


























© BIOCOM AG, Berlin 2024
European Biotechnology Science & Industry Guide 2024 (Vol. 14)
Published by:
BIOCOM Interrelations GmbH
Jacobsenweg 61
13509 Berlin, Germany
Tel. +49-30-264921-0
Fax +49-30-264921-11 service@biocom.de www.biocom.de
In cooperation with the European Biotechnology Network AISBL, Brussels www.european-biotechnology.net
Editor: Andreas Mietzsch
Executive Producer: Wolfgang Gutowski
Production: Martina Willnow
Graphic Design: Michaela Reblin
Printed at: KOPA, 54469 Kaunas, Lithuania
This book is protected by copyright. All rights including those regarding translation, reprinting and reproduction reserved. No part of this book covered by the copyright hereon may be processed, reproduced, and proliferated in any form or by any means (graphic, electronic, or mechanical, including photocopying, recording, taping, or via information storage and retrieval systems, and the Internet).
The copyright for the printed photos is held by the presented companies.
ISBN: 978-3-928383-91-2
No amount of fussing will help: The general economic sentiment at the start of 2024 is lousy almost everywhere in Europe. Disruptions and disentanglements in the global economy all around. The megalomaniac would-be tsar in the Kremlin is only the biggest single problem. (But congratulations, dear Poles, on the new, rational and Europe-friendly government!) War is not only raging in Ukraine, the Middle East is also burning in several places. In Germany, which has been rather pacifist since the Second World War, a term that had been completely forgotten has returned: “war-capable”. The German Defence Minister Pistorius brought it up and presumably he means “capable of defence”. Why do we have to deal with this in a biotechnology book? Because mankind is facing a global climate catastrophe and must now spend billions to secure peace and freedom. Necessary, but from the point of view of our endangered biosphere a blatant misallocation of investment.
What’s more, it is precisely in bad times that the failings of the comfortable good days become glaringly apparent. The industrialised nations have ignored the interests of the young countries in the South for far too long – even though the modern age of migration began many years ago. Instead, we have woven an ever denser web of regulations and social benefits that is becoming increasingly unaffordable and is now making us increasingly immobile in the global competition for talent and innovation. The situation is certainly gradually different in the many various European countries, but overall the European economic area is under pressure globally.
If we now have to look at how we want to earn our living in the global sharing of labour in the future, we should be looking at Biotechnology right now: We still have excellent research, but the fragmented industry is only capable of global market disruption in exceptional cases – for example mRNA vaccines. In the field of Industrial Biotechnology, which would be particularly important for the circular economy and climate protection, we are shackling ourselves over and over. CRISPR/Cas and Biofuels are the saddest recent examples, with slight glimmers of hope. Added to this are the underdeveloped capital markets. The industry, which shows excellent examples of its capabilities in this book, can do something about this misery itself: Anyone who revolutionises industrial processes with modern biology (just think of the latest beacon of hope, “precision fermentation”) should tell the public, politicians and, above all, the capital markets clearly in one key term what they are doing: Biotechnology! Only together will we be taken seriously as an industry with great growth opportunities. The stakes are high!
 Andreas Mietzsch President of the European Biotechnology Network
Andreas Mietzsch President of the European Biotechnology Network


With more than 38 years of experiences in the life sciences, BIOCOM ensures skilled management and targeted communication for projects such as the EU Framework Programme for Research and Innovation.
Communication & dissemination: strategy development, digital & print communication, public relations, website development, film & animation, social media
Memorable experiences: exhibitions, innovative workshops, hands-on labs
Stakeholder & public engagement: networking hubs, living labs, public dialogue formats
Capacity development: communication trainings, educational toolkits
Analysis & consulting: country and market studies, evidence-based policy advice
For more information, just head to www.biocom.eu/comdis or contact Christin Boldt at c.boldt@biocom.eu
European biotech companies continue to demonstrate strong innovative capacity. However, structural and regulatory obstacles in critical areas of innovation have increasingly made it challenging to tap into their full potential. In the future markets of plant breeding/new genomic techniques (NGT), cellular agriculture and digitalisation/clinical trials, in particular, Europe’s bureaucratic processes lag behind the more nimble approaches in Asian countries and the USA. In 2024, the commercialisation of biotech innovations developed in Europe threatens to shift to more modern locations, despite well-intentioned yet poorly implemented EU legislative amendments. The EU Commission is thus failing to achieve its goal of increasing Europe’s resilience and self-sufficiency in key areas of climate protection and the healthcare market. In two areas that are important for the future of European biotech companies, the European Commission has resolutely pushed ahead with regulatory amendments this last year: in deregulating the strict EU genetic engineering law, and, as part of the Pharma Regulation, accelerating the multi-centre clinical trials that previously had to be approved individually by the member states. In a third, young biotech market segment – cellular agriculture – the need for action has at least been identified. However, it is up to the newly elected European Parliament and the new EU Commission to decide which improvements are made.
In June 2023, after three years of preparation, the EU Commission under President Ursula von der Leyen, who will be in office until the end of 2024, proposed a draft EU regulation aimed at modernising the strict EU genetic engineering law for plant varieties. In light of the rapid development of climate-adapted seeds and the desired reduction in CO 2 emissions and environmental pollution caused by chemical pesticides and fertilisers, the aim is to speed up the approval of breeds designed with cis-genic, new genomic techniques (NGT). Technologies such as targeted CRISPR-Cas directed mutagenesis offer an alternative to achieving results that could, albeit more slowly, be
obtained through traditional breeding techniques like chemical or radiation mutagenesis. The prerequisite for this is the determination of equivalence according to so-called equivalence criteria, which allow up to 20 non-transgenic changes in the plant genome (NGT1 breeds). For the 6% of NGT2 plants in development, which have major non-transgenic modifications, a less bureaucratic approval procedure is to apply, which roughly corresponds to the current EU genetic engineering law. The proposed regulation would be one of the most research-friendly in the world. Unlike in the biotech Mecca of the USA, where genetically modified varieties are deregulated and approved without labelling if only a single trait has been specifically bioengineered, the EU proposal goes further. It allows up to 20 modifications, a move considered by observers to be exceptionally supportive of both research and companies, making it unique worldwide.
The draft faced criticism from GMO-sceptical NGOs, the European Greens and some member states for its lack of product labeling, absence of co-existence rules to protect organic farming and because it only provides for a simple safety check as part of the variety approval process. These groups are also calling
 Picture: © Krischi Meierstock.adobe.com
Picture: © Krischi Meierstock.adobe.com
Source:
for a ban on patents, as they believe that patents on seeds would promote seed monopolisation.
Under the Spanish EU Council Presidency, it became apparent that reaching an agreement before the European Parliament (EP) elections in June 2024 would be a close call. In the Council of Ministers, two voting rounds failed to achieve the required qualified majority as the EU member states supporting the draft represented just under 57.6% of the EU population, falling short of the 65% threshold needed. The EP voted in favour of the proposal at the end of early February – albeit with the provison of a ban on patenting and a product labeling requirement for NGT1 breeds. The Commission seemed unwilling to agree to this, particularly the patent ban, at least until midFebruary 2024. Before a second vote by the agriculture ministers, the EU Council had not endorsed the patenting ban. However, by then, 16 EU states, representing just under 60% of the population, agreed. While the seed industry lobby group Euroseeds emphasises that patents for biotechnological inventions, as provided for in EU Directive 98/44, must coexist with the prevailing system of plant variety protection
(breeder’s exemption), the EU biotech association EuropaBio is clearly opposed to sector-specific regulations on patent protection.
Whether an agreement will be reached under the current EU Commission was still open at the end of February. If the Council does not agree on a regulation by then, there will be little time for the EU Commission, the European Parliament and the Council to reach a decision before the new EP elections. As the EU Commission will also be newly elected, it remains to be seen whether the legislative initiative, which promised European plant research access to a market worth billions after decades of stagnation, will be pursued further.
Given that Asian countries and the USA have permitted approvals on a case-by-case basis for several years, closing market access in Europe could hinder academic research by removing incentives to invest in these technologies.
According to leading contract research organisations (CROs), which conduct around 60% of all clinical trials
worldwide, the Commission’s 2012 decision to accelerate the approval of multi-centre clinical trials across all participating member states through a centralised, digital approval system (EC 536/201) is to be applauded. However, pharmaceutical, biotech and CRO associations criticised its instability and dysfunctionality even before the mandatory digital reporting obligation via the digital approval portal CTIS came into force on 31.1.2023. Insiders in the associations have reported that essential documents could not be uploaded and other necessary information could not be selected or entered.
Although significant improvements have been made, the system is still unstable and extremely labour-intensive. According to several national CRO associations, it does not allow changes to be made after submission without a new application (which triggers six-figure fees) and often crashes. This causes unnecessary delays and expenses, prompting some major pharmaceutical clients to consider pulling out of Europe. Despite this, the European Medicines Agency, which is responsible for the maintenance of the system, does not intend to make any significant changes.
According to industry experts, there is a risk that European clinical trials registered under the old EUDract system will be relocated to regions with simpler and more efficient reporting systems as early as April 2024. For example, in the USA, companies can create a study plan at significant cost, but only need to do so once, not for each individual study as is the case in Europe. Provided they adhere to this plan, they face no further bureaucratic hurdles until approval. In addition, the US system allows changes without resubmission.
According to industry experts, the first studies under the so-called transition period will expire in April, after which studies registered under the old EUDract system must be transferred to the digital CTIS system and subsequently be resubmitted. According to Martin Krauss, CEO of FGK Clinical Research GmbH, the
transfer takes just as long as a new application in CTIS. Numerous study sponsors apparently do not want to go along with this. According to the German CRO association VDMA, around 2,000 to 4,000 studies will be affected by January 31, 2025, when the transition period ends.
If study sponsors will be closing clinical trials in Europe and recruiting the missing subjects in Asia and the USA, the resulting exodus will not only affect the biopharma and CRO business in Europe, but also downstream sectors such as the global growth market of contract manufacturing by CDMOs. Europe faces a potential billion-euro decline in sales due to the anticipated exodus of studies. According to market analyses, the CDMO market alone is set to grow from US$76.6bn to US$127.3bn by 2028. Although the CRO market is growing at a slightly slower rate of 9% annually compared to the CDMO market, there is still money at stake. While sales in Europe were US$21.7bn in 2023, they are expected to grow to just under US$39.5bn by 2030. It remains to be seen how severe the downturn will be.
While Singapore, the USA and Israel can already boast approved products from cell-based agriculture, Europe is still in need of skilled personnel and regulatory adjustments for participating in this billion euro market. Since 2000, milk, fish and meat products produced microbially or by cell culture have received market approval in Singapore, a city state that is poor in agricultural land and therefore dependent on food imports. Here, and since 2023 in the USA and 2024 in Israel, the authorities have been supporting young companies to get their fermenter-brewed foods approved. In the EU, the birthplace of these technologies, the situation is different. Cultured meat, eggs and dairy products are covered by the Novel Food Regulation, which does not yet have any specific rules for this kind of land and climate-friendly production processes. In addition, EFSA has so far been reluctant to enter into an exchange with manufacturers on specific rules for their products that they wish to authorise under the Novel
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2024
› Guide to German Biotech Companies 2024
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2024
› Guide to German Biotech Companies 2024
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)


Food Regulation in the EU. If the EFSA has given a positive assessment, the market approval, which has cost several 100,000 euros at this point, could still be overturned. This is because, after the EFSA food safety check, the Paff Committee of the permanent representatives of the EU member states decides whether and how a product can be marketed. Interest groups such as GFI Europe are expressing their concern that its decision-making criteria are not transparent. In addition, the agricultural lobby appears to exert a great deal of influence. This became evident at the end of 2023 when Italy became the first country in the world to ban cell-based meat in order to protect its domestic agriculture and food traditions. A few months later, a coalition of 13 member states led by Austria, Italy and France’s Républicains, which together collect around 70% of the CAP agricultural subsidies of around
€55m per year, joined forces with the aim of making it more difficult to approve the new cell-based foods.
There is an opportunity for a sustainable, animalfriendly market worth billions. A large percentage of companies active in this field are based in Europe, especially in the pioneering countries of the UK and the Netherlands, with more companies in Europe than in the USA. The UK is investing £2bn in the biomanufacturing sector over the next 10 years. The Netherlands has introduced unbureaucratic tastings of cultured foods before market authorisation to allow consumer feedback, akin to Singapore, where market approval typically takes less than 12 months (EU 24-48 months).
However, companies such as Formo Bio, Solar Foods and Meatable have their products approved in Singapore and are already seeking funding outside the EU, where new technologies are more readily adopted. The EU Commission is already issuing internal warnings about an impending exodus of companies, but can hardly control the powerful agricultural lobby. Meanwhile, more than 50% of European consumers show interest in the new products available and are eager to try them. Among those under 25, the figure rises by an additional 30%. A new spirit is needed in Europe’s offices to ensure that the enthusiasm of the young EU founders is not stifled.
Some industry experts look back on 2023 with relief, stating, ‘Luckily, it’s over.’ Following a peak in 2021, the industry experienced a sharp decline in 2022 and an even more pronounced rebound to pre-pandemic levels in 2023. Several factors contribute to this notably harsh downturn. Certainly, too many early-stage companies have gone public in the US and were faced with limited cash reserves combined with limited access to fresh capital, resulting in 200 listed biotech companies worldwide (out of a total of 850) trading below their NPV by autumn 2023 (i.e. negative enterprise value).
Beyond those numbers, a few key events dominated. The rapid collapse of Silicon Valley Bank in March was Picture:
a low point for many founders. ECB interest rates, which have risen from a record low of -0.5% in summer 2022 to a record high of 4% at the end of 2023, have starved the riskiest companies of capital. Adopting strategies for a more streamlined operation became a routine practice for many start-ups.
Sinking interest rates have an immediate impact on market behaviour and cash flows. Signs of this may have already appeared in the last quarter as biotech equity indices rallied again. But is it sustainable, especially in the year of a highly uncertain US election? The volatility of the US market will impact Europe one way or the other, casting a shadow of uncertainty over 2024. Despite this difficult financing environment, a number of follow-on financings have recently been successfully launched, and mergers and acquisitions activity has intensified. Some biotech companies have secured funding from existing investors, often through private investments in public equity, but these options are unattractive or unattainable for many smaller companies at current valuations. Thus, experts like Marcus Wieprecht, Stifel Europe Bank, remain cautious. Wieprecht commented in E urop E an B iot E chnology M aga-
zin E : “The financing environment for biotechnology needs more creativity. We need more creativity in deal structuring, including alternative financing options such as licensing deals, structured financing for clinical trials, an expanded role for private equity, convertible bonds and even non-traditional venture debt financing”. Inflation will affect the ability of biotech companies to raise capital from external investors. Alternatively, funding may be sourced through M&A. The pharmaceutical industry is increasingly on the hunt for innovation as the patent cliff is about to collapse with hundreds of billions at stake by 2030.
There are political measures being installed for the sector, as the UK showed in December with a £2bn “National Vision for Engineering Biology”. Will the EU follow? A recent paper assessed the EU’s economic security in terms of further risks and identified four technology areas that are likely to pose the most sensitive and immediate risks in terms of technology security and technology leakage, namely advanced semiconductors, artificial intelligence, quantum technologies. And: biotechnologies.
Georg GThomas Gabrielczyk, Georg Kääb


Name ›
3PBIOVIAN
Manufacturing site
Address/P.O. Box › Postal Code/City › Country ›
Polígono Mocholi, Plaza Cein, 1 31110 Noáin
Spain
Manufacturing site
Address/P.O. Box ›
Postal Code/City › Country ›
Tykistökatu 6A, Biocity 20520 Turku
Finland
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Areas of Activity ›
Commercial office 53 State Street, Suites 503 504 02109 Boston MA (USA)
Elena Erroba (Group Chief Commercial Officer)
+34-948-34-64-80 +35-8-50-3650973
withinus@3pbio.com
business@biovian.com www.3pbiovian.com
I
550+
| Biotechnology | Biomedicine
CDMO | Biologics
GMP
| Recombinant Proteins
Cell Therapy | Viral Vectors
Fill & Finish
3PBIOVIAN is a globally operating Contract Development and Manufacturing Organisation (CDMO) formed from the joined forces of 3P Biopharmaceuticals and Biovian.
3PBIOVIAN offers unparalleled end-to-end development and manufacturing services for all protein expression systems and viral vectors, both for Drug Substance and Drug Product, from preclinical to clinical development and commercial phases. Leveraging a joint 40-year track record, expertise, capabilities, and financial strength, the Group positions itself as a leading pan-European independent biologics CDMO, aiming to spearhead the development of drugs tackling patients’ unmet clinical needs.
Our end-to-end service offering spans across multiple platforms (microbial and mammalian expression, adenoviruses, adeno-associated viruses, cell therapy, and plasmids) and offers enhanced production scale flexibility via a diverse range of bioreactor sizes. This comprehensive set of services targets a global base of biopharma clients looking for scientific expertise, premium quality, and outstanding customer service.
3PBIOVIAN offers GMP manufacturing services for microbial and mammalian protein expression platforms, viral vector production for adenoviruses and adeno-associated viruses, cell therapy, and plasmid DNA production.
› Plasmid DNA production
› Cell line development, GMP cell banking and Virus Seed Stock
› Technology transfer, process development and optimization
› Analytical methods development, qualification, and validation
› Formulation development
› Scale-up and cGMP manufacturing
› Fill and Finish
› Quality Assurance and Quality Control
› DS & DP release




› Cell therapy products
› Tissue-engineered products
› Biomaterials (scaffolds or membranes)
› Gene therapy products
› Mammalian, single use bioreactors: 50L, 200L, 400L, 2000L
› Microbial, stainless-steel bioreactors: 10L, 100L, 200L, 500L, 1000L
› Viral Vector, single use bioreactors: 10L, 25L, 40L, 200L
› Plasmid DNA, stainless-steel bioreactors: 40L, 200L
In Turku, Finland, our manufacturing site boasts a workforce of 200 employees and spans an area of 5,100 sqm. Our facilities feature dedicated capacities and capabilities for GMP manufacturing, analytical methods development, quality control services, and fully automated aseptic fill and finish processes.
In Pamplona, Spain, our manufacturing facility employs 350 people and encompasses an expansive area of 10,500 sqm. Here, we house GMP manufacturing capabilities and recently inaugurated state-of-the-art equipment and advanced technology for developing analytical methods and conducting quality control assessments.
Our flexible facilities, certified by EMA and inspected by the FDA, are continually expanding to accommodate the evolving landscape of cutting-edge biopharmaceutical manufacturing requirements.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
AGC Biologics
Czernyring 22
69115 Heidelberg
Germany
Ben Clauberg +49-1515-7528832
bclauberg@agcbio.com
www.agcbio.com
F I
2,500+
2018
Technology Transfer, Process Development, Analytical Development, cGMP Manufacturing, Quality Control and Quality Assurance, and Process Validation
Areas of Activity ›
Mammalian and Microbial-based Therapeutic Proteins, Recombinant DNA, Plasmid DNA (pDNA), Messenger RNA (mRNA), Viral Vectors, and Genetically Engineered Cells.
Request for ›
Further Collaborations
We are currently seeking additional collaborations and have capacity available at all of our sites in Europe.
AGC Biologics is a global CDMO (Contract Development and Manufacturing Organisation) providing pharmaceutical development and manufacturing services for protein-based biologics and cell and gene therapies. With seven facilities and teams of scientists across three continents, we have the resources and available capacity you need to bring your next project to life. From development to clinical trials to full-scale commercialisation, we can help you reach your goals at any stage in your drug product’s journey.
We specialise in the following modalities and substances: mammalian and microbial-based therapeutic proteins, recombinant DNA, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cells.
Our sites offer services you need at any stage of product development and manufacturing, including Technology Transfer, Process Development, Analytical Development, cGMP Manufacturing, Formulation Development, Quality Control and Quality Assurance, and Process Validation.
We have several sites near Germany that can service small, medium, and large biotech companies, global pharmaceutical organisations, and emerging advanced therapies organisations working on cell and gene therapies.
Heidelberg, Germany
The Heidelberg facility serves as a hub for microbial systems and services for our global customers. The site has scientists with several decades of protein expression experience, which has enabled AGC Biologics to establish a centre for custom pDNA and mRNA service lines at this site. We have just completed an expansion in 2023, adding a new line to offer more pDNA and mRNA projects, at a faster pace.
The facility’s segregated line design allows for capacity and technological flexibility while ensuring compliance with the current global guidelines required for cGMP compliance.




The Copenhagen site is one of the most active biologics facilities in the AGC Biologics network.
Onsite, we operate multiple mammalian and microbial cGMP manufacturing lines at a variety of scales, and the facility also offers a great depth of technological flexibility. The core teams of scientists have been working together to develop and manufacture biologics for two decades. This expertise and experience mean that your product is managed by some of the best in the business.
This site is finalising an expansion to extend its capabilities and more than double its single-use bioreactor mammalian cell-culture capacity. We are in active discussions to fill this space now before it opens in mid-2024.
AGC Biologics Milan specialises in cell therapy and viral vector development and manufacturing. The site works with any cell type and lentiviral, retroviral, and adeno-associated viral vectors. This site expanded in 2023 to offer more more vector manufacturing capacity for clinical and commercial projects.
The facility was the first cell and gene therapy site approved in Europe for GMP manufacturing of clinical and commercial supplies. The core scientific team has 25 years of expertise in the field and unique knowledge you will not find anywhere else.
The Milan team has developed 3 commercial products including, before joining the AGC network, bringing its own product to market (Zalmoxis ®). This experience means they understand the processes, procedures, and painstaking work involved in developing and bringing these treatments to market.
With one site in Japan and three in the U.S., one of the unique things about AGC Biologics is we can produce products on three continents. If you need something done outside of Europe, we have the network and global supply chain to meet your needs.
The company also announced a new cell therapy, mRNA and mammalian facility in Yokohama Japan in 2024, which will be operational in 2026.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
External ›
Collaborations
AiCuris Anti-infective Cures AG
Friedrich-Ebert-Str. 475/ Building 302
42117 Wuppertal Germany
Dr Katharina Nothelfer (PR/IR), Evgenii Bondarenko (BD) +49-202-317-63-0
+49-202-317-63-11-77 info@aicuris.com
www.aicuris.com
I 67 2006
R&D antiviral therapeutics for immunocompromised patients
Merck & Co., Inc., Rahway, NJ, USA (MSD in Germany), Hybridize Therapeutics, Deutsches Zentrum für Infektionsforschung (DZIF)

AiCuris is a privately held, innovative, late-stage biopharmaceutical company dedicated to delivering novel antiviral treatment options to patients with weakened immune system, e.g., those afflicted by immune deficiencies or undergoing immune-suppressive treatments. Leveraging its anti-infective research and clinical development expertise to advance a highly differentiated product portfolio, AiCuris aims to bring game-changing, high growth potential antiviral product candidates to the market – and to those patients in need.
AiCuris’ global headquarters is located in Wuppertal, Germany, with its United States subsidiary, AiCuris U.S., Inc., located in Marlborough Massachusetts. Led by a highly experienced international management team with deep roots in science and commercialization, AiCuris is on its way to become a fully integrated biopharmaceutical company covering the entire value chain from research and development to commercialization.
Proven track record:
AiCuris’ first-in-class non-nucleoside cytomegalovirus (CMV) inhibitor, PREVYMIS ® (letermovir), prevents life-threatening CMV reactivation and is approved for prophylactic use in adult immunocompromised patients who have undergone allogeneic hematopoietic stem cell or kidney transplantation. The drug is commercialized by license partner MSD (tradename of Merck & Co., Inc., Rahway, N.J., USA), with increasing revenues.
AiCuris receives royalty payments from sales of PREVYMIS® and is cash-flow positive. In addition, the Company announced milestone payments of EUR 45 million over the past year, further supporting pipeline development.

Candidate with fast track to market:
Pritelivir, currently in Phase 3 clinical development, is AiCuris’ lead proprietary product candidate for oral treatment of acyclovir-resistant herpes simplex virus (HSV) infections in immunocompromised patients. It obtained FDA breakthrough therapy designation in 2020 and launch preparations have been initiated.
Innovative pipeline of anti-virals:
AIC468, an anti-sense oligonucleotide (ASO) to prevent BK virus (BKV) reactivation in kidney transplant patients, is expected to enter clinical Phase 1 this year. AIC468 may also have potential for the treatment of BKV-induced life-threatening hemorrhagic cystitis in hematopoietic stem cell transplant patients. AiCuris’ additional preclinical programs are designed to combat human adenovirus (ADV) infections, including systemic infections in pediatric transplant patients and highly contagious epidemic keratoconjunctivitis (EKC). Despite the high medical need, there are currently no approved treatment options available for any of these indications.
Partnering opportunities:
AiCuris is not limited to one proprietary platform or technology and always aims to identify novel approaches. In-licensing interests comprise novel antivirals and antifungals in clinical development, which could significantly improve the quality of life of patients with weakened immune defenses.
A strong 3rd generation HBV Capsid Assembly Modulator and a resistance-breaking anti-bacterial with novel target in lipid A biosynthesis are available for out-licensing.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Altasciences
575 Armand-Frappier Blvd. Laval, QC
H7V 4B3
Canada
Sophie Dubois
+1-450-973-6077
contact@altasciences.com
www.altasciences.com
I
+1,800
1992
Full-service CRO/CDMO offering pharmaceutical and biotechnology companies a proven, flexible approach to preclinical and clinical pharmacology studies, including bioanalysis, formulation, manufacturing, and analytical services.
Altasciences transforms the traditional outsourcing paradigm by simplifying and streamlining drug development solutions to offer pharmaceutical and biotechnology companies a proven, integrated approach to preclinical and clinical pharmacology studies. Altasciences’ full-service solutions include preclinical safety testing, clinical pharmacology and proof of concept, bioanalysis, programme management, medical writing, biostatistics, clinical monitoring, and data management, all customisable to specific sponsor requirements. With nine locations, Altasciences helps sponsors get better drugs to the people who need them, faster.
Areas of Activity ›
CRO, CDMO, preclinical safety testing, clinical pharmacology and proof of concept, bioanalysis, formulation, drug manufacturing, programme management, medical writing, biostatistics, data management, small molecules, biologics, CNS, ophthalmology, gene therapy.
Altasciences offers an all-in-one early-phase drug development solution as a combined CRO/CDMO, valuing the heritage and culture of European and North American companies. Our firm grasp of the nuances inherent in drug development across Europe and North America enables us to provide streamlined decisionmaking tools, empowering optimal utilisation of time and resources.
Our mission is to offer clients a simple outsourcing solution, whether for one study, entire programme, or something in between, with a focus on customer service that removes the need for numerous service providers during the early stages of drug development.
Proactive Drug Development is Altasciences’ innovative approach that aligns our decades of expertise around delivering a streamlined, synchronised early phase drug development pathway, supported by proprietary designed platforms, processes, and a unique organisational structure. Our services and teams are fully integrated for optimal communication, collaboration, and scheduling to safely save sponsors up to 40% in early phase drug development time.
Whether for one study or a complete programme, we offer you a customised and synchronised approach to CRO and CDMO services for your IND/CTA submissions.





Preclinical Services:
› 4 facilities for a total of 585K sq. ft.
› 700+ safety studies conducted annually
› 500+ team members
› Full range of in vivo non-GLP and GLP safety evaluation studies, for large and small molecules
› Experience with both rodent and non-rodent species
› Multiple routes of administration
Clinical Services:
› 3 facilities in the United States and Canada
› 500+ beds
› 285+ clinical trials completed annually
› 400k+ participants in our database
› First-in-human to end of Phase II
› Specialty trials
› Healthy normal volunteers (HNVs), patients, and special populations
Bioanalytical Services:
› 3 laboratories across North America
› From discovery to preclinical to Phase IV
› 260+ highly trained regulatory bioanalysis specialists
› Capacity for 720,000+ study samples per year
› Assays covering 650+ molecules
› 25+ research and development scientists
› Small and large molecules
› Mass spectrometry, ligand binding, or hybrid assays
Manufacturing and Analytical Services:
› Small molecules
› Formulation development
› Phase I through commercial manufacturing
› ICH stability testing
Research Support Services:
› Centralised project and programme management
› Scientific, regulatory, and strategic guidance for your IND/CTA submissions
› Clinical protocol development
› Data management
› Biostatistics
› Medical writing
› Clinical reporting
› CDISC
› Archiving
› And more!

