Innovation issue:
The Bubble-PAPR: reinventing respiratory personal protective equipment
CloudGuard® – a novel leak-free tracheal tube
Innovation with the Exovent team
Curating the voice of healthcare

Medical Device Regulations –where are we?
Veni, video, vici: the slow retreat of the direct laryngoscope

May 2023 ISSN 0959-2962 No. 430 www.anaesthetists.org @Anaes_News


Ultramed www.ultramed.co What does an innovative POA solution look like to you? Book a demo: info@ultramed.co
®
Welcome

Welcome to this Innovation Issue of Anaesthesia News. This year the 11th Association of Anaesthetists Innovation, Critical Care and Pain Awards had fierce competition, resulting in a winner and two highly-commended runners up. These innovations were presented at the Winter Scientific Meeting and are showcased in this issue.
The outright winner was a reinvention of respiratory PPE by Brendan McGrath’s team from Manchester, born out of the COVID pandemic in recognition that most respirator masks were designed for industrial use. We all experienced the discomfort, noise, injuries and communication problems that these caused. Bubble-PAPR, consisting of a protective PVC collared hood and a filtered air intake fan, was designed for repeated and prolonged wear in the healthcare setting in order to avoid these problems.
CloudGuard®, a redesign of the tracheal tube cuff that is under development, won highly commended. Aspiration past a standard cuff commonly occurs during anaesthesia and may predispose to postoperative pneumonia; in ICU this is associated with ventilatorassociated pneumonia, the leading cause of nosocomial mortality in this situation. CloudGuard provides an improved seal against flow either into or out of the airway, without increasing tracheal mucosal pressure.
Also highly commended was Exovent, featured in Anaesthesia News and Anaesthesia last year. Exovent is a re-invention of the iron lung with the technology of the 21st century; extra-thoracic negative pressure ventilation provides comfort and a more physiological evenly-distributed opening of the alveoli without reducing cardiac output as with positive pressure ventilation, and might in some patients avoid the need for tracheal intubation and resulting complications.
Two articles relate to problems around procurement of suitable devices that we see on a daily basis. The start-up Malue has a mission to help us and those involved in purchasing to improve choice, selection and review of our equipment through digital technology innovation. Paul Sim from Barema discusses the impact of changing medical device regulations; hopefully this will bring improved safety in time, but in the short term has created bottlenecks and cost in the regulatory systems that may result in some established devices being discontinued and perhaps challenges to developing new devices. Coincidentally a correspondence item from Ardeshana et al. gives a real-world example of clinical risk from behind-the-scenes equipment purchase decisions.
Jan Hansel provides a succinct summary and discussion of his team’s extensive systematic Cochrane review comparing videoand direct laryngoscopy – perhaps a sign of the future as we, as a specialty, embrace technological improvements and march towards perhaps the inevitable of routine videolaryngoscopic intubation.
Contents
3 Introduction
5 The Bubble-PAPR: reinventing respiratory personal protective equipment
8 CloudGuard® – a novel leak-free tracheal tube
10 Innovation with the Exovent team – our continuing story of re-introducing negative pressure respiratory support
12 Curating the voice of healthcare
16 Medical Device Regulations – where are we?
18 Veni, video, vici: the slow retreat of the direct laryngoscope
20 A deeper plane. The Self – a QIP in a different direction
22 Learn, network and socialise at Trainee Conference 2023, Leeds
24 Your letters
27 Your membership – Maximise your benefits
29 Anaesthesia Digested
30 Poetry corner
Association of Anaesthetists 21 Portland Place, London W1B 1PY Telephone: 020 7631 1650 Website: www.anaesthetists.org
Editor: Mike Kinsella
Address for all correspondence, advertising or submissions:
Editorial Assistant: Kate Mooney
Email: anaenews@anaesthetists.org
Design: Chris Steer
Digital Designer
Peter Young Innovation and Industry Lead, Association of Anaesthetists

