOTECanada ici :


The Hon. Jean-Yves Duclos (Minister of Health), Bettina Hamelin (Ontario Genomics), Jean-Pierre Baylet (Sanofi Canada), Oliver Technow (BIOVECTRA and Member of the BIOTECanada Board of Directors) participate in a panel discussion on building ecosystem success, and the next steps on the partnership with government moderated by Rosemary Thompson (Coalition for a Better Future)
L’honorable Jean-Yves Duclos (ministre de la Santé), Bettina Hamelin (Ontario Genomics), Jean-Pierre Baylet (Sanofi Canada) et Oliver Technow (BIOVECTRA, membre du conseil d’administration de BIOTECanada) participent à une séance de discussion sur la mise en valeur des écosystèmes de biotech et les prochaines étapes en matière de partenariat avec le gouvernement, séance animée par Rosemary Thompson (Coalition pour un avenir meilleur)
IN AN ERA WHERE BIOTECHNOLOGY PLAYS A PIVOTAL ROLE IN SHAPING THE FUTURE, Ottawa is set to welcome policymakers, industry leaders, and stakeholders to BIONATION 2024. The annual event, hosted by BIOTECanada is the only national event showcasing the vibrant biotechnology network found throughout Canada and has become key for fostering a comprehensive understanding of Canada’s biotech landscape; promoting a more integrated policy environment conducive to attracting investment and modernizing regulatory decision making; and supporting a healthy biotech ecosystem leading to more research and development for company creation and growth.
Originally launched in the Spring of 2020, BIONATION has continued its steady momentum demonstrating the interconnectivity of the Canadian biotechnology ecosystem and providing attendees an in-depth look at the industry’s core. The event advocates for increased public awareness amongst policymakers of biotech’s critical value, emphasizing the economic benefits of a robust domestic biotech ecosystem in Canada.
This year, BIONATION will be held from September 24th – 25th in Ottawa. Over the course of 2 days, event programming will primarily focus on three key areas: the biotech ecosystem, regulatory affairs, and the growing

David Lee, dirigeant principal de la réglementation, Santé Canada, Christopher Johnstone, sous-ministre adjoint, Santé Canada et Darryl Patterson, directeur général, Innovation, Sciences et Développement économique Canada
À UNE ÉPOQUE OÙ LA BIOTECHNOLOGIE OFFRE DE RÉELLES PERSPECTIVES, Ottawa s’apprête à accueillir des décideurs politiques, des chefs d’entreprise et divers intervenants à l’occasion de BIONATION 2024. Cet événement annuel, organisé par BIOTECanada, est le seul événement national qui mette en valeur le dynamique réseau biotechnologique réparti à travers le Canada. Il s’agit d’une réunion devenue essentielle, où se rencontrent : une vue d’ensemble du paysage biotechnologique canadien; la promotion d’un environnement réglementaire plus intégré, propice à attirer des investissements, et la modernisation du processus décisionnel en matière de réglementation; la valorisation de la santé de l’écosystème biotechnologique, favorable à la recherche-développement et, ultimement, à la création d’entreprises et à leur évolution.
À la suite de la première édition du printemps 2020, BIONATION a continué sur sa lancée. Agissant comme un révélateur de l’interconnectivité de l’écosystème biotechnologique canadien, c’est un événement qui permet aux participants de découvrir l’industrie de l’intérieur. L’objectif est de sensibiliser les décideurs politiques à l’importance de la biotechnologie, en mettant l’accent sur les avantages économiques pour le Canada de pouvoir compter sur un écosystème biotechnologique en plein essor.

Andrew Casey, President & CEO, BIOTECanada
Andrew Casey, président et chef de la direction de BIOTECanada

Norma Biln, CEO, Augurex and Member of the BIOTECanada Board of Directors
Norma Biln, chef de la direction d’Augurex et membre du conseil d’administration de BIOTECanada

Canadian Olympian & Physician: Hayley Wickenheiser delivers the keynote speech to BIONATION attendees
Le physicien et olympien canadien Hayley Wickenheiser prononce le discours liminaire de la rencontre BIONATION
economic opportunity for Canada, the event will also underscore BIOTECanada’s main policy priorities, namely to:
• Establish a more competitive and efficient regulatory environment; and
• Grow the availability and impact of investment capital in the sector
Establishing a competitive policy environment that supports the growth of companies, attracts additional investment, and extends the effectiveness of capital will allow more companies to scale up and become commercial in Canada. BIONATION stands as a testament to this vision, delivering an engaging platform where pivotal discussions and partnerships can flourish, and advancing Canada’s position in the global biotech arena.

