
3 minute read
Affimed GmbH
Name ›
Address/P.O. Box › Postal Code/City › Country › Contact Person › Telephone › Fax › Email › Website › Social Media › Number of Employees › Founded (year) ›
Areas of Activity ›
Biological Patents ›
External ›
Collaborations
Request for ›
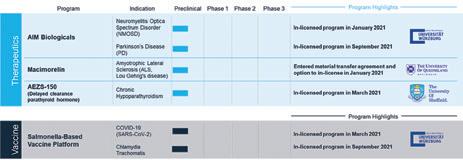
Further Collaborations Aeterna Zentaris GmbH
Weismuellerstr. 50 60314 Frankfurt am Main Germany Dr Eckhard Guenther +49-069-42602-0 +49-069-42602-3444 info@aezsinc.com www.zentaris.com
IL F
16 2001
Endocrinology, autoimmune and CNS diseases, vaccines, COVID-19, Chlamydia trachomatis, rare and orphan diseases, growth hormone deficiency
Several patents and patent applications covering our products and projects filed or licensed in
Big and medium-sized pharmaceutical companies together with academic institutions
We are interested in in-licensing late stage preclinical or clinical opportunities in our areas of activity and are potentially seeking development partners for our projects.
In Pursuit of Medical Innovations
Aeterna Zentaris (NASDAQ and TSX: AEZS) is a publicly listed, specialty biopharmaceutical company commercialising and developing therapeutics and diagnostic tests.
Zentaris was founded in 2001 as a spin-off from ASTA Medica, the pharmaceutical division of Degussa AG, later acquired by Aeterna Laboratoires in Quebec, Canada, which led to the foundation of Aeterna Zentaris Inc. Aeterna Zentaris GmbH is the wholly owned German subsidiary located in Frankfurt am Main. The GmbH is the R&D and operations centre of the group, whereas the legal headquarters is in Toronto, Canada.
In the past, Aeterna Zentaris successfully developed Cetrotide® for in-vitro fertilisation, Impavido® for the treatment of Leishmaniasis, and Lobaplatin for the treatment of solid tumours. All three products have been commercially available through global and local pharmaceutical companies for many years and have improved the lives of patients around the world.
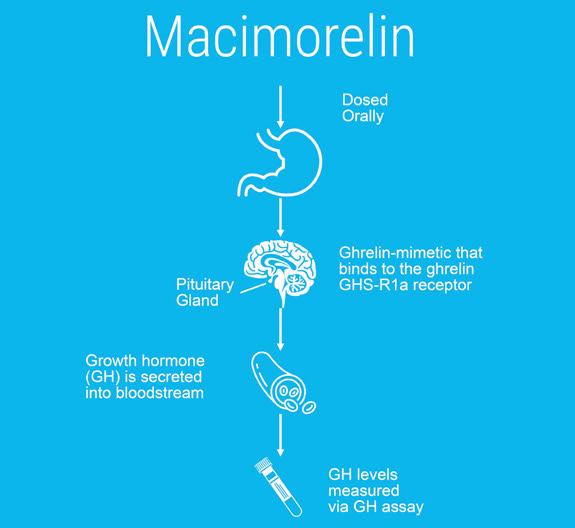
At present, our lead product macimorelin, a ghrelin receptor agonist, is the first and only approved oral drug indicated for diagnosis of adult growth hormone deficiency (AGHD) in the US and EU. Macimorelin stimulates the secretion of growth hormone from the pituitary gland into the circulatory system. Macimorelin has the potential to displace current testing procedures as a diagnostic standard.
Our commercial and development partner for Macrilen™ for the US and Canada is Novo Nordisk, a global leader in endocrinology. In 2020 we entered into commercial agreements with Megapharm Ltd. to commercialise macimorelin in Israel and Palestine, and with Consilient Health Ltd., to commercialise macimorelin in Europe. Recently we entered into commercial agreements with Er-Kim Pharmaceuticals for Turkey and NK-Meditech Ltd. for Korea.
Our business development efforts are ongoing to secure commercialisation partners for macimorelin in Asia and ROW.

We are leveraging the clinical success of macimorelin to develop it also for the diagnosis of childhood-onset growth hormone deficiency (CGHD) to expand the existing indication. We have started a multinational pivotal Phase 3 efficacy study in Q4 2021 jointly with Novo Nordisk.
Our Expertise
AEZS has strong scientific R&D expertise in endocrinology, CNS, and cancer, with a focus on small molecules, natural products, phospholipids, peptides, and proteins.
Pipeline Expansion Opportunities
We are seeking growth opportunities as part of our efforts to re-establish a diversified, yet focused, development pipeline to leverage our expertise and experience. We have recently signed licensing, and R&D agreements with the University of Würzburg, The University of Sheffield, and The University of Queensland.
CEO, Dr Klaus Paulini: “Aeterna Zentaris was established with strong expertise in research and development, which I believe is an incredible asset to the Company and one that we plan to leverage moving forward. Apart from investigating opportunities for new therapeutic applications of macimorelin, we are pursuing newly in-licensed projects with high unmet medical need and very interesting commercial potential. With our capabilities and specific know-how, we believe we are well-positioned to rapidly move promising projects into clinical development.”
Management Dr Klaus Paulini Dr Eckhard Guenther Giuliano La Fratta Dr Nicola Ammer Dr Michael Teifel