Membership Services Committee: Robert Self (Chair), Tei Sheraton (Vice President), Kenneth Barker (Elected Member), Benjamin Fox (Elected Member), Jonathan Harrison (Elected Member). Paul Bourke (Convenor, Scottish Standing Committee), David Honan (Convenor, Irish Standing Convenor), Ben Evans (Trainee Committee), Reshma Khopkar (SAS Committee) and Officers of the Association (ex-officio).
Telephone: 020 7631 8803
Email: chris@anaesthetists.org
Copyright 2023 Association of Anaesthetists
The Association cannot be responsible for the statements or views of the contributors. No part of this magazine may be reproduced without prior permission. Advertisements are accepted in good faith. Readers are reminded that Anaesthesia News cannot be held responsible in any way for the quality or correctness of products or services offered in advertisements.
3 Anaesthesia News | May 2023 | Issue 430 Back to contents
Follow Anaesthesia News on Twitter @Anaes_News
Who’s looking out for you?
Protection that fights your corner
Outstanding careers deserve uncompromising support. That’s what we’ve always believed.
Membership gives you the right to request indemnity for cases arising in a range of different situations. And with the largest medicolegal team of any medical defence organisation, we’re always ready to fight for your reputation.
For just £549 can feel safe in the support membership brings. We also offer competitive, tailored prices for consultants working in Private Practice.
See how we’r
The Medical Protection Society Limited (“MPS”) is a company limited by guarantee registered in England with company number 00036142 at Level 19, The Shard, 32 London Bridge Street, London, SE1 9SG. MPS is not an insurance company. All the benefits of membership of MPS are discretionary as set out in the Memorandum and Articles of Association. MPS® and Medical Protection® are registered trademarks. 2111122727 12/21 medicalprotection.org
Association of Anaesthetists Innovation in Anaesthesia, Critical Care and Pain Awards 2023, Winner
The Bubble-PAPR: reinventing respiratory personal protective equipment
Design
The COVID-19 pandemic highlighted the need for better PPE for frontline healthcare staff. Respirator masks adopted from industrial use are not designed for repeated or prolonged healthcare use. People with characteristics that vary from the ‘norm’ used by facemask manufacturers in terms of face shapes and sizes, or the presence of facial hair, can struggle to find a safe mask type, requiring multiple time-consuming 'fit-tests'. Even correctly fitting filtering face piece (FFP) masks can cause pressure sores when worn for long or repeated stints; they can be claustrophobic, limit vision, and significantly impair communication with patients and colleagues. Powered air-purifying respirators (PAPRs) offer increased comfort and greater protection than FFP masks by delivering filtered air into a hood using a fan, but also have significant limitations including being heavy, noisy and costly; they were also limited in their availability at the start of the pandemic. Bubble-PAPR is the product of an innovative project where we took the PAPR concept and re-designed it to offer high level protection, specific to healthcare needs, that could be equitably available to all staff.
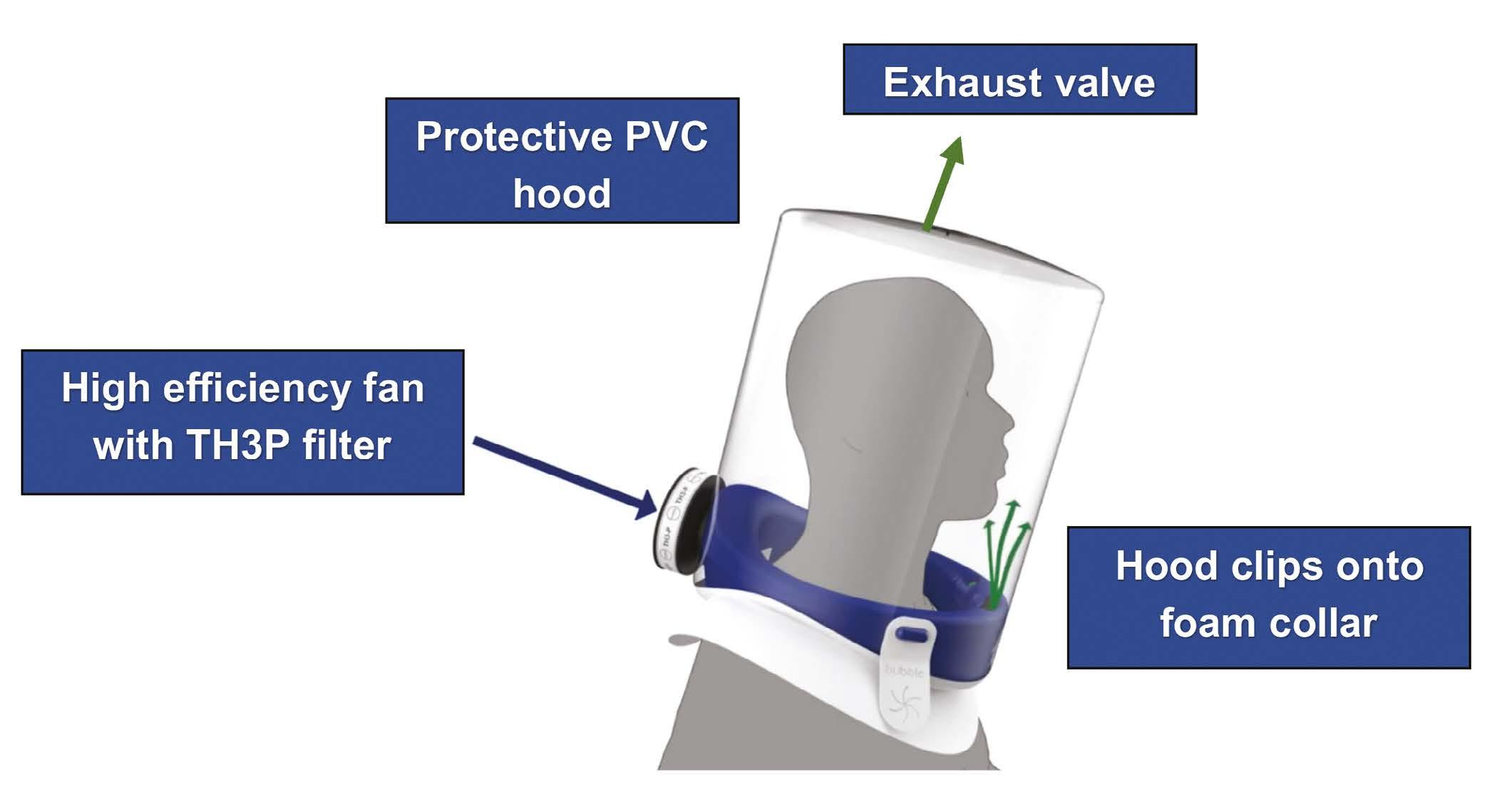
The Bubble-PAPR consists of two parts. A protective hood, made from recyclable PVC, is securely attached to a collar made from medical foam. An air intake fan and TH3P filter are housed within the collar, which incorporates a flow safety gauge. Filtered air is delivered into the hood, and an exhaust valve at the top prevents excessive pressure building up (Figure 1). New PVC hoods are stored flat to save space.
User evaluation
We carried out a study to evaluate Bubble-PAPR when used by healthcare staff in a range of clinical settings from a simulation suite to a high-risk ICU [1]. A Trial Safety Committee was established to oversee the results of laboratory and bench testing of the prototype, initial safety data, usability, and adverse event data at each stage of the evaluation. The Bubble-PAPR demonstrated a mean fit factor (concentration of challenge aerosol outside respirator divided by concentration inside secondary to leakage) of 16,961 during quantitative fit testing, exceeding safety standards; in comparison, FFP3 masks require a fit factor of 100 [2].
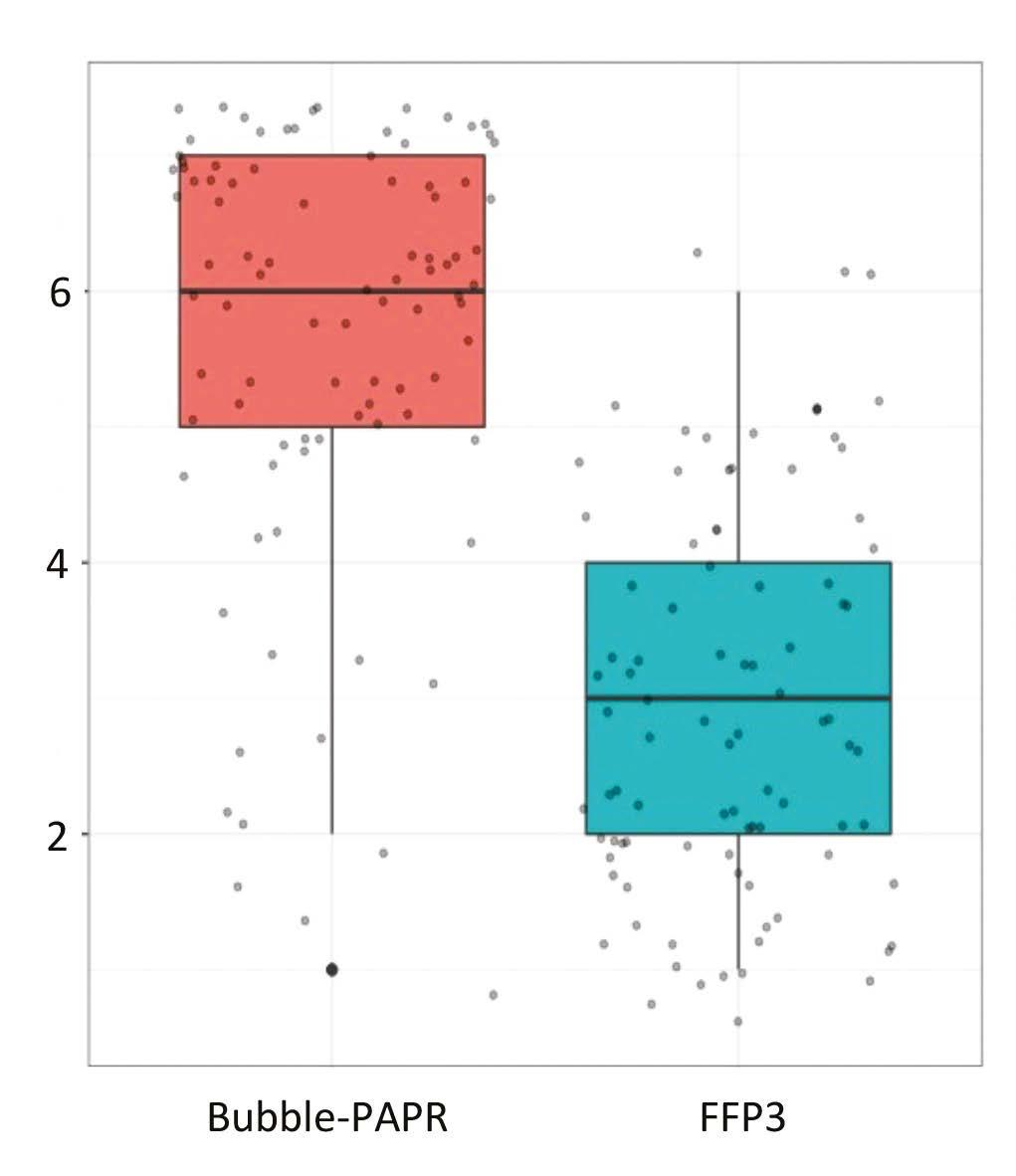
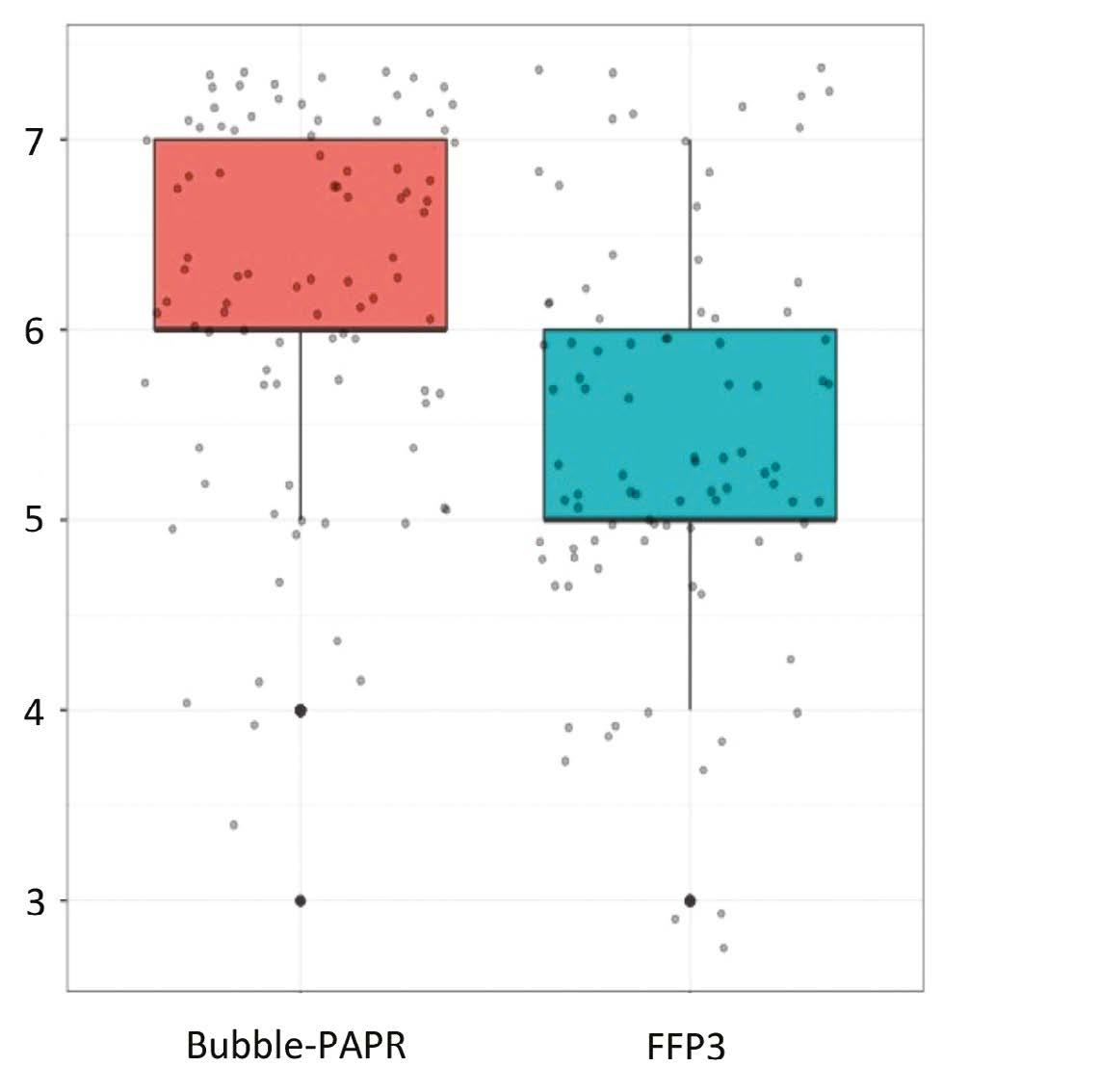
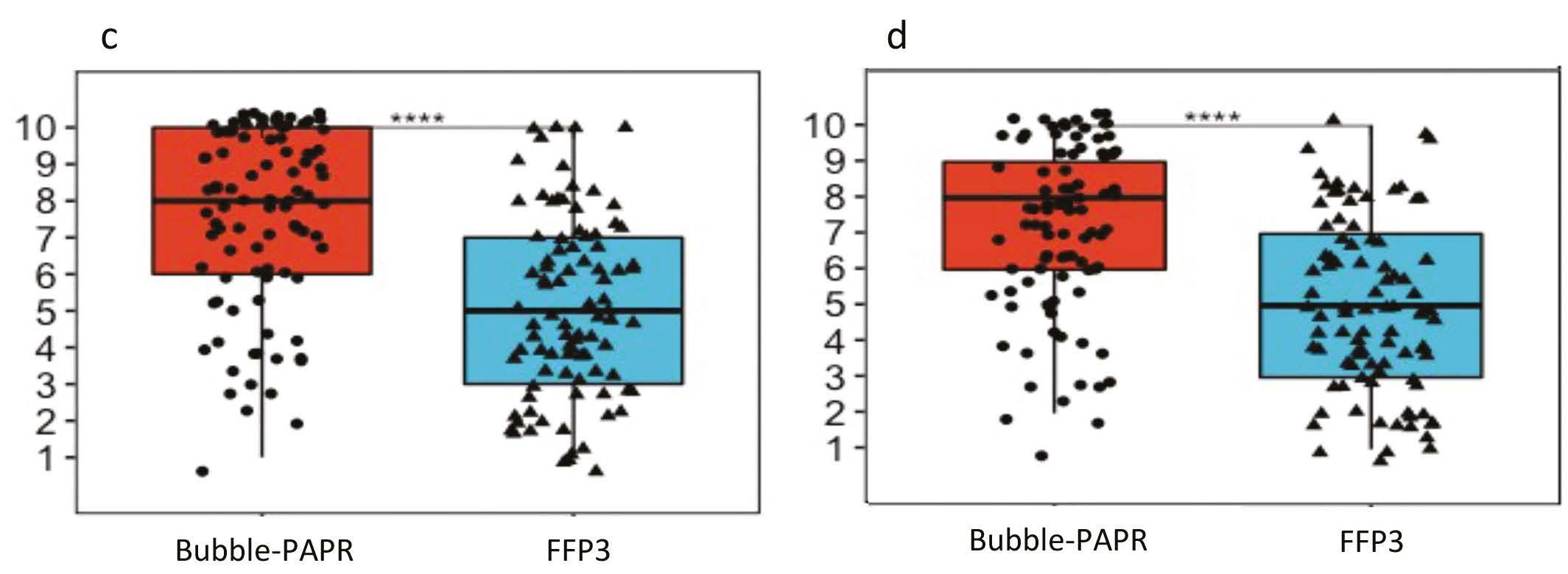
Staff focus groups helped us to capture all the potential frontline activities undertaken while wearing Bubble-PAPR. Likert scales were used to evaluate user experiences comparing Bubble-PAPR with conventional FFP3 masks from 91 diverse multidisciplinary participants. The average use time was 2 h. Bubble-PAPR felt more comfortable (Figure 2a; mean difference 2.68, p < 0.001) and safe (Figure 2b; mean difference 0.73, p < 0.001) than FFP3. Bubble-PAPR was considered to improve communication with patients and staff versus FFP3 (Figure 3).
Environmental sustainability
Between February and August 2020, the UK produced 591 tonnes of waste from PPE [3]. Conventional FFP3 masks are mostly single use, with limited potential for recycling waste streams. We recognised the potential environmental impact of Bubble-PAPR at an early stage. The collars are UK-made, designed with the intention to be refurbished and re-used, and contain simple engineering. We commissioned the Centre for Sustainable Healthcare to model the carbon footprint of the PAPR, using a bottom-up (cradle-to-grave) process-based
5 Anaesthesia News | May 2023 | Issue 430 Back to contents
analysis [4], comparing disposal by incineration versus closed loop recycling of virgin PVC (Table 1). Based on a typical 2-hour period of use, we estimate that the carbon footprint of BubblePAPR would be 93 g greater than conventional PPE (comprising one single-use FFP3 mask and one face shield per hour of use), and would be 142 g lower if closed-loop PVC recycling was available. However, the capacity for recycling of potentially infectious PVC waste is by no means universal, and requires minimum waste volumes to be commercially viable.
Considerations for the future
The COVID pandemic exposed the lack of PPE specifically designed for healthcare, as well as the preparedness and resilience of our systems and equipment for protecting staff. Bubble-PAPR was designed primarily for use during this pandemic but could provide a high-quality PPE option for frontline staff, particularly for workers undertaking long or continuous stints in high-consequence environments. BubblePAPR can be used by anyone, regardless of gender, ethnicity, and body habitus, and the collar can be shared between users after cleaning. By understanding and evaluating the environmental impact of Bubble-PAPR, we believe that we have set a new benchmark for consideration for all PPE manufacturing and disposal.
Acknowledgements: many of the people involved gave their time and expertise without payment, and without their contributions the Bubble-PAPR project would not have been possible: frontline staff at Manchester University NHS FT; Designing Science Ltd; University of Manchester Mechanical Aerospace and Civil Engineering; Manchester University NHS FT Innovation team.
Andrew Song
Specialty Trainee in Anaesthesia
Wythenshawe Hospital, Manchester
Clifford L. Shelton
Consultant Anaesthetist, Wythenshawe Hospital, Manchester Senior Clinical Lecturer, Lancaster Medical School
Professor Brendan McGrath
Consultant in Anaesthesia & Intensive Care Medicine, Wythenshawe Hospital, Manchester Honorary Professor, Manchester Academic Health Science Centre
Twitter: @BrendanAMcGrath; @DrCliffShelton
References
1. ClinicalTrials.gov. Evaluating Bubble-PAPR for healthcare workers (BubblePAPR), 2022. https://clinicaltrials.gov/ct2/show/NCT0468136 5?term=bubble+papr&draw=1&rank=1 (accessed 15/3/2023).
2. Health and Safety Executive. Guidance on respiratory protective equipment (RPE) fit testing, 2019. https://www.hse.gov.uk/pubns/ indg479.pdf (accessed 15/3/2023).
3. World Health Organization. Global analysis of health care waste in the context of COVID-19, 2022. https://www.who.int/publications/i/ item/9789240039612 (accessed 15/3/2023).
4. Pinder A, Fang L, Hilson R, et al. The carbon footprint of BubblePAPR: a novel item of personal protective equipment. Journal of the Intensive Care Society 2023; in press.
6 Anaesthesia News | May 2023 | Issue 430 Back to contents
Incineration Closed loop recycling Non-variable elements 0.06 0.06 Manufacture of hood 0.62 0.48 Disposal of hood 0.11 0.01 total 0.79 0.56 Total including electricity 0.80 0.57
Figure 1
Table 1: Carbon footprint analysis of different disposal methods for Bubble-PAPR collar and hood. Values are kgCO2 per use.
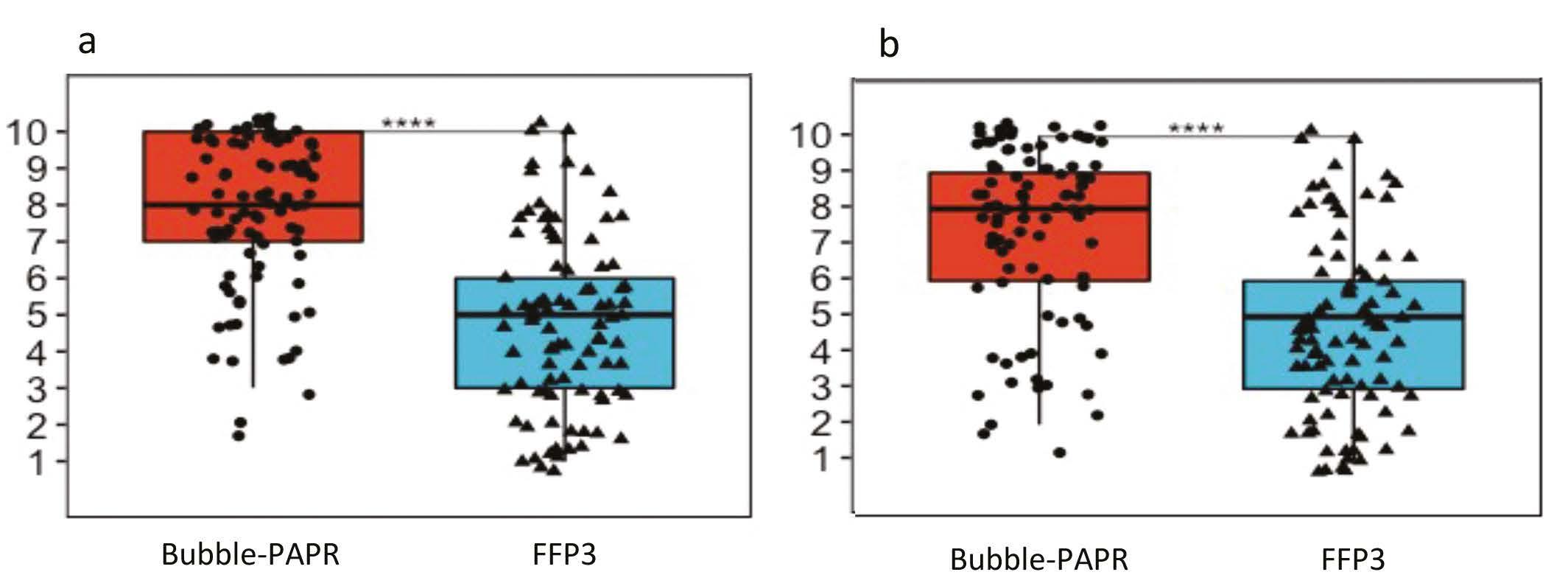
7 Anaesthesia News | May 2023 | Issue 430 Back to contents Figure 2a. Comfort
Figure 3. Communication: a Speaking to patient b Hearing patient c Speaking to staff d Hearing staff
Figure 2b. Safety
Association of Anaesthetists Innovation in Anaesthesia, Critical Care and Pain Awards 2023, Highly Commended
CloudGuard® – a novel leak-free tracheal tube
From time to time, an innovation comes along that causes a paradigm shift. The supraglottic airway device, propofol and point-of-care ultrasound, to name but a few, have changed the face of daily practice and massively improved patient safety. To achieve a similar impact is the goal of all clinical innovators – perhaps lofty, ambitious, or even arrogant. But this determination and a desire to strive to improve care for patients is an attribute that we need to foster in the next generation of Anaesthetists if we are going to discover the next ‘LMA.’
When Mick, a long-time ODP colleague showed me his concept drawings for the CloudGuard® tracheal tube, my initial reaction was “How has this not been done before?” How can we ensure that this is as successful as those preceding examples, rather than consigned to the dustbin of history along with quirky ideas such as the laryngeal tube or the Intubating LMA? The product brief is simply to be a better product than that which is on the market, at no additional cost. Any product that offers safety improvements without drawbacks or a cost premium should surely supersede what comes before it?
The clinical problem
The introduction of high-volume, low-pressure tracheal tube cuffs has been a significant development, but can be seen as an evolution rather than a revolution of design. Cuff leak is a potentially disastrous consequence that can lead to patient harm: the effects can be immediate and obvious, such as aspiration of gastric contents or sudden inability to ventilate effectively; equally serious, but invisible and insidious in nature, is micro-aspiration leading to ventilator-acquired pneumonia.
The design
The unique CloudGuard design incorporates two flaps and three mini cuffs, ensuring multiple points of contact with the tracheal mucosa at all times and reducing the risk of leaks. The conical flaps at the proximal and distal ends provide additional protection by closing shut in response to both positive and negative pressures from either direction. The CloudGuard cuff can permit pressures significantly greater than the cuff pressure to be delivered to the lungs if required; higher pressures actually improve the seal quality!
Development
The development process from concept to production took more than 10 years. The initial design, as with all great inspirations, started on the back of an envelope in a bar. The first prototype was handmade out of wood, followed by balloons, and subsequently rolled metal utilising computer-assisted design (Figure 1). A weakness was identified at this point when it was found that the central cuff was too wide, resulting in a pivot point that made the cuff more likely to unseat or migrate. This problem is prevalent with current tracheal tube cuff designs, but the updated CloudGuard design mitigates against this. Figure 1 shows the prototype cuff (without pilot balloon) mounted on a tracheal tube made from siliconised PVC. The patented production method will potentially allow the cuff to be attached onto any existing tracheal tube type on the market.
The product has undergone extensive in-vitro testing in Belgium, where production is based. With a standard cuff inflation pressure of < 30 mmHg confirmed with an electronic cuff monitor, the ventilator pressure was incrementally increased. There was not a single instance of cuff leak at pressures < 100 mmHg. There was also no greater pressure transmitted to the tracheal mucosa or oesophagus when compared with a standard cuff.
8 Anaesthesia News | May 2023 | Issue 430 Back to contents
A highly leak-resistant cuff is particularly important for: infectious patients who require high ventilator pressures (cuff leak leads to loss of recruitment, failure to deliver desired ventilator volume, environmental contamination with viral particles); prevention of pulmonary soiling and ventilator-acquired pneumonia on ICU; patients undergoing surgery who are at high risk of regurgitation; lung isolation techniques (with the cuff attached to a double lumen tube or bronchial blocker).



Assessment


CE rating has been attained through manufacturing rights, so the CloudGuard tracheal tube is a stepping-stone away from being ready for clinical use. It will be available as a single use item in sizes from 5-10 mm internal diameter including half sizes, and incorporates a Magill tip, Murphy eye, an X-ray visible strip and a subglottic suction lumen. I would like to see the Association of Anaesthetists Innovation Award being a way to encourage hospitals across the UK to consider participation in independent trials. If the clinical claims can be verified by independent research, then I see no reason why this design cannot supersede current tracheal cuff design, both in the UK and further afield, in the next 5-10 years.


Acknowledgements: Michael Barnes and Kristof Braem from Medical Technology 4 Life (MT4L; www.mt4l.com); Jan Mulier from AZ Sint Jan Brugge-Oostende, University of Ghent, Belgium; Association of Anaesthetists Heritage Museum for their support in producing the CloudGuard poster.
Gareth James Consultant Anaesthetist
Hywel Dda University Health Board, Carmarthen
Twitter: @garethiwanjames
Video link here
9 Anaesthesia News | May 2023 | Issue 430 Back to contents
Figure 1
Association of Anaesthetists Innovation in Anaesthesia, Critical Care and Pain Awards 2023, Highly Commended
Innovation with the Exovent team –our continuing story of re-introducing negative pressure respiratory support

The story so far
The May 2021 Issue of Anaesthesia News contained two articles from the Exovent team of engineers, doctors and nurses describing the beginning of our innovation journey and the reasons for developing a new lightweight, patient-friendly, negative-pressure respiratory support device [1, 2]. The global pandemic stimulated our group to form a charity and produce a new device in record time, working alongside the UK’s largest privately-owned aerospace and defence company Marshall ADG. Only positive-pressure devices were considered by the UK ventilator challenge so we were unable to receive fast track support, but we remain undeterred as we believe that the physiological benefits of negative pressure respiratory support will be applicable to a wide range of acute and chronic respiratory diseases besides COVID-19. We expect our proposed devices to be used in homes, hospital wards and within respiratory HDUs and ICUs, as they provide simple technology with low cost implications.
Trials and tribulations
As anyone knows who has tried to introduce an innovative medical device, there are many trials and tribulations to work through. Publication of the results of our initial testing (voted one of the top 10 publications in Anaesthesia 2021 [3]) demonstrated the need for some improvements from the initial prototype, and our excellent new working colleagues at Portsmouth Aviation have taken over the development and subsequent manufacture of the first adult Exovent devices. The whole team at Portsmouth Aviation are enthusiastically committed to the project, and many enjoyable, often humorous, meetings and discussions continue apace. Marshall ADG kindly assisted at the outset with all necessary information to enable the transfer. Full technical files with all the necessary information are being prepared for regulatory approval from MHRA, and we anticipate our clinical trials will commence later this year.
Multiple grant applications and financial requests are in the pipeline with tremendous assistance from Dr Dave Brearley, Consultant and Head of Research in Intensive Care at University College London Hospital, in conjunction with Professor Mike Grocott, Professor of Critical Care and Head of Biomedical Research at Southampton University Hospital. We are actively seeking financial investment –if anyone reading this has £500,000 to put to an excellent cause, then please underwrite the costs of our clinical trials at Southampton University Hospital under the leadership of Mike Grocott and Dr Sophie Fletcher, Consultant in Respiratory Critical Care!
10 Anaesthesia News | May 2023 | Issue 430 Back to contents
Our volunteers and charity
Product development is continuing with the design and production of a low-cost global device for LMIC countries, and specific paediatric devices. Our website, www.exovent.org and charity structure are undergoing continuous development with weekly 2-hour Zoom meetings, supplemented by a very large WhatsApp group and email traffic between Exovent team members! All team members contribute voluntarily, and the charity bank account is mainly comprised of fresh air. We do not waste time worrying about this – the physiological advantages of negative pressure respiratory support are undeniable – the grants and other financial support will happen.
We have been gifted a wonderful laboratory space in Brentford, West London, courtesy of Professor Monty Mythen, UCL Professor of Anaesthesia, and Howe and Co., eminent international solicitors who assist disadvantaged people worldwide.
Approximately 90% of all medical innovations do not make it into production and use in the NHS, let alone elsewhere in the world, but we now have some of the most brilliant, open minded, and progressive engineers and clinicians in the world working on our project. We will succeed. Our story reminds me so much of personally assisting Archie Brain in the 1980s when he experienced a multitude of problems during his development of the Laryngeal Mask Airway. Around 50% of all general anaesthetics in the UK are now administered using a supraglottic airway device, and worldwide the laryngeal mask has been used in millions of patients.
International collaboration
The Exovent team have achieved some wonderful international collaborations in Bangladesh, Ghana, Canada and Pakistan. In particular, we must mention the Alsons Engineering Company in Karachi, Pakistan and the Bangladesh team who have both rapidly developed their own lightweight negative pressure, torso-only devices. The Bangladesh team made theirs of composite material, and have already undertaken their initial clinical trials and submission to their health service and regulator.
Awards
The Exovent Team were awarded the 2021 Verena Winifred Holmes Award from the Institute of Mechanical Engineers for commitment to diversity, inclusivity of engagement, and the development of technology specifically designed to benefit disadvantaged communities and people in the underdeveloped world.
At this year’s Association of Anaesthetists Winter Scientific Meeting we worked with the Heritage Manager to create a poster covering the history of technological innovations and developments that paved the way for Exovent. We displayed the ventilator in the technology exhibition area of the meeting, creating considerable interest amongst anaesthetists, critical care and respiratory physicians. There were some interesting
and amusing discussions relating to perceptions of this ‘old’ technology, confirming that the advantages of negative pressure ventilation have to be re-introduced to the newest generation of doctors; the concept is indeed old, in that all hominid species, and indeed all mammals going back approximately 170 million years, breathe by negative pressure!
Conclusion
A lightweight, torso only, negative-pressure device that is effective, easy to use, and low cost to manufacture has enormous potential to re-introduce negative pressure respiratory support as an alternative to the sophisticated and often expensive modern positive pressure devices. Exovent can provide an alternative to continuous positive airway pressure by delivering continuous negative extra-thoracic pressure (CNEP). Exovent does not require pressurised air or oxygen to function, markedly reducing the demands on oxygen supplies in both wealthy and LMIC countries.
Acknowledgements: More than 50 engineers, doctors, nurse and scientists have voluntary input into the Exovent Development Group (Exovent UK Charity no.1189967).
David Howard
Honorary Consultant, Imperial NHS Trust Hospitals, London
Malcolm Coulthard
Translational and Clinical Research Institute, Newcastle University, Newcastle
Heather Lambert
Retired Consultant Paediatric Nephrologist, Great North Children’s Hospital, Newcastle
Diane Ackerley
Retired GP, Guildford
References
1. Joesbury IR, Howard D, Roberts J. Exovent: an accelerated innovation journey in 2020. Anaesthesia News 2021; Issue 406: 8-9.
2. Coulthard MG, van Egmond J, Patel A. Exovent: a new development from old technology. Anaesthesia News 2021; Issue 406: 10-2.
3. The Exovent Development Group. Exovent: a study of a new negative-pressure ventilatory support device in healthy adults. Anaesthesia 2021; 76: 623-28.
11 Anaesthesia News | May 2023 | Issue 430 Back to contents
Curating the voice of healthcare

Between 2020 and 2022, anaesthetists the world over spearheaded a response to a global pandemic that was characterised, from a clinical perspective, by rapid innovation. A period of ‘having to do things differently’ during the pandemic, combined with the broad adoption of IT into healthcare, has led to a plethora of new systems that have been developed to improve the efficiency of healthcare provision. We present key steps in the journey of Malue Ltd, a funded start-up. We are developing a system that provides operational decision support by capturing, aggregating, analysing and sharing the collective experience of healthcare professionals and patients.
Historically, healthcare innovation has largely been focused in the two areas of basic science and clinical research, and medical device and pharmaceutical development. In contrast, innovations in healthcare systems have lagged. Now because of economic, social, and sustainability challenges, there is a greater imperative to improve these systems. This change in focus, coupled with emerging policy initiatives, has meant that investors are now more likely to support solutions focusing on improving healthcare delivery through integrating life sciences and technology.
Malue collects the wisdom of healthcare professionals, and the insights gained, to support improved operational decisions, so while we are a ‘tech’ company our purpose is focused on healthcare professionals ‘fixing’ healthcare. Maybe unsurprisingly people (including many anaesthetists), rather than technology, have been the most valuable resource in developing the platform’s core capabilities.
A recent event illustrates a common scenario: “For an unclear reason, our anaesthetic facemasks, which we had used for many years, were changed to an alternative brand that had a poor seal. Most of the anaesthetists in our department were unhappy with the new brand. A consensus was reached, after a lot of staffroom chats and sharing of opinions on WhatsApp, to source a product that was closer to the original model. Utilising a product like Malue would have given both the clinical and procurement teams the different perspectives they needed to make a quick and effective decision the first time, and would have prevented the initial switch to a clinically inferior product.”
Malue has spent the last three years developing a ‘decisionsupport’ tool to help drive improvements in effectiveness, efficiency, and sustainability by engaging across the breadth of health and social care. In doing so we have developed
an understanding of some of the unique challenges and opportunities of delivering innovation to healthcare systems. Here are some of the key areas that we believe contribute to a robust business:
• Build an effective team: investors consider the quality of the team ahead of anything else.
• Establish a space that is safe for creativity: many a great idea has been scuppered before it finds its legs because of a dysfunctional team environment.
• Engage the market: to deliver a durable innovation, you must listen to your intended users.
• Accept feedback and respond to changing user requirements: we have found this process to be the most valuable and motivating element of our journey. A huge number of healthcare professionals have shared their experiences, knowledge, and time to guide us on our journey. To the co-founders of Malue, these are the unsung heroes of innovation.
• Find your purpose: bring value to everything you do.
• Understand your customers: know the environment within which you will be operating. You will need to identify those who make the decisions, who regulate, who purchase and who fund.
• Know your competition: before you invest significant time and effort in developing your idea, ensure that your ‘innovation’ does not already exist. If a version of your idea does exist, then ensure that whatever you propose is better, cheaper or more sustainable.
• Acknowledge bias: assume confirmation bias and challenge it. Build an environment that challenges and addresses bias.
• Utilise the bodies that exist to support innovation: in our journey, we have received enormous support from the NHS Clinical Entrepreneur Programme (part of the Accelerated Access Collaborative (AAC)), the Academic Health Science
12 Anaesthesia News | May 2023 | Issue 430 Back to contents
Network (AHSN), and a number of NHS Trusts. Find the organisations that exist to support your specific innovation.
• Don’t forget grants: although competitive, they help you hone your pitch and presentation. They also improve your credibility, as future funders will appreciate that you have successfully demonstrated early-stage potential.
• Set up an operational business: focus on the basics! Set up a company, clarify relationships between partners (plan for both success and failure). Get an accountant, understand tax opportunities for innovation (SEIS/EIS) as these help enormously with initial investment.
• Protect your equity: it’s very tempting in the early stages of development, when you are operating on zero capital, to reward help with equity. Avoid this as much as possible; there will be occasion later when there is no choice but to release equity, but until then hold on to everything you can.
• Identify and protect your Intellectual Property: IP includes every element of your innovation. Capture everything, create a collaborative space and use it, no matter how frustrating that may be – it will be invaluable in the future.

• Know your partners and funders: don’t jump at an opportunity for funding without having a deep understanding of who you are involving. If possible, ensure any new partner also brings complementary expertise.
• Develop an excellent pitch: if you are continuing to work in a healthcare environment, you will have considerable opportunities to speak to people who could be very influential for progress. Assume you only have half a minute of their time to present your idea before they move on, and develop a pitch that defines the problem and provides a solution in 30 seconds. This will open the door for more detailed engagement.
• Build a business plan: you will need one, and it will need to be built by someone who has relevant expertise.
• The NHS is the place to innovate: it’s the world's largest integrated healthcare system, and offers unique opportunities to develop and introduce innovative products and systems. We believe that the wisdom that will drive innovation and change in healthcare lies within the organisation, in its people, in you. If you have an idea that might make a difference in your environment, know there is support to turn your ideas into reality.
Acknowledgements: we would like to thank Buckinghamshire NHS Trust, Oxford AHSN, British Orthopaedic Association, NHS Entrepreneur Programme and our advisors, the unsung heroes. Interested readers may find out more about Malue Ltd at: www.malue.co.uk
Paul Southall
Consultant Anaesthetist, Worcestershire Acute Hospitals NHS Trust, Worcestershire
Carlos Kidel
Consultant Anaesthetist, Royal Free London NHS Foundation Trust, London
Simon Withey
Consultant Plastic and Reconstructive Surgeon, Royal Free London NHS Foundation Trust, London
13 Anaesthesia News | May 2023 | Issue 430 Back to contents
malue wisdom captured
Would

SCReaM
Topics




Calling ALL Theatre staff...
to live in a world where
daily work is easier than it is right now?
you like
your
is an accredited course that could help…
include: Systems Thinking, Human Performance,
of Safety, Psychological Safety and Non Technical Skills (stress, comms, leadership & teamwork) SCReaM Human Factors & Team Resource Management Accredited Human Factors Training @SCReaMSafety Established fellowship opportunity available www.medisimulation.org/scream ✉ rsch.scream@nhs.net
Models
Webinar Invite


Artificial Intelligence in Regional Anaesthesia: The New Kid on the Block











Wednesday 10 May

15:00 GMT / 10:00 EST



































Scan










Dr. Nabil Elkassabany, MD, MSCE, MBA

Anaesthesiology Vice Chair of Clinical Operations, University of Virginia, USA.















Choice Challenge Change

Dr. David Burckett-St.Laurent, FRCA, MBBS
International Keynote Speakers
A/Professor
Gunisha Kaur Professor Jennifer Weller Dr Vanessa Beavis

International ‘Virtual’ Keynote Speakers Professor



















































































Robert Hahn Professor Elizabeth Malinzak Professor Ramani Moonesinghe
Next Generation Keynote Speakers
A/Professor Jai Darvall
A/Professor Lachlan Miles

A/Professor Julia Dubowitz


The Australian Society of Anaesthetists annual National Scientific Congress will be held in Melbourne in October 2023. This in person Congress will include a fantastic scientific program featuring distinguished international keynote speakers. Non-Scientific program includes Welcome party on 4 Oct, Gala dinner on 6 Oct, laneway tours and more. It is the perfect opportunity to meet research and prize winners and collect Practice Evaluation CPD points that will meet the new CPD program, all while networking and socialising with colleagues. International speakers in addition to Keynote Speakers include Prof. Elizabeth Malinzak, Prof. Ramani Moonesinghe and Prof. Robert Hanh.
www.asansc.com.au


Melbourne Convention and Exhibition Centre
Clinical Lead for Regional Anaesthesia, Royal Cornwall Hospital Trust, UK. the QR code to register and learn more
Medical Device Regulations – where are we?

Medical devices were placed on the UK market under the jurisdiction of the European Medical Devices Directives (MDD) in June 1998. This changed in May 2017 when the European Medical Device Regulations (MDR) – EU 2017/745 were enacted in the EU and associated countries. Both the MDD and MDR are classified as ‘new approach regulations’; as such manufacturers are responsible for demonstrating the ‘safety and performance’ of the medical device. This work is then subject to review and examination by a notified body, a commercial entity that has applied and been approved by the EU member state Competent Authority. Before Brexit the role of the UK Competent Authority was undertaken by the Medicines & Healthcare Products Regulatory Agency (MHRA).
Medical Device Directive Review


















After the MDD had been in use for many years the European Commission started a detailed analysis and review in 2010, including taking account of the experience gained – both good and not so good. This review covered many different topics including reclassification of devices, requirements for more clinical investigation/ analysis, increased requirements for post market surveillance, and inclusion of devices with no clinical purpose (e.g. cosmetic and coloured contact lenses with no corrective vision capability). The performance of notified bodies, expertise within notified bodies, greater surveillance of notified bodies by a competent authority, and overall increase in the requirements for placing a medical device on the market were also reviewed. This work resulted in the MDR being published and adopted in 2017. Requisite transition provisions were established; initially the date for application was May 2020, and May 2022 for the Invitro Diagnostic Medical Devices Regulation –EU 2017/746 (IVDR). The proposal submitted in December 2022 to extend the transition period to May 2027 for Class IIb and III devices, and May 2028 for Class IIa and Class I, was formally enacted and published on the 20 March 2023 in the Official Journal of the EU as Regulation (EU) 2023/607.
Impact of the new Medical Device Regulations
The increase in requirements and the overall approach requires additional resources, expertise, funding, etc across the competent authorities, notified bodies and the manufacturers who wish to supply the European market wherever they are located globally. The increase in requirements demands greater effort from manufacturers and notified bodies. Greater surveillance of notified bodies has led to a significant reduction in the number within the European system. Under the MDD there were just over 80, now under MDR there are around 40, many of which do not have full scope. For a notified body to have the capability to review a particular type of device, for example ‘anaesthesia systems’, they must employ resources with that specific competency and expertise. The competent authority has oversight of the notified body designation process; part of this process is to determine the scope of designation, which is achieved in part by the use of Notified Body Oversight Group Codes (e.g. MDA 0201 – Active non-implantable imaging devices utilising ionizing radiation). As noted above, there has been a significant reduction in notified bodies, and in addition many of the current notified bodies now hold limited scope. This is one of
16 Anaesthesia News | May 2023 | Issue 430 Back to contents
the factors that led the EC to extend the transition provisions. It is also important for clinicians and procurers to be aware that changing from MDD to MDR compliance is not cost effective for the manufacturer in some instances. Manufacturers have already completed an exercise to review their medical device portfolio to determine their commercial and compliance strategy. Typical factors considered include: MDD CE Certification expiry date, product life cycle, cost associated to move from MDD to MDR compliance, date of their next notified body audit, and ISO 13485 Quality Management System certificate expiry. From this analysis, manufacturers will already have developed plans for their product folio. Manufacturers are able to revise or amend MDD devices; however if this is undertaken there are requirements as specified in the MDR, for example no change to ‘intended purpose’, no major changes, no specification change, and devices are subject to the post market surveillance requirements as specified in the MDR.
The regulatory environment in the UK
Following the decision to leave the EU, the UK could no longer utilise the European MDR. MHRA specified particular requirements based on the existing European MDD regulations, and from this the UK enacted legislation to adopt the UK Medical Device Regulations as amended 2002. Additionally, the decision was taken to develop UK-specific regulations. This process commenced when the Government published a consultation document in 2021 taking comments from various stakeholders, with the Government’s response published in June 2022. This provided the basis for the development of the UK MDR that MHRA are currently developing; the outlined timetable is that regulations will be: notified to the World Trade Organization in early 2023; laid before Parliament Spring 2023; and come into force in July 2024 with transitional provisions effective from July 2024. In October 2022 MHRA acknowledged there had been issues because of the limited number of UK ‘approved bodies’ that perform a similar function to the EU notified body. At that time there were only four approved bodies, some of which had limited scope, causing a considerable bottleneck for manufacturers seeking approval to the existing MDR.
It is also important to be cognisant of the major restructure that MHRA has been subject to during this very critical period. The regulations have yet to be published, so it is not possible to comment in detail, but suffice to add that MHRA have indicated that the transitional provisions will be quite generous in order not to place additional burdens on the sector. MHRA have taken into consideration the latest changes to the transitional provisions in Europe, and how they will have an impact on the continued availability and supply of European CE-marked devices for the UK market.
Conclusion
From MHRA briefings it is very clear that the new regulations are intended to be aligned where possible with other jurisdictions, for example the USA FDA and other global programmes. Where possible there is a hope of minimising the regulatory burden on the sector, and promoting the opportunity for innovation and research into new therapies, technologies and devices. It is also clearly the intent to facilitate more patient involvement. The Cumberlege Report emphasised First do no harm and provided a number of strategic recommendations, including the appointment of a national Patient Safety Commissioner [1].
As users of medical devices, it is important that you are aware of these changes and the potential impact they may have on your clinical practice. Once the regulations have been published I believe that the situation will become clearer, but in the meantime please contact me at paul.sim@bsigroup.com should you have any questions.
Paul Sim Barema Council Member/ BSI Medical Devices Knowledge Manager
References
1. Webarchive.org.uk. First do no harm. The report of the Independent Medicines and Medical Devices Safety Review, 2020. https://www.webarchive.org.uk/wayback/ archive/20200721101148mp_/https://www.immdsreview. org.uk/downloads/IMMDSReview_Web.pdf (accessed 23/3/2023).
17 Anaesthesia News | May 2023 | Issue 430 Back to contents
There has also been a major restructuring of the MHRA Regulation for manufacturers is more onerous
in the
The MDD is in transition to the MDR
Currently there are delays
regulatory system Manufacturers
may be ‘orphaning’ established products New innovations may face additional hurdles, delays and costs
Veni, video, vici: the slow retreat of the direct laryngoscope
Almost a year has passed since the Cochrane Anaesthesia Research Group's systematic review update titled Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation first saw the light of day [1]. With the entire formatted manuscript spanning just north of 590 pages, most readers would be forgiven for not committing to a deep dive into the nitty gritty of forest plots and subgroup analyses comparing various devices used for intubating the trachea during a leisurely weekend away. In brief, our work reaffirmed and strengthened the findings of the original review by Lewis et al. from 2016 [2]; videolaryngoscopy reduces overall intubation failure, improves firstpass success, improves laryngeal views, reduces trauