
2 minute read
ProJect Pharmaceutics GmbH
Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Email › Website › Social Media › Number of Employees › Founded (year) › Type of Laboratory ›
Areas of Activity ›
ProductLife Group
14 rue Gerty Archimède 75012, Paris France Adrien Decheix +33-787-603-325 adecheix@productlife-group.com https://www.productlifegroup.com/
F I
650 1993 Consulting & outsourcing services
regulatory affairs, quality and compliance, market access, pharmacovigilance, and medical information
PLG at a glance
ProductLife Group’s mission is to improve human health by delivering regulatory compliance services for the safe and effective use of medical solutions. For almost 30 years, PLG has supported clients through the entire product life cycle, combining local expertise with a global reach spanning more than 140 countries, with HQ in France (Paris) and offices in UK (Cambridge), Germany (Haan, close to Düsseldorf), Belgium (Ninove), Italy (Milan), Switzerland (Basel), Czech Rep. (Prague), etc. See https://www.productlifegroup.com/worldwidepresence/. PLG provides consulting and outsourcing services in the areas of regulatory affairs, quality and compliance, market access (pricing & reimbursement), vigilance, and medical information, covering both established products and innovative therapeutics & diagnostics. With the goal of continuously improving the value delivered to people and customers, PLG is committed to long-term partnership, innovation, flexibility, and cost efficiency.
PLG BIOTECH solutions
Discovering and developing treatments that mean alleviating suffering, extending life, and improving its quality requires time, top professionals, significant resources, collaboration across sectors and borders, and complex and risky processes with no guarantee of success in the search for new breakthroughs. PLG has set up a team of biopharmaceutical and medtech experts with extensive experience and a strong understanding of bio-pharmaceutical product development, registration, market access, health economics, and commercialisation.
PLG provides flexible and tailored pre-clinical, clinical, and market access solutions designed to help mid-size and small biopharmaceutical and medtech companies move forward in their effort to get treatments to patients
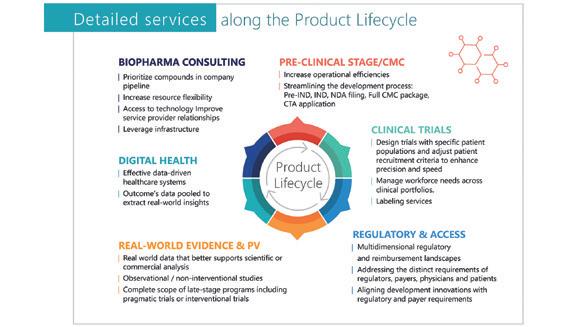

– from helping to define the development plan, to implementation of all stages of product development, through to launch and commercialisation – in order to deliver compelling results to regulatory authorities, investors, and stakeholders.
PLG assists the companies with: 1. reaching product development milestones faster, and 2. effectively managing the development programme from pre-clinical to post-marketing and beyond.
PLG also assists biopharmaceutical companies
PLG also offers specific support to biopharmaceutical start-ups through all phases of product development, market access, and post-marketing:
Geography Expanding activities in the US, Europe, and Asia. The US is the leading player in the healthcare field. Northern Asia is making more rapid progress in advanced therapy R&D than the US and Europe. Regulatory New regulatory pathways for emerging diseases and technologies (specialised medicines, curative medicines, combination therapies, personalised therapies, CAR-T, DC- vaccination…) Clinical outcomes Defining the criteria of clinical outcomes with biopharmaceutical companies is a new need in order to balance with the HTA. HTA The HTA (Health Technology Assessment) needs to take into account the growing numbers of EU and US agencies. Pricing Pricing payment models in line with the development of new and innovative technologies. Digital health Digital health (AI) is integrated into new medicine development (time and cost savings).