Name ›
BED BusinessPark
Ehingen (Donau) GmbH
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Talstraße 14-21
89584 Ehingen (Donau)
Germany
Prof. Dr Michael Gaßner
+49-7391-781-89-10
mail@businesspark-ehingen.de
www.businesspark-ehingen.de
I I
< 10 2014
Commercial property, office building
existing offices and laboratories, meeting and conference rooms, a modern InnovationLab
The BED BusinessPark Ehingen (Donau) offers the highest customer benefits, great flexibility, and a successful combination of modern office culture and a creative working environment.
Additionally, there are expandable areas available as building grounds for laboratories, clean rooms, and other technological facilities, tailored to specific needs. With 18,000 square meters of office and laboratory space, as well as conference rooms, a modern InnovationLab, and coworking areas, the BusinessPark Ehingen provides a wide range of options. From individual offices to entire building wings spanning several hundred square meters, the BusinessPark flexibly meets all space requirements, not only for start-ups but also for established companies seeking a representative location. The BusinessPark is easily accessible by transportation, offering ample parking, including electric charging stations, bicycle parking, and public transportation connections.
The more than 60 companies that have opted for the BusinessPark in recent years include established companies as well as start-ups, service providers, companies from the biopharma and health-care sector, technology companies, educational institutions, schools, and universities. The Ulm | Alb-Donau | Biberach Digitalisation Centre, public authorities and a notary’s office are also represented here. Several hundred people work in the BusinessPark.
The players in BioPharma Cluster South Germany include prominent companies, universities, and other research-related institutions in the immediate vicinity of the BED BusinessPark Ehingen (Donau). Boehringer



Ingelheim Pharma GmbH and Co KG, Rentschler BioPharma, Sartorius, Teva Biotech, Vetter Pharma International GmbH, the University of Ulm, and Biberach University of Applied Sciences stand out here.
All companies benefit from an excellent infrastructure: BED service contacts are on hand with help and advice. Post office and parcel station, janitor service, secretarial services and other services can be booked. A fitness center and a physiotherapy practice perfectly complement the opportunity to combine work, leisure, and health.
All tenants have access to a sophisticated IT system and a cloud telephone system that enables modern, location-independent working with virtualisation. Thanks to a fiber optic connection to every floor, 1,000 Mbit/s symmetrically is available everywhere. Reliable firewalls and managed subnets ensure a high level of data connection security. Space is available for special applications in an in-house data center, where the power supply is backed up by a UPS system.
The large number of companies on site and the cooperation with the Ulm | Alb-Donau | Biberach Digitalisation Centre creates synergies. Joint events also help people to get to know each other and network. The Ehingen Business Forum, which takes place twice a year, also serves this purpose - a platform for news, innovation, knowledge transfer and networking.
In addition to the city of Ehingen and Donau-Iller-Bank, partners of the BusinessPark Ehingen include TFU Ulm, InnoSüd, bwi Baden-Württemberg International, bwcon Baden-Württemberg Connected and, in particular, BIOPHARMA CLUSTER South Germany.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Biaffin GmbH & Co KG
AVZ 2, Heinrich-Plett-Str. 40
34132 Kassel
Germany
Dr Bastian Zimmermann
+49-561-8044668
+49-561-8044665
info@biaffin.com
www.biaffin.com
www.proteinkinase.biz
F I Q
8 2001
Analytical, ISO 9001:2015 certified
Service provider for biomolecular interaction analysis using SPR Biacore technology; supplier of recombinant proteins and reagents for kinase research and signal transduction
External › Collaborations
Request for ›
Further Collaborations
Collaborations with academic research groups and industrial partners
Biaffin is looking for strategic partners interested in complementing their analytical portfolio with SPR Biacore technology.
Biaffin GmbH & Co KG is an ISO 9001:2015 certified bioanalytical service provider specialised in the application of biosensors based on surface plasmon resonance (SPR) for biomolecular interaction analysis (BIA). BIA technology is ideally suited for highly accurate kinetic characterisation of any interacting molecules in real time in a label-free state. Biaffin has extensive knowledge and many years of experience in various application areas and supports biopharmaceutical drug development programme of pharma, biotech, and generic drug companies as well as scientific institutions worldwide as a competent partner. Our services are performed by a highly specialised team (PhD level scientists) in a laboratory well equipped with several state-of-the art SPR instruments (5x Biacore T200, 1x Biacore 8K+). Sufficient capacity of SPR instruments allows Biaffin to start interaction studies on short notice, quickly run multiple projects in parallel, and receive results in a timely manner. A qualified Biacore T200 instrument is available for SPR assay validation and GMP-like SPR analysis. The company’s expertise is documented by many peerreviewed publications in renowned scientific journals.
For the development of therapeutic antibodies, biopharmaceuticals, and biosimilars, Biaffin offers reliable SPR services for a highly accurate, quantitative kinetic characterisation, semi-quantitative kinetic ranking, or rapid qualitative screening of drug candidates. SPR assays have already been established for many target proteins and can be developed quickly by application of established protocols for reversible and site-directed coupling of biomolecules using specific capture surfaces. These assays can be applied for comparability and stability studies and batch-to-batch comparison during process optimisation. Available assay formats include dual-potency assays, pair-wise epitope mapping, and determination of active concentration based on binding activity (calibration-free methods). Assays can be validated and conducted under GMP-like conditions on a qualified Biacore instrument.

ADCC SPR assays are established for interaction analysis of antibodies with Fc gamma receptors (CD16, CD32, CD64) including receptor variants and different species, neonatal Fc receptor (FcRn), and complement C1q binding.
Biaffin’s services in small molecule drug development comprise customised SPR assay development for defined target proteins, fragment screening, hit validation, secondary screening, kinetic profiling, competition analy sis, and lead optimisation of compounds to improve specificity, selectivity, and potency. Early ADME studies (serum protein binding) and studies on mode of action, cofactor requirements, and thermodynamic binding analysis further support the drug development process.
Apart from analytical services, Biaffin offers a range of products serving customers’ needs in kinase research and exploring cellular signal transduction pathways. Biaffin’s product portfolio, available from the online shop www.Proteinkinase.biz, comprises recombinant proteins (kinases, cytokines, receptors, CD antigens), peptide substrates, specific inhibitors, and a biochemical ATP determination assay for customers’ in-house research.
Biaffin has successfully implemented and maintains a Quality Management System certified according to the ISO 9001:2015 international standard, enhancing our ability to supply high-quality products and provide reliable, innovative services according to the highest quality standards, which consistently meet the needs and expectations of our clients.
Name ›
BIOCOM Interrelations GmbH
– a BIOCOM AG company
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Jacobsenweg 61
13509 Berlin
Germany
Andreas Macht
+49-30-264921-0
+49-30-264921-11
service@biocom.de
www.biocom.de
30 1986 | Consulting | Publishing
| Events
| Video production
Information and communication for the biotechnology, content-driven and results-oriented – that has been BIOCOM’s self-formulated mission statement since it was founded 38 years ago. A lot has developed scientifically and technically in these four decades, but the core task is more topical than ever. In times of looming climate catastrophe and a rapidly ageing population in many countries, biotechnological solutions are the means of choice. Yet reffectively informing all stakeholders in the economic, political, and social spheres is now more crucial than before. At BIOCOM, a dedicated team of young as well as more experienced staff is ready to tackle this informational challenge through various different channels.
Our journals |transkript (German) and European Biotechnology Magazine (English), which are published both in print and online, are the two leading B2B publications in Europe in our programme. Plus various books, such as this European Biotechnology Guide. BIOCOM’s media are useful for the reader and attractive for companies. From advertisements to advertorials and online campaigns to corporate publishing and films, we offer a wide range of communication options are conceivable.
Comprehensive information platforms on behalf of the public sector are a very successful business area for BIOCOM. An excellent example is biooekonomie.de: This is the leading information portal on the bioeconomy in Germany and worldwide. Here everyone can find news, portraits, interviews and reports, videos and podcasts, innovative multimedia stories as well as databases on funding programmes, researchers and research institutions. In addition, exhibitions, information stands and on-site events help to communicate the bioeconomy to a broad target group.
Member of
European Biotechnology Network
BIOCOM organises large and small events, such as the INDUSTRIA BIOTEC. At this conference, industrial biotechnology solutions are discussed and presented to a wider public.



BIOCOM produces market, financial and technologyspecific studies with the highest user value for its clients. It conducts qualitative and quantitative analyses, developed closely with customers and delivering focused, accurate data. The result is clear, informative dossiers with high-quality information designed to effectively support professionals in industry and politics.
Consistent and internationally comparable statistics are a valuable tool for political decision makers. BIOCOM has long-standing experience in sector-specific surveys of both industry and academia. Based on global definitions such as the OECD’s biotechnology statistics framework, we have so far performed surveys in several European countries. Survey results are kept in proprietary databases of the European life science sector and are aggregated and processed for publication.
BIOCOM compiles targeted communication for projects such as those under the EU Framework Programme for Research and Innovation (e.g. BIO2REG, RIBES, COMBINE). Our communication expertise ranges from strategy development, digital and print communication, public relations, website development, film and animation, and social media. Besides network management and secretariat organisation (e.g. for the International Advisory Council on Global Bioeconomy), we create memorable experiences in the form of inspiring conferences, exhibitions, innovative workshops and dialogue formats. Beyond this, we draw on years of experience in policy analysis and evidence-based policy advice and capacity building.
BIOCOM offers the full range of services for the creation of audiovisual content: from consulting and conception, filming and post-production to strategies for the distribution. The portfolio includes image and corporate films, recruiting videos, product and explainer videos, documentaries and portraits as well as animations in 2D and 3D.

Name ›
Address/P.O. Box ›
BioCopy AG
Switzerland Innovation Park Basel Area AG
Novartis-Campus, Gebäude (Building) WSJ-210
Lichtstrasse 35
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
4056 Basel
Switzerland
Dr Matthias Wiedenfels, CEO +49-7641-9377870 info@biocopy.com biocopy.com
I 35 2019
l Antibody Discovery and Development
l AI-driven closed-loop platform
l Automated Lab
The BioCopy Group is headquartered in Switzerland and includes an R&D hub in the south of Germany. BioCopy is breaking new ground in drug discovery and development with precision screening and engineering. Core of the BioCopy technology is a unique AI-driven automated platform for the development of next generation cancer drugs.
The BioCopy platform is designed to streamline and optimise the antibody drug discovery and development process. It combines AI-driven workflows with real biological data and advanced digital design in a closedloop feedback system.
BioCopy can provide a constant flow of molecules through the automated laboratory. The BioCopy molecules are optimised for multiple parameters that are crucial for the pre-clinical and clinical development stages. They carry the highest probability of success.
The BioCopy workflow starts with a signature target molecule of a specific cancer variant. In parallel, BioCopy’s AI-driven process can seamlessly start identifying target-specific antibodies. These antibody candidates enter the automated lab. Screening and optimisation happens simultaneously and in ultra highthroughput, such that the molecular designs can be improved rapidly and efficiently whilst in process.
The automated laboratory is embedded into a specialised AI environment to ensure streamlined and standardised data analysis and digital molecular design.




Unlike standard iterative R&D, the BioCopy workflow is automated and parallelised. This significantly increases throughput and efficiency. It reduces the long and arduous journey from concept to candidate to a short sprint.
BioCopy keeps the entire value chain in focus. By testing for developability parameters and biological activity at the same time, we are able to carefully balance all the relevant parameters and ensure an optimal outcome for a complex molecule, ready for entering the next definitive stage.
BioCopy paves the way for a lean, efficient and highly effective drug discovery and development programme from initial molecular concept to final drug candidates with a high probability of success.
We design next-generation cancer drugs outrunning the pharma produc tivity crisis.
At BioCopy, we herald a revolution in drug discovery and development redefining time-to-market benchmarks.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Bioengineering AG
Sagenrainstrasse 7
8636 Wald Switzerland
Marc Bachmann +41-55-256-81-11
info@bioengineering.ch
www.bioengineering.ch
140
1972
l Engineering & design
l Service
l Plants
l Bioprocess control
l Lab & pilot
l Components
The visionary idea to provide the biotechnological industry and research community with equipment for excellence led to the foundation of Bioengineering in 1972. This idea has been put into practice by designing and constructing fermentation systems, including all components for large and small scales. By expansion of our product portfolio, based on the latest advancements in biotechnology, we are carrying this founding concepts into the 21st century.
Our company has been independent and autonomous since it was established. This approach has little in common with current corporate mind sets and the vast array of management models, which allegedly have answers for everything. It gives us the liberty for result-orientated commitment to the process specifications. Our research and design teams know how to set customers’ ideas on the road to implementation and turn them into practical reality, based on optimised organisational structures and – not least – sound financial planning.
We build plants for our customers that meet their specific requirements. We do this at Bioengineering by having a team of dedicated engineers and designers working on every stage of a project. From the idea to the finished plant, there is a strong culture of innovation. Our many years of experience in project management enable us to implement fast-track projects efficiently.
The support we offer goes beyond the planning, construction, and commissioning of plants. We provide expert knowledge, servicing, inspection, and documentation to assist with the operation of your plants. We perform two roles in one: that of an expert and a reliable partner.
We design and build complete bioreactor and fermenter systems for well-known pharmaceutical and food companies. These systems are used in laboratories



and production facilities to manufacture vaccines, biosimilars, starter cultures, and other products. It is no accident that our plants serve as a reference for an entire industry. With more than 45 years of experience in building plants, we have the experience, the knowledge, and the innovative capacity to realise even the most complex of plants.
We develop complex automation solutions with software and hardware for the plants and are thus in a position to offer a wide range of architectures and designs. This helps our customers to manage and monitor their plants perfectly and reliably.
We develop and build fermenters and bioreactors for laboratories and production facilities across the world. These range from small expandable modular bench-top devices for research and development departments to scalable big plants for the manufacturing industry.
We develop and build components for our customers’ plants which perform very specific tasks depending on the application and the requirements. For example, components which ensure that cleaning is carried out in compliance with strict hygiene regulations and which have been reliably carried out in our plants for many years.
Every customer enjoys the advantages of our modular design approach: cost-effective transport, rapid installation, and streamlined testing and quality control. Our quality-assurance manual is the control system that ensures unified monitoring and documentation of the entire production sequence, with precise specifications, instructions, and prescribed procedures.
But the commitment to our customer does not end, once the system is installed and qualified. For us quality assurance also means providing our customers with aftersales services and replacement parts for many years to come. We have honoured that commitment for every facility built by Bioengineering since 1972.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
BIOINGENIUM SL
Baldiri Reixac 10 08028 Barcelona
Spain
David Resina +34-93-402-02-40 info@bioingenium.net www.bioingenium.net
I 20 2008
| Alternative proteins
| Biologics
| Biomanufacturing
| Biomedicine
| Bioprocesses
| Biotechnology
| CDMO (preclinical)
| Full upstream and downstream bioprocess
| Industrial enzymes
| Natural Ferments
| Recombinant proteins
Bioingenium is a preclinical Contract Development and Manufacturing Organization (CDMO) specialised in protein expression. Bioingenium has more than 15 years of experience in designing and developing tailor-made bioprocesses, recombinant therapeutic proteins and natural ferments based on microbial systems and animal cells. We bridge the gap between R&D and GMP production.
› Biologics for biopharmaceuticals
› Diagnostics
› Active ingredients for cosmetics
› Alternative proteins and industrial enzymes for food sector
Biopharmaceuticals / Biotech / IVD companies
› Recombinant protein production, cell line and strain development.
• Full process development at the preclinical phase: upstream processes from the scratch and its optimisation.
• Design of customized purification processes.
› Bioprocess scale-up and production. We have protein production plant with capacity up to 200 liters fermentation.
› Preclinical batch production.
› Quality control analysis.
› Sterile Reagents production:
• Proteins (cellular differentiation factors) for Cell Therapy,
• DNA modifying Enzymes (restriction enzymes, polymerases) for Gene Therapy,
• mRNA Manufacturing enzymes (Restriction enzymes, polymerases) for mRNA Vaccines.
› Production of proteins and antigens for IVD companies.
Cosmetics
› Development and production of active ingredients based on fermention.
Food industry
› Development of alternative proteins, precision fermentation.
› Industrial enzymes: proteases, lipases, amylases, transglutaminases and catalases.




Our technology platforms/expression systems
› Yeast: Pichia pastoris.
› Bacterial: E.coli.
› Mammalian cell: CHO & HEK.
Our achievements
We have experience with more than 150 different proteins, enzymes, antibodies, antibody fragments, fusion proteins, and vaccines. We have successfully produced strains and processes for recombinant protein vaccines: single-protein, multi-protein, and VLPs vaccines. We have also developed and produced new innovative active ingredients for the leading cosmetic companies for anti-aging, regenerative, and whitening purposes. We create test samples, develop manufacturing processes, and supply the actives.
Our value proposition
Quality
We are reliable partner and want to deliver the highest quality. Our dedication to excellence is reflected in our recombinant protein expression expertise and uncompromising quality. We provide our services under the ISO 9001:2015.
Innovative customized solutions
We tailor-made each project to fit our client´s unique needs and goals. We produce proteins from scratch in our state-of-the-art facilities in the Barcelona Science Park.
Talented team
We have experienced and talented team, driven by passion and dedication.
Strong know-how
We have profound knowledge, more than 15 years of experience in the field of recombinant proteins.
Value-added partner
Our priority is to be a strategic and value-added partner, understanding our client´s needs.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Biomay AG
Ada-Lovelace-Strasse 2
1220 Vienna
Austria
Dr. Angela Neubauer
+43-1-7966296-100 info@biomay.com
www.biomay.com
I
110+
1984 CDMO
GMP manufacturing / aseptic filling of plasmid DNA, mRNA, recombinant proteins; supply for clinical phases I-III, market
All from a single source: the CDMO based in Vienna has been committed to this principle for 15 years as a GMP manufacturer of biomolecules. Today, Biomay operates two state-of-the art, fully integrated biomanufacturing facilities for client biomanufacturing projects, one of them FDA-inspected. Specialising in manufacturing and aseptic filling of mRNA, plasmid DNA and recombinant proteins from microbial fermentation, the company has a track record of well over 200 successfully released GMP products.
Biomay offers fully scalable processes for GMP manufacturing in five parallel production lines in bioreactor scales 30L, 50L, 150L and 750L. Routinely, manufacturing up to several hundred grams per batch of starting material, drug substance or drug product is achieved in a solvent-, antibiotics- and animal component free process. With this setup, Biomay can provide the material quantities and quality required for use as advanced therapeutics, like gene editing / gene therapy products, mRNA-, plasmid DNA- and protein vaccines.
For precision medicine applications, an integrated plant for the manufacturing of personalised (patient-specific) tumour vaccines is available: plasmid DNA vaccines are produced in a 3L bioreactor scale in multiple parallel clean room suites. This process is optimised in terms of turnaround times and offers unprecedented speed while maintaining highest quality.
Principal feasibility studies and process development using representative down-scale models guides the setup of the GMP-process and ensures a smooth transition from the beginning of a project to GMP-batch release. Analytical development and phase-appropriate qualification and validation of in-process control analytics and release methods is offered as well as stability testing.




Due to a globally increased demand, Biomay recently expanded its GMP biomanufacturing facilities significantly to strengthen the company’s focus on mRNA activities. After adaptation, additional floor space of 2000 sqm houses five GMP-commissioned clean rooms for upstream- and downstream-processing and aseptic filling.
For mRNA processing, Biomay applies state-of-the-art enzymatic run-off transcription based on linear DNA templates that are manufactured in-house. Several options for 5’-capping (co-transcriptional, enzymatic), 3’-poly-adenylation and nucleotide modification are offered. The mRNA process is fully GMP qualified, including all starting and raw materials, is free of animal components and employs mainly single-use equipment. Furthermore, a comprehensive method set for mRNA analytics is available in-house.
Biomay’s claim to accompany their client’s complete project life cycle from clinical phases I-III to market readiness and supply, has been strengthened by the successful approval of the first CRISPR/Cas9 based gene editing therapy, CASGEVY, for which Biomay manufactures the nuclease Cas9.
During the Covid-19 pandemic, the company has contributed a substantial amount of linearised plasmid template for Pfizer-BioNTech’s mRNA Vaccine Tozinameran/Comirnaty.
With sustained growth and expansion options on the company grounds, Biomay is able to fulfill any present or future demand for tailor-made manufacturing solutions.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Moritzburger Weg 67
01109 Dresden
Germany
Dr Norman Gerstner
+49-351-8838-400
+49-351-8838-403
info@biotype.de
www.biotype.de
www.modaplex.biotype.de
www.custom-solutions.biotype.de
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
80
I Q
1999
S1, BSL-2
ISO 13485 & ISO 9001 certified
Areas of Activity ›
In-vitro diagnostics
Molecular diagnostics
Contract development
Contract manufacturing
External ›
Collaborations
Strong network with numerous industry and academic partners
Member of the Molecular Diagnostics Group (MDG); contributing solutions for healthcare, personalised medicine & data management
BIOTYPE is an innovative solution provider for molecular precision diagnostics, headquartered in Dresden, Germany. We are committed to advancing precision medicine through our products and services for robust analysis and interpretation of biomarker signatures.
BIOTYPE offers a strong portfolio in molecular cancer diagnostics for applications in hemato-oncology, liquid biopsy, and molecular profiling of solid tumors. The portfolio includes in vitro diagnostic (IVD) assays for clinical diagnostics and research use only (RUO) assays for translational and clinical research.
In addition, BIOTYPE offers high-quality and IVD compliant contract development and manufacturing services. Our proprietary MODAPLEX platform is a benchtop system for automated molecular profiling and modular multi-gene testing.
Founded in 1999 and privately owned since then, BIOTYPE’s core business for many years has been PCR multiplex applications for forensic and professional laboratories in various industries. Since then, BIOTYPE has gained extensive expertise in providing high-quality molecular diagnostic products and services to professional laboratories, diagnostic, pharmaceutical and biotech companies, and research institutions worldwide. Today, BIOTYPE is a molecular diagnostics company providing the highest quality molecular testing systems, including assays, proprietary biochemistry, instrumentation and software.
BIOTYPE‘s quality management system is certified according to ISO 9001:2015 & ISO 13485:2016.

BIOTYPE provides a robust portfolio of molecular cancer diagnostics to support hemato-oncology, liquid biopsy, and molecular profiling of solid tumors. The portfolio comprises IVD assays for clinical diagnostics, RUO assays for translational and clinical research, and a benchtop system for automated molecular profiling. Professional laboratories use the molecular test systems to diagnose cancer patients accurately, determine the best treatment option, and timely identify actionable biomarkers for molecular-guided therapy.
BIOTYPE offers a unique system for molecular profiling. The MODAPLEX platform merges Polymerase Chain Reaction (PCR) and Capillary Electrophoresis (CE) into one automated workflow. Thus detection, differentiation, and quantification of all clinically relevant molecular markers and all known genetic alterations can be consolidated onto one platform.
Focus on your core business and get things done. Our contract development & manufacturing services cover all challenges from the idea up to the realisation of a commercial product. This allows companies to outsource defined tasks or projects, thus improving efficiency, success rate, and scalability. We are committed to high-quality standards and accommodate individualised product specifications for our international customers.
› Contract development
› Contract manufacturing
› Custom packaging
› Multiplex assay development
› Regulatory consultancy

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Biovet AD
Peshtera/ Petar Rakov Street 39 4550 Peschtera
Bulgaria
Spas Petkov/Alfred Stiefel
+35-988-722-44-26/ +49-173-4162105
spas.petkov@huvepharma.com alfred.stiefel@huvepharma.com
www.huvepharma.com
1500
1961
S1; S2
Contract development and manufacturing organisation of fermentation driven products and biologics;
Large scale manufacturing of enzymes; flavors; fragrances, food ingredients and pharmaceuticals; vaccines; others.
Annual turnover ›
Request for ›
Further Collaborations
€270m
Biovet offers services to companies with need of fermentation driven products
Biovet AD is the manufacturing arm of Huvepharma. Huvepharma is a leading and fast growing top 10 animal health company, focused on veterinary products, feed additives, and vaccines for the live stock segment. Biovet is amongst the largest fermentation companies in Europe and the world, operating in excess of 10,000,000 Liters of fermentation capacity. In addition to manufacturing fermentation derived animal health products for Huvepharma, Biovet manufactures and is licensed to manufacture by fermentation food ingredients and enzymes, flavours and fragrances, nutritional and vitamin products. Biovet holds GMP for animal and human health products from both the EU and USFDA. The food-related products and ingredients are manufactured according to the highest-quality standard for food products (FSSC 22000 quality system) and could also be Kosher and Halal certified. This allows the company to service the global food markets. Biovet has a proven track record in managing investments in large production and energy-related projects.
We offer our clients process development and scaleup services for biotechnology products. We provide contract manufacturing service for industrial-scale production of fermentation-driven products including different down-stream/purification possibilities such as filtration, ion-exchange, extraction, drying etc. We offer process development and pilot scale production from 20 litres up to 10.000 litres. Biovet can offer industrial fermentation scales from 15 m³ up to 160 m³ and related DSP capacities.




Our fully integrated services are underscored by our commitment to quality and the operational excellence that flows through our best-in-class process and cGMP manufacturing capabilities, meeting FDA, EMA, ISO and FSSC standards.
Biovet has been certified according to cGMP by FDA and EU. Biovet has implemented an integral management system for quality management and environmental protection.
The company is focused and committed to continuously reducing the carbon footprint by various initiatives. Currently 25% of energy utilised comes from own renewable sources. Biovet is committed to reach carbon neutrality by 2030.
Biovet can offer contract manufacturing, partnerships and other types of cooperation to ensure successful development and industrial-scale production.
Name ›
Boehringer Ingelheim Biopharmaceuticals GmbH
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Binger Strasse 173
55216 Ingelheim
Germany
Matthias Reinig
+49-6132-77-0
+49-6132-72-0
bioxcellence@boehringer-ingelheim.com
www.bioxcellence.com
around 53,000 in 2022
1885 in Ingelheim, Germany S1
Focus on human pharmaceuticals, animal health and biopharmaceutical contract manufacturing
Boehringer Ingelheim is working on breakthrough therapies that transform lives, today and for generations to come. As a leading research-driven biopharmaceutical company, the company creates value through innovation in areas of high unmet medical need. Founded in 1885 and family-owned ever since, Boehringer Ingelheim takes a long-term, sustainable perspective perspective. More than 53,000 employees serve over 130 markets.
Boehringer Ingelheim has pioneered biotechnology since the 1980s and is today one of the leading companies for the research and development of highly complex, innovative biopharmaceutical molecules and their production in a large scale for global markets. In 2022, Boehringer Ingelheim achieved net sales of about 24.1 billion euros.
Net Sales ›
Relevant R&D Budget ›
Request for › Further Collaborations
24.1 billion in 2022
5.0 billion in 2022
Boehringer Ingelheim BioXcellence™ is a dedicated partner for biopharma companies, providing comprehensive development and GMP manufacturing services. Renowned for its reliability, the company supports clients throughout the lifecycle of their products, thus transforming biologic innovations into commercial realities.
Boehringer Ingelheim BioXcellence™ is a leading biopharmaceutical contract manufacturer – a reliable partner accompanying our customers along the life cycle of their products.
With over 40 years of biotechnology experience and dedicated and highly trained employees in our global network we have helped our customers to bring more than 40 biopharmaceutical medicines to the market serving patients all over the world. This achievement is accompanied by an exceptional regulatory track record.
Wherever you are, we are by your side. Our network of manufacturing facilities spans the globe, from Fremont, California in the United States to Biberach in Germany, Vienna in Austria and Shanghai in China. Based on our experience with biopharmaceuticals from external partners and Boehringer Ingelheim, we deliver technological expertise, process excellence, and superior quality from this global production network.
Global capacity for cell culture: ~430 commercial
Global capacity for microbial and yeast fermentation: > 12 kL commercial




We are your trusted partner for patient supplies.
For many of our customers we are the preferred partner for the production of biopharmaceutical medicines, ranging from mammalian cell cultures to microbial and yeast fermentations. We have comprehensive experience with a variety of different host cells, producing an equally broad variety of molecules according to cGMP manufacturing guidelines and covering all stages of development including Aseptic Filling. Our focus is on producing value for our customers with robust processes, optimal preparation for launch, and global commercial supplies.
Aside from our extensive experience and technical expertise, there are several other good reasons to choose us as your trusted contract manufacturing partner: At Boehringer Ingelheim, we have learned over the generations that the challenging path towards health innovation requires long-term commitment, resilience, and transformative action.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person ›
Telephone ›
Email › Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Distretto Campania Bioscience
Via L. De Crecchio, 7 80138 Naples Italy
Amleto D’Agostino, Roberta Lauro +39-081-5667677
direttore@campaniabioscience.it r.lauro@campaniabioscience.it
www.campaniabioscience.it
I 12 2013
| Industrial Biotechnologies
| Innovative Technologies for the Biomedical Industry
| Oncology
| New Therapies
| Genetics and Genomics
| Cosmeceuticals, Nutraceuticals, and Functional Foods
External › Collaborations
| ALISEI –National cluster on life sciences
| CEBR –Council of European bioregions
| EBN –European Biotechnology Network
Request for ›
Further Collaborations
| Boosting collaboration with other EU clusters
| EU projects collaboration
| Exchanging best practices
(as of 2021)
European Biotechnology Network Member of
Campania Bioscience operates as a regional institutional cluster, gathering together competences and facilities in the fields of health, biotech and agri-food with the commitment to valorising life sciences regional ecosystem. Our mission is to generate a positive impact within our national community by sharing innovative products and services and strenghtening regional investment attractiveness and competitiveness, also by contributing to the training of highly qualified professionals in the field of life sciences.
The cluster is composed of 7 research entities, 47 companies and 1 technology transfer agency.
Campania Bioscience possesses extensive expertise in making significant contributions to European projects aimed at fostering innovation and research in the life sciences industry. In pursuit of this goal, Campania Bioscience has forged meaningful and enduring partnerships with some of the most experienced and skilled clusters across Europe.
We firmly believe that our initiatives play a crucial role in terms of social impact, and for this reason we foster a seamless debate with local and national institutions and stakeholders on how to tackle contemporary challenges with our know-how, innovation and passion.
› Cosmeceuticals and nutraceuticals
› Diagnostics, biosensors, biomedical
› Design and testing of new therapies
Campania Bioscience is responsible for the management and implementation of R&D projects, with an overall value of €70 million; a few examples are given below:
› Innovative therapeutic approaches to drug-resistant cancer
› Preclinical development of new technologies and innovative strategies to produce molecules with pharmacological action
› Preclinical and clinical evaluation of molecules with pharmaceutical action and new therapeutical indication for molecules already approved


Main Competences
› Pharmacogenomics
› Bioinformatics
› Industrial biotechnologies
› Gene expression and proteomics
› Nutraceuticals, cosmeceuticals and functional foods
› Bioremediation and biodegradation
› Molecular dignostics
› Gene Therapy

Thematic Networks
Council of European Bioregion (CEBR)
CEBR is a network of life science clusters across Europe, with approximately 40 subscription members and hundreds of cluster partners across the world. The goal is to improve support for cluster planning and management through sharing good practice and joint initiatives between regions.
ALISEI - National Technological Cluster
ALISEI gathers key national players in the field of life sciences, including regional clusters, indistrial associations and national research centres. ALISEI aims to introduce and launch, in Italy, an innovative strategic approach to the creation of new products, technologies and services, as well as new business opportunities.
Diagnostics, biosensors and innovativ e technologies for the biomedical industry
› Development of innovative diagnostic tools for risk detection and early diagnosis of metabolic diseases
› New strategies for medical and molecular diagnostics
› High efficiency diagnostic methods for osteoarticular patients
› Smart materials and new devices in the biomedical sector
Development and production of nutraceuticals and cosmeceuticals
› Recovery strategies of bioactive compounds from waste biomass from the agri-food sector
› Design, development and production of functional food
› Experimentation and development of molecules with nutraceutical and cosmeceutical action
› New strategies for food tracking and monitoring
We support our members to foster the green and digital transition and competitiveness in Europe trough the participation in several related projects. EPICENTRE is an European project funded by the European Commission under the call Joint Cluster Initiatives - Euroclusters in which 3 European clusters - Clúster Digital de Catalunya (Spain - ICT Sector) - Distretto Campania Bioscience (Italy – Health Sector) and the Lithuanian Association of Food Exporters (Lithuania - Food Sector) aim to play their role in the innovation ecosystem.
The main objective of the project is the realisation of new cross-sectoral value chains through the creation, in an Open Innovation perspective, of an SME – CLUSTERCORPORATE ecosystem in the following sectors: ICT, Fintech, Health and Food. It is a programme of mentoring, acceleration, validation and exploration of a potential transfer to the market of innovative solutions starting from the needs of large companies.

Name ›
CG Pharma & Biotech Business Unit of CG Chemikalien GmbH & Co. KG
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Ulmer Straße 1
30880 Laatzen Germany
Dr. Frank Velte, Head of CG Pharma & Biotech +49-511-87803-0 info@cg-chemikalien.de www.cg-pharma-biotech.com
I
65 dedicated CG Pharma & Biotech 1962
| Manufacturing of customised, sterilefiltered (bio)pharmaceutical process solutions and excipients for upstream and downstream applications
| Standard catalogue products for several pharmaceutical applications
| Scalable from small scale to commercial production
| Ready-to-use sterile filtered solutions in single-use bags
| GMP cleaning solutions for CIP and SIP applications
| Processing of hazardous substances in the cleanrooms (corrosive and flammable)
| In-house production of WfI and AP
As a developer, manufacturer and supplier of critical raw materials, we understand the challenges of our partners and compliance with regulatory requirements. We accompany our partners on the path from the development stage to commercial production. Our services & products are tailored to the needs of our clients and can be selected and combined on a modular basis:
C-Made: Manufacturing of customised formulations for use in upstream, downstream and formulation. PharmProve ®: Multi-compendial production chemicals for use as an ingredient, excipient or API.
Our customers’ most important applications and products include biotechnologically produced active ingredients and drugs, e.g. vaccines, ATMPs, cell culture media and biosimilars, as well as biological drugs - e.g. plasma-derived medical products, enzymes and heparins.
› Development and production of customised formulations
› In-house R&D department, from laboratory scale to commercial production
› Agile project management, consulting and planning
› In-house chemical/physical analytical laboratory
› Testing of starting materials according to pharmacopoeia (e.g. EP, BP, USP, JP, ChP)
› Finished product testing (e.g. appearance, pH,conductivity, density, refractive index, osmolality, assay)
› Microbiological testing (e.g. sterility, endotoxins, RNAse, bioburden)
› Stability testing



› Manufacturing authorisation according to German Drug Law AMG §13
› Quality assurance agreements with customers and suppliers
› Batch release by authorised person
› Risk Base Management | Change control CAPA / OOS System
› Robust Risk management system acc. to ICH Q9
› Product Quality Review: Batch record review, Supplier qualification system, Audit and training system
› Qualification and validation acc. to Annex 15 EU GMP: Process-, cleaning and method validation, Room- and equipment qualification, Software validation
› 800 m2 cleanroom area, divided into areas liquid and solid
› Clean corridor concept
› Production facility free of animal components (AOF)
› Personnel locks
› Weighing of raw materials in cleanroom class C&D
› Compounding and filling in cleanroom class C&D
› 3 filling rooms for liquid media cleanroom class C&D
› 2 filling rooms for solid media cleanroom class C&D
› Automatic transport conveyor system with integrated weighing technology
› SIP/CIP system for sanitisation and cleaning with validated cleaning processes
› In-house quality control including microbiology
› Washroom for cleaning production equipment
› In process use of nitrogen and ultra-pure steam
› Multi-purpose equipment with validated cleaning procedures

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
COHESION BUREAU
Chemin des Voirons 21
1224 Chêne-Bougeries
Switzerland
Chris Maggos +41-79-367-62-54
chris.maggos@cohesionbureau.com www.cohesionbureau.com
15
2022
Our experienced team offers insightful strategic consulting and services, specializing in investor relations, (social) media relations, and business development (licensing / M&A). We have a dedicated editorial unit producing corporate content, including, writing, graphic design, video and photography.
We aim to level the playing field for European companies
Our mission is to enable European companies to compete on a global scale and increase their engagement with the investment community and relevant media across Europe and the United States.
We are committed to helping our clients to overcome unique challenges and achieve their full potential through tailored strategies.
Our deep expertise in corporate strategy, investor relations, press relations, and business development enables us to provide tailored solutions that drive growth for European life science companies. We are passionate about helping our clients achieve their goals. Our panEuropean and trans-Atlantic presence gives us a unique perspective on the global business landscape.
We work with firms that are
› Venture stage - Series A to IPO
› Preparing IPO in Europe or U.S.
› Listed (Europe & U.S.)




Your business is not limited by national boundaries, cultural differences, or language – and nor are we.
Frank Schwarz
Frankfurt
frank.schwarz@cohesionbureau.com
Chris Maggos Genève
chris.maggos@cohesionbureau.com
Frank Hoerning Andersen København
frank.hoerning@cohesionbureau.com
Douwe Miedema Luxembourg
douwe.miedema@cohesionbureau.com
Giovanni Ca’ Zorzi Milano
giovanni.cazorzi@cohesionbureau.com
Sophie Baumont Paris
sophie.baumont@cohesionbureau.com
Tomer Assis
Tel Aviv
tomer.assis@cohesionbureau.com
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Coriolis Pharma Research GmbH
Fraunhofer Straße 18b
82152 Martinsried/Munich
Germany
Bettina von Klitzing-Stückle
Bettina.klitzing@coriolis-pharma.com
www.coriolis-pharma.com
I
200+
2008
Contract research and development
Formulation excellence for biopharmaceuticals, incl.:
| Liquid and lyophilised formulations
| Lyophilisation process development
| Analytical services for biologics
| Aggregate & particle analysis
| Particle identification
| Higher-order structure analysis
| Forced-degradation studies
| Long-term and in-use stability
| Pre-formulation screening
| Developability assessment
| Supply of TOX-material
| Level: R&D, GMP, S1 (BSL1), S2 (BSL2)
Coriolis – Your partner for drug product development services, manufacturing support and contract analytics
Coriolis Pharma is a global contract research and development organisation (CRDO) and one of the world leaders in formulation research and development of (bio)pharmaceutical drugs, including cell and gene therapy products and vaccines. The company’s offerings include liquid and lyophilised drug formulation development from early stage to market approval and beyond, lyophilisation process development, and analytical development under R&D and GMP.
Hundreds of clients – from start-ups to big pharma –already rely on Coriolis’s scientific expertise. For each and every project, our highly skilled scientists develop customised study designs that align with our customers’ drug development strategies.
Formulation development services for liquid and lyophilised products including biosafety level S1 and S2
› Pre-formulation and candidate selection
› Early-stage formulation development
› Late-phase formulation development
› Lyophilisation process development and optimisation
› Scale-up and transfer studies
› Developability / manufacturability assessment
› Support in container closure system selection
› In-use stability studies
› Stability testing including forced degradation studies
› R&D and cGMP-compliant contract analytical services
› Orthogonal analysis of subvisible aggregates and particles
› Analytical testing of S1 and S2 GMO material
› Polysorbate analytics
› Biosimilarity studies
› Supply of preclinical Tox-material



We combine technologies, knowledge, and experience to add significant financial value to projects by generating IP for the clients’ pharmaceutical compound and managing the patent lifecycle in later stages.
Coriolis can draw from a unique analytical portfolio of more than 100 different techniques – performed entirely in-house – to obtain orthogonal and meaningful data.
With the in-house lyophilisation development centre, our scientists can obtain time-saving and robust lyophilisation cycles at lab, pilot, and production scale. Dedicated GMP facilities enable us to conduct lot-release analysis and generate supportive data for market approval and allow for tailored “enhanced R&D” level studies. Coriolis – as a trendsetter – provides state-of-the-art as well as promising new and emerging technologies in the field of:
› Particle characterisation (from nanometre to visible size range)
› Particle identification
› Aggregate analytics
› Physico-chemical characterisation of lyophilisates
› Surfactant characterisation
› Higher order structure analysis
› Chemical changes
We provide cutting-edge services and tailor-made solutions for our clients, conducted by our interdisciplinary team of highly skilled scientists and an international expert scientific advisory board. This scientific advisory board contains some of the most renowned specialists in biopharmaceutical formulation development and particle characterisation, who are actively involved throughout each client project and contribute with independent advice to a successful outcome.
The scientific expertise of our project teams is driven by our internal research unit – composed of post-docs, PhD candidates, and students – who continuously publish exciting articles in high-impact journals.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
A Relva s/n Torneiros
36410 O Porriño
Spain
Mercedes Rodriguez +34-986-330400
mercedes.rodriguez@czvaccines.com
czvaccines@czvaccines.com
https://czvaccines.com
I
600+ (Zendal Group)
1993
| Biotechnology
| CDMO
| Development
| Manufacturing
| Vaccines
| Viral & microbial vaccines and cell therapy
| Aerobes and anaerobes, yeast
| Covid-19 vaccines
| Tuberculosis vaccines
| One-Stop Shop
| Biomedicine
| Aseptic filling
| Lyophilisation
| GMP upstream & downstream processes
| Biotech production sites in Spain and Portugal
Relevant R&D Budget ›
11% revenue
CZ VACCINES is a full service provider. We cover the entire value chain from contract development through clinical phases I to III to commercial production of vaccines and biological products.
CZ VACCINES has extensive scientific experience in developing new and ambitious projects that include different categories and vaccine platforms:
› Human vaccines and animal vaccines
› Against viral, bacterial, and parasite diseases
› Conventional vaccines
› Genetic engineering of live attenuated vaccines
› Subunit vaccines (recombinant protein)
› Latest generation DNA vaccines
› Live and inactivated bacterial and viral vaccines up to BSL-2
› Roller bottles, cell factory and wave bioreactor
› Single-use and stainless steel bioreactors
› Aseptic processing systems
› Centrifugation, and ultrafiltration and diafiltration
› Chromatography (ÄKTA ready)
› Aseptic filling into vials
› Lyophilisation (clinical trial material and large scale)
› Process development / optimisation
› Quality control testing: microbiological, sterile / nonsterile, chemical-physical, and biological
› Storage: (2º-8ºC), (-20ºC), (-30ºC), (-80ºC)




CZ VACCINES has large manufacturing capacity located in several GMP certified biotech production plants in Spain and Portugal
cGMP-production capabilities
Upstream:
› Single-use bioreactors: 2x50L + 3x250L + 3x2,000L
› Stainless steel bioreactors: 100L + 300L+ 2x2,500L
Downstream:
› Single-use mixers: 500L, 650L and 1500L
Media:
› Single-use and stainless-steel bioreactors: From 200L up to 3,000L
Purification:
› State-of-the-art purification processes: chromatography (ÄKTA ready), centrifugation, ultrafiltration, diafiltration, tangential flow filtration
cGMP fill and finish capabilities
› Liquid and lyophilised products up to BSL-2
› 4 filling machines: 6,000 vials/hour - 2x12,000 vials/ hour – 24,000 vials/hour
› 2 freeze dryers: up to 24,000 vials per run, and up to 180,000 vials per run with automatic loading and unloading system
› Component size: 2R-10R-20R
› Manual and automatic visual inspection
› Labeling and packaging

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Enzymaster Deutschland GmbH
Neusser Straße 39
40219 Düsseldorf
Germany
Dr Thomas Daussmann +49-211-158-216-10
+49-211-158-216-12
info-europe@enzymaster.de
https://enzymaster.de/
I Q
260
2013
| Biocatalysis Research
| Enzyme Panel Screening
| Enzyme Engineering: Computer-Aided Directed Evolution of Enzymes
| General and Customised Enzyme Screening Kits
| Immobilisation of Enzymes
| Reaction Process and DSP Development
| Fermentative Enzyme Preparation and Formulation
| Biocatalytic Manufacturing of Fine Chemicals, Intermediates, APIs, and other Compounds (Lab to Industrial Scale)
Enzymaster provides a one-stop solution for the development and commercialisation of innovative and sustainable enzyme catalysis technologies. With our proprietary BioEngine ® and BioNavigator ® platforms as well as our long-term experience, we offer R&D services combined with the establishment of complete technology transfer packages and manufacturing collaborations to fine chemical, pharmaceutical, and other industries. Our portfolio includes enzyme identification, enzyme panel screening, smart enzyme engineering, process development, enzyme preparation by fermentation, and biocatalytic manufacturing. In addition to these services, we also offer general enzyme kits that represent various enzyme classes as well as customized enzyme kits that fit to the individual biotransformation needs of our customers. Enzymaster Deutschland GmbH, a subsidiary of Enzymaster (Ningbo) Bio-Engineering Co. Ltd., is your reliable partner in the international market for enzyme applications and products manufactured by biocatalytic processes.
Biological Patents ›
Comprehensive international patent portfolio mainly in the field of novel engineered enzymes for biocatalysis
External ›
Collaborations
| JingJing Pharmaceutical
| Austin Chemicals
| Lake Chemicals and Minerals
| ThermoFisher Scientific
| Research Centre Jülich
Enzymaster strives to contribute to a greener environment and improve manufacturing processes by helping organisations leverage the benefits of biocatalysts, decreasing dependence on toxic and often inefficient, purely chemical syntheses. Flexible fee-for-service models moving in lockstep with R&D programmes, high success rates, commitment to confidentiality, and the avoidance of one-size-fits-all IP policies allow Enzymaster to advance biocatalysis innovation rather than impede it, ultimately fuelling the future of manufacturing. Green Magic Happens Here!

Enzymaster has developed BioEngine ®, a proprietary directed evolution platform with integrated computational enzyme engineering. The implementation of computational hot spot identification, in silico enzyme library screening, and in silico recombination into the directed evolution cycle allows us to shift most of the screening efforts from the laboratory into the computer and cover a large sequence space of the target enzyme, all while accounting for the real chemical process conditions. Based on the computational results, we construct smart, focused, and small libraries of enzyme variants, which can conveniently be screened via HPLC or GC to characterise a comprehensive range of desired properties for these variants. This enables us to reach the desired biocatalytic process target in typically only 3-6 rounds of combined in silico and experimental directed evolution. Therefore, BioEngine ® can significantly speed up the process of bringing your idea to the market.
The BioNavigator ® platform is part of BioEngine ® and has been developed by Enzymaster as a proprietary in-house computational toolbox for sequence- and structure-guided combinatorial library design and enhanced diversity generation. BioNavigator ® is a set of state - of-the -art methods for computational enzyme identification, in silico activity and selectivity prediction, and stability calculation under process conditions. This includes bioinformatics-driven hot spot identification, focused combinatorial library design, and in silico loop design. Artificial intelligence (AI) and structure - based recombination algorithms are applied to in silico prescreening to enhance the combinatorial sequence diversity coverage beyond traditional random and sitesaturation mutagenesis approaches, thereby increasing the sequence space coverage and identifying all important sequence areas for fast and efficient enzyme engineering.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Enzymicals AG
Walther-Rathenau-Str. 49b
17489 Greifswald
Germany
Dr Rainer Wardenga
+49-3834-515470
info@enzymicals.com
www.enzymicals.com
I 20
2009
| S1 Biotech
| Chemistry & Analytics
| Process Engineering
| Kilo Lab & Small-Scale Manufacturing
With 15 years of industry expertise, Enzymicals is your trusted partner for industrial biocatalysis, offering endto-end solutions from catalyst lead-finding to process optimisation and scale-up, spanning from gram- to ton-scale.
Areas of Activity ›
| Development and scale-up of chemoenzymatic processes incl. DSP
| Customer-specific solutions in enzyme technology and biotransformation
| Development and production of enzymes
| Applications in pharma, fine chemicals, F&F
Patents ›
IP protected by international patent applications and granted patents
External ›
Collaborations
| Broad international academic R&D collaborations, most notably Greifswald University, Bornscheuer group
| 4Synth Network
| Cluster Industrielle Biotechnologie
| Aminoverse B.V.
| ChiralVision B.V.
| Candidum GmbH
| SpinChem AB
| Allozymes Pte Ltd.
| CMO and CDMO network
| EU upPE-T
Our Commitment: At Enzymicals, we champion in vitro biosynthesis technologies for industrial implementation, driving a shift towards biology-inspired production concepts, which is a global trend. Being part of a strong EU-based partner network ensures highest regard for your IP and supply chain security. We are a CRO that presents our clients with an enticing business model, granting them full ownership of all new intellectual property. This empowers clients to devise strategic IP plans and ensures freedom of action, without cumbersome license agreements.
Our Expert Team: Backed by a highly skilled interdisciplinary team of 20 chemists and biologists, Enzymicals specialises in complex chemical synthesis and performs comprehensive services encompassing biocatalysis, enzymology, tailor-made cloning and expression in diverse hosts, along with fermentation development for protein manufacturing.
Comprehensive Enzyme Services: Enzymicals is a premier source of enzymes for diagnostics, research, and commercial production. With access to one of the world’s largest off-the-shelf collections of biocatalysts, totalling over 3,500 enzymes from 50 classes, we offer catalyst screening services to identify high-performing enzymes tailored to specific research needs. What is not found in those collections, we can access by expression of suitable genes in various microbial host systems centered around E. coli, Pichia and Bacillus, but we have experience with other, more exotic systems. This allows us to supply almost any biocatalyst of interest at analytical and commercial scale.




Seamless Transition from Screening to Synthesis: Our chemical expertise seamlessly translates screening hits into optimised processes and kilogram-scale syntheses through bespoke development programmes. We employ scalable strategies, encompassing feasibility studies, DOE-based process intensification (4x 0.5 L for chemistry and 4x 1.0 L for fermentation) and scaleup of both enzyme production (up to 30 L) and the chemo-enzymatic process (up to 10 L glass) including downstream processing, ready for tech transfer. Our industry-exclusive approach spans from traditional batch reactions to enzymatic flow and rotating bed reactors.







Enzyme Technology Alliance: Enzymicals is a founding member of the Enzyme Technology Alliance, a collaborative effort involving six independent partners. This alliance addresses complex development projects, integrating in-silico catalyst discovery, AI-supported enzyme engineering, biocatalyst immobilisation, process intensification, and scale-up of both enzyme production and chemo-enzymatic processes.
Network Capabilities: Within our network, we offer processes in up to 85 m 3 fermenters or multi-kilogram- to ton-scale for chemical processes, both under non-GMP or GMP. The combination of superior catalysts, optimised chemistry, and integrated processing enables us to expedite the delivery of effective solutions.



Let’s Create Value Together: Join us in creating value for you through innovative, sustainable, and efficient biocatalysis. Enzymicals is your dedicated partner for shaping a future where industrial processes align with the principles of green chemistry.
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
European Biotechnology Network
AISBL
Avenue Louise 65, Box 11
1050 Brussels
Belgium
Margarita Milidakis
+32 2 588 70 71
office@european-biotechnology.net
www.european-biotechnology.net
3 2008
Information and communication
The European Biotechnology Network is dedicated to facilitating cooperation between all European professionals in biotechnology and the life sciences all over Europe. The non-profit organisation provides information about business, politics, science, jobs, funding and grants, events, initiatives and many other opportunities to work together anywhere in Europe. Its more than 2,200 members are united by the vision to contribute to a better world through biotechnology. The network is registered as an official non-profit organisation in Belgium. Its legal domicile is in Brussels.
Biotechnology as a solution to the ecological crisis of our time? It is difficult for society in general and politicians or financiers in particular to answer this question with Yes, as long as almost no company dares to write proudly about its product: Biotech inside! Obviously, the term does not have a positive enough connotation after the longstanding debate about genetic engineering in agriculture.
The European Biotechnology Network wants to change this with a unique branding and has developed a biotech label that manufacturers can proudly print or stick on their products. The subtitles “Nature based” and “Bioscience for life” are intended to signal to consumers at a glance what this means. In addition, there is an individual subline that informs potential buyers the particular benefits or advantages of this specific product compared to conventional competing products that may be next to it on the shelf.
To avoid „greenwashing“ and to be allowed to use the label, manufactures will have to meet stringent conditions. A top-class panel of experts at the Network monitors compliance with these criteria. More information at biotech-label.net
Proposals from consumers and companies for products that could bear the Biotech label in the future are highly welcome by the European Biotechnology Network.

CO2 compensation is sometimes considered a new form of medieval selling of indulgences. So what? As long as mankind does not succeed in changing its habits and living climate neutrally, CO2 compensation is better than nothing. Our Biospheria Project promotes climaterelevant activities within our limited financial resources. But it is better to take small steps than to stand still.
Of course, the European Biotechnology Network would prefer to actively participate in the realisation of a large biotechnological project on climate protection. The organisation is always open to suggestions from members and supporters. In the meantime, volunteers plant trees.
The Biospheria Project No. 1 is a classic CO 2 sink, namely forest. It is located about 100 meters above sea level and is crossed by the European long-distance hiking trail E10, which will at some point lead from the north of Finland to the south of Spain. In Central Europe, it runs from the German island of Rügen to Bolzano in northern Italy.
Network volunteers have removed a former pine wood plantation from management and are accompanying the forest on its transformation into a natural, sustainable mixed wood land. Careful interventions such as the cutting of small clearings should support and accelerate this natural process. However, it is important that the forest is permanently protected and that the CO2 bound here remains on site as part of natural regeneration.
Many European countries are lacking qualified employees, which is a significant barrier to economic growth. Concomitantly, many well-qualified people at different places are seeking for a job in various European countries. Unfortunately, the european labour markets differ extensively. How can I find a new job or a new employee? This will now be easier: with eurobiotechjobs.net, the Europe-wide job market for biotechnology and the life sciences.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person ›
Telephone › Email › Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity › External › Collaborations
Request for ›
Further Collaborations
FGK Clinical Research GmbH
Heimeranstr. 35 80339 Munich Germany
Martin Krauss, Dr Edgar Fenzl +49-89-893-119-0 martin.krauss@fgk-cro.com rfp@fgk-cro.com www.fgk-cro.com;
I 240 2002
Full service CRO for conduct of clinical studies, offering support in
| Regulatory Affairs
| Project Management/Monitoring
| Medical Safety
| Data Management/Biostatistics
| Medical Writing
| eSolutions
| Quality Assurance all over Europe.
Our daughter companies create special added value for our clients:
| “FGK Representative Service”: EU Representation Services which are legally required for non-European sponsors conducting clinical studies in the EU(EEA).
| “FGK Pharmacovigilance”: Post-Market PV services including QPPV and PMSF.
BVMA - Federal Association of Contract Research Organisations
Small to mid-sized biotech, device or pharmaceutic seeking flexible support in their clinical development
European Biotechnology Network Member of
FGK Clinical Research is an owner-managed CRO of an ideal size for cooperation with smaller and mid-sized biotech, medical device or pharmaceutical companies. Covering all phases and areas of clinical development in Europe, we have experience in every major medical indication, including oncology, cardiology, neurology, dermatology, and gastroenterology. Broad knowledge in rare diseases, ATMPs and Cell Therapy completes our expertise.
Most staff is located in our headquarters in Munich and our branch office in Berlin, Germany. We have operational subsidiaries in Czech Republic, Hungary, Poland and the UK – where FGK recently acquired the mid-sized CRO Clinicology.
The main philosophy for FGK is to prepare and conduct studies in close cooperation with the sponsor. Our operational team works hand-in-hand with all other departments involved. This also applies for the project manager, who is the central contact person delivering all required information to the sponsor.
Timely approvals and efficient troubleshooting are achieved through a combination of centralized project management and local monitoring, as well as local expertise and regulatory submissions within the country of study conduct.
› Project management, primary liaison for sponsor communication, status reports, etc.
› Feasibility, site management, monitoring, etc.







Regulatory Affairs
› Consulting on regulatory topics
› Review of study documents (e.g. protocol, informed consent form, labels)
› Clinical Trial Application with submission to EU and non-EU regulatory bodies
› Legal representative – also visit www.fgk-rs.com
Medical Safety/Pharmacovigilance
› Adverse Event Management and assessment/reporting
› Drug safety, medical monitoring and coding of medical terms
› Pharmacovigilance – also visit www.fgk-pv.com
Medical Writing
› Investigator’s brochures, study protocols, ICF and subject information
› Clinical expert reports, clinical publications, IMP and submission dossiers
Data Management
› CRF design and review, clinical trial databases
› Data validation, processing and cleaning, external data handling, CDISC SDTM
Biostatistics and Programming
› Study design, sample size calculations
› Statistical consultancy, analysis plan, programming and reporting
› CDISC ADaM
Quality Assurance
› Audits of investigator site, database and system audits, internal audits
› SOP composition and implementation
eSolutions
› eCRF/ePRO, RTSM, eTMF, CTMS, DCT elements
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
External › Collaborations
Formycon AG
Fraunhoferstr. 15
82152 Martinsried/Planegg Germany
Sabrina Müller
+49-89-864-667-149
+49-89-864-667-110
sabrina.mueller@formycon.com
www.formycon.com
I Q
230
2012
Biosimilar Development
Formycon aims to develop its biosimilar candidates to near-market development stages before transferring them fully or partially into partnerships for global commercialisation. Project and marketing rights for FYB206, FYB208, and FYB209 are fully owned by Formycon. For FYB202, Formycon entered into a global commercialisation partnership with Fresenius Kabi. FYB203 is in a licensing partnership with Klinge Biopharma GmbH. Formycon owns a 50% stake in the already approved and launched biosimilar FYB201, which is marketed by Coherus BioSciences Inc. in the US, by Teva Pharmaceutical Industries Ltd. in Europe and other territories, as well as MS Pharma in the MENA region.
Formycon is an expert company for the development of Biosimilar Medicines. The company’s pipeline includes FYB201 (reference medicine Lucentis ® ), which has already been approved in the US, Europe and further territories, the late-stage biosimilar candidates FYB202 (reference medicine Stelara ®) and FYB203 (reference medicine Eylea®), as well as FYB206 (reference medicine Keytruda®), a biosimilar candidate in an advanced preclinical development phase. The two preclinical biosimilar candidates FYB208 and FYB209 complete Formycon’s biosimilar pipeline.
A Biosimilar Medicine is a biological medicine highly similar to another already approved biological medicine (the “reference medicine”). Biosimilars are approved according to the same standards of pharmaceutical quality, safety, and efficacy that apply to all biological medicines.
Through their proven efficacy, cost efficiency, and high standard of quality, Biosimilar Medicines are already making a major contribution towards improving patient access to essential medical treatments. Thus Biosimilars help patients around the world, while helping to ease the financial strains on the world’s healthcare systems.





Formycon develops Biosimilars for the rapidly growing disease areas of ophthalmology, immunology, and immuno-oncology, as well as for other key chronic diseases. Formycon covers all stages of biopharmaceutical development from analytics and cell line development to preclinical and clinical studies, all the way to the preparation of regulatory submission documents.
We develop Biosimilar Medicines to meet the high standards of the world’s most regulated markets: European Union, United States, Canada, Japan, and Australia.
Our unique advantage lies in the vast expertise of our international scientists, our management, and our board members. Through their long and distinguished careers, these scientific and business professionals have already, in their past work, successfully brought the world’s first Biosimilars to market and thus bring comprehensive experience and expertise spanning all stages of development – from market analysis and protein analytics, to the development of production processes, to clinical trials and the regulatory approval process.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
FyoniBio GmbH
Robert-Rössle-Str. 10
13125 Berlin
Germany
Dr. Ulrike Scheffler
+49-30-9489-2500
contact@fyonibio.com
www.fyonibio.com
30+
2019
ISO 9001 certified and GCLP compliant S1 and Bio2
Service business with own technology platform in cell line development, USP/ DSP development, MS-based PTM & glycoanalytics, bioassays, and clinical bioanalysis under GCLP
FyoniBio is a contract development organisation (CDO) that offers its clients 20 years of experience in biopharmaceutical development, from mammalian cell line development through process development to clinical bioanalysis and bioassays.
We are an ISO 9001 certified company located on the biotech campus Berlin Buch and operate in part under GCLP.
We provide:
› Customised clone development to RCB using our high performing CHOnamite ® (up to 9 g/L) and GlycoExpress® cell lines for complex glycoproteins
› Own CHOFlow ® cell line in place: a Fut8-KO cell line established for the production of afucosylated and therefore ADCC-enhanced mAbs for potency increase
› Robust USP/DSP Process development to transfer to the GMP facility of your choice
› Outstanding expertise in protein glycobiology (analytics and cell biology)
› Bioassay development for a wide range of proteins
› Excellent know-how in PK, biomarker and immunogenicity analysis of clinical samples under good clinical and laboratory practice (GCLP)
› Development of highly sensitive and advanced oligonucleotide PK and ADA assays
› Excellent track record: Delivering high quality in service and products
› Professional and dedicated project management and customer care
› Experienced project team
Glycosylation is fascinating and complex, and it’s amazing how much it can be influenced by the host cell line! FyoniBio offers a cutting-edge cell line development service that utilises our versatile expression platform, which includes the human GlycoExpress ® (GEX®) cell lines, as well as our CHOnamite ® platform technology for unparalleled productivities and CHOFlow ® for ADCC-enhanced mAbs.
We offer a wide range of CMC services including upstream, downstream, formulation, and analytical development to create robust and transferable processes! Our upstream process development is based on mammalian cell culture systems such as GlycoExpress ® , CHO-K1, CHO-GS, CHO-DG44, HEK, and others, for fed-batch, perfusion, and intensified fed-batch processes.
We are highly skilled in analysing PTMs, including N- and O-glycosylation, using state-of-the-art and advanced high-resolution analytical UPLC-MS methods for various biomolecules!
Within clinical bioanalysis our core competencies are the evaluation of PK and PD characteristics of biotherapeutics (proteins and oligonucleotides) as well as their immunogenic and neutralising potential. Assays are established and validated according to the current ICH, FDA and EMA guidelines. Samples are analysed under “Good Clinical Laboratory Practice” (GCLP). Analysis of SNPs is another service of ours to select and stratify patients in clinical trials.
Adequate bioassays are needed during all stages of drug development.
We establish cell-based assays using different target and effector cells including human primary immune cells. Assay validation and application within clinical trials is our daily business.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
GALAB Laboratories GmbH
Am Schleusengraben 7
21029 Hamburg
Germany
Dr Jürgen Kuballa
+49-40-36-80-77-0 +49-40-36-80-77-401 info@galab.com
www.galab.com
F I I
250
1992
Research and contract laboratory for bio-analytics, S1 according to GenTSV, S2 according to BioStoffV
GALAB Biotechnology is specialised in the biocatalysis of valuable products for a broad range of industrial applications. One of our main focused products are human milk oligo-saccharides (HMOs). Our interdisciplinary experts develop customised bioprocesses with synthetic biology and complex multi enzyme cascades for sustainable and high-yield productions. Moreover, with our GlycoKat ® and NucleoKat ® technologies, we provide innovative, high-quality products and services for our customers.
Areas of Activity ›
| Synthetic biology
| Biocatalysis
| Enzyme production
| Enzyme immobilisation
| Cofactor regeneration
| Process development
We apply synthetic biology and metabolic engineering for precise modifications of cells to initiate and enhance the production of target compounds. One of our competencies at GALAB Biotechnology is the in vivo synthesis of valuable HMOs with our patented strains. Our engineered Corynebacterium glutamicum and Escherichia coli strains produce high titer of fucosyllactose and sialyllactose. CRISPR/Cas9 is one of our powerful tools for precise genome editing and modifying metabolic pathways. It allows the fast addition of new genes, modification of existing genes or the knockout of specific genes to optimise the synthesis of our target products.
We offer a complete process development service for in vitro synthesis, from genes to products. Our proprietary expression strains are used in high cell density fermentation, which have been developed and optimised over the last 10 years to achieve high enzyme yields. In order


to develop a tailor-made production process for each enzyme, we offer a variety of enzyme purification and preservation methods. Furthermore, our unique enzyme immobilisation technology enables us to produce highly stable enzymes that can be used in different reactor systems. We have developed a technology that simplifies the implementation of multi-enzyme cascades by using our stable immobilised enzymes in packed bed reactors in continuous flow reactor setups.
Modular process design makes it easy to implement multi-enzyme cascades. Our GlycoKat ® technology enables the continuous synthesis of a wide range of glycosylated oligosaccharides, with simple product changes by replacing individual modules. The addition of our NucleoKat ® technology takes glycosylation to a whole new level. The NucleoKat® technology is a module that enables the regeneration of expensive nucleotide cofactors and can be integrated into other processes for all types of nucleotides.
› Synthetic Biology to produce HMOs in efficient E. coli and C. glutamicum strains
› CRISPR/Cas9 technology for precise genome editing and modifying metabolic pathways
› Production of pure, active, and stable biocatalysts through formulation- and immobilisation techniques
› Process development in different reactor concepts, e.g. continuous flow operation with packed bed reactors
› Model development of multi-enzyme cascades
› GlycoKat ® and NucleoKat ® modular technology for efficient synthesis and cofactor regeneration

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Galimedix Therapeutics Inc.
3704 Calvend Lane Kensington, MD 20895 USA
Am Klopferspitz 19
82152 Planegg/Martinsried Germany
Alexander Gebauer, MD, PhD Cofounder and Executive Chairman info@galimedix.com galimedix.com
I <10 2018
Small molecules, amyloid beta neuropathologies, dry age-related macular degeneration, glaucoma, Alzheimer’s disease
External › Collaborations
Request for ›
Further Collaborations
We are looking for licensing/codevelopment partners for GAL-201 (oral) for the treatment of Alzheimer’s disease in all regions and for GAL-101 (topical/oral) for the treatment of ophthalmic indications in Greater China and Asia Pacific.
Galimedix is a Phase 2 clinical-stage private company developing novel oral and topical neuroprotective therapies with the potential to revolutionize the treatment of serious eye and brain diseases. Founded by a seasoned and highly dedicated team of bio-entrepreneurs, pharmaceutical executives and scientists, Galimedix’s groundbreaking small molecules offer the hope of changing the course of disease where amyloid beta (Aβ) plays a role, such as in dry age-related macular degeneration (dAMD), glaucoma and Alzheimer’s disease (AD) - Galimedix’s initial areas of focus. Galimedix has a licensing agreement with Théa Open Innovation (TOI), a sister company of ophthalmic specialty pharmaceutical company Laboratoires Théa, for the development and commercialization of GAL-101 for the topical and oral treatment of dAMD, glaucoma and other ophthalmic indications with high unmet medical need, in Europe, the Americas, the Middle East and Africa.
Normal A β monomers are important to neuronal function, but misfolding of these monomers can lead to the development of toxic forms of A β – oligomers and protofibrils – that can be highly damaging to retinal and brain cells. Many studies have indicated that these oligomers and protofibrils are an underlying cause of neurodegenerative diseases of the eye, such as dAMD and glaucoma. Recent approvals and promising Phase 3 results of anti-A β drugs also have validated them as a key target in AD. All three diseases are chronic, age-related disorders with no known cure and lead to irreversible loss of function.
Galimedix aims to stop neurodegeneration at its source by blocking this key step in the neurodegenerative process, namely, preventing the formation of toxic A β oligomers and protofibrils. This is in contrast to most products in development and on the market, which aim to remove already formed toxic species.

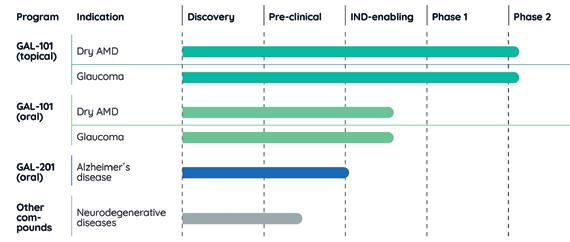
Galimedix is developing cutting-edge A β aggregation modulators that target the beginning of the A β peptide aggregation cascade. Product candidates GAL-101 and GAL-201 act upstream to most other A β -targeting approaches on the market and in advanced development. This unique mode of action, which not only removes already formed toxic species, but prevents their formation, too, could enable GAL-101 and GAL-201 to effectively impact disease progression without disturbing normal neuronal function.
Compelling pre-clinical data support the potential of Galimedix’s product candidates to slow or stop neurodegeneration and also restore lost neuronal function. A Phase 2 proof-of-concept study in dAMD with topical GAL-101 is in preparation as well as a FIH study with the oral GAL-101 formulation. Clinical studies in other indications are planned. GAL-201 is in preclinical development.
Galimedix’s approach could offer potentially gamechanging oral and topical neuroprotective therapies for the treatment of serious eye and brain diseases that impact millions of people.


Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Founded (year) ›
Areas of Activity ›
Genosphere Biotechnologies
52 rue du Moulin de Pierre 92140 Clamart
France
Dr M. Benamira, CEO
+33 -142-717-021
info@genosphere-biotech.com
https://www.genosphere-biotech.com
www.biomodul.de
1996
| Custom peptide synthesis
| Custom polyclonal antibody development
| Custom monoclonal antibody development
| Custom recombinant protein expression
Genosphere Biotechnologies is an independent and trusted service organisation for the development and production of customised peptides, custom polyclonal and monoclonal antibodies, and custom recombinant proteins. Customers from universities, research & development industrial groups, and high-tech startup companies benefit from our expertise and from our individual and flexible solutions for each customer requirement.
We support our customers in their success. Our goal is to provide reliable services at competitive prices. With over 25 years of experience in organic chemistry, peptide chemistry, immunochemistry, and recombinant technologies, we are able to provide excellent service with highly reproducible quality.
Peptides are essential in a large variety of biological processes. A number of peptides have been shown to play a key role in specific biochemical processes as well as disease. Hence, a trustworthy partner in chemical peptide synthesis has become critical to many laboratories across the life sciences in both basic and industrial R&D.
Over the past 25 years, Genosphere Biotechnologies has produced complex peptides using total chemical synthesis, incorporating any of the 20 standard L- amino acids or other unnatural amino acids (D-form, glycosylated, azide-containing, methylated, etc). Additionally, we can engineer structural modifications, such as gamma-peptide bond branching or epsilon-peptide bond branching. Other popular structural changes to the linear peptide chain include disulfide bridges or Nter to Cter lactam cyclisations.
Genosphere Biotechnologies has been synthesising custom peptides for research and development laboratories since 1996 from proof-of-concept to preclinicalmass production steps and is one of the most trusted group of peptide chemists in the industry. The company’s peptide synthesis group will support you in the design of your peptides and peptide derivatives.



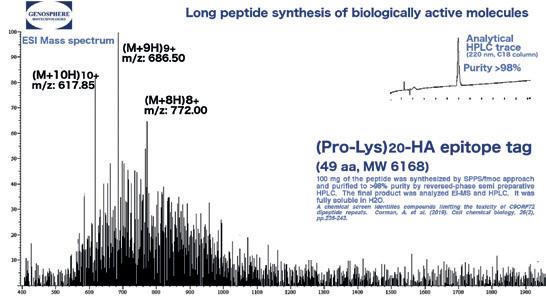


Genosphere Biotechnologies offers complete polyclonal antibody production services packages, including peptide or protein antigen synthesis, choice of host immunisation, antibody purification, and quality control analyses.
Our polyclonal antibody production services are designed to meet a variety of experimental requirements. Multiple purification methods are available, including protein A and antigen affinity purification.
Our polyclonal antibody development services are based on years of process optimisation and a strong reputation for reliable antibody production services. We have been a preferred partner to various institutions worldwide for antibody development and antibody production services for over 25 years.
Mouse monoclonal antibodies have broad applications as therapeutics, diagnostic tools, and research reagents. Genosphere Biotechnologies provides comprehensive mouse monoclonal antibody development services based on our well-established hybridoma platform. We provide complete support for all steps of mouse monoclonal antibody development, from antigen preparation to animal immunisation, fusion and screening, subcloning, and antibody production, which allows the identification of clones with the desirable qualities. We provide free consultation for target antigen design and expression, and our experienced project managers and scientists will support you directly throughout the process.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Founded (year) ›
Type of Laboratory ›
Brenneckestraße 20 – ZENIT
39120 Magdeburg
Germany
Dr Erdmann Rapp (CEO & CSO)
+49-391-6117-251
+49-391-6117-255
info@glyxera.com
www.glyxera.com
2011
| Glycomics
| Glycoproteomics
| Proteomics
Areas of Activity ›
High Performance Glycoanalytical & (Glyco)Proteinanalytical Services & Products
glyXera GmbH is a Max Planck Society spin-off, specialised in high performance glycoanalysis. We are utilising separation- and mass-spectrometry-based glycoanalytical tools and have substantial experience in providing glycoanalytical products & services tailored to the specific needs of our customers. Worldwide exclusively glyXera provides the high-performance glycoanalysis system glyXboxTM
Our clients include leading pharmaceutical, biotechnology and food companies throughout the world. We offer you our sophisticated technology platforms and our expertise with respect to glycoanalytical services, tailored to your specific needs. We guide you for better decisions and help you to perform better and faster during discovery phase, R&D, (clinical) trials and approval of your innovative products (biopharmaceuticals (originators & biosimilars), vaccines, food additives, functional food, etc.), and QA/QC in your production stages.
glyXera GmbH is specialised in glycoanalysis, utilising state-of-the-art separation- and mass-spectrometrybased glycoanalytical tools and has a strong background in providing glycoanalytical services. We run modern analytical and synthesis labs, equipped with state-of-the-art instrumentation.
glyXera offers high performance glycoanalysis to its clients, providing sophisticated technology platforms and expertise with respect to glycoanalytical services, tailored to the specific needs of the variety of its customers from academia, clinics, pharmaceutical and food industries. We have substantial experience in glycomics, glycoproteomics and proteomics of biotechnological (e.g. recombinant glycoproteins (mAbs, fusion proteins, factors, hormones, etc) or viral membrane glycoproteins) and clinical (blood, milk and other body fluids) samples.
glyXera provides exclusively worldwide fast and reliable glycoanalysis utilising a “real” high-throughput system (method/software/database) with superior performance and capacity compared to other glycoanalysis tools available on the market.




Our patented system glyXboxTM, based on multiplexed capillary gelelectrophoresis with laser induced fluorescence detection (xCGE-LIF) enables generation and comparison of glyco-fingerprints from large sets of samples and structural analysis of glycans within these samples.
High throughput, high resolution, high sensitivity, high reproducibility, high reliability:
› Up to 96 capillaries in parallel enable “real” high throughput
› Up to 3500x more sensitive and 450x faster, compared to LC
› An order of magnitude higher separation power, compared to LC
High-performance glycoanalysis on 3 levels:
› Glycofingerprinting: Glycosylationpattern analysis & comparison
› Standard Glycoprofiling: Identification of glycans in complex mixtures via database matching
› Extended Glycoprofiling: Extended structural analysis of glycans in complex mixtures using exoglycosidase sequencing in combination with repeated glycoprofiling.
Our services
› Glycofingerprinting & Glycoprofiling by xCGE-LIF & HILIC-FLR
› MALDI-MS based Glycoprofiling
› LC-MS based profiling of N- and/or O-glycans
› Monosaccharide (Composition) Analysis
› Site Occupancy Analysis
› Proteomics
› N- & O-Glycopeptide Mapping
› Intact Mass Protein Analysis (Glycoform Determination)
› General (Glyco)-Protein Characterisation by Protein Gel Electrophoresis Pattern Analysis
Our products
› glyXbox CE : High performance glycoanalysis system based on xCGE-LIF (incl. glyXtoolCE software)
› glyXprep: Sample preparation kit for glycoanalysis
› Tailored standards & kits for glycoanalysis
Name ›
HealthCapital – Cluster Healthcare Industries
Berlin-Brandenburg
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Fasanenstr. 85
10623 Berlin
Germany
Dr Kai Bindseil, Clustermanager HealthCapital
Telephone ›
Email ›
Website ›
Social Media ›
Areas of Activity ›
Berlin-Brandenburg +49-30-46302-463
info@healthcapital.de
www.healthcapital.de
I #HealthCapitalBB
l Technology transfer between science and industry
l Support for technology-oriented start-ups
l Funding support for innovative project concepts
l Providing and presenting regional life sciences information
l Initiation and support of networks
l Establishing contacts between experts from all disciplines
l Organisation of events and seminars, Public Relations work for the life sciences region
The Berlin-Brandenburg region is one of the leading, international locations for life sciences. The area concentrates on clinical research via a dense and compact healthcare network, and boasts a state-of-the-art IT infrastructure. It is no coincidence that the region is Germany’s ‘health capital’ as it is home to both the German government, as well as the centre for healthcare industries.
External › Collaborations
l Member of the Council of European Bioregions (CEBR)
l Member and contact point in Berlin for Enterprise Europe Network (EEN)
l Collaborations with European Life Science clusters and SMEs in several European projects and other activities
l Bio Deutschland
l Global Health Hub Germany
The region’s distinction is anchored in its unique research and clinical landscape, as well as its ability to closely link the key players in the life sciences and healthcare. Biotechnology, in particular, is a strong driving force within the Berlin-Brandenburg healthcare industries cluster – HealthCapital – generating innovation and growth there and beyond. Through networking the pharmaceutical, diagnostics and medical technology sectors completely new fields of business are created. Around 280 biotechnology and more than 30 pharmaceutical companies are located in the German Capital Region. They include market leaders like Bayer, Bausch & Lomb, B. Braun Melsungen, Berlin-Chemie, Pfizer, Sanofi and Takeda. Along with the sector’s many small and mid-sized companies, they benefit from close cooperation, both with science and with 140 hospitals – above all, one of Europe’s largest university hospitals: Charité – Universitätsmedizin Berlin. Customers from research and industry have access to patient cohorts of urban and rural populations of about 180 ethnicities, covering all medical indications. The many technology parks and networks in the various branches of modern biotechnology create the excellent infrastructure and technological support that characterize the Berlin-Brandenburg life sciences area. Focus of biotech activity within the region are biomedicine and diagnostics, therapeutics and regenerative medicine as well as industrial biotechnology.

Active cooperation in the networks of Europe’s BioRegions has played a central role in the internationalisation efforts in the region. The region is a member of the Council of European Bioregions (CEBR).
The central contact and coordination office for all issues concerning life sciences in the German Capital Region is HealthCapital. At the interface of business, science and clinics, the HealthCapital cluster management drives networking and the technology transfer and supports companies interested in relocating to the region. Berlin Partner for Business and Technology and the Economic Development Agency Brandenburg (WFBB) are responsible for managing the cluster.
› BIO Europe Spring | March 18-20, Barcelona, Spain
› DMEA 2024 | April 9-11, Berlin, Germany
› Deutsche Biotechnologietage | April 16-19, Berlin, Germany
› Swiss Biotech Day 2024 | April 22-23, Basel, Switzerland
› BIO International Convention | June 3-6, San Diego, CA, USA
› BIO Europe | November 4-6, Stockholm, Sweden
› MEDICA | November 11-14, Düsseldorf, Germany

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Biological Patents ›
External ›
Collaborations
IBA Lifesciences GmbH
Rudolf-Wissell-Str. 28
37079 Göttingen
Germany
Dr Mike Rothe +49-551-50672-0 +49-551-50672-181 info@iba-lifesciences.com www.iba-lifesciences.com
I
~50 1996 S1
l Protein Production & Assays
l Strep-Tactin®XT
l Twin-Strep-tag®
Numerous in- and out-licensing contracts
Strep-tag ® - the leading affinity tag in recombinant protein production
IBA Lifesciences GmbH is a biotechnology company that develops innovative products for life science research in both academic and industrial settings. Our unique Strep-tag® technology opens up a wide range of application possibilities in the purification and analysis of recombinant proteins.
Strep-tag ® technology
The Strep-tag ® technology exploits one of the strongest non-covalent interactions in nature; the interaction of biotin and streptavidin. Strep-tag® II and its tandem equivalent Twin-Strep-tag ® are peptide sequences exhibiting intrinsic affinity towards the biotin-binding pocket of two specifically engineered streptavidin variants, Strep-Tactin ® and its high affinity version StrepTactin ®XT. The wide range of affinities as well as the reversibility of the binding interaction make Strep-tag ® the leading affinity chromatography system.
From protein purification...
Strep-Tactin®XT is the high affinity variant for the purification of strep-tagged fusion proteins, providing binding affinities in the picomolar range for Twin-Strep-tag® while still maintaining binding reversibility and mild recovery of immobilized proteins. It is suitable for efficient protein purification independent of protein class and size, including challenging proteins as well as low abundant proteins.
Fused to magnetic beads, MagStrep ® Strep-Tactin®XT, is the ideal tool for complex identification via pull-downs and fast small-scale batch purification in reaction tubes or 96-well plates.
In particular, Strep-Tactin ® XT 4Flow ® high capacity provides superior performance for the purification of strep-tagged proteins from diluted cell extracts and facilitates intensive wash procedures with large volumes of wash buffer. Strep-Tactin ®XT 4Flow ® high capacity resin is stable over a wide range of pH and compatible with various buffer conditions, and can be reused at



least 100 times without losing performance. Due to this frequent reusability, experimental costs can be significantly reduced.
The near covalent affinity of Strep-Tactin ®XT to TwinStrep-tag® expands the range of use towards analytical applications such as high throughput screening, assay development and protein kinetic studies. You can obtain Strep-Tactin ® XT conjugated to microplates, fluorophores, or chips.
Strep-Tactin®XT coated microplates ensure convenient diagnostic assay and high-throughput screenings with high stability and antibody-free protein immobilization. Moreover, immobilized biomolecules are presented to interaction partners in a uniform manner, which results in reliable and highly reproducible assay formats.
The picomolar affinity is particularly valuable for surface plasmon resonance (SPR) analysis and bio-layer interferometry (BLI) and supports measurements with long dissociation times and slow off-rates. Moreover, Strep-Tactin®XT biosensors can be easily regenerated.
Key features:
› Highly selective binding properties leading to unparalleled protein purity (> 95 %)
› Bioactive target proteins due to rapid one-step purification under target specific conditions
› Variability in buffer conditions, e.g. high salts, detergents, metal ions, chelators or reducing agents
› Favorable for protein-protein interaction studies due to mild elution conditions that preserve natural protein structure
› Products covering the entire workflow from cloning, purification to analytical applications (ELISA, FACS, SPR, Microscopy, Western Blot, etc.)

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Ideogen AG
Hurdnerstrasse 119
8640 Hurden
Switzerland
Sanjeet Telang / Murat Goker
Office: +41-43 3115252
S Telang: +41-79-277-3626
M Goker: +41-79-676-3628
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
sanjeet.telang@ideogen.com
murat.goeker@ideogen.com
www.ideogen.com
I
88 2013
Specialty Pharmaceuticals
Specialty Pharma / Rare & Orphan Diseases / Salvage & Chronic Care Therapies / Managed Access Programmes (MAP) / Oncology, Haematology, Cell & Gene Therapy, Hospital Sales
External › Collaborations
Pre-clinical to clinical development. Multiple ongoing collaborations with research hospitals, CROs, disease associations and patient organisations
Request for ›
Further Collaborations
Exclusive in-licensing of specialty innovative assets with/without Orphan Drug Designation (ODD) / unlicensed or licensed commercialisation for the EU & MET regions
Ideogen is a Swiss, privately owned, specialty pharmaceutical disease management company focused on providing products that address unmet medical needs in the areas of specialty, salvage , and chronic care in oncology, haematology, and rare/orphan diseases, among numerous other disease areas.
We have a robust track record of success in driving the commercialisation of unlicensed products through Managed Access Programmes (MAP) to licensed sales of specific assets, while covering all aspects of the value chain from regulatory pathways, market access, pricing, and reimbursement to commercialisation in Europe, Turkey, and the Middle East.
We are headquartered in Switzerland, and have subregional affiliates in Austria, the Netherlands, and Spain, along with a standalone affiliate in Turkey that caters to the Turkish and Middle East markets.
Our business model and success to date are based on strong in-house medical and clinical expertise as well as experienced key account management teams, in addition to established partner relations in specialised settings across multiple territories that require geography-specific solutions.

Our unique selling proposition is to manage the streamlined roll-out and execution of early and managed access programmes for relevant unregistered assets.
We also commercialise registered assets while managing the market access, pharmacovigilance, supply chain deployment, customer service, data collection, and reporting activities in the geographies in which we operate.
Ideogen is increasingly looking to diversify its scope of activities by entering the high-value, injectables manufacturing space with a view to entering cell and gene therapy, focusing on in vivo gene therapies.
This would cement the company’s position as a vertically integrated, specialty pharmaceutical organisation with relevant CDMO capabilities that can cater to the emerging needs of the next generation of personalised medicine.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Immunic Therapeutics
Lochhamer Schlag 21 82166 Gräfelfing
Germany
Headquarter: New York City, USA
Jessica Breu
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
(Vice President of Investor Relations and Communications) +49-89-2080477-09 info@imux.com
www.imux.com
F I
80 2016
| Immunology
| Chronic inflammatory and autoimmune diseases
External ›
Collaborations
| Ludwig-Maximilians-University (LMU) Munich, Germany: Department of Pharmacy: Prof. Dr. Daniel Merk
| City of Hope Duarte, California, USA: Department of Molecular Imaging & Therapy, Research in Cancer and Immunity, Mechanisms Controlling T-cells: Prof. Zuoming Sun, Ph.D.
| Universitätsklinikum Erlangen, Germany: Institute of Clinical and Molecular Virology: Prof. Dr. Manfred Marschall
Immunic (Nasdaq: IMUX) is a biotechnology company developing a clinical pipeline of orally administered small molecule therapies for chronic inflammatory and autoimmune diseases: Vidofludimus calcium (IMU-838) is being developed as a next-generation treatment option for patients with multiple sclerosis (MS) and other chronic inflammatory and autoimmune diseases. IMU-856 has demonstrated clinical proof-of-concept in celiac disease patients and may represent a completely new treatment approach for gastrointestinal disorders, as the mechanism of action targets the regeneration of bowel wall architecture and restoration of intestinal barrier function, while maintaining full immunocompetency. IMU-381, currently in preclinical testing, is a next generation molecule specifically addressing the needs of gastrointestinal diseases.
Immunic’s lead development programme, vidofludimus calcium, is an orally available, next-generation selective immune modulator that acts as a potent, first-in-class nuclear receptor related 1 (Nurr1) activator, in addition to its known mode of action as a dihydroorotate dehydrogenase (DHODH) inhibitor. Nurr1 is a neuroprotective transcription factor and an emerging target in neurodegenerative diseases.
Vidofludimus calcium is currently being investigated in the twin phase 3 ENSURE trials in relapsing multiple sclerosis (RMS) and in the phase 2 CALLIPER trial in progressive multiple sclerosis (PMS). An interim biomarker analysis of the CALLIPER trial demonstrated a clear separation from placebo in serum neurofilament light chain (NfL) levels. CALLIPER top-line data is expected in April 2025. Data from an interim futility analysis of the ENSURE programme is expected in late 2024; the read-out of the first of the ENSURE trials is anticipated in the second quarter of 2026.


Immunic believes that vidofludimus calcium has the potential to demonstrate medically important advantages versus currently approved MS treatments, due to its combined neuroprotective, anti-inflammatory, and anti-viral effects as well as its established favourable safety and tolerability profile.
The molecule has a targeted effect on hyperactive immune cells without suppressing the normal immune function. It also showed a robust MRI lesion suppression and initial signals for improved rates of confirmed disability worsening in phase 2 clinical testing, while also decreasing serum neurofilament light chain (NfL), a biomarker for axonal damage. The broad-spectrum antiviral effect of vidofludimus calcium may support lowering the rate of viral infections and reactivations, including Epstein-Barr virus (EBV) reactivation, potentially resulting in slowing EBV-related neurodegenerative processes.
IMU-856 is an orally available and systemically acting small molecule modulator that targets Sirtuin 6 (SIRT6), a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium.
In 2023, Immunic announced positive results from a phase 1b clinical trial in patients with celiac disease. IMU-856 demonstrated positive results in four key dimensions of the disease’s pathophysiology: histology, disease symptoms, biomarkers and nutrient absorption. IMU-856 was also observed to be safe and well-tolerated in this trial. Clinical phase 2 testing of IMU-856 in patients with ongoing active celiac disease is in preparation.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Indivumed GmbH
Falkenried 88 – Bldg. D
20251 Hamburg Germany
Prof. Dr. Hartmut Juhl (CEO) +49-40-4133-83-0 +49-40-4133-83-14
info@indivumed.com
www.indivumed-therapeutics.com
I
~100
2002 S2, S1
Drug development partnerships
| Precision cancer medicine R&D
| Pharma and biotech partners
Proprietary cancer database
| Multi-omics data based on highquality tissue samples
| Extensive clinical data based on longitudinal patient follow-up
Target Discovery
| Multivariate, biomathematical analytics
Target Validation
| 2D and 3D patient based cellular models
External ›
Collaborations
Indivumed Global Clinical Network
| Collaboration with leading cancer clinics in Europe, Asia, and North and South America
Academic Partnerships
| Salk Institute
| Georgetown University
| University of Rochester Medical Center
| A*STAR Institute for Molecular and Cell Biology
Indivumed Therapeutics is a global oncology company with a focus on data-driven R&D. Our vision is a world in which precise therapeutic treatments exist for every cancer patient. With this goal in mind, we enhance R&D activities and launch new discovery programs, providing expertise, capabilities, and insight knowledge throughout the cancer drug development process. Through a global network of selected partner clinics, we have created and maintain a unique database of proprietary multi-omics and longitudinal clinical datasets that reflect the molecular reality of cancer at an unparalleled level. By combining these proprietary datasets with multivariate biomathematical analytics and patient based cellular models, we drive target discovery and validation for novel therapies.
The basis of our activity is a strict clinical standard, to which we have been adhering for the past 20 years. Biospecimen and associated data quality is crucial to making all relevant details of a cancer disease transparent. To ensure this, our global clinical partners apply numerous detailed standard operating procedures (SOPs) to the collection and processing of biospecimens, achieving an ischemia time for tissue of ≤10 minutes. Extensive longitudinal patient data covering around 300 individual data points complete the sets. We have developed and refined these procedures through years of experience working alongside surgeons and pathologists in our network.



The high quality of our biospecimens allows us to use a full multi-omics approach. Using next-generation sequencing and mass spectrometry technologies, we extract genomics, transcriptomics, miRNA, proteomics, and phosphoproteomics from both normal and tumour tissue samples – in-depth molecular information that feeds into our comprehensive cancer database. This is where our holistic discovery and validation process nRavel ® begins. We discover new therapeutic targets by using advanced biomathematical algorithms to uncover hidden patterns and useful correlations in the vast amounts of data.
To achieve the most accurate target validation results, we use 2D and 3D cellular models derived from the original patient cohort used in discovery. These models provide a highly detailed recapitulation of the patient’s physiological processes and represent the characteristic features of the disease.
This approach ensures end-to-end comparability and increases the likelihood of successful therapeutic development further down the pipeline.
Our expertise in target identification benefits pharmaceutical companies, with whom we partner to drive precision oncology therapeutics forward. The exceptional quality and consistent comparability of our data makes our approach unique in the industry and gives our partners a higher chance of new drugs reaching the market.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
iOmx Therapeutics AG
Fraunhoferstr. 22
82152 Martinsried
Germany
Dr Apollon Papadimitriou +49-89-8999-7090-0
info@iomx.com
www.iomx.com
I 50
2016 | Next-generation cancer immunotherapies
| Drug development
| Immuno-oncology
iOmx is a clinical-stage biotech company that harnesses deep tumour and myeloid biology insights along with its proprietary iOTarg™ target screening platform, to generate novel treatments for the most prevalent solid tumour indications.
The company is translating unexplored immune evasion biology into a growing pipeline of biomarker-enabled drug programs. Focused on developing drugs with single agent activity, iOmx is creating new backbone therapies in a modality-open fashion. By applying its comprehensive drug discovery & development expertise, iOmx is committed to shaping the future of cancer therapy.
Immunotherapy has become an essential pillar in cancer treatment. Currently, the field is dominated by drugs targeting the PD-1/PD-L1 pathway. However, these agents represent only the beginning of cancer immunotherapy and show variable efficacy across tumour indications. In the majority of cases, they fall short in meeting the needs of cancer patients, leaving a gap in immunooncology treatment options.
iOmx aims to close this gap with a differentiated approach that follows the route of new immune-evasion biology discovery and drug development. The company is developing first-in-class therapies with single-agent efficacy, focusing on targets that are biologically distinguished and orthogonal to the PD-1/PD-L1 suppression pathway.
iOmx prioritizes defining a biomarker-driven patient enrichment strategy from the very beginning of the drug development program.


In brief, iOTarg™ is a high-throughput target discovery & validation platform that can be flexibly applied to cell types of interest in the tumour microenvironment (e.g., tumour cells or myeloid cells) with the aim to identify the immune-regulatory potential of genes that can modulate the activity of co-cultured effector immune cell (e.g., T cells). Novel and druggable immune-regulatory targets are prioritized to run through an extensive suite of tailored and well-defined target validation & characterization assays, ultimately leading to the initiation of a drug discovery campaign.
The lead candidate, OMX-0407, is a first-in-class, spectrum-selective SIK kinase inhibitor, currently being investigated in a first-in-human Phase I study in multiple solid tumours. The program will move seamlessly into Phase Ib expansion cohorts in selected priority indications, including exploratory biomarker evaluation for patient selection.
The mode of action of OMX-0407 combines immune sensitization and modulation of the tumour microenvironment with direct tumour cell-killing effects, thereby driving tumour eradication. In preclinical studies, the lead candidate demonstrated striking monotherapy activity in multiple syngeneic anti-PD-1 non-responsive tumour models.
The company’s second program, IOMX-0675, is a best-in-class antibody, that targets two key immunosuppressive receptors in the tumour microenvironment simultaneously. Neutralization of these receptors results in a pronounced anti-tumour immune response by retuning the tumour microenvironment and activating T cells against cancer cells. IOMX-0675 is currently advancing through IND-enabling studies.
In addition, iOmx is applying its proprietary highthroughput target discovery & validation platform iOTarg™ to enhance its pipeline of drugs against novel immune escape targets.
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
ITM Isotope Technologies Munich SE
Lichtenbergstrasse 1
85748 Garching / Munich Germany
Dr Konstantin Zhernosekov +49-89-329-8986-6000
+49-89-329-8986-6061 businessdevelopment@ itm-radiopharma.com www.itm-radiopharma.com
I
more than 650 2004
Radiopharmaceutical biotech company
| Oncology
| Precision Medicine
| Targeted Radionuclide Therapy
| Nuclear Medicine
Targeted radiodiagnostics and therapeutics for cancer treatment
ITM, a leading radiopharmaceutical biotech company, is dedicated to providing a new generation of radiomolecular precision therapeutics and diagnostics for hardto-treat tumors. We aim to meet the needs of cancer patients, clinicians and our partners through excellence in development, production and global supply. With improved patient benefit as the driving principle for all we do, ITM advances a broad precision oncology pipeline, including two phase III studies, combining the company’s high-quality radioisotopes with a range of targeting molecules. By leveraging our two decades of pioneering radiopharma expertise, central industry position and established global network, ITM strives to provide patients with more effective targeted treatment to improve clinical outcome and quality of life.
Radiopharmaceutical Therapy (RPT) is an emerging class of cancer therapeutics, which seeks to deliver radiation directly to the tumor while minimizing radiation exposure to normal tissue. In contrast to conventional external radiotherapy, Radiopharmaceutical Therapy is defined by the intravenous infusion of a radiopharmaceutical. Targeted radiopharmaceuticals are created by linking a therapeutic radioisotope to a targeting molecule (e.g., peptide, antibody, small molecule) that can precisely recognize tumor cells and bind to tumorspecific characteristics, like receptors on the tumor cell surface. As a result, the radioisotope accumulates at the tumor site and decays, releasing a small amount of ionizing radiation, thereby destroying the tumor. The highly precise localization enables targeted treatment with minimal impact to healthy surrounding tissue.
Often, the targeting molecule can be used in a “theranostic” approach for both therapeutics and diagnostics. For diagnosis, the targeting molecule is linked with a diagnostic radioisotope, for therapy with a therapeutic radioisotope. This allows tumors and metastases to be both precisely localized at an early stage, and subsequently treated following the same mechanism.



ITM is developing a proprietary portfolio and precision oncology pipeline of targeted treatments in various stages of clinical development, which address a range of cancers such as neuroendocrine tumors, glioblastoma, osteosarcoma and bone metastases, prostate cancer, as well as folate receptor α positive tumors like lung, ovarian or breast cancer. To validate the Radiopharmaceutical Therapy approach, efficacy and safety of the lead candidate n.c.a. 177Lu-edotreotide is currently being compared to standard treatments for patients with neuroendocrine tumors of gastroenteric or pancreatic origin in two Phase III clinical trials, COMPETE & COMPOSE.
The unique combination of ITM’s longstanding expertise, high-quality radioisotopes and global supply network enables ITM to develop new Radiopharmaceutical Therapy based treatment options for hard-to-treat cancer indications. ITM strives to positively impact the treatment algorithm currently in place for solid tumors and make a tangible impact in improving the treatment outcomes and lives of cancer patients.
For more information about ITM, please visit: www.itm-radiopharma.com

Name ›
Fördergesellschaft IZB –Innovations- und Gründerzentrum Biotechnologie mbH
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Number of Firms ›
External ›
Collaborations
Am Klopferspitz 19
82152 Planegg/Martinsried Germany
Dr Peter Hanns Zobel
+49-89-5527948-0
+49-89-5527948-29 info@izb-online.de
www.izb-online.de
36 1995
S1 labs
l Biotechnology
l Life Sciences
over 40 start-ups
l Collaborations with the scientific faculties on the Campus Martinsried
l Munich Life Science Pitch Day with the High-Tech Gründerfonds Management GmbH
l IZBrunch - Science meets Science
l Law Lab - law for biotech businesses
l Biotech Press Lounge
The IZB, founded in 1995, is the operating company for the Innovation and Start-up Centers for Biotechnology located in Planegg-Martinsried and Freising-Weihenstephan, and has developed into one of the top biotechnology centers in the world. Currently, in an area totaling 26,000 m2, more than 40 start-up biotech companies with over 700 employees are located. An essential criterion for the success of the IZB is its close proximity to the cuttingedge research at the Ludwig Maximilians-University (LMU) and renowned biotechnology research institutions on the Campus Martinsried, such as the Max PlanckInstitutes für Biochemistry and Biological Intelligence.
Since 1995, the Planegg-Martinsried location, now covering 23,000 m2, has accommodated start-ups focusing on medical biotechnology. Founded in 2002, the IZB in Freising-Weihenstephan with its 3,000 m2 area offers ideal conditions for start-ups in the field of life sciences. At both locations, young entrepreneurs and company founders will find an excellent infrastructure for transforming their products or services into business ideas, within a competence cluster for life sciences that is one of the best in the world. S1 labs for fair rental prices, internal property management, close contact to the venture capital scene, as well as joint location marketing are also factors for our success, as is the flexibility to adapt space requirements to the development of our tenants.
Today, the scientific and business Campus Martinsried is one of the largest centers in Europe where teaching, basic scientific and clinical research, as well as technology innovation are combined on one campus. Located in the direct vicinity are other institutes such as the Max Planck Institutes for Biochemistry and Biological Intelligence, the Helmholtz Zentrum Munich Hematology Unit and the following institutes of the LMU: the Clinical Center Grosshadern, the Faculty of Biology, the Faculty for Chemistry and Pharmacy, the Munich Center





for Neurosciences, the Center for Neuropathology and Prion Research (ZNP), the Gene Center, the Biomedical Center, the BioSysM Bavarian Research Network for Molecular Biosystems and the Institute for Chemical Epigenetics.
The close proximity of the scientific institutes around the Campus Martinsried is a major competitive advantage. Young scientists can profit from incorporating expertise in science and research into their own business enterprises; short distances promote interaction and cooperation between biotech companies – in terms of globalization both factors are essential requirements for successfully entering world markets.
The 28-meter high IZB Residence CAMPUS AT HOME is the architectural and communicative center point of the Campus Martinsried. The sevenstorey hotel with 42 rooms and its own restaurant accommodates visiting scientists from all over the world. The core element is the Faculty Club G2B. Its purpose is to promote the transfer of research results into marketable products and services, and intensify the dialogue between top class scientists.
IZB – in brief
› 26,000 m2 laboratory and office space for start-ups and growing companies
› Home for over 40 start-ups
› Business development support
› In-house estate management
› Center of an impressive research campus
› Access to an international network
› Flexible lab and office structures
› Close contacts with investment partners
› Joint location marketing
› Modern conference rooms, also for external booking
› IZB Residence CAMPUS AT HOME (42 Rooms)
› Faculty Club G2B
› Restaurants: SEVEN AND MORE and THE BOWL Food Lounge
› 2 day care centers (Bio Kids & Bio Kids 2)

Name ›
LabWare Ltd.
European Headquarters
Address/P.O. Box ›
Postal Code/City ›
Country ›
Telephone ›
Email ›
Website ›
Social Media ›
Founded (year) ›
Areas of Activity ›
Denzell Lodge, Denzell Gardens, Dunham Road
WA14 4QF / Altrincham, Cheshire United Kingdom
+44-161-927-5600
infoEU@labware.com
https://www.labware.com/industries/ biopharma
F I I
1987
| Digital lab data management
| Instrument results interfacing
| Robotics process automation
| LIMS/ELN/LES for bioprocessing
| Data analytics
| Mobile device (iOS/Android) lab automation
| Inventory management
| Cell banking
| Equipment management
| Consultancy and implementation services
With LabWare, customers do not have to choose between a rigid LIMS system or juggle a narrowly focused point solution. LabWare is a holistic LIMS that covers the entire biopharma manufacturing spectrum from R&D, scale-up to manufacturing, and supporting ANSI/ISA S88 standards.
QC and R&D laboratories in the biopharmaceutical industry trust LabWare to bring their products to market safely and efficiently. Our solutions have been equally deployed across the R&D continuum offering tremendous flexibility and rapid configurability, as well as in Quality Control, ensuring sustainable regulatory compliance and quality.
LabWare offers a scalable LIMS software with consulting services to guide you from initial formulation through trials, testing, and manufacturing.
Prebuilt Workflows: With little staff and no internal IT resources, LabWare can get you up and running in under 30 days. Out-of-the-box workflows enable you to start collecting data and running reports immediately.
Regulatory Compliance: The LabWare platform is fully compliant with all technical controls of industry regulations, such as 21 CFR Part 11 (Electronic Records and Electronic Signatures), GxP, and regulatory requirements from many international regulatory agencies. The LabWare Platform has an extensive audit trail and can be configured very easily to support your company’s interpretation of audit reason prompting and electronic signature.
Customer Success: Training & Support – LabWare users say that it is easy and quick to learn the system. Our training and support team is with you at every step of the journey, as needed. LabWare allows to onboard quickly with best-in-class features and then evolve as your business grows and your needs expand.


LabWare for Enterprise Biopharma Labs
Digitally transform your biopharmaceutical laboratory with the #1 Enterprise Laboratory Platform in the world.
Configurable Enterprise Laboratory Platform: One of the most compelling attributes of the LabWare solution is its ability to be rapidly adapted through configuration, not customisation, to meet your specific needs in the lab, institutional requirements, and system interfaces to business software and in-house databases.
The LabWare platform offers unmatched out-of-the-box functionality in the market. LabWare’s advanced software architecture enables the solution to be extended without requiring you to move to the next version or revalidate the system, an overwhelming advantage that most LabWare customers find genuinely compelling.
Data Science Engine: LabWare’s Data Science Engine can be configured to answer questions related to your company setup. Whether you are analysing DNA sequences, comparing bioreactors with thousands of data points, or trying to maintain continuous bench flow, LabWare’s analytics capabilities can answer those questions affordably and quickly.
Integration with Business Systems: LabWare eliminates silos of information with an open architecture and powerful interoperability tools that enable exchange of information with other enterprise information systems via web services, HL7, ASTM, file transfer, and direct database communications.
About LabWare
LabWare is recognised as the global leader of Laboratory Information Management Systems and Instrument Integration software solutions. The company’s Enterprise Laboratory Platform combines the award-winning LabWare LIMS™ and LabWare ELN™, which enables its customers to optimise compliance, improve quality, increase productivity, and reduce costs.
LabWare also provides digital lab management consultancy, professional implementation & validation services, training, and world-class support to ensure customers get the maximum value from their LabWare engagement.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Schildbach 119
8230 Hartberg
Austria
Werner Kobenter
+43-3332-61000 +43-3332-64218 info@lugitsch-strasser.at www.lugitsch-strasser.at 9
1990
Lugitsch-Strasser GmbH provides services in the field of medical engineering.
We carry out installation works and maintenance/service works on steam sterilisers, disinfectors, etc. Our customers are hospitals, veterinary medical institutions, as well as pharmaceutical research laboratories. Another important focus of our activities is the production of wastewater sterilisers. Areas of application for this type of sterilisers are, for example, research laboratories of universities, biological laboratories, research facilities of pharmaceutical companies, etc.
The washing water steriliser L500 as well as the wastewater steriliser L500S have been developed for the thermal inactivation of infectious and biologically contaminated effluents. The units are especially designed for decentralized use in BSL3/BSL4 laboratories. After treatment (sterilisation) the water can be disposed in an environmentally-friendly way. Both system can be directly connected to already existing effluent lines or storage tanks on site.
On request, both systems can be supplied with comprehensive device documentation, e.g. IQ/OQ test forms. sterilisation capacity: 600 – 800 L/day





Process steps
› heating
› sterilisation/disinfection
› draining and cooling of the sterilised effluent
› rinsing of the sterilising vessel with demineralised water (type L500S)
› cycle end /stand-by mode
Safety features
› PLC controlled programme sequences
› permanent monitoring of the sterilising parameters to ensure maximum process safety
› automatic leakage test of the drain valves
› password-protected programme editing
We take care of your infectious waste water
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Company ›
Areas of Activity ›
Maiwald Intellectual Property
Elisenstraße 3, Elisenhof
80335 Munich
Germany
Dr Eva Dörner
+49-89-747266-0
+49-89-776424
info@maiwald.eu
www.maiwald.eu
I Q
260
1995
Law Firm
Sectors:
| Pharma & Biotech
| Organic Chemistry & Polymers
| Inorganic & Construction Materials
| Food & Agriculture
| Electrical & Mechanical Engineering
| Communication & Information Technology
| Mobility & Energy
| Displays & Light
| Measuring & Process Technology
| Medical Technology & Imaging
| Artificial Intelligence & Digitalization
Legal Areas:
| Patents & Utility Models
| Supplementary Protection Certificates
| Trademarks & Designs
| Copyright
| Competition & Antitrust Law
| Compliance
| Contract Law
| Employee Invention Law
| Pharmaceutical Law
| Data Protection
| Plant Variety Protection
| UPC
Strategy:
| IP Search
| IP Consulting
As one of Germany’s largest and best-known firms in the field of intellectual property, Maiwald’s foremost aim is to achieve the best possible solution for each client in each particular case. Maiwald employs about 250 people working out of Munich and Düsseldorf. The interdisciplinary teams work closely with each other and with foreign associates, always with an eye to the client’s particular needs, whether start-up, medium-sized firm, or large corporation, across all industrial sectors. Maiwald’s team does its utmost to ensure that all IP matters are handled with competence and care, irrespective of the client’s physical location, whether in Germany, Europe, or beyond.
Client-oriented solutions and personal consultation are at the heart of Maiwald’s professional approach. About 100 highly qualified patent attorneys, attorneys-atlaw, and technical experts, an in-house patent search department, and numerous client-specific teams of paralegals put their extensive range of skills and experience at the clients’ disposal in order to arrive at the best possible customised IP solutions. Client satisfaction is evidenced by the long-standing business relationships with many prestigious corporations.
Maiwald advises and represents domestic and international clients from all technical fields across the entire spectrum of intellectual property law. When appropriate, an interdisciplinary exchange of information and expertise can take place between various teams, ensuring that the optimal solution is achieved in each particular case.
Maiwald’s team of patent attorneys combine their expertise across various technical fields and advise and represent clients across a wide range of industries.
Maiwald’s patent attorneys have extensive experience in the management of international patent portfolios. Services include drafting and filing patent applications
and coordinating worldwide prosecution as well as defending, enforcing, and contesting IP rights. Patent attorneys also prepare freedom-to-operate (FTO) opinions based on thorough research and analysis to establish whether existing IP rights of third parties could potentially stand in the way of a client’s particular product or manufacturing process. Clients also benefit from the exceptional expertise in opposition and appeal proceedings before the German and European Patent Office as well as in infringement and nullity proceedings before the national courts.
Maiwald’s team of attorneys-at-law can boast many years of experience in trademark, design, copyright, and contract law as well as patent infringement litigation, particularly in the conduct and coordination of international litigation proceedings.
Close cooperation between patent attorneys and attorneys-at-law is especially important in patent infringement litigation, since this type of proceedings generally calls for a comprehensive overview of the various national regulations as well as a thorough grasp of the particular technology. The interdisciplinary teams provide a comprehensive package of services to guarantee the successful conduct of legal proceedings in an international arena, also in cases where speed is of the essence, such as in preliminary injunction proceedings or border seizure procedures.
However, Maiwald’s attorneys-at-law would be the first to acknowledge that litigation is not always the best solution. They are skilled advocates in arbitration and mediation proceedings and are trusted experts when it comes to negotiating licensing contracts

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Medicon Valley Alliance
Arne Jacobsens Allé 15, 2 2300 Copenhagen Denmark
David Munis Zepernick, Director Member Engagement and Communication
+45-70-20-1503
mva@mva.org
https://mva.org/ I
12
1997
Events, networks, business development, your gateway to the binational Danish-Swedish Medicon Valley region
Medicon Valley – EU’s largest life sciences region
Medicon Valley is the bi-national life sciences cluster spanning the island of Zealand in Eastern Denmark and the Skåne -region of Southern Sweden, with more than 1150 life sciences companies and more than 65,000 privately employed life sciences professionals.
Medicon Valley is the crucible of Scandinavian life sciences. Located at the gateway to Denmark and Sweden it has a vibrant ecosystem and deep talent pool underpinned by world-class life sciences universities and research infrastructure.
Set in a competitive business environment with the Scandinavian quality of life close at hand, Medicon Valley is an attractive location for both businesses and people.
Scandinavian innovation is globally recognised, and our life sciences reflect this. Within Medicon Valley we have a rich life sciences heritage and pioneering spirit that continues to attract many successful companies. Companies like Novo Nordisk, Ferring, Genmab, Baxter Gambro, McNeil AB, PolyPeptide, and recently merged Novozymes and Chr. Nansen – now Novonesis – are representative, but so too are the many smaller and medium-sized innovative Danish and Swedish life sciences companies that continue to energise the area. Many of them choose to become members of the regional network organisation Medicon Valley Alliance and help strengthen the regional life sciences cluster. Furthermore, Medicon Valley is home to five universities, which supply life science-related education, and research facilities such as MAX IV Laboratory and European Spallation Source.



Founded in 1997, Medicon Valley Alliance (MVA) is today a non-profit membership organisation in the Danish-Swedish life sciences cluster Medicon Valley. Our 300+ members – including more than 60 biotech companies – represent the region’s triple helix and include universities, hospitals, human life sciences firms, regional governments, and service providers. We exist to develop Medicon Valley into the leading life sciences cluster in Northern Europe and make the region an attractive destination for the best talent within the field. We create value for our growing number members from both inside and outside the Nordics by launching and driving initiatives that put Medicon Valley firmly ahead in the global race for talent, and we connect, develop, and promote scientific strongholds in the region.
On average MVA hosts and organises 30+ annual network meetings, seminars, conferences, and other events, which serve as meeting and market places for member companies and organisations both within and outside the region or with an interest in exploring the potential of Medicon Valley and reaching out to potential new partners and customers.
Learn more at www.mva.org
As a member of Medicon Valley Alliance you can introduce yourself and your company’s expertise to the region using the MVA Good Morning Meeting concept, which allows you to co-organise awareness-raising events with MVA at the reduced member price. MVA Good Morning Meetings are typically 1½ hour events – online or physical – where you get the opportunity to demonstrate your expertise in problem-solving in an educational manner to a relevant audience of potential partners and customers. These events are typically hosted and marketed by MVA, and a qualitatively and quantitatively successful turnout is practically guaranteed.
Learn more on www.mva.org/events/good-morningmeetings/

Name ›
Meissner Bolte Patentanwälte
Rechtsanwälte
Partnerschaft mbB
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Widenmayerstr. 47
80538 Munich
P.O. Box 860624, 81633 Munich
Germany
Dr Lukas Bischoff, Dr Felix Letzelter +49-89-2121860 +49-89-21218670
mail@mb.de
www.meissnerbolte.com
I Q I
350 1908
Fields:
| Organic and Inorganic Chemistry
| Polymers
| Pharma
| Biotech
| Food and Agriculture
| Plant varieties
| Bioinformatics
| Artificial Intelligence
| Computer Implemented Inventions
| Medical technology
Legal Areas:
| IP Prosecution
| IP Litigation
| Patents
| Utility Models
| Supplementary Protection Certificates
| Plan Variety Protection
| Trademarks
| Designs
| Employee Invention Law
| Inspection Proceedings
| UPC
Services:
| IP Portfolio Management
| IP Search
| Freedom to Operate analyses
Developing new chemical products and medications is often a lengthy process involving very high costs. In order to ensure that the high investments ultimately pay off, most chemical and pharmaceutical companies rely on the monopoly granted by patents on end products.
Our qualified team of Patent Attorneys, specialised in chemistry and pharmaceuticals, routinely support multinational and medium-sized companies, start-ups, and scientific institutions in protecting the results of their research and development.
We are ready and able to advise on obtaining, enforcing, and defending protective rights in the chemical and pharmaceutical field. Areas in which we have proven expertise include the fields of: detergents, cosmetics, food and dietary supplements, textiles, tyres, alternative fuels, paints and coatings, adhesives, nanotechnology, polymers, construction chemicals, paper, packaging, classical active pharmaceutical ingredients, as well as pharmaceutical formulations and antibody drugs.
As the modern field of life sciences merges increasingly with the field of computer science to provide bioinformatics tools or artificial intelligences for solving biological problems, we offer an interdisciplinary team of computer- and life-scientists in order to best advise in this rapidly developing field of technology. We are here to assist you in the preparation of patent applications and support and accompany you on the road to a patent grant and to further represent and support you in opposition, nullity, and infringement proceedings. Expert opinions, FTO analyses, and due diligence examinations in the chemical and pharmaceutical sector are also part of our daily business. In the pharmaceutical sector, we also handle applications for supplementary protection certificates (SPC).
Our Patent Attorneys specialising in chemical, in biotech and pharmaceutical patents look forward to your contact at any time!



Meissner Bolte is a fully integrated IP law firm providing legal services in all areas of intellectual property. Filing more than 2,000 patent applications per year, Meissner Bolte is one of the largest specialised IP boutique firms in Germany.
Boasting eleven offices throughout Germany and one office in the UK, our professionals combine close contact to clients with short lead times. With more than 60 patent attorneys and a total of 350 employees, Meissner Bolte handles patent portfolios of all sizes.
Our strong team of attorneys at law works in close cooperation with our patent attorneys to resolve all IP disputes, making use of the full know-how and technical expertise available. Our attorneys are currently handling over 50 cases before all major patent courts, with our clients benefitting from detailed knowledge of both the proceedings and judges at these courts. All patent attorneys are qualified to represent clients before the novel Unified Patent court.
We further have an in-depth knowledge of German inspection proceedings, having successfully concluded dozens of cases to date.
Well-known “Of Counsel” support at Meissner Bolte:
› Rainer Engels, presiding judge BPatG, retired
› Dr Hans-Peter Felgenhauer, Member of the Board of Appeal, EPO, retired
Native speaking experts, including of English and Japanese, provide valuable support to many of our international clients.
Industrial strengths:
› Specialist Chemistry/Pharmaceutical/Biotech Department
› Special department for handling computerimplemented inventions

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Microcoat Biotechnologie GmbH
Am Neuland 3
82347 Bernried Germany
Dr Ingrid Wanninger (Business Development)
+49-8158-9981-0
+49-8158-9981-10
info@microcoat.de
www.microcoat.de
I > 200
1992
S1/S2, GLP, GCP, GMP, ISO 9001, EN ISO 13485
| Contract manufacturing
| Custom development
| Laboratory services
| Endotoxin and pyrogen testing service
External ›
Collaborations Request for ›
Further Collaborations
Business-to-business with industrial partners in the pharmaceutical industry, diagnostic industry and life science industry
Microcoat seeks joint projects and service partnership with pharmaceutical, biotech and diagnostic companies, as well as with players in the field of food safety testing, crop science and instrumentation (medical devices).
Microcoat offers a wide range of individual and specialised services for the diagnostic and pharmaceutical industry. In close cooperation with our customers, we aim for best performance, building on a complete range of advanced technologies and uncompromised quality standards.
For pharmaceutical companies, we leverage our profound expertise in in-vitro diagnostics and assay development in order to become a preferred partner in early development, pre-clinical and clinical phases of drug development and patient sample testing.
For food safety, environmental testing and other specialised diagnostic fields, Microcoat aims to be the preferred OEM-partner for the development and manufacturing of reliable test components and kits.
Microcoat is an approved original equipment manufacturer (OEM) for diagnostic test kits, bulk and finished components. We manufacture according to ISO and IVD standards. Since all critical test components are produced and modified in house, we are able to control quality at all stages of the production process.
Production technologies:
› Fermentation (30 litre scale)
› Downstream processing
› Protein chemistry
› Microplate and particle coating
› Dispensing/filling/labelling
› Freeze-drying
› Kit assembly
Product categories:
› Coated microplates
› Lateral flow test strips
› Recombinant proteins/antigens
› Antibody/protein conjugates
› Liquid components
› Dried/lyophilized components
› Diagnostic kits





Microcoat offers unique expertise in diagnostic assay and product development. A broad spectrum of methods, skilled staff and intensive communication with the customer are the cornerstones of successful project execution. Development projects are based on detailed milestone plans and follow standardised development guidelines through the five project phases (feasibility, development, verification, validation and manufacturing).
We develop for you:
› Immunological assays
› Molecular assays
› Sample and matrix preparation protocols
› Depletion protocols
In our certified facilities, we conduct a broad spectrum of GLP/GCP services to support drug development starting from early discovery to post-marketing surveillance. As an independent contract research organisation and preferred provider we serve pharma and life science industries worldwide. Based on long lasting experience in endotoxin and pyrogen testing, Microcoat offers a set of proprietary methods and skilled scientific personnel for GMP release testing as well as non-routine projects. Services are run as flexible customer-specified projects including the search for root causes, exploration of realization alternatives, development of product-specific adaptions and GMP compliant validation of newly established methods.
Our services include:
› Biomarker testing
› Immunogenicity/PK assays
› Endotoxin and pyrogen testing
› Assay development and validation
Microcoat is focused on business-to-business with industry customers, seeking a reliable and long-lasting partnership for outsourcing projects such as contract manufacturing (OEM), assay and product development, and bioanalytical testing in the context of pre-clinical and clinical studies.
Name ›
Subsidiaries ›
Microsynth AG
Microsynth Seqlab GmbH
Microsynth France SAS
Microsynth Austria GmbH
Ecogenics GmbH
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Schützenstrasse 15
9436 Balgach
Switzerland
Dr Markus Schmid, Christof Wunderlin (Co-CEOs)
Dr Christoph Grünig (Head of Contract Research)
Johanna Brändli
(Head of Quality Management)
Telephone ›
Email ›
Website ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
+41-71-722-83-33
info@microsynth.ch
www.microsynth.com
www.ecogenics.ch
1989
S1/S2, GMP/GLP
| Oligonucleotide synthesis
| Sanger sequencing
| Next generation sequencing
| DNA/RNA isolation
| PCR, qPCR, digital PCR
| Genotyping
| Contract research/outsourcing
Contract Research ›
| Assay Development
| Assay Validation (ICH Guidelines)
l GxP analysis of customer test items (Sanger, NGS, qPCR & dPCR)
| Process outsourcing
| Genomics research
Microsynth is a leading European company in the field of nucleic acid synthesis and analysis. Its main activities involve oligonucleotide synthesis, DNA/RNA analysis and sequencing, as well as contract research. For over three decades, the company’s goal has been to serve its customers by delivering products and services of the highest quality, on time, while offering outstanding service – and all this at competitive prices. Microsynth has subsidiaries in Germany (Microsynth Seqlab GmbH), France (Microsynth France SAS), Austria (Microsynth Austria GmbH), and Switzerland (Ecogenics GmbH). In total, Microsynth employs a staff of more than 130 people.
Microsynth is a premier expert in oligonucleotide manufacturing, providing solutions for diverse applications. From research primers to diagnostics and therapeutic oligos (ASOs, siRNAs) for drug discovery and preclinical testing, we meet your needs. Choose from various backbones (DNA, RNA, 2’-MOE, 2’-OMe, LNA, PTO), >250 modifications (MGB, GalNac, Palmitate), and purification options. Backed by decades of expertise, we drive innovation through technology and protocols. High-throughput platforms and rigorous processes ensure quality, while skilled chemists optimize strategies. Whether it’s a specific project or complex demands, trust Microsynth for tailored oligonucleotide solutions.
With over 30 years of experience, Microsynth is a leading provider of DNA Sanger sequencing services in Europe. Our strategically located Sanger sequencing laboratories in Switzerland, Germany, France and Austria enable efficient and environmentally friendly sample collection. Our commitment to innovation is exemplified by Ecoli Nightseq®, a groundbreaking service offering faster and more cost-effective E. coli plasmid sequencing.
Microsynth’s expertise extends beyond Sanger sequencing. We embrace next-generation sequencing technologies and offer comprehensive support for Illumina and ONT platforms. Our services cover a wide range of applications, from DNA and RNA sequencing

for various organisms to plasmids and cosmids. We accommodate projects of all sizes, from single loci to complex metagenomic studies. Our commitment to excellence doesn’t end with data generation; our customised bioinformatics pipelines and comprehensive support ensure accurate and user-friendly results. In addition to sequencing, we excel in nucleic acid isolation, PCR (from classic to digital), fragment length analysis, and genotyping by sequencing.
Over the past decade, Microsynth has evolved into a globally renowned contract research organisation, offering cGMP services in assay development, qualification, validation, and sample testing. Our team has extensive knowledge and experience working with scientists, QA/QC professionals, and project managers from top notch pharma and biotech companies to achieve critical milestones cost-effectively and on time. Particularly, in the burgeoning domain of advanced therapy medicinal products, including gene, cell, and RNA therapies, we have carved a distinctive niche. Here, we have excelled in the development and application of nucleic acid-based analytical methods, establishing a noteworthy reputation.
Microsynth prioritizes continuous production process enhancement with a focus on regulatory compliance. We have ISO 9001:2015 certification across all branches. Moreover, our NGS, Sanger, and fragment length analysis (FLA) departments at Balgach and Vienna (Sanger and FLA) are ISO/IEC 17025:2017 accredited (STS 0429), showcasing our commitment to precision. All our genetic analyses platforms are certified by Swissmedic as GMP-compliant for quality control (chemical, physical, biochemical and biological) of medicinal products as contract laboratory. The scope includes transplant products (TpP), gene therapy drugs (GT), as well as medicinal products involving genetically modified organisms (GMOs) or containing GMOs, all of them with intended use in humans. Lastly, we maintain ISO 13485:2016 certification for production and distribution of nucleic acids and components for medical use and provision of associated activities.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Molecular Devices
660-665 Eskdale Road RG41 5TS
United Kingdom
Janet Graystone
+44-118-944-8000 infoboxeu@moldev.com moleculardevices.com
F I
1,000+
1983
We are the innovation partner that empowers scientists with nextgeneration technology to advance discoveries, improving the quality of life everywhere.
| Cell Line Development
| CRISPR Gene Editing
| Drug Discovery
| 3D Biology
| Laboratory Automation
Since 1983, our mission has been to drive innovation in the life sciences sector, focusing on enhancing human, plant, and animal life globally. We empower researchers with breakthrough technology, simplifying the complexities of biological systems to pave the way for new therapeutic developments. As a key part of the Danaher consortium, we leverage unparalleled science and technology leadership to enhance our solutions, broadening our impact and reach. Serving as a trusted partner, we offer comprehensive solutions across a wide range of scientific disciplines to academia, biopharma, and government research sectors, enabling transformative solutions for some of the planet’s most urgent challenges.
Our commitment to advancing scientific research is particularly evident in our focus on cell line development and clonal screening. These areas are pivotal for accelerating the time to market for life-saving therapeutics, including monoclonal antibodies and genomic medicines. Our innovative technology and expertise enables groundbreaking research in cell and gene therapies, genetic engineering, and precision medicine. Through automated clonal screening, we play a crucial role in the drug discovery process, ensuring the development of effective, safe therapeutics.
Our technology platforms, such as the QPix® Microbial Colony Picker and DispenCell™ Single-Cell Dispenser, are at the heart of our efforts to enhance laboratory efficiency. These systems not only improve the accuracy and throughput of workflows but also ensure the integrity and traceability of data. By automating critical processes, we enable researchers to focus on innovation without the worry of data loss or inefficiency.

We understand the importance of high-throughput screening in today’s research environment. Our Lab Automation services are tailored to meet the unique needs of each research project, integrating the latest technologies for maximum efficiency. By offering customised, automated solutions, we help researchers optimise their time and resources, accelerating the path to discovery and development.
With a presence in over 21 countries, we are committed to supporting the global scientific community. Our efforts in cell line development and clonal screening have positioned us as a key player in the life sciences industry, empowering research that leads to innovative therapeutics. Our solutions are designed to meet current research needs while being adaptable for future challenges, ensuring our partners can continue their vital work without limitations.
The importance of cell line development and clonal screening cannot be overstated, especially with the advent of new technologies like mRNA vaccines and CRISPR-based gene editing. These areas are critical for the future of drug discovery and personalised medicine, offering promising avenues for treating infectious diseases, cancer, and genetic disorders. As we look forward, the integration of artificial intelligence in our systems will further revolutionise the field, enhancing our ability to select and identify the most promising therapeutic candidates.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Molzym GmbH & Co. KG
Mary-Astell-Str. 10
28359 Bremen
Germany
Marina Linow
+49-421-69-61-62-0
+49-421-69-61-62-11
info@molzym.com
www.molzym.com
I 35 2003
Manufacturer and supplier of products:
| CE IVD kits for infection diagnosis
| Microbial DNA isolation (manual and automated solutions)
| DNA-free PCR reagents, assays and polymerases
| Customized master mixes
| OEM business
Request for ›
Further Collaborations
Molzym is interested in the following partnerships or collaborations:
| Development and production of individualized, pre-analytical and analytical solutions, including automation.
| Service provider or companies in the field of Next Generation Sequencing, e.g., to establish and improve NGSbased workflows for pathogen identification directly from clinical samples.
Nucleic acid extraction is an important step in molecular microbiological processes as it significantly influences the entire downstream workflow. Molzym focuses on culture-free detection of infectious agents that can cause severe diseases and developed a unique technology for targeted enrichment and extraction of microbial DNA from clinical samples.
Since 2003, Molzym’s sole aim has been to avoid the GIGO effect and to provide laboratories with solutions for highly sensitive and broad detection and identification of pathogens.
Molzym relies on 2 processes that ensure the most efficient extraction: (1) the depletion of non-target human DNA from clinical samples and (2) the production of ultra-pure reagents.
Based on these special characteristics, various highquality products for molecular microbial analysis by PCR- and NGS-based methods are available in over 30 countries.
From CE IVD-marked broad-range molecular diagnostic kits, host DNA depletion technology, MolYsis™, to ultra-clean PCR reagents and assays, Molzym is one of the leading providers of culture-independent molecular solutions.
Our technology enables the detection of microbes directly from body fluids, tissues, and swabs using PCR or NGS-based methods. Pathogens can be identified within hours, significantly improving the management of patients with rapid, pathogen-targeted antimicrobial therapy.



MolYsis™ is Molzym’s proprietary technology of host DNA depletion allowing targeted isolation of microbial DNA from a great variety of samples. Ultra-pure, DNA-free PCR reagents and master mixes allow for the precise detection of microbes from human samples and animal models. Complete assays for the broadrange amplification of selected regions of the 16S and 18S rRNA genes of bacteria and fungi are available. Basic and dye master mixes can be used with custom primers for PCR and Real-Time PCR analysis.
The latest development is a walk-away robotic system, SelectNA™plus, for the fully automated host DNA depletion and extraction of microbial DNA from clinical and other material. SelectNA™plus conveys utmost technical advances to the demands for contamination-free and low-hands-on processing of samples.
With SepsiTest™-UMD and Micro-Dx™, Molzym offers two CE-marked in vitro diagnostic solutions for the culture-independent detection of pathogens causing various diseases. Both kits include reagents for the depletion of human DNA and the targeted isolation of microbial DNA from a variety of clinical samples, e.g. whole blood, CSF, BAL, joint aspirates, tissue biopsies, abscesses and other specimens. Manual DNA isolation can be accomplished with SepsiTest™-UMD and with Micro-Dx™ the process is fully automated on the SelectNA™plus robot. Precise detection is performed with broad-range 16S/18S PCR and sequencing analysis to identify the pathogens down to the species level. Both assays are especially applicable for samples which were negative after culturing, e.g. due to prior antibiotic treatment or fastidious growth requirements.
The Molzym team appreciates your interest and is at your disposal for further information about our products and developmental projects. Please call at: +49-421-69-61-62-0 or send your inquiries to info@molzym.com.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Neovii Biotech GmbH
Am Haag 6+7
82166 Gräfelfing
Germany
Sebastian Hofmann +49-89-898888-0 +49-89-898888-16
info.germany@neovii.com
www.neovii.com
ID-Number: ATM.00003-2.0
120
2003
BioStoffV S3**
cGMP certified production facilities, EU (Annex 1 EU-MP guideline) up to grade A respectively ISO5 (according to EN-ISO 14644-1)
The company Neovii Biotech GmbH – as part of the Neovii Group – is dedicated to delivering targeted biopharmaceutical treatment options in transplantation medicine and haematological oncology. The focus of its activities is the development and commercialisation of immunologically active biopharmaceutical therapeutics based on innovative antibody technologies. All the manu facturing activities are located in Gräfelfing/ Germany.
Neovii has a presence in more than 40 countries worldwide.
For further details please visit:
www.neovii.com
Areas of Activity ›
| Transplantation Medicine
| Haematological Oncology
External ›
Collaborations
Academic research institutions and other companies
Neovii supports research and development activities in the fields of solid organ transplantation, stem cell transplantation, and immune and haemato-oncological disorders. Neovii actively seeks in-licensing and acquisitions opportunities. We are looking to expand our portfolio of products with novel life-transforming therapies that address severe unmet medical needs, in particular in the areas of transplantation, haemato-oncology, and immune disorders.
Since its inauguration Neovii Biotech has been manufacturing and commercialising a medicinal product for the prevention of graft-versus-host-disease after stem cell transplantation and prevention/treatment of acute organ rejection following solid organ transplantation.


The main fields of our operations are:
› Stem Cell Transplantation
› Solid Organ Transplantation
› Aplastic Anaemia
› Autoimmune Diseases
Transplantation: ATLGs (Anti-human T-lymphocyte immunoglobulins) – polyclonal antibodies
ATLGs are antibody-based medicines for the targeted suppression of immune responses (immunosuppressants).
They suppress immune responses by multiple mechanisms, such as immune cell depletion, inhibition of T-cell function, and induction of regulatory immune cells. Their immunosuppressive properties are used mainly in solid organ transplantation and stem cell transplantation. In stem cell transplantation (SCT) polyclonal antibodies are indicated for the prevention of graft-versus-hostdisease (GVHD).
GVHD is recognised as a severe complication following SCT that negatively impacts on the patient’s quality of life and is a major cause of morbidity and mortality. In GVHD, transplanted immune cells attack the recipient’s body, causing tissue and organ damage. By suppressing these immune reactions, ATLGs significantly lower the incidence of GVHD (Finke J, et al‚ Lancet Oncot 2009;10(9)1855—64).
In solid organ transplantation (SOT), polyclonal antibodies are indicated either for the prevention of organ rejection or to suppress active, acute rejection reactions. The rejection of a transplanted organ is primarily caused by immune cells of the recipient that start attacking the grafted organ, because it is recognised as foreign. By suppressing these immune reactions, ATLGs limit organ damage and prevent graft loss (Kaden J, et al. Ann Transplant. Jan 10 2013;18:9-22).

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Founded (year) ›
New England Biolabs GmbH
Brüningstr. 50 – Geb. B852
65926 Frankfurt am Main
Germany
Dr Carsten Lanwert +49-69-305-23140
+49-69-305-23149
info.de@neb.com
www.neb-online.de
F I I
1981
Founded in the mid-1970s as a collective of scientists committed to developing innovative products for the life sciences industry, New England Biolabs is proud to be a world leader in the discovery and production of enzymes for molecular biology applications.
Created by scientists for scientists, NEB prioritizes the advancement of science, stewardship of the environment, and giving back to the world around us in everything we do. Since our establishment in 1974, we have remained committed to developing high quality, innovative products that not only empower your research but also our own. Our profits have always funded an extensive research program, which we believe is critical for staying connected to our customers and helping to drive scientific breakthroughs. From our founding principles to our unique corporate culture, NEB’s philosophy can be distilled down to three core values: passion, humility and being genuine.
NEB offers the largest selection of recombinant and native enzymes for genomic research. While restriction enzymes remain part of our core product portfolio, our ever-expanding catalog also includes products related to PCR, gene expression, sample preparation for next generation sequencing, synthetic biology, glycobiology, epigenetics and RNA analysis. Additionally, we are also focused on strengthening alliances that enable new technologies to reach key market sectors, including molecular diagnostics development and nucleic acid vaccines.
We believe that basic research and the cultivation of scientific knowledge is critical for us to stay connected with our customers and to drive scientific breakthroughs. At NEB, over 30 labs participate in research projects, which are aided by post-doctoral fellows and students in Masters and Ph.D. programs. NEB researchers have authored or coauthored over 1,450 publications to date, the vast majority of which are in peer-reviewed journals. With a dual focus on basic and applied research, NEB’s culture is both collaborative and academic – Our scientists actively participate in collaborations with their peers and share their research through speaking engagements and poster presentations.

Our outstanding expertise in protein engineering and evolution has led to the creation of unrivaled enzymes and unique workflows in the library preparation and target enrichment for the NGS space. These products set the benchmarks for optimal performance and ease-of-use in modern molecular biology laboratories. Moreover, NEB’s specialised offerings for alternative DNA assembly, isothermal amplification as well as novel reagents for CRISPR/Cas9 based genome editing makes us a firstchoice supplier for today’s molecular biologists.
NEB is dedicated to providing research products of the highest quality. We are committed to processes that ensure the protection of the environment and our integrated quality and environmental management systems are certified to meet the requirements according to the standards ISO13485, ISO9001, and ISO14001.
NEB’s OEM business unit has been delivering tailor-made enzyme formulations and packaging solutions to biotech, pharma and diagnostic customers for over 25 years. Building on this extensive experience, we have recently expanded our GMP-grade manufacturing capabilities to offer a range of customized products at scale, enabling commercial customers and partners to use NEB’s worldclass products in their specific applications and to support them in their efforts to access regulated markets. Recently, we established our new subsidiary NEB Lyophilization Sciences – experts in the design, development and manufacture of innovative solutions for ambient store products.
The NEB subsidiary in Germany represents the service hub for Central Europe. From our location in Frankfurt, we serve scientists and industry customers in Germany, and Austria and support our network of distribution partners across Europe.
For up-to-date information please visit our website at www.neb-online.de.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone › Email › Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
External › Collaborations
nNano AG
Bodmerstrasse 12 3645 Thun
Switzerland
Sabrina Dällenbach +41-33-334 24 00 sales@nnano.com
nNano.com
I ~10
2024
Sales and support for micro-dispensing technology
Fritz Gyger AG
nNano AG was started on the 1st of January by the Gyger family and offers the ever-growing business with and around the CERTUS FLEX an enterprise to make sales and service even better, with structures perfectly designed for this purpose. Outsourcing the sales and service for the CERTUS FLEX offers great advantages – the customer has designated contact persons, better service and all information about the Certus-Family bundled at hand. Fritz Gyger AG is once again given more space to advance groundbreaking inventions in the field of microtechnology/micromechanics. Both companies are wholly owned by the Gyger family and are run by the 3rd generation.
Our products find their application, where micro technical thresholds are reached. Nearly impossible requirements regarding quality, miniaturization and integration density are the drivers for our innovative solutions. Our core competencies are microfluidics in the areas of genomics, proteomics, cancer research and drug discovery with applications like cell dispensing, biochemical & immunoassays, NGS, PCR, HTS and peptide synthesis.
Our liquid dispenser CERTUS FLEX optimizes low volume liquid handling using the patented state-of-the-art Gyger microvalves, which allow a maximum of accuracy and precision in contact-free multichannel dispensing at high speed.




Unique flexibility is provided by parallel operation of up to 8 independent channels dispensing different liquids at various volumes in a straight or angled manner depending on the valve head. Due to its unlimited programming possibilities, the dispenser can be employed in a broad range of applications to transfer fluids from bottles or syringes onto various plate types or by integrating the CERTUS FLEX into your automation workflow via the SiLA interface.
[Image 1 +2]
Based on the needs of our customers, we continuously offer new modules that offer application-specific benefits for the users. The latest development improving especially the dispensing of cells, beads and other dissolved particles, is the Magnetic Stirrer System, which can easily be attached onto the dispensing head of the CERTUS FLEX and ensures a homologous suspension while dispensing.
[Image 3]
The CLEANING STATION provides a quick and simple way to automatically clean your microvalves, saving you precious time while achieving the optimal cleaning results. Using 3 different cleaning solutions, up to 8 microvalves can be simultaneously cleaned with various cleaning programs to meet your specific requirements.
[Image 4]

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Northway Biotech
Mokslininkų 4
08412 Vilnius
Lithuania
Prof. Vladas Algirdas Bumelis (CEO) +370-5-210-2247
bd@northwaybiotech.com www.northwaybiotech.com
I
180-200 (Vilnius site)
2004
Biopharmaceuticals, Monoclonal Antibodies, Recombinant Therapeutic Proteins, Contract Development, Contract Manufacture, cGMP Manufacture, Analytical Services/ Testing
External ›
Collaborations
Northway Biotech cooperates with numerous biotech companies and also with some medium-sized companies and three Big Pharma companies.
Northway Biotech is a flexible biologics CDMO headquartered in the EU member state of Lithuania, a rapidly-growing biotech hub with a state-subsidised university system that supports a highly-educated scientific workforce. Northway Biotech is exceptionally well-versed in all aspects of the biopharmaceutical value chain, from cell line development to commercial production of drug substances (antibodies and recombinant therapeutic proteins) and aseptic fill-finish. The company partners with small biopharma companies, as well as large pharma companies providing the highest quality of customised services. Services are available from early to late phase clinical trial stages as well as scale-up and commercial manufacturing. Over the years, the company has worked with over 90 clients and has completed over 160 projects. Typical projects range from gene cloning to final drug product manufacturing, but Northway also handles, for example, a variety of tech transfer projects in different clinical phases. Most recently, Northway Biotech expanded to the Greater Boston Area, US, offering process development and cGMP manufacturing services at this second site as well.
Cell Line Development Capabilities:
› Single-cell cloning with analytical confirmation and stability studies for the development of mammalian cell lines;
› Development of bacterial and yeast strains with stable expression.
Upstream Process Development:
› Optimisation of media and feeding strategy at lab scale;
› Small-scale qualification and process characterisation studies;
› DOE experiments for yield and quality improvement;
› Scale-up and confirmation runs (up to 200 L SUB).




Downstream Process Development:
› Harvesting (centrifugation, depth-filtration) and cell disruption;
› Isolation and solubilisation of inclusion bodies followed by refolding of target protein;
› Protein purification at different scales using various chromatography techniques;
› Small-scale qualification and process characterisation studies;
› Rapid technology transfer and scale-up to cGMP manufacturing scale.
Analytical and Formulation Development Capabilities:
› Development, qualification, and validation of physicochemical, biochemical, and immunological analytical methods and cell-based bioassays according to ICH guidelines;
› Characterisation, monitoring, and optimisation of product’s quality attributes across all stages of development;
› Release and in-process control testing;
› Formulation development services for liquid and lyophilised drug product in vials and pre-filled syringes;
› Stability studies.
cGMP Manufacturing:
› GMP certified facilities
› Master and Working Cell Bank (MCB and WCB) manufacturing for microbial and mammalian cell cultures;
› Microbial fermentation up to 3000 L working volume in stainless steel reactors;
› Mammalian cell culture up to 4000 L working volume in single-use reactors;
› Fill and finish of sterile drug product into vials and pre-filled syringes (PFS);
› Lyophilisation of aseptic drug product into vials.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Nova Biomedical Corp
200 Prospect Street
Waltham, MA 02454-9141
United States
Matt McRae
+1-781-894-0800
+1-781-894-5915
info@novabio.com
novabiomedical.com
F I Q
1,500
1976
Cell culture analysis, bioprocess monitoring and optimisation, bioprocess automation, in-vitro diagnostics, point-of-care testing, blood gas analysis, critical care, glucose reference
Nova Biomedical develops, manufactures, and commercialises analytical systems for cell culture and whole blood testing. The company is headquartered in Waltham, MA, USA. Worldwide, more than 1,500 people are employed by Nova, which operates direct subsidiaries in 11 countries and factory-trained distributors in more than 110 countries.
Our commitment to scientific innovation and product quality has made Nova Biomedical a world leader in the development of whole blood analyzers for clinical applications and cell culture/fermentation analyzers for biopharma for more than 40 years.
GMP-compliant cell culture analysis made easy Nova offers the latest in maintenance-free cell culture analyzer technology – BioProfile ® FLEX2. FLEX2 provides fully automated analysis of key chemistries and gases, cell density, cell viability, and osmolality with one sample and a single data output stream in under 4.5 minutes. The full 16- test menu includes: Gluc, Lac, Gln, Glu, NH4+, Na+, K+, Ca++, pH, PCO2, pO2, total cell density, viable cell density, viability, cell diameter, and osmolality.
The system can be flexibly configured to meet diverse and changing needs with the optional and field upgradeable pH/gas, CDV and osmolality modules. sampling from 96-well plates, syringes, or a 24-position external “load-and-go” sample tray provides maximum workflow flexibility and efficiency for cell culture monitoring.
FLEX2 is the only cell culture analyzer designed for online sampling from micro-scale vessels through large-scale commercial manufacturing SIP/CIP systemsand integration with the ambr ® 15 and 250 cell culture systems. An optional Sample Retain Collection system automatically collects cell culture samples from the On-Line Autosampler and stores them in a refrigerated environment to enable further offline testing. IQOQ support can be offered by the local Nova representative.




BioPro fi le 300 series analyzers offer a total of nine measured tests, including glucose, lactate, phosphate or glycerol, acetate, ammonium, pH, sodium, and potassium, plus calculated osmolality. The analyzer is offered in two versions: the BioPro fi le 300A which includes a phosphate assay, and the BioPro fi le 300B with glycerol testing. These systems offer measurement ranges speci fi cally suited to monitor bacterial and yeast cultures. A conversion kit is available for easy interchange between the 300 A and B versions.
BioProfile FAST CDV is a high-throughput, fully automated viable cell density and viability analyzer with a throughput of 45 samples per hour on 100µL of sample volume. The analyzer performs all sample dilutions internally, enabling cell culture samples up to 140e6 c/ mL to be analysed without any external sample dilution. Cell culture samples can be analysed via the external 32 position load-and-go tray or via an innovative 96-well plate option.
FLEX2 Basic analyzers provide multiple test menus for chemistry analysis in cell therapy, gene therapy, vaccine development, and alternative foods applications. The FLEX2 Basic A test menu consists of Gluc, Lac, Gln, Glu, NH4+, Na+, K+, Ca++, pH, PCO2, pO2 with automatic dilutions and an extended range. The FLEX2 Basic B provides the same test menu with standard ranges. Flex2 Basic C offers an 8-test menu of Gluc, Lac, Na+, K+, Ca++, pH, PCO2, pO2 with standard ranges.
Nova Primary Whole Blood Glucose Reference Analyzer
Nova Primary fills the need for a replacement to the discontinued YSI STAT PLUS 2300 Glucose and L-Lactate analyzer. Nova Primary uses a single, reusable glucose electrochemical sensor based on glucose oxidase and has a measurement range of 20-900 mg/dL. It uses a small, 25 µL whole blood or plasma sample and provides results in under 2 minutes.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Omicron-Laserage Laserprodukte GmbH
Raiffeisenstrasse 5e
63110 Rodgau
Germany
Natalie Stühler
+49-6106-8224-0 +49-6106-8224-10 sales@omicron-laser.de www.omicron-laser.de
F I I
99 1989
Laser, LED, Light Sources, Diode Lasers, Medical Lasers, Medical Systems, CW Light Sources, Customised Laser Systems, Photonics, Laser Development, Laser Solutions, Customised Light Sources, Biotech, Life Sciences, Microscopy, Spectroscopy
Omicron-Laser has been developing, manufacturing, and distributing innovative laser and LED light sources since 1989 and is one of the leading manufacturers for demanding applications in biotechnology, microscopy, microlithography, medicine, and many more.
Based in Germany, Omicron is specialised in customised solutions in addition to its broad product portfolio. Product development and production comply with European and US guidelines and are conducted according to the ISO9001 and ISO13485 quality standards. Omicron offers CW light sources as well as high-speed modulated systems in the nanosecond and picosecond range, both as single and multi-wavelength solutions.
Important developments based on ISO 9001 and ISO 13485 include:
› the LightHUB® laser beam combiner series
› the LedHUB® high-power LED light engine
› the QuixX® picosecond pulsed diode laser
› the PDT medical laser system for cancer treatment
› the BrixXLAB multichannel desktop laser for laboratory applications
As a symbiosis of the proven LuxX+® diode lasers and a desktop housing, the LaserNest® is an easy-to-use plug and play laser light source for science and research. It is equipped with one laser module with wavelengths from the UV to the near IR range and offers rapid analogue intensity modulation with up to 3MHz and rapid digital modulation with up to 250MHz. An additional electronic shutter function provides complete on/off modulation with a switching time of <1μs and frequencies up to 500kHz.The light output can be either single-mode (PM) fibres, multi-mode fibres, or liquid light guides. The LuxX+® laser modules within the LaserNest ® are available with up to 500 milliwatts of optical output power and more than 30 different wavelengths between 375




and 1550nm. Suitable applications include microscopy, fluorescence analysis, and analytical processes.
Omicron’s innovative LightHUB+® and LightHUB Ultra® laser light engines represent a new form of laser light sources for science, research, and industry based on the ultra-compact LuxX (LuxX+) laser series. The highperformance systems can be equipped with one to six (LightHUB+®) or seven (LightHUB Ultra®) laser modules of different wavelengths from UV to the near IR range and offer rapid analogue intensity modulation with up to 1.5MHz (3MHz and 250MHz digital) and digital full ON/ OFF modulation with a switching time of <1µs for each channel. The individual laser modules can easily be exchanged and added by the user. Hence the systems can be adapted to changing application requirements and are future-proof.
The Omicron LedHUB® is a high-power LED light source for biotech, industrial, and analytical applications. With up to 6 different wavelengths between 340 and 940nm it can be used in applications like widefield microscopy, optogenetics, chemical analysis, forensics, and many more. The modular principle of the LedHUB® provides the possibility to start with only one or two wavelengths initially and upgrade by further wavelengths at a later stage. The capability of rapid switching between wavelengths and high-speed analogue modulation of intensity is a key feature for demanding applications.
Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Contact Person ›
Telephone ›
Fax ›
Email › Website ›
Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Annual Turnover › External › Collaborations
Request for ›
Further Collaborations
OPIS s.r.l.
Via Matteotti 10 20832 Desio Italy
Giovanni Trolese
+39-0362-633-1
+39-0362-633-633 office@opiseurope.com
www.opiseurope.com
www.clinical.net
I ca. 500 1998
Premium support for clinical drug and device development
€ 30 m
Pharma | Pharma
| Biotech
| Medical device
| Food supplements
OPIS is a Global Full-Service Clinical CRO providing premium clinical trial management for clinical trials. Phase I-IV drug trials, pre and post-marketing clinical investigations for medical and diagnostic devices, nutraceutical and food supplement studies.
Founded in 1998, OPIS is a Global CRO, with over 500 employees. The company operates from eighteen offices located around the world (Italy, Spain, France, Germany, UK, Belgium, Sweden, Poland, Switzerland, The Netherlands, United States, Taiwan, Australia, Hungary, Serbia, Bulgaria, Japan, South Korea).
In 25 years of combined medical, regulatory, statistical and technological expertise, OPIS has supported a huge number of different clients with interventional and observational trials.
1500 clinical studies managed to date, covering a wide range of therapeutic areas, including various rare disease indications and pediatric studies. 40% of the trials are in of oncology and onco-hematology. We have covered all therapeutic OPIS has established solid collaborations with KOL all around the world leveraging an important network of experts.
We draw on our know-how, experience, professionalism, and ethical values to assist our partners in developing drugs in compliance with laws and regulations while achieving objectives at a quick pace. Our strength lies in the passion for what we do and in the quality with which we deliver. We believe that clinical studies, particularly those incorporating scientific and methodological innovation, can significantly contribute to medical progress and a better quality of life.
Founded by medical doctors from the Pharma Industry, OPIS provides clinical research services and drug development consultancy to pharma and biotech. From early-phase clinical trials through proof of concept and up to late-phase and post-market research, projects are executed in compliance with regulatory requirements

and ICH-GCP E6 guidelines, with therapeutic-specific attention and high-quality training for top-quality services. Operational staff, including medical writers, regulatory experts, project managers, and monitors, as well as data managers, biostatisticians, and SAS programmers, assist sponsors with full-service study management of multi-country trials.
The clinical trial data handling solution Clinical.net is fully FDA 21 CFR Part 11-compliant and modular in design to digitally manage EDC, protocol deviations, patient randomization, and study drug management, innovative Medical Monitoring module and ePRO/eCOA.
Study types
Phase I to IV Clinical Trials • Healthy Volunteers studies • PK and PD studies • Medical Device Clinical Investigations • IVD Clinical Investigations • Nutraceutical studies • Bioequivalence Clinical Trials • Biosimilars Clinical Trials • Observational studies • Real World Evidence studies • Investigator Initiated Trials • Expanded Access Programs • Patient Name Programs • Compassionate Use Programs • Human Challenge studies
Services
Project Management • Pharmacovigilance • Regulatory • Trial Start-up • Audit • Investigators Meeting Organization • Vendor Management • Pharmacovigilance • Regulatory • Scientific Advice with EMA • Data Management • Pre-IND and New Drug Applications • All FDA Type of meetings and Interactions • CDISC Standards • Orphan Drug Designation • Functional Service Provider • Real World Evidence • Data Monitoring and Data Analytics • Monitoring Activities and Site Management • Medical Writing • Management of Trial Documentation • Interactive Voice Response System • Statistical Analysis • Medical Review and Medical Monitoring • Clinical.net EDC • ePRO/eCOA • Early Access Program/ Expanded Access • Patient Name Program • Compassionate Use • Protocol Writing • Trial Design • Strategic Regulatory • Medical Feasibility • Legal Representation • Steering Committee • Safety Review Committee • Independent Data Monitoring Committee Meetings • Data Safety Monitoring Board

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Pivot Park
Kloosterstraat 9
5349 AB Oss
The Netherlands
Emiel Westerhof
+31-4128-460-10
joinus@pivotpark.com
www.pivotpark.com
I 25
2012
| State-of-the-art laboratories
| Turnkey office space
| Open access laboratories with shared equipment
| Ultra-High-Throughput Screening centre
| GMP-certified pilot plants
| On-site analytical and entrepreneurial support
| Facilitate community building
| Minor study programme, summer school, lectureship, masterclasses and more!

Europe’s foremost biopharmaceutical campus
Pivot Park, located in Oss, the Netherlands, was founded in 2012. Today, Pivot Park is the hub for companies and knowledge institutes in the biopharma industry. Here, we do all in our power to ensure that the entrepreneurs at Pivot Park have every opportunity to accelerate their growth. Guided by the UN’s Sustainable Development Goals, we facilitate companies that specialise in innovative drug development in the fields of immunology and manufacturing technology. We further focus on entrepreneurship, community building and the availability of talent.
By investing in sustainable real estate, a world-class biopharmaceutical infrastructure and being integral to Europe’s best-connected life sciences and health ecosystem, we create the perfect conditions to enable start-ups and scale-ups to grow. Factor in the history of pharma in Oss, and the wealth of knowledge available locally, from R&D to manufacturing. Everything that is required to be Europe’s foremost biopharmaceutical campus for nurture a dynamic, pharma-based knowledge community that will together improve global health.
Today at Pivot Park 1,000 highly qualified people are working at 60 start-ups, scale-ups and established companies. We support their work with customised facilities, state-of-the-art laboratories, routes to finance, gateways to knowledge and access to outstanding scientific and entrepreneurial expertise. We make sure they have what they need to succeed.




Pivot Park facilitates community building. This includes helping our customers to stay abreast of the latest scientific developments, helping them secure access to funding and grants and offering skills-related learning. We also run and support activities that further intra-campus connections and provide businessoriented support for entrepreneurship.
Be inspired by interacting with others informally, every day, and at regular Pivot Park-wide events. Discover new opportunities for exciting collaborations. Leverage the diverse backgrounds and experiences of smart men and women from dozens of countries around the world.
Pivot Park is located in entrepreneurial Oss, the spiritual home of the Dutch pharmaceutical industry and the largest pharmaceutical production cluster in the Netherlands today. This is the ideal place to attract talent, spark new ideas and support growth, whilst simultaneously offering an unmatched work-life balance.
Learn more at www.pivotpark.com

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Pivot Park Screening Centre
Kloosterstraat 9
5349 AB
The Netherlands
Katja Stellingwerff +31-412-846050
info@ppscreeningcentre.com
www.ppscreeningcentre.com
I
25
2012
Screening Centre
biochemical, biophysical and cellular assay development, ultra-high Throughput Screening, hit-to-lead biological profiling
Pivot Park Screening Centre accelerates drug discovery by offering tailored services for early drug discovery. Specializing in lead discovery, we combine biochemical, biophysical, cellular assay development and miniaturization expertise with fully automated lab operations for ultra-high Throughput Screening (uHTS) and hit-to-lead biological profiling.
Having completed over 200 full-deck screening campaigns, numerous assay development solutions and hit-to-lead profiling projects across various target classes and disease areas, we continuously expand our capabilities to accelerate drug discovery programs of our customers.
› The largest screening infrastructure footprint in Europe
› State-of-the-art uHTS setup and experienced screening service provider
› Rapid generation of high-quality hits; All major readout modalities available in-house, including FLIPR, High-Content Imaging and Mass Spectrometry
› Specialist in miniaturizing assays to 1536-well format
› Handling of large customer compound libraries; bring your library or use our in-house 300k diversity-based compound collection
› Broad knowledge of different label-free and labelbased assay development solutions, including Affinity Selection Mass Spectrometry (ASMS)
Our scientists have longstanding and proven experience in assay development for various phenotypes and target classes ranging from kinases, GPCRs and ion channels to nuclear receptors, proteases and protein interactions with other proteins or nucleic acids in many therapeutic areas.
We collaborate with you to deliver tailored and highquality assays. No matter how challenging your target




is, our scientists will ideate out-of-the-box solutions and propose the best-fitting approach to develop the right assays suitable for target evaluation, uHTS, hit triaging and hit-to-lead biological profiling.
We provide access to a wide range of label-based and label-free biochemical, cellular, and biophysical assays optimized to deliver high-quality and robust data. Our scientists have ample experience in a variety of readout technologies, including the common label-based biochemical and cellular readouts such as Fluorescence, FRET/HTRF/TR-FRET, FP, Luminescence, absorption, FLIPR-based, high-content, phenotypic, as well as label-free MALDI-TOF mass spectrometry biochemical, affinity-based and cellular fingerprinting assays.
By means of miniaturization, we can reduce sample volumes and screen more compounds per plate, shortening hit discovery timelines and minimizing compounds and reagents use while maintaining reliability, robustness, and reproducibility.
Bring your library or use our 300.000 in-house IP-free compound library
Pivot Park Screening Centre has a diversity-based collection of over 300,000 high-quality drug-like compounds and offers ample flexibility to accommodate your library or request for specific vendor sets. Our fully automated robotic system can process up to 350,000 data-points per day and store up to 2.5 million compounds for on-the-fly screening.
By outsourcing your project to our passionate team, you can benefit from our longstanding experience with the most challenging targets and assays, our pharmastandard infrastructure and operations, all of which contribute to accelerating your lead discovery program. Our team can also facilitate hit prioritization by coupling the biochemical, cellular, and biophysical data generated in projects with in-depth medicinal chemistry knowledge and advanced cheminformatics tools to deliver the most promising and tractable hit series.
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
PlasmidFactory GmbH
Meisenstr. 96
33607 Bielefeld
Germany
Dr Martin Schleef/Dr Marco Schmeer +49-521-2997-350
+49-521-2997-355
info@plasmidfactory.com
www.plasmidfactory.com
I
49 2000
| Contract Manufacturing of plasmid and minicircle DNA in different quality grades for research up to clinical applications
| High Quality (HQ) Grade plasmid and minicircle DNA as starting material for GMP productions, e.g. of mRNA or viral vectors, also in gram scales
| In Stock Service for AAV Helper & Packaging Plasmids (pDG/pDP) and for further plasmids and minicircles (e.g. reporter genes)
| Analysis of plasmid topology
Biological Patents ›
PlasmidFactory owns any relevant know-how, patents, and licences for plasmid and minicircle manufacturing
PlasmidFactory is the leading contract manufacturer of plasmid and minicircle DNA and the driving force in the development of gene vectors for gene and cell therapy and genetic vaccination. PlasmidFactory’s research and development as well as all services are located in Bielefeld, Germany.
PlasmidFactory’s individual manufacturing service is frequently used by researchers from the fields of transfection and drug delivery, virus production, nanobiotechnology, gene therapy, cell or tumor therapy, and RNA or DNA vaccination. The company offers the production of plasmid and minicircle DNA in several quality grades: Research Grade and ccc Grade qualities for research purposes and pre-clinical applications, HQ Grade as starting material for e.g. GMP production of RNA, viral vectors and CAR-T cells. Full GMP will be available from 2024.
Our In Stock products are deliverable immediately “offthe-shelf” – e.g. reporter genes (gfp, lacZ, luc), AAV Helper & Packaging plasmids (2-Plasmid-System, pDG/ pDP family, several serotypes) or pEPI/pEpito plasmids (containing S/MAR elements).
External ›
Collaborations Request for ›
Further Collaborations
Fruitful long-term cooperations with renowned academic and industrial institutions in the fields of gene and cell therapy and vaccination
PlasmidFactory is always interested in working in long-term and strategic collaborations with pharmaceutical and biotech companies that require plasmid or minicircle DNA for (pre-) clinical applications.
On request, plasmid DNA storage and logistics can be organized, supplementing the company’s service portfolio.
PlasmidFactory’s proprietary method for quantification of the structural diversity of plasmid DNA by means of capillary gel electrophoresis (CGE) is the only reliable method able to determine the stability of plasmid DNA, e.g. during storage.
The
Customized plasmid & minicircle production
g Extensive expertise in manufacturing of minicircles e.g. for CAR-T cell production
High Quality Grade DNA for GMP production of viral vectors & RNA
QC including CGE service
pDG/pDP plasmids for AAV production
2 plasmid system: AAV packaging and AdV helper genes in a single construct
Various serotypes including AAV8 and AAV9
GFP transfer plasmids for ss- and sc-AAV
Dedicated QC for ITR sequence integrity –ITRRESCUE®
In Stock service PlasmidFactory.com

Minicircle – a safe vector system (MC) are circular DNA molecules that are generated e.g. by an intramolecular (cis-) recombination from a parental plasmid (PP). The difference between MC and standard plasmid vectors for gene therapy or nucleic acid vaccination is that the MC contains neither the bacterial origin of replication (needed only in bacteria for the amplification of plasmids in cell division) nor any antibiotic resistance markers or other selection systems to keep the plasmid in high amounts within the producer cell.
Hence, MC are the most promising tools to achieve both increased efficacy as well as regulatory requirements for future clinical applications.
PlasmidFactory is the exclusive owner of all relevant patents and IP in this field and provides service production of these supercoiled monomeric constructs, according to clients’ requirements.
As a special type of linear protected DNA vector PlasmidFactory established the production of MIDGE®-DNA.
In particular, the HQ Grade production of plasmid DNA as a starting material for the production of RNA vaccines has gained in importance, especially in the context of the COVID-19 pandemic, as RNA is a promising vaccine candidate for the prevention of certain virus infections, with the additional advantage of not integrating into the genome of the cell and thus remaining as a potentially effective molecule in a patient’s body in the long term.
PlasmidFactory has developed and established the process for the production of the corresponding plasmid DNA and is able to preserve long polyA stretches.

Name ›
Process Sensing Technologies PST GmbH
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Einsteinstrasse 17-23
76275 Ettlingen
Germany
Roland Scheurich, Managing Director
Telephone ›
Email ›
Website ›
Social Media ›
Founded (year) ›
Areas of Activity ›
Christoph Arnswald, Key Account Manager +49-7243-383-250 info@rotronic.de
www.processsensing.com
I
1965
Measurement Instruments and Environmental Monitoring Solutions
FDA 21 CFR Part 11/EU Annex 11 compliant GxP services including qualifications (thermal mappings), ISO17025 calibrations and validations.
Process Sensing Technology (PST) brings together well-established brands, each of which are trusted for the precision and reliability of their products, strong innovation and focus on customer service. Rotronic was founded in Switzerland in 1965 and is part of PST since 2017.
Within the Group, Rotronic is positioned as the competence centre for humidity & temperature measurement and environmental monitoring systems. This enables PST to offer its customers a uniquely broad range of measurement technology products that perfectly meet the requirements of biotechnology.
Biotechnology companies are subject to strict regulatory requirements and guidelines to ensure the safety and quality of their products. Environmental monitoring helps comply with regulations by tracking and documenting environmental conditions that could impact product quality and safety. Accurate and comprehensive environmental monitoring data is essential for maintaining data integrity and traceability.
Environmental monitoring of a laboratory and laboratory equipment is an integral part of the quality management system. It helps promote the quality and validity of data generated during the testing process and prevents fraudulent practices.
Trust in PST’s real-time monitoring experience and our wealth of knowledge of all critical environmental parameters to comply with the GLP/GSP/GMP/GDP regulations and ICH/FDA guidelines.
› Fridges
› Freezers
› Incubators

› Autoclave
› Cryogenic storage
› Laboratories & Cleanrooms
› Rooms in general
› Storage & Transport


Measurement types
› Temperature range: -200...850 °C
› Relative humidity
› Carbon dioxide CO2
› Oxygen O2
› Differential pressure
› Particle counting
› Light (lumens, PAR, lux, etc.)
› Door monitoring (open/closed)
› Webcams (pictures)
Additional services
› Calibration
We offer a range of ISO-17025 and traceable calibration services, both within our facilities and onsite.
› Qualification
PST offers qualification services for rooms and equipment to help ensure the perfect environment throughout all facilities. The qualification will ensure the placement of the sensors within the various environments to ensure the highest quality of measurement data.
› Validation
Validation includes the provision of documented evidence that a system was planned/produced according to quality guidelines, is tested against specifications and has been operated in a qualified manner since it was introduced. The PST real-time monitoring solutions are validateable and PST offers complete documentation, including IQ/OQ and PQ, to fully validate the systems.
› Consulting
PST offers a range of consulting services from the start of a project, including the URS, through the complete validation master plan, including developing SOP’s and risk analysis. Maintenance and support ensure the long-term stability and accuracy of your system.
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
PROGEN
Maassstrasse 30
69123 Heidelberg Germany
Caroline Odenwald
+49-6221-8278-0
+49-6221-8278-24
info@progen.com
www.progen.com/ I
42 1983
S1, S2, L2 class laboratories, DIN ISO 13485 certification for the production of in vitro diagnostic kits and research reagents.
PROGEN helps life scientists worldwide to make new discoveries and to conduct breakthrough research. We also support the gene therapy community in developing safe and reliable treatments. Our mission is to advance research processes and new therapies by providing high quality analytical tools – quickly and affordably.
As a manufacturer and provider of Adeno-AssociatedVirus (AAV) analytical tools, research antibodies, density gradient media and phage display technologies, we strive to understand what scientists need in research and gene therapy development. It is our goal that our products offer solutions for the manifold challenges in academia, biotech, and pharma along the complete value chain – from basic research to manufacturing and quality control, thereby enabling progress.
Areas of Activity ›
and antibodies, antibodies and reagents for biomedical research, in vitro diagnostics, ELISA, antibody phage display technology, density gradient media
Annual Turnover ›
External ›
Collaborations
Request for ›
Further Collaborations
€12.49m
Strong network with industrial and academic institutions
PROGEN is actively seeking partners for the advancement and distribution of its gene therapy and antibody-based products & services.
Labs attempting to find treatments for genetic disorders worldwide face obstacles such as a limited availability of accurate & reliable techniques for the characterization of AAV vectors. PROGEN is a leading manufacturer and exclusive provider of AAV antibodies and analytical tools for AAV titer determination, which facilitate the development of safe and efficient gene therapies. The density gradient media provided by us ensure a high yield purification of viral particles during production, while our AAV antibodies enable multi-step monitoring in vector manufacturing resulting in superior vector preparations. Exclusive AAV ELISA kits and controls allow an accurate capsid titer quantification that is critical for dose assessment. The pre-screening of patient sera for AAV-neutralizing antibodies using our AAV particle antibodies improves clinical safety and efficacy.
High-quality antibodies which allow the generation of reliable and reproducible data are indispensable tools for the life science community. The four PROGEN founders came together in 1983 to fulfil exactly that requirement – high-quality antibodies for biomedical research, which can generate reproducible data time and time again. Initially a spin-off from the German Cancer Research Center and the University of Heidelberg, PROGEN has expanded to become a DIN ISO 13485 certified company, which to date continues to serve the life sciences community with over 600 high-quality antibodies worldwide.
Starting as a pioneer antibody manufacturer, PROGEN has now become a globally operating biotech company and a reliable partner for academia, biotech and pharma. What still drives us after all these years is the continuous dialogue with researchers so that we can offer advanced products that truly make life science better.
PROGEN is a 100% subsidiary of R-Biopharm AG, based in Darmstadt/Germany, a developer of test solutions for clinical diagnostics and food & feed analysis. This corporate affiliation provides continuity for the growth of our product & service portfolio, which has been established for the last 38 years. CEOs Katja Betts & Maik Lander continue to expand their broad network and join forces with academia and industry to develop new solutions that add value.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Founded (year) ›
Type of Laboratory ›
ProJect Pharmaceutics GmbH
Fraunhoferstrasse 22
82152 Martinsried Germany
+49-89-45-22-89-700
+49-89-45-22-89-717
info@project-pharmaceutics.com www.project-pharmaceutics.com
I
2010
Contract research and development (CRO); cytotoxic (≤OEL4); BSL-1; BSL-2; fee-for-service business model; 2-4 weeks start up time
Areas of Activity ›
Biological Patents ›
External ›
Collaborations
Analytical characterisation; preformulation screening; formulation development; freeze drying cycle development; lyophilisation process and scale-up; pre-clinical batch manufacturing; technology transfer Several patents in freeze drying and formulation
| Network for seamless development from pre-clinic to fill/finish and market.
| Formulation specialist for DS and DP CMOs worldwide.
| Process specialist for scalable drug products ensuring manufacturability.
| Cross-country competence hub with business partners in Asia.
| Credited laboratory for Wyatt Technology in Europe.
Request for ›
Further Collaborations
| Small biotechnology/mid-size pharma companies, large top players in pharma/biotechnology.
| From DSP development to early and late drug product phases up to life cycle management.
| Customised solutions for challenges in formulation, analysis or process and tailored solutions for unmet needs not yet supported by the industry.
ProJect Pharmaceutics is a leading independent European CRO specialised in formulation and manufacturing process development for injectables: biologics (rec. proteins, antibodies, fusion proteins), peptides, ADCs, cytotoxics, small molecules, generics, viral therapeutics, ATMPs, VLPs and other nanoparticular drug delivery systems. We apply innovative, quality-by-design based, rational concepts of pharmaceutical development according to ICHQ8 and other guidelines. We support our global customers in developing a consistently high-quality pharmaceutical product that is transferable, scalable and manufacturable under GMP conditions. ProJect Pharmaceutics is managed by experts with >30 years of experience in the pharmaceutical industry. In about 850 m2 we operate special laboratories for biologics, cytotoxics and a BSL-2 unit, equipped with dedicated industrial HOF pilot freeze dryers.
› Predictive formulation analytics: a QbD-based highthroughput approach for accelerated development
› Pre-formulation, early state & late phase formulation
› Liquid & lyophilised formulation (DoE-based)
› Low viscosity formulation for s.c. application
› UF/DF process development
› Syringeability evaluation
A deep understanding of the challenges when processing highly potent drugs, such as limited solubility in water and rapid degradation, paired with long-lasting experience and comprehensive know-how of lyophilisation from various organic solvents enables us to provide specific solutions for high potency & cytotoxic drugs.
› Predictive, high-throughput formulation development
› High-end freeze-drying technology
By combining protein formulation and process know-how with cytotoxic drug expertise the company holds unique assets to develop ADCs. On top we offer complementary formulation and process development of intermediate antibody & linker-payload bulk.





Peptide formulation
› Formulation and lyophilisation cycle development
Innovative liposome technology
› High encapsulation efficiency
› Hydrophilic & lipophilic APIs
› GMP-compatible manufacturability with standard equipment
Viral therapeutics
› Lyophilised formulation and cycle development
Downstream process
› Smart optimisation of UF/DF & purification steps
› Targeting a common BDS and DP formulation
Freeze drying
› In vials, dual chamber systems, syringes, cartridges, bulk trays or bags, containers in nest&tub configuration
› Out of organic solvents
› Rational cycle development (robust, collapse-safe, cost & time efficient, tailored to the formulation)
› Bulk lyophilisation (solid or powder)
› SEM structural analysis of lyo cake
› Time lapse video & IR thermal camera monitoring
› Aseptic pre-clinical batch manufacturing
› Robustness & design space evaluation
› Smooth technology transfer to GMP
Process development and manufacturing
› Container closure system compatibility
› Forced stress, in-use & accelerated stability studies
› Detergent & excipient quantification
› Sterile filtration study
› Manufacturability assessment
› Fill&finish process mock-up
› Aseptic pre-clinical batch manufacturing
Fill&finish
As state-of-the-art development experts we are teamed up with state-of-the-art contract manufacturing experts to provide high-quality parenterals from pre-clinical to clinical and commercial scale.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Telephone ›
Fax ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Promega Corporation
2800 Woods Hollow Road Madison, WI 53711 USA
+1-608-274-4330
+1-608-277-2516
www.promega.com
F I I
1800+
1978 by William A. Linton
| High quality reagents & kits for all types of DNA, RNA and protein analysis; cell-based & biochemical assays for analysis of cellular processes; MSI diagnostics; cell systems for therapeutic antibodies including LOT release assays.
| Integrated Systems including instruments, chemistries and personalized support that simplify the process of automating research in the lab.
| Custom manufacturing
| Onsite Stocking
| Automation Support
| Tailored R&D Solutions
Annual turnover ›
Biological Patents ›
$775m USD
Promega holds hundreds of patents in the areas of:
| Bioluminescence
| Cellular Analysis
| Cell biology
| Drug Discovery

Fuelled by curiosity. Powered by exploration. Promega Corporation is a global leader in biotechnology, biochemistry, and cellular and molecular biology, developing innovative technologies for the Life Sciences. Based on bioluminescent technology innovations, Promega offers a broad portfolio of functional reporter bioassays, primary cell-killing assays, and immunoassays, used for R&D or QC batch release of therapeutic antibodies and cell/gene therapies. Promega’s services are designed to accelerate your biologics drug discovery and development workflows.
The company’s portfolio of over 4,000 products supports a range of life sciences work across areas such as cell biology; DNA, RNA, and protein analysis; drug development; human identification; and molecular diagnostics. For over 40 years, Promega’s products have been used in labs for academic/government research, forensics, drug discovery, clinical diagnostics, as well as agricultural and environmental testing.
Let’s work together to power ideas for tomorrow and the next 100 years.
Promega supports research and development worldwide with innovative products, technical advice, and service. As a partner of pharmaceutical and biotechnology companies, Promega offers special fillings and formulations of enzymes as well as the production of specialised materials such as reporter cell lines, e.g. CRISPR-modified cell lines based on luminescent Promega technologies. From simple changes in product size or packaging to the development of custom assays and high-throughput solutions, Promega’s custom offerings cover a breadth of capabilities in manufacturing, technology development, assay automation, and inventory management. (www.promega.com/custom-solutions/)
Since 2008, Promega has reported on its commitment to and progress regarding sustainability. In addition, Promega has been an active participant in the UN Global Compact for almost a decade. Sustainability is a core value across the entire company, from the way Promega



designs its buildings and develops its products, to how Promega cultivates its vision of meaningful business. (www.promega.com/corporate-responsibility-csr/)
Promega is headquartered in Madison, WI, USA with branches in 16 countries and over 50 global distributors.
Our Branches in Europe:
Promega GmbH, Walldorf, Germany (covering Germany, Austria, Poland, and Eastern-Europe)
Tel: + 49-6227-6906-0
Email: de_custserv@promega.com
Promega Biotech Iberica SL, Madrid, Spain (covering Spain and Portugal)
Tel: +34-916621126
Email: esp_custserv@promega.com
Promega Italia S.R.L., Milan, Italy
Tel: +39-2-54-05-01-94
Email : marketing.it@promega.com
Promega UK Limited, Southampton, UK
Tel: +44-23-8076-0225
Email: ukmarketing@promega.com
Promega AG, Dübendorf, Switzerland (covering Switzerland and Liechtenstein)
Tel: +41-44-878-90-00
Email: ch_custserv@promega.com
Promega Benelux, Leiden, Netherlands (covering Benelux and Rwanda)
Tel: +31-71-532-42-44
Email: benelux@promega.com
Promega Nordic, Nacka, Sweden (covering Nordic countries and Estonia)
Tel: +46-8-452-24-50
Email: sweorder@promega.com
Promega France, Charbonnières-les-Bains, France
Tel: +33-800-488-000
Email: FR.support@promega.com

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Rentschler Biopharma SE
Erwin-Rentschler-Str. 21 88471 Laupheim
Germany
Dr Latika Bhonsle-Deeng (Head of Communications)
+49-7392-701-0
+49-7392-701-300
communications@ rentschler-biopharma.com
www.rentschler-biopharma.com
I
1,400
1927
S1
| Contract development and manufacturing organization (CDMO) for biopharmaceuticals, including adeno-associated virus (AAV) gene therapies
Rentschler Biopharma is a leading global contract development and manufacturing organization (CDMO), focusing exclusively on client projects. The company’s end-to-end service offering includes process development and cGMP manufacturing of biopharmaceuticals, including adenoassociated virus (AAV) gene therapies, as well as related consulting activities, project management and regulatory support. The family-owned company is headquartered in Laupheim, Germany, with a second site in Milford, MA, USA. A third site in Stevenage, UK, is dedicated to cell and gene therapies. Rentschler Biopharma’s high quality is proven by its long-standing experience and excellence as a solution partner for its clients.
Rentschler Biopharma is highly experienced in the development and cGMP manufacturing manufacturing of therapeutic proteins and AAV gene therapies, in compliance with international standards.
External › Collaborations
Strategic alliance with Leukocare AG, located in Munich, Germany, for bestin-class formulations
Strategic collaboration Xpert Alliance, with Vetter, headquartered in Ravensburg, Germany, for high quality aseptic filling and secondary packaging
Request for ›
Further Collaborations
Rentschler Biopharma offers bioprocess development and cGMP manufacturing solutions, from concept to market, for pharma and biotech companies in long-term collaborations and strategic collaboration settings
Working collaboratively with its clients, Rentschler Biopharma provides customized solutions with optimized work packages for each development stage. State-of-the-art cGMP facilities and cell culture processes are leveraged for manufacturing for both, clinical studies and commercial supply. Consistent monitoring of the international regulatory landscape and in-depth understanding of the necessary regulatory documentation (CMC), ensures that each project is properly documented in accordance with international regulatory requirements. In order to offer excellent solutions across the entire value chain, the company has entered into a strategic alliance with Leukocare for formulation development. Another strategic collaboration is the Xpert Alliance, with Vetter, for aseptic manufacturing and packaging. The desired goal of the collaboration with Vetter is the alignment of manufacturing approaches that enable clients to bring their products to patients more easily and faster.



› Fast and efficient supply for multiple candidate screening
› Robust and scalable CHO cell lines for cGMP manufacturing
› Efficient cell culture and purification processes
› Well-established analytical methods
› Advanced formulation development exclusively by Leukocare AG
Rentschler Biopharma’s strategic alliance with Leukocare offers best-in-class formulation development considered at every step of the biopharmaceutical development and manufacturing process.
Leukocare’s approach combines formulation expertise with data science to achieve superior drug product formulation. Bioinformatics and Artificial Intelligence are strategically combined to explore a broader design space than conventional approaches and to reach optimal formulations in less time at lower costs.
› Multiple stainless steel bioreactor lines, including a twin system with 2x 3,000 L
› Flexible single-use bioreactor lines up to 2,000 L
› State-of-the-art purification processes
› Guaranteed product quality and purity in accordance with cGMP guidelines
› Four new 2,000 L single-use bioreactors will be operational at the new, state-of-the-art Rentschler Biopharma Manufacturing Center at the site in Milford, MA, USA, in the second half of 2024
As a world-class solution provider, Rentschler Biopharma ensures optimum time-to-clinic and time-tomarket by accelerating timelines to create competitive advantage for their clients. For seamless market approval, they consult and assist clients with regulatory queries, develop optimised strategies, and generate complete documentation for approval of clinical studies and market launch.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Suhrenkamp 59
22335 Hamburg Germany
+49-40-55290-801
+49-40-55290-888
BusinessDevelopment@ richter-helm-biologics.eu
www.richter-helm.eu
I
>350 1987
Contract development and manufacturing of biopharmaceuticals derived from microbial systems
Biological Patents ›
Several patents on manufacture of recombinant proteins
External › Collaborations
Request for ›
Further Collaborations
Richter-Helm is working with biotech and pharma companies worldwide
Richter-Helm’s Business Development team will be pleased to learn more about your needs for development and GMP manufacturing services to bring your project one step further. For more detailed information please contact us.
GMP-compliant microbial production of biopharmaceuticals –Flexibility makes the difference
Richter-Helm is a successful and constantly growing Germany-based GMP-compliant contract manufacturer of biopharmaceuticals specialised in products derived from bacteria and yeasts.
Over the past 35 years Richter-Helm has gained substantial experience in the development and manufacture of a variety of products including therapeutic proteins and peptides, antibody-like scaffolds (e.g. VHH), bacterial vaccines, and plasmid DNA (pDNA).
The company currently operates a development facility and two cGMP manufacturing facilities with bioreactor capacities of up to 1,500 litres. The addition of two highly flexible production trains with capacities of 300 and 1,500 litres and fully separated flows in a new facility building opens up capacity for new and further projects, enabling GMP manufacturing of material for phase I to III and commercial products.
With flexibility, commitment to good manufacturing practice, and total transparency our experienced team offers highly specialised contract development and manufacturing services to support the global pharmaceutical and biotechnological industries.
With its solid experience and strong expertise in the field, Richter-Helm is the partner of choice for pDNA production with the platform process RHB-pART to supply pDNA in different grades, quantities, and for different applications. We further provide an established analytical platform specifically for pDNA products, including a generic and validated quality control testing approach.
Richter-Helm ensures the highest pharmaceutical quality standards, as verified by major regulatory bodies including EMA, FDA, PMDA, ANVISA, Health Canada, and MFDS as well as by numerous customer audits.



The highly motivated and experienced teams at RichterHelm have excellent expertise in development and GMPcompliant production of biopharmaceuticals. To ensure efficient and transparent work, we apply professional project management methods.
Our process development team possesses extensive experience in developing and optimising fermentation and purification processes. For initial strain development, a variety of proprietary E. coli expression systems are available to support strain selection. Small-scale models of existing processes provide helpful tools for trouble shooting, process characterisation, and validation. Furthermore, small-scale models also ensure a smooth scale-up to large-scale GMP-compliant manufacture.
The quality system at Richter-Helm meets the strictest standards of quality in biopharmaceutical production. Our in-house quality control covers development and validation of a variety of analytical methods (incl. biological assays), performance of stability studies, and characterisation of reference standards.
Richter-Helm offers end-to-end manufacturing services, including:
› Establishment of cell banks (MCB/WCB)
› GMP manufacturing for phases I to III
› Commercial GMP manufacturing
› Fill & finish services
Thanks to the broad knowledge of Richter-Helm’s experts and many years of experience gained from different kinds of projects, Richter-Helm works out tailor-made solutions for its worldwide customers, which include large pharmaceutical companies as well as advanced biotech companies. We treat each of our customers’ projects individually and with the highest flexibility – guaranteeing a successful partnership.

Name ›
Secarna Pharmaceuticals GmbH & Co. KG
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person › Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Am Klopferspitz 19
82152 Planegg/Martinsried Germany
Alexander Gebauer, MD, PhD; CEO & Managing Director
Konstantin Petropoulos, PhD, MBA; CBO & Managing Director info@secarna.com www.secarna.com
I 20 2015
Discovery and development of highly specific, safe, and efficacious 3rd generation ASOs to address areas of high unmet medical need in a range of indications incl. immune-oncology, CNS and fibrotic/inflammatory diseases.
External › Collaborations
| Denali Therapeutics, Inc.
| Evotec SE
| Firebrand Therapeutics, Inc.
| First Affiliated Hospital at Guangzhou
| Lipigon Pharmaceuticals AB
| SciNeuro Pharmaceuticals
| Sun Yat-sen University (SYSU)
Request for ›
Further Collaborations
Secarna strives to become the global partner of choice for all (target- and organ specific) ASO drug discovery and development programs and is open to new partnerships. Partnering is possible for new targets as well as for selected programs from Secarna’s proprietary pipeline.
Founded in 2015, Secarna Pharmaceuticals has established itself as Europe’s leading independent antisense oligonucleotide (ASO) company. Secarna has several in-house programs in various stages of preclinical development for indications such as immuno-oncology and fibrotic/inflammatory diseases. With its proprietary ASO platform, the Company is developing molecules with significantly improved efficacy and a comprehensive safety profile, addressing targets that are difficult to reach with conventional approaches. Several technologies have been successfully integrated to enhance delivery at the target organ or cell type.
Using their state-of-the-art Oligofyer™ bioinformatics system, Secarna’s antisense molecules are precisely engineered to specifically bind to the targeted RNA of the gene of interest. In addition, they are prescreened and selected because of their high potential for activity. In parallel, the AI-based technology allows to de-select sequences based on expected safety concerns. This ensures high-quality candidates for clinical development with little to no need for lead optimization, which significantly speeds up the development process compared to conventional approaches.
Secarna’s platform has been validated in various inhouse projects as well as in ten commercial partnerships such as with Denali Therapeutics and Lipigon Pharmaceuticals, who have just received the approval of the Swedish authorities to conduct a phase II study with Lipisense, an ASO derived from Secarna’s platform.
ASOs bind sequence-specific to a target RNA and modulate protein expression through several different mechanisms. The ASO field is a promising area of drug development that targets the disease’s source at the RNA level and offers a promising alternative to therapies targeting downstream processes. Evidenced by the recent increase in market approvals and high value partnering transactions, ASO-based therapeutics are becoming more and more popular, filling a position not or insufficiently addressed by more traditional approaches. The ability to combine ASOs with targeted modalities, such as antibodies and peptides, further broadens the


potential of Secarna’s technology to now offer targeted antisense therapeutics to numerous clinically validated targets in indications with high unmet need.
Secarna’s lead asset SECN-15 is an ASO that targets Neuropilin 1 (NRP1), a protein that is involved in various tumour-promoting functions. ASOs, in contrast to other drug modalities, have the potential to block all functions of NRP1 simultaneously at the pre-mRNA level, that is, a step prior to the formation of the protein.
SECN-15 has demonstrated that it can selectively and with high potency target NRP1, showing a higher than 90 % knockdown of the protein expression in vitro.
Secarna has gone one step further and has tested the effects of SECN-15 in in vivo disease models, showing that the levels of NRP1 were successfully reduced in different cell types within the tumour, including immune cells with immunosuppressive functions. This translated into a potent anti-tumour activity in vivo. A striking suppression of tumour growth rates were achieved, together with an increase in survival. In many cases, complete and durable tumour eradication was seen when treatment with SECN-15 was combined with an anti-PD-L1 antibody. Animals that were tumour-free after treatment were exposed a second time to the cancerous cells but did not develop a tumour, indicating the establishment of anti-tumour immunity in treated mice.
Secarna is paving the way for a new generation of compounds that give patients hope for innovative therapies for difficult-to-treat diseases. Specifically, Secarna seeks to establish ASOs as the third pillar in drug development, next to small molecules and antibodies, expanding the universe of druggable targets. The Company strives to become the partner-of-choice for ASO drug discovery and development programs and to form strategic partnerships leveraging its collaborators’ as well as Secarna’s own strengths. To learn more about Secarna and the competitive edge of its ASO platform, get in touch: partnering@secarna.com.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone › Email › Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Socorex Isba SA
Chemin de Champ-Colomb 7a
1024 Ecublens
Switzerland
Yves Lachavanne (Marketing & Sales Support Manager)
+41-21-651-6000
socorex@socorex.com
www.socorex.com
F I
60
1963
| Liquid handling instruments
| Laboratory consumables
| Calibration services for all brands
Socorex Isba SA, based in Switzerland, manufactures highly reliable laboratory instruments for manipulating liquids in a large number of applications. Manual and electronic micropipettes, single- or multi- channel pipettors, repeater pipettes, dispensers, pipette controllers, and re-usable syringes, along with consumables, and accessories, constitute the heart of the programme. Each precision instrument bears a unique serial number and undergoes a strict performance control attested by an individual ISO 8655 QC certificate.
With over 60 years in the industry, Socorex distributes its products worldwide via a carefully selected distributor network. Visit our website to find your closest provider and test the products yourself.
Acura ® manual micropipettes offer superior performance, comfort, and fun, along with a 3-year warranty. Key features include:
› Exceptional ergonomics
› Patented Justip™ shaft height adjustment system for versatile tip fitting
› Swift-set easy user calibration system with an integrated key and locking mechanism
The handle and positioning of the large-surface, low activation-pressure tip ejector button, are designed to eliminate hand stress. The adjustment button allows easy volume setting, even when wearing a glove. The soft springs and tightness ring with PTFE sleeve reduce friction and make plunger activation smoother than ever.
Made from virgin polypropylene and free from DNAse, RNAse, pyrogens, endotoxins, proteases, and ATP, the Qualitix® tips offer high purity grade. Available in sterile, filtered, or unfiltered models, they ensure accurate liquid delivery and are compatible with Socorex and all major micropipette brands.

To minimize the laboratory plastic waste production, a smart, re-closable tipfill ™ solution was introduced. A stack of 10 96-tip inserts in an elegant cardboard packaging, uses 60% less space than corresponding number of conventional packages and generates much less plastic refuse. Many of the Qualitix® tips are also available in bulk bags.
The Calibrex™ dispenser line offers safe, consistent liquid distribution, with three models in sizes from 0.1-1 mL to 10-100 mL:
› Calibrex™ universal 520 for all common laboratory reagents
› Calibrex™ organo 525 for organics and solvents
› Calibrex™ solutae 530 for salt solutions, weak and strong acids and bases
Volume selection is effortless with a smooth, springloaded cursor and dual scale. The optional stopcock allows for reagent recycling without loss or contamination. Cleaning the dispenser is simple – it quickly disassembles or can be autoclaved fully assembled at 121°C. Calibration is straightforward with the integrated calibration key under the plunger cap. The QR code printed on the device provides instant access to the chemical resistance chart.
At our state-of-the-art Service Centre, a dedicated team of experts takes care of micropipettes and dispensers regardless the brand. We offer:
› Calibration service in a ISO 17025 accredited laboratory, according to revised ISO 8655:2022 standard
› Quick and comprehensive services - Service Express as fast as 48h (excl. transportation)
› Highly customizable services to best fit your needs
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Telephone ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
External › Collaborations
Swiss Biotech Association
Löwenstrasse 11
8001 Zürich
Switzerland
+41-44-455-56-78 info@swissbiotech.org
www.swissbiotech.org
5 (FTE)
1998
National cluster development powered by Swiss Biotech Association
National and international stakeholders of the Swiss biotech industry
The Swiss Biotech Association represents the interests of the Swiss biotech industry. To support its members in a competitive market, the Swiss Biotech Association works to secure favourable framework conditions and facilitate access to talent, novel technologies, and financial resources. To strengthen and promote the Swiss biotech industry, the Swiss Biotech Association collaborates with numerous partners and life science clusters globally under the brand Swiss Biotech™.
The association’s core objective is to ensure that the value generated by the Swiss biotech industry continues to grow and that the industry contributes to the well-being of the socio-economic ecosystem, thereby enabling Switzerland to be a key player at the forefront of bioscience innovation.
To this end, the Swiss Biotech Association is dedicated to supporting Swiss biotech companies by:
Developing favourable and competitive framework conditions
› Creating awareness of the biotech industry’s needs and interests with policy makers
› Advocating a competitive tax system alongside lean and pragmatic regulations
› Fostering life science education, technology transfer, and intellectual property
Attracting talent, know-how, and financial resources to drive innovation and growth
› Promoting and facilitating access to funding opportunities
› Connecting investors and attractive biotech investment opportunities
› Facilitating access to national and international talent

Fostering networking and collaboration through strategic, national, and international partnerships
› Connecting industry stakeholders and foreign life science clusters with the Swiss biotech ecosystem trough the Global Village platform
› Organising and co-promoting national and international life science events
› Providing access to privileged information through industry platforms and working groups
Promoting the accomplishments of the Swiss biotech industry
› Disseminating the value-creation of Swiss biotech companies
› Visualising the diversity and competitiveness of Swiss biotech companies
› Presenting innovative products and technologies and their contribution to quality of life
As a Swiss Biotech Association member you profit from our engagement with national and international stakeholders, receive privileged information, share knowledge, improve your network, and optimise business efficiency. You also gain more visibility and receive discounts at events, as well as favourable rates with business solution providers and for promoting your company.
Contact us at swissbiotech.org/team to explore more ways we can collaborate.

Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
Tosoh Bioscience GmbH
Im Leuschnerpark 4
64347 Griesheim
Germany
Regina Roemling
+49-6155-7043700
+49-6155-8357900
Info.tbg@tosoh.com
www.tosohbioscience.de
>20
1989
Supplier of chromatography media, HPLC and UHPLC columns
Application support in analysis and purification of biomolecules.
Tosoh Bioscience is an acknowledged global leader in the field of liquid chromatography with a focus on bioseparations. For more than 30 years, Tosoh Bioscience is providing cutting-edge solutions to meet the needs of customers developing and producing new biologics, biosimilars or biobetters, such as plasma products, monoclonal antibodies, recombinant proteins, vaccines and oligonucleotides.
Our product portfolio encompasses SEC instruments and a comprehensive line of media and prepacked process development, HPLC, and UHPLC columns. These products are popular in the biotech and biopharmaceutical industry and used in R&D, downstream processing, and quality control. Typical applications comprise the purification of therapeutic proteins in lab, pilot, and commercial scale, as well as their characterisation by HPLC or UHPLC. Latest developments comprise SkillPak columns pre-packed with best-in-class TOYOPEARL resins for quick and reproducible development and scale-up of purification methods and a holistic multicolumn chromatography solution for DSP intensification, the Octave BIO instrument and dedicated SkillPak BIO columns. For antibody characterization, we introduced novel TSKgel size exclusion columns suited for native separation with MALS or MS detection and a hydrophobic interaction column for ADC analysis.


Headquartered in Griesheim Germany, in the Frankfurt/ Rhine-Main Metropolitan Region, Tosoh Bioscience’s European operations offer extensive technical support like application development, on-site training and workshops. One of the services that stand out in the industry is the Tosoh Chromatography Workshop Series providing a comprehensive background to the chromatographic purification of biomolecules.
Tosoh Bioscience is part of the Tosoh Group, a Japanese chemical and speciality products and materials group, founded in 1935, which comprises over 100 companies worldwide and a workforce of more than 12,000 people.
Our brands TOYOPEARL® and TSKgel® are renowned for their quality and reliability. The stationary phases cover all common modes of liquid chromatography, including ion exchange, hydrophobic interaction (HIC), mixedmode, reversed phase, hydrophilic interaction (HILIC), size exclusion (SEC) and affinity.
In 2021, our renowned HPLC column brand TSKgel celebrated its 50th birthday.

Name ›
Address/P.O. Box ›
Postal Code/City › Country ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone › Email ›
Website › Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
Annual turnover ›
External › Collaborations
Valneva SE
Registered seat
6 rue Alain Bombard
44800 Saint-Herblain
France
R&D center
Campus Vienna Biocenter 3
1030 Vienna
Austria
Laetitia Bachelot-Fontaine
VP Global Communications and European Investor Relations
+33-6-4516 7099
communications@valneva.com
www.valneva.com
F I
~700
2013
Vaccines
€153.7m in 2023
Pfizer for Lyme disease vaccine candidate
We are a specialty vaccine company that develops, manufactures, and commercialises prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialised and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions.
We have a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently market three proprietary travel vaccines, including the world’s first and only chikungunya vaccine, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, as well as vaccine candidates against the Zika virus and other global public health threats.
We want to make a difference in people’s lives by applying our innovative and pioneering science to address potential vaccine preventable diseases. Our pipeline includes vaccine candidates for infectious diseases with major unmet needs, where no vaccines are currently available.
Valneva – Advancing vaccines for better lives



Valneva and Pfizer are developing VLA15, a Lyme disease vaccine candidate. There are currently no approved human vaccines for Lyme disease, and VLA15 is the most advanced Lyme disease vaccine candidate currently in clinical development, with two Phase 3 trials in progress.
This investigational multivalent protein subunit vaccine uses an established mechanism of action for a Lyme disease vaccine that targets the outer surface protein A (OspA) of Borrelia burgdorferi, the bacteria that cause Lyme disease. The vaccine candidate covers the six most common OspA serotypes expressed by the Borrelia burgdorferi sensu lato species that are prevalent in North America and Europe.
VLA15 is an alum-adjuvanted formulation, administered intramuscularly and has demonstrated a strong immune response as well as satisfactory safety profile in preclinical and clinical trials so far.
VLA1601 is a highly purified inactivated, adjuvanted vaccine candidate against the mosquito-borne viral disease caused by the Zika virus (ZIKV). Disease outbreaks have been reported in tropical Africa, Southeast Asia, the Pacific Islands, and, since 2015, in the Americas. Zika virus transmission persists in several countries in the Americas and in other endemic regions. To date, a total of 89 countries and territories have reported evidence of mosquito-transmitted Zika virus infection; however, surveillance remains limited globally. There are no preventive vaccines or effective treatments available and, as such, Zika remains a public health threat.
VLA1601 is being developed on the original manufacturing platform of Valneva’s licensed Japanese Enceph alitis vaccine, which was further optimised to develop the Company’s inactivated, adjuvanted COVID-19 vaccine. Valneva plans to re-initiate clinical development.

Name ›
VelaLabs GmbH
A Tentamus Company
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Brunner Strasse 69/3
1230 Vienna
Austria
Dr Andreas Nechansky
+43-1-890-597911
+43-1-890-597910
office@vela-labs.at
www.vela-labs.at
I Q
95
2006
Analytical services, contract laboratory for pharma and biotech from drug development to product release (GLP, GCLP, GMP)
VelaLabs combines a focused, customer-oriented approach, a highly motivated team, and broad expertise in analytical development and quality control. VelaLabs supports pharma and biotech companies, starting from early drug development to non-clinical & clinical phases up to product commercialisation.
We support, with in-depth analytical characterisation, the product development of pharmaceuticals such as biopharmaceuticals, biosimilars, monoclonal antibodies, hormones, growth factors, and conjugated proteins. VelaLabs offers methods in accordance with relevant ICH guidelines from method development to validation. The analytical portfolio of VelaLabs includes:
Areas of Activity ›
Protein characterisation, ligand binding assays, cell-based assays, microbiological / sterility analysis, non-clinical and clinical services, physico-chemical analysis, compendial methods
Annual turnover ›
External ›
Collaborations
€ 9.5-10.5 m
NBS-C, Protagene, Eurofins-DiscovRx; MSD MesoScaleDiscovery
› Physico-chemical methods (HPLC, UHPLC, gel electrophoresis…)
› Cell-based (customised) bioassays (potency, cytotoxicity, proliferation…)
› Reporter gene bioassays (RGA)
› Ligand binding studies (Biacore/SPR, ELISA, MSD)
› Lectin microarrays / glycoprofiling
› Bio-analytics for non-clinical and clinical testing
› Pharmacokinetic (PK)
› Pharmacodynamic (PD)
› Immunogenicity testing (anti-drug antibodies (ADA))
› Bio-safety (BSL 2) testing capabilities
The analytical portfolio covers a wide range for proteins. Standard methods include gel electrophoresis, Western blot, HPLC, and compendial (Ph. Eur., USP) methods. For complex molecules like antibodies, our extended portfolio covers the Fab part (e.g. target binding by ELISA, SPR, FACS) and the Fc-related part (e.g. Fc gamma receptor binding by SPR, C1q binding by ELISA, and biological assays related to the mode of action of antiproliferation, CDC, ADCC, apoptosis). For the analysis of glycosylation patterns of proteins, VelaLabs offers an exploratory method using lectin microarray technology.



In addition, VelaLabs can also offer microbiological services, e.g., sterility testing in accordance with EMA requirements, as well as environmental control and germ identification using different approaches. Endotoxin measurements with various methods including recombinant factor C (rFC) or low endotoxin recovery (LER) testing is part of the portfolio, as well as Bioburden and TOC testing.
VelaLabs can carry out stability studies in stability chambers (WEISS) and has qualified equipment for storage ranging from liquid nitrogen (LN2), and ultra-deep freezers (-80°C) to walk-in cold storage rooms (-20°C/ +4°C). Consultancy for GMP storage of test materials
VelaLabs offers consultancy regarding current Good Manufacturing Practice (cGMP) and current Good Clinical Laboratory Practice (cGCLP), with a main focus on analytics.
VelaLabs is located in Vienna with a total area of 2,600 m² (laboratory and office spaces), of which approximately 1,200 m² are GxP laboratory spaces including a stateof-the-art bio-safety level 2 (BSL2) laboratory. We are looking forward to supporting your analytical needs and will be happy to advise you personally:
+43 189 059 7911
Contact information:
VelaLabs GmbH, Brunner Strasse 69/3, 1230 Vienna, Austria
+43-1-890597911; +43-1-890597910
velabd@vela-labs.at
https://www.vela-labs.at/
Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Vetter Pharma International GmbH
Eywiesenstrasse 5 88212 Ravensburg Germany
Andrea Wesp +49-751-3700-0
EU and other international inquiries: info@vetter-pharma.com
US inquiries: infoUS@vetter-pharma.com
Asia Pacific inquiries: info@vetter-pharma.com
Website ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
www.vetter-pharma.com
6,300
1950
Drug Product Development, Aseptic clinical and commercial fill and finish, Device Assembly and Packaging, Analytical Services, Regulatory Support, Logistic services
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 70+ years of history. We do not manufacture our own drugs – we focus on providing highly skilled support and state-of-the-art manufacturing resources. We support our customers from the initial phases of clinical drug product development and filling to commercial manufacturing, device assembly and packaging, and lifecycle management. Over 80% of our active projects are biologics.
Our portfolio of services includes dedicated resources for clinical development and commercial manufacturing as well as assembly and packaging, and more. We provide tailored solutions to meet your product’s specific market needs.
External ›
Collaborations
Strategic collaboration with Rentschler Biopharma SE, headquartered in Laupheim, Germany, for bioprocess development and API production (since July 2020)
› Drug Product Development
Support in navigating key early decisions in your product’s evolution with scalability, quality and efficiency in mind.
› Aseptic Filling & Visual Inspection
Comprehensive expertise in manufacturing injectable drug products and related visual inspection systems.
› Device Assembly & Packaging
Strategic, technical and packaging support for patient-friendly combination products.
› Analytical Services
Robust, customizable testing methods for drug products including evaluation and quality verification.
› Regulatory Support
Support to achieve milestones related to compliance during clinical development, market authorization and beyond.
› Logistic Services
Cutting-edge solutions that maximize efficiency, transparency and precision of a vital supply chain.



› Headquartered in Ravensburg, Germany
› Commercial production sites in Ravensburg and Langenargen, Germany
› Dedicated clinical production facilities in Rankweil, Austria, and Chicago, USA
› Branch offices for Asia Pacific in Singapore, Japan, South Korea, and China
› More than 6,000 employees worldwide
› Global specialist in the aseptic production of prefilled drug delivery systems
› International expertise with regulatory authorities including FDA, EMA, PMDA (Japan), and RP (Germany)
› Service offerings for pharma and biotech firms of all sizes and locations
› Numerous patents including technologies for protection against tampering and counterfeiting
› Lyophilization (freeze-drying) and siliconization specialist
› CO 2 neutral at all corporate sites since 2021
For more information, please scan the QR code.


Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Rudi-Conin-Strasse 4
50829 Cologne Germany
Olivia F. Hoffmann, Sales & Marketing Manager
Andreas Fichtner, Head Biostatistical Services
Ilka Strehlau, Head Clinical Data Management
Jasmin Atarodi, Managing Director
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees › Founded (year) ›
Areas of Activity ›
+49-221-337716-0
+49-221-337716-33 business@x-act-cologne.com
https://x-act-cologne.com
I Q
35+
1994
| Specialised service provider
| Clinical data management
| Biostatistical services
Passion for evidence since 1994!
We are an experienced partner to the life science industry, obtaining reliable study results for the development of safe and innovative therapies.
› Owner-managed CRO.
› Established 1994 and located in Cologne, Germany.
› Successful conduct of more than 450 interventional (Phase I to IV), non-interventional and medical device studies.
› 35 + seasoned and technophile team members contribute to the success of your project.
Biostatistical Services
› Statistical consulting, assuring that your scientific questions are answered.
› Delivering sample size determinations and statistical input to your study protocols.
› Development of a randomisation plan. Performing randomisation according to your study requirements.
› Developing Statistical Analysis Plans with a clear focus on the statistical programming of key results and analyses.
› SDTM and ADaM Mapping supporting the submisson of your study results.
› Biostatistical reporting as part of your clinical study / investigational report, publication or dossier.

› Strategic consulting to find the best eClinical solution for your project.
› eCRF implementation according to CDSIC | CDASH supporting submission.
› Development of all essential DM documents for your successful project conduct.
› Implementing Good Clinical Data Management Practices throughout your project.
› The conduct of all DM activities is supported by the excellent procedures of our SOPs.
› Targeted data review and data cleaning in close collaboration with your CRA team.
› Benefit from expressive status reports providing transparency and overview to the study team.
Curious about our special services? Please contact: business@x-act-cologne.com


Name ›
Address/P.O. Box ›
Postal Code/City ›
Country ›
Telephone ›
Fax ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Areas of Activity ›
YMC Europe GmbH
Schöttmannshof 19
46539 Dinslaken
Germany
+49-2064-427-0
+49-2064-427-222
info@ymc.eu
www.ymc.eu
I Q
50 (Europe) 485 (worldwide)
1993
| Glass Columns for Lab and Pilot Scale
| Prepacked Glass Columns
| Laboratory Services
| Preparative Liquid Chromatography
| Analytical Liquid Chromatography
As a research-driven chromatography specialist, YMC provides chromatographic solutions for compounds such as peptides and proteins, mAbs, and ADCs, as well as oligonucleotides from R&D scale through scale-up to production and final quality control in your lab. We supply innovative bio-chromatography resins and columns for preparative processes, as well as highly reproducible BioLC-(U)HPLC-columns for SEC, IEX, RP, & HIC.
Furthermore, YMC glass columns in laboratory as well as pilot scale are designed to meet bio-chromatographers’ demands. You can choose them empty as well as prepacked according to your requirements by YMC’s in-house packing service. In addition, you can make use of on-demand services for application support, screening, analytical, or preparative method development. Trainings on different chromatographic topics complement the products and services.
The most recent and interesting developments include:
› The new standard for columns for bio purification suitable for self-packing: YMC PilotPLUS
› Next generation RP phase for peptide purification: YMC-Triart Prep Bio200 C8
› Innovative solutions for BioLC: YMC-Triart Bio (U)HPLC columns, also available in bioinert hardware
The broad and continuously growing portfolio of stationary phases especially designed for the separation of biomolecules provides high flexibility for the chromatographer and ensures successful lab results, day after day. Additionally, the availability of particle sizes from analytical to preparative scale guarantees smooth and easy scale-up processes.

YMC products are available worldwide through a dedicated support network that guarantees qualified and meaningful practical assistance with regard to method development, method transfer, method validation, purification services, and custom column packing – locally, personally, and efficiently. It is not only product specifications that represent YMC quality, also but the positive energy and dynamics of our employees, who provide competent and consistent performance together with pronounced reliability even for the most difficult and demanding separations. The YMC network consists of highly focused product specialist teams to provide active support for chromatographers.
Since all YMC processes and working procedures are thoroughly monitored and documented, YMC resins and glass columns are fully compliant with requirements. Full technical documentation is available to show compliance with all applicable regulations.

Name ›
YUMAB GmbH
Science Campus Braunschweig-Süd
Address/P.O. Box ›
Postal Code/City ›
Country ›
Contact Person ›
Telephone ›
Email ›
Website ›
Social Media ›
Number of Employees ›
Founded (year) ›
Type of Laboratory ›
Areas of Activity ›
Inhoffenstr. 7
38124 Braunschweig Germany
Alexander Ehm
+49-531-481170-0
info@yumab.com
www.yumab.com
I
30 2012
S1, S2
| Fully human antibody discovery & lead development
| Antibody Libraries
| Engineering & Humanisation
| R&D services (CRO)
| Partnered innovation
Request for › Further Collaborations
YUMAB seeks clients & partners for human antibody development in the biopharma, biotech, & diagnostic industries.
YUMAB is a contract developer of fully human antibodies for the global biotechnological and biopharmaceutical industry. Starting in 2012 with the goal to bridge the gap between innovative research and novel therapies, the company has since become a global player that advances medicine by accelerating the development of next-generation biotherapeutics.
Our advanced discovery platform provides access to challenging targets, enables new formats, and accelerates the development of human antibodies at a high success rate. From target discovery to fully characterised lead – our experienced team leads biotech and pharma teams worldwide through the development process as your trusted, de-risking partner.
The YUMAB ® R&D platform delivers fully human antibodies, the closest to natural germline among those on the market, using fast and reliable in vitro discovery technologies. The company’s highly diverse, human antibody libraries contain 1011 natural antibody sequences and specificities to all types of antigens. Unlike animalderived, chimeric, humanised, or synthetic antibodies, each YUMAB® antibody sequence has been shaped in the human body, which maximises epitope diversity and overcomes restriction by in vivo immune responses while minimising immunogenicity and potential adverse effects in clinical development.
Our advanced in vitro selection technology is also efficient for rare and difficult target antigens and allows pre-design of epitope specificity, interspecies X-reactivity, affinity, stability, and other properties early in the discovery and development process. First antibody candidates are identified swiftly within a few weeks and are highly developable into all types of antibody drug formats such as full-length IgGs, Fabs, scFvs, bispecifics, CARs, fusion proteins, and ADCs.
Our contract research and partnered development has been proven successful by more than 200 projects over the past decade with over 120 clients and partners worldwide.

When the Corona pandemic hit in 2020, YUMAB collaborated with the University Braunschweig and other partners to develop antibodies against SARS-CoV-2, generating leads in only four months. The awarded fast track collaboration led in 2020 to the spin-out of CORAT Therapeutics GmbH, which is pursuing the clinical testing of the antibody drug candidate COR-101 (phase Ib/II) in hospitalised patients with moderate to severe COVID-19. COR-101 is designed not to induce elevated immune responses that contribute to lung damage, which enables treatment of patients with high viral loads.
Trusted partner
YUMAB provides tailored service and partnering concepts to jump-start translation from research innovation to drug development with affordable entry costs, flexible licensing options, and no additional third-party obligations. Our expert team values close communication with customers and partners, whom we consult and guide through the entire process of antibody development –from target to lead.
Human antibody discovery platform
› Natural fully human libraries for low immunogenicity
› Optimised in vitro selection to address difficult targets
› Rapid discovery within weeks
Direct link to lead development
› Advanced antibody lead development & optimisation
› All antibody formats, all disease areas
› Robust process, high success rates
YUMAB® platform
› Fully integrated discovery & development platform from target to optimised lead
› Customised project plans with flexible entry & exit points
› Smooth translation from bench to bedside
Flexible services, collaborations & partnering
› CRO: fee-for-service & flexible licensing options
› No technology access fees
› Collaborative development