left to right:
Ramsay, VP and
Member of the BIOTECanada Board of Directors, Deputy Minister, Health Canada, Dr. Stephen Lucas, Deputy Minister, Innovation, Science and Economic Development Canada, Simon Kennedy
La v.-p. et directrice générale d’AbbVie Canada et membre du conseil d’administration de BIOTECanada, Tracey Ramsay, le sous-ministre, Santé Canada, Stephen Lucas, Ph. D., sous-ministre, Innovation, Sciences et Développement économique Canada, Simon Kennedy
BIONATION se tiendra cette année les 24 et 25 septembre à Ottawa. Le programme de l’événement, réparti sur deux jours, sera principalement axé sur les trois points suivants : l’écosystème biotechnologique, les questions réglementaires et les perspectives économiques croissantes pour le Canada. Ce sera également l’occasion de souligner les principales priorités politiques de BIOTECanada, qui sont les suivantes :
• Mettre en place un environnement réglementaire plus concurrentiel et plus efficace.
• Accroître la disponibilité et la portée des capitaux d’investissement dans le secteur
La mise en place d’un environnement réglementaire concurrentiel, qui stimule la croissance des entreprises, attire davantage d’investissements et accroît le rendement des capitaux, permettra à un plus grand nombre d’entreprises d’évoluer vers la commercialisation de leurs produits depuis le Canada. BIONATION incarne cette vision : la rencontre offre une tribune stimulante où des débats peuvent avoir lieu, et des partenariats, se nouer, ce qui renforce la position du Canada dans la sphère mondiale de la biotechnologie.
For more information, visit:

In line with this spirit of innovation and collaboration, BIOTECanada is pleased to announce AbbVie, adMare BioInnovations, AstraZeneca, Fonds de solidarité FTQ, Novartis, and Stem Cell Network as the initial sponsors and partners for BIONATION 2024. Scan the QR code for organizations interested in partnership and sponsorship opportunities, or to request an invitation to BIONATION.
Dans cet esprit d’innovation et de collaboration, BIOTECanada a le plaisir d’annoncer que AbbVie, adMare BioInnovations, AstraZeneca, le Fonds de solidarité FTQ, Novartis, et le Réseau de cellules souches sont les premiers commanditaires et partenaires de BIONATION 2024. Les organisations qui souhaitent explorer les possibilités de partenariat et de commandite ou demander une invitation à BIONATION sont invitées à lire le code QR.
Pour obtenir de plus amples renseignements, veuillez lire ce :


WHEN CONSIDERING THE MOST SIGNIFICANT CHALLENGES FACING CANADA’S BIOMANUFACTURING SECTOR, access to the right talent is at the top of the list.
Fueling the bioeconomy requires a steady stream of skilled workers to meet production demands, to innovate, and to navigate the next inevitable health crisis. A 2021 study of Canada’s labour market1 projects the bioeconomy will require more than 65,000 additional workers by 2029, with some of the highest demand in biomanufacturing. However, it’s fair to say that companies across the sector are already feeling the HR pinch.
The Canadian Alliance for Skills & Training in Life Sciences (CASTL) in partnership with Upskill Canada, powered by Palette Skills, is working to bridge the talent gap with CASTL Elevate. This national six-week training program is designed for young workers, new Canadians, and others seeking out opportunities in Canada’s biomanufacturing sector.
“CASTL Elevate is a critical pathway to bring new workers to this fast-growing sector,” says Penny WalshMcGuire, CASTL’s Executive Director. “Developed in consultation with industry, it provides the sector with skilled workers who can hit the ground running, reducing the usual onboarding interval and laying the groundwork for the industry’s next generation workforce.”
The Elevate program is delivered through a blend of self-directed and instructor-led online learning as well as practical hands-on experience at a state-of-the-art CASTL training facility. It teaches the requisite technical competencies to fill entry-level biomanufacturing technician roles in areas that include laboratory, manufacturing, production, and process development. Coursework focuses on developing a strong foundation in aseptic processing; following Good Manufacturing Practices (GMPs), Standard Operating Procedures (SOPs), and Good Documentation Practices (GDPs); developing proficiencies in the use of biomanufacturing equipment; and understanding the various roles and processes in a biomanufacturing environment.
The program also cultivates the professional skills needed to connect with employers. Learners are coached in writing effective resumes, job search techniques, networking, and interviewing skills.

“We’ve taken the program to the next level to close the loop between talent development and employment,” says Walsh-McGuire. “We offer networking sessions and other HR services to connect prospective employers with prospective employees.”
CASTL Elevate is part of a larger national initiative to help companies in key growth sectors access talent while creating new career pathways for workers.
“Thanks to the support of the Innovation, Science and Economic Development Canada’s (ISED) Upskilling for Industry Initiative, Upskill Canada, powered by Palette Skills, is working to reverse the underutilization of the Canadian workforce by upskilling workers to unlock their full potential and move them into new and better jobs in the biomanufacturing sector,” says Rhonda Barnet, CEO of Palette Skills.
Over the next year, Elevate will train up to 400 new workers in 20 cohort sessions. With a maximum of 20 individuals enrolled per session, participants will benefit from the small group learning environment, which fosters enhanced peer collaboration, more feedback from instructors, team-building, and greater learner confidence. It will play a key role in establishing a skilled talent pipeline that will keep Canada at the forefront of a global industry that is continually innovating.
Canadian biomanufacturing companies are invited to learn more about the Elevate program at www.castlcanada.ca/en/ elevateprogram. For more information about networking and other HR opportunities with Elevate graduates, please contact Jennifer Lenentine at jennifer@castlcanada.ca.
The Canadian Alliance for Skills and Training in Life Sciences (CASTL) is a national skills and training organization formed to address the talent needs of the Canadian life sciences sector. Specializing in biopharmaceutical manufacturing, CASTL delivers on the economic and sectoral demand for individuals who have the technical skills to enter, thrive and meet the needs of the fast-growing Canadian biomanufacturing industry castlcanada.ca
1 BioTalent Canada (2021). National Report: Close-up on the bio-economy. Labour Market Intelligence. www.biotalent.ca/wp-content/uploads/ BioTalent-Canada-LMI-National-Report-13OCT2021-1.pdf
• Industry-informed hands-on training in GMP-like facilities
•
•
GMP and aseptic technique-focused technical training
Flexible delivery of onsite, online, on-demand and blended course options
Biomanufacturing Training Facility coming Fall 2024


• Customized training packages and group rates available
•
•
Global trusted Canadian delivery partner of the National Institute of Bioprocessing Research and Training (NIBRT) courses
Focus on risk reduction and building confidence through quality training





L’entreprise académique : un pilier de l’écosystème d’innovation en santé
IN THE LIFE SCIENCES, DRUG DEVELOPMENT IS A COMPLEX AND COSTLY UNDERTAKING. Each new scientific discovery offers considerable potential for improving human health, but this potential cannot be realized without adequate funding. Yet the journey from a promising drug discovery to a tangible therapy available to patients is often compared to crossing the metaphorical “valley of death,” the critical point at which promising discoveries come to a halt for lack of financial support.
Affiliated with the Institute for Research in Immunology and Cancer (IRIC) at the Université de Montréal, IRICoR has, since its inception in 2008, provided a vital financial springboard for researchers, enabling them to cross this formidable divide and bridge the gap between academic research and the pharmaceutical industry.
With government grants totalling close to $50 million, IRICoR has been able to design innovative therapies, found several spin-off companies and establish licensing agreements worth $2.2 billion. These agreements have already generated over $107 million in revenues, demonstrating a significant return on investment despite declining government subsidies.
At the heart of IRICoR’s success is the link between the public and private sectors. Public-private partnerships (PPPs) are key to bringing therapies to market faster. These collaborations not only inject capital, but also share knowledge and expertise, fostering efficient innovation and the creation of intellectual property.
“The IRIC-IRICoR pairing is a unique model that is transforming the way we add value to research. It embodies this culture of collaboration, promoting the exchange of ideas and the sharing of resources within Quebec’s scientific community, and helping to strengthen Quebec’s leading position,” emphasizes Elizabeth Douville, President and CEO of IRICoR.
Yet IRICoR’s impact is not limited to its financial contributions. It plays a leading role in project
DANS LE DOMAINE DES SCIENCES DE LA VIE, LE DÉVELOPPEMENT DE MÉDICAMENTS REPRÉSENTE UNE ENTREPRISE COMPLEXE ET COÛTEUSE. Chaque nouvelle découverte scientifique offre un potentiel considérable pour améliorer la santé humaine, mais ce potentiel ne peut être réalisé sans un financement adéquat. Or le parcours qui mène d’une découverte prometteuse de médicament à une thérapie tangible disponible pour les patients est souvent comparé à la traversée de la métaphorique “vallée de la mort,” moment critique où des découvertes prometteuses s’arrêtent faute de soutien financier.
Affilié à l’Institut de recherche en immunologie et en cancérologie (IRIC) de l’Université de Montréal, IRICoR offre, depuis sa création en 2008, un tremplin financier vital aux chercheurs, leur permettant de franchir ce redoutable fossé et arrime le milieu de la recherche académique et l’industrie pharmaceutique
Avec des octrois gouvernementaux totalisant près de 50 millions de dollars, IRICoR a su concevoir des thérapies innovantes, fonder plusieurs sociétés dérivées et établir des ententes de licence d’une valeur de 2,2 milliards de dollars. Ces ententes ont déjà généré plus de 107 millions de dollars de revenus, démontrant un retour sur investissement significatif malgré le déclin des subventions gouvernementales.
Au cœur du succès d’IRICoR se trouve le maillage entre le secteur public et privé. Les partenariats publics-privés (PPP) sont essentiels pour accélérer la mise sur le marché des thérapies. Ces collaborations permettent non seulement d’injecter des capitaux, mais aussi de partager des connaissances et des expertises, favorisant ainsi une innovation efficiente et la création de propriété intellectuelle. « Le couple IRIC-IRICoR constitue un modèle unique qui transforme la façon de valoriser la recherche. Il incarne cette culture de collaboration, favorisant l’échange d’idées et le partage de ressources au sein de la communauté scientifique québécoise et contribue à renforcer la position
management, the search for commercial partners, and the implementation of intellectual property protection strategies. IRICoR also fosters the creation of innovative companies, feeding Quebec’s entrepreneurial ecosystem and stimulating regional economic growth. These include ExCellThera, whose patented UM171 molecule stimulates the expansion of stem cells to treat leukemia patients. Epitopea, is developing a therapeutic cancer vaccine that could target both solid and liquid tumors.
In conclusion, academic enterprise is a key driver of prosperity and competitiveness for a country’s economy, and funding translational research is essential to accelerate the discovery of new medicines and improve human health. It is therefore crucial that governments, academic institutions, the pharmaceutical industry and private investors continue to work together to overcome financial challenges and create a favorable ecosystem to transform scientific discoveries into effective therapies. “By embracing collaboration and investing appropriately in human capital, infrastructure and research we will catalyze the emergence of therapeutic solutions. Together, we are generating and will continue to generate sustainable wealth in human and financial capital for Quebec and the rest of Canada.”
de premier plan du Québec. » souligne Elizabeth Douville, présidente – directrice générale d’IRICoR.
Pourtant, l’impact d’IRICoR ne se limite pas à ses contributions financières. Il joue un rôle prépondérant d’encadrement dans la gestion des projets, la recherche de partenaires commerciaux, et dans la mise en place des stratégies de protection de la propriété intellectuelle. IRICoR favorise également la création d’entreprises innovantes, alimentant ainsi l’écosystème entrepreneurial québécois et stimulant la croissance économique régionale. Parmi elles, ExCellThera dont la molécule brevetée UM171 stimule l’expansion des cellules souches pour traiter les personnes atteintes de leucémie. Epitopea, met au point un vaccin thérapeutique contre le cancer qui pourrait viser les tumeurs solides et liquides.
En conclusion, l’entreprise académique est un moteur essentiel de la prospérité et de la compétitivité pour l’économie d’un pays, et le financement de la recherche translationnelle est indispensable pour accélérer la découverte de nouveaux médicaments et améliorer la santé humaine. Il est donc crucial que les gouvernements, les institutions académiques, l’industrie pharmaceutique et les investisseurs privés continuent à travailler ensemble pour surmonter les défis financiers et créer un écosystème favorable afin transformer les découvertes scientifiques en thérapies efficaces. « En adoptant la collaboration et en investissant adéquatement dans le capital humain, les infrastructures et la recherche nous catalyserons l’émergence de solutions thérapeutiques. C’est ensemble que nous générons et continuerons de générer une richesse durable en capital humain et financier pour le Québec et le reste du Canada. »

$2.2B in potential licensing revenues
$107M in revenues from collaborations focused on the research and development of new therapies
$69M in non-dilutive research grants
5 spin-off companies
5 new drugs currently in clinical trials
60 IRIC scientists committed to drug discovery, making significant contributions to projects
11 specialists with expertise in science and business development
2,2 G $ en revenus potentiels de licences
107 M $ en revenus de collaboration axée sur la recherche et le développement de nouvelles thérapies
69 M $ en subventions de recherche non dilutives
5 entreprises dérivées
5 nouveaux médicaments actuellement en essais cliniques
60 scientifiques de l’IRIC attachés à la découverte de médicaments, qui contribuent significativement aux projets
11 spécialistes détenant une expertise en sciences et développement des affaires

country’s overall health
THE PRIDE CANADIANS FEEL TOWARD THE COUNTRY’S HEALTHCARE SYSTEM HAS DECLINED IN RECENT DECADES. Beyond the well-documented doctor shortages and overcrowded hospitals, patients are experiencing lengthy delays in getting access to the innovative medicines they need.
Canadians wait two years or longer before getting access to Health Canada-approved new treatments and vaccines through public drug plans. That’s almost double the wait times of other developed countries around the world.
As a result, Canada ranks last in the G7 nations and 19th out of 20 Organisation for Economic Co-operation and Development (OECD) member countries in the time it takes for approved new medicines to become accessible to patients.
“For rare disease patients, the numbers are even more staggering,” says Alison Sargent, Chief of Staff and Vice President of Operations at Innovative Medicines Canada. “Only 60 per cent of rare disease treatments make it into Canada, and most get approved up to six years later than in the US or Europe.”
For Sargent and many of her industry peers, the importance of improving Canada’s healthcare takes on a more personal meaning. Sargent’s 11-year-old daughter suffers from phenylketonuria, a rare disease which can lead to serious brain injuries when not properly managed by a strict diet. As the mother of a child suffering from a rare disease, Sargent fully understands the importance of being able to access cutting-edge new treatments, quickly.
Canada’s innovative pharmaceutical industry can be a key part of the solution to the healthcare challenges we face, according to Sargent. Health stakeholders, governments, and industry can collaborate on policies and regulations that help position Canada as an ideal destination for healthcare innovation and life sciences investments.
The industry continues to strive toward improving quality of life for all Canadians while contributing to the country’s total health.
“Not only are we developing treatments and vaccines that help improve the health and wellbeing of the population, but we also contribute significantly to the
health of our economy and research ecosystem,” says Sargent. “Our industry contributes nearly $16 billion to the Canadian economy, supports more than 100,000 highvalue jobs, sponsors over 2,000 clinical trials, and invests $2.4 billion in research and development each year.”
After a diagnosis, having access to state-of-the-art treatments early can potentially save the lives of patients in need.
“Beyond the constant danger that hovers over the people we love, new treatments offer hope for a better quality of life. For me, it’s about my child being able to hope for a normal life,” says Sargent. “The wait can be devastating for patients and their families who know that a treatment exists, but they can’t access it because they live here in Canada.”
For parents like Sargent and other caregivers, the innovative pharmaceutical industry is working tirelessly to improve access to life-changing and life-saving treatments. When stakeholders come together and work toward solutions that put patients first, the benefits extend far beyond an individual—and this is what Canadians deserve.


The innovative pharmaceutical industry enhances the TOTAL HEALTH of Canada by supporting high-value jobs, contributing to the economy, investing in research, and sponsoring thousands of clinical trials for Canadians.
Innovation is at the heart of our industry – it’s what drives our work every day. Because of this, we’re well-positioned to work with health leaders across the country to drive needed change.
When we bring in the whole of Canada, united towards broader solutions that put patients first, THIS is total health. And it’s what Canadians deserve.

JN NOVA PHARMA IS DEVELOPING A NOVEL THERAPEUTIC CLASS WHICH OFFERS A UNIQUE POTENTIAL THERAPEUTIC SOLUTION for Long Covidrelated severe pulmonary symptoms, and beyond, for ARDS (acute respiratory distress), IPF (Pulmonary fibrosis) and subsequently in AKI (acute kidney injury). Our drug candidates were developed in collaboration with Canada’s National Research Council – Human Health Groups (NRC-HHT) in Montreal and Ottawa, Canada, to which JN Nova Pharma has exclusive worldwide rights.
The December 2023, Canadian COVID-19 Antibody and Health Survey reported 2.1 million Canadian adults were still experiencing long-term symptoms following a COVID19 infection, termed post-acute sequelae of SARS CoV-2 infection (PASC). Long COVID has emerging definitions and health problems which vary among individuals, with symptoms including lingering fatigue, breathlessness, brain fog and muscle weakness.
Almost half of those still experiencing symptoms reported they have not seen any improvement in symptoms over time. Proportionally with Canada, 20 million people across the United States, and more than 100 million worldwide are also experiencing this. Novel therapeutic agents for Long Covid are therefore a vital need, as no current precise mechanistic diagnosis and no current therapeutic is available.



The proprietary drug of JN Nova Pharma has dual mechanism; potent viral Spike protein antagonism, thereby blocking the viral infection mechanism and providing novel enzyme replacement to protect acute organ injury. Also, the company’s lead candidate has a unique therapeutic profile to block the virally-independent pathological features of the Spike protein. Spike induces severe inflammatory mediators, also it stimulates vascular endothelial dysfunction and complement activation, all of which are crucial contributors to ongoing pulmonary thromboinflammatory damage.
Recent studies show some Long COVID patients are presenting with an ongoing circulating presence of the viral Spike protein, which is very likely to be contributing to Long Covid severity and may also serve to identify Long COVID patients have who ongoing presence of SARS-CoV-2 in the body—which is not readily detectable.
JN Nova Pharma has completed the Clinical Trial Application package for Health Canada submission. “We will evaluate the safety and efficacy of JN2019-M5.2 in patients with COVID and Long-COVID-19-related diseases, in particular, a group of Long COVID patients, who are affected by respiratory damage, using an inhaled delivery device,” stated Dr. John Gillard, CEO, JN Nova Pharma Inc.
Our goal is to develop an anchor company in Canada to develop innovative biological medicines. This precision medicine approach allows us to develop novel therapeutics in an accelerated manner and to collaborate with Canadian public and private biotech and the AI ecosystem to identify and precisely monitor patients during clinical studies. Molecular diagnostics, linked with specific therapeutic responses to novel biologicals is the future of drug development, with exact patients, reduced time and cost. “We are interested in collaborating with partners to bring these novel therapeutics to approvals for the global marketplace,” mentioned Dr. Nathan Yoganathan, CSO, JN Nova Pharma Inc.
For additional information, please contact: john@jnnova.com or nathan@jnnova.com
We dream of a day when patients no longer need opioids following surgery
AmacaThera is transforming how therapeutics get delivered



AMT-143 is AmacaThera’s lead non-opioid asset for treating post-surgical pain.
Designed for localized delivery via our unique hydrogel platform, which would be directly applied in the surgical site, AMT-143’s novel, slow-release formulation would potentially offer longer-lasting pain relief for surgical patients.










And avoiding an opioid prescription could make a significant difference in patient lives.
Grounded in the research of world-renowned biomaterials scientist, Dr. Molly Shoichet, AmacaThera is developing products at the intersection of biomaterials and pharmaceuticals.
AmacaThera is pursuing regulatory approval for AMT-143. As a result, this product is not yet commercially available in any market. Visit amacathera.ca to learn more.






The Canadian Life Science and Technology Park will fuel the growth of startups while advancing cutting-edge research at all levels. Join us in shaping the future.
This project exemplifies the power of collaboration in our industry. By leveraging the collective expertise of IPS, Allyant, and the Canadian Life Science and Technology Park, we’re driving innovation and excellence in research and development that will positively affect patients globally.
IPS is proud to be a strategic partner with Allyant and the Canadian Life Science and Technology Park. Together, we are focused on the advancement of the design and engineering of this state-of-the-art life science, healthcare, and technology park tailored for specialized turnkey startups, commercial manufacturing, and comprehensive support facilities.

21 SITES | 826,000 SQ. FT.
There is a unique desire among life science, healthcare and technology entities to cluster together in a campus ecosystem, converging right infrastructures + right ideas + right people to drive efficiencies, to recruit and retain top talent, to attract strategic capital, and to ensure patient saving products transition from idea to commercial realization.
Safa’a Al-Rais / President & CEOHost a grad through the Health Research BC-Mitacs internship program




























To an entire ecosystem.












We believe “what’s possible” can always go further. That’s why we do things differently at IQVIA – by bringing the science of healthcare together with data science, advanced analytics and expert knowledge. It’s how we look beyond what’s expected in healthcare to see what’s possible.



Others may offer a way forward. IQVIA gives you a way further.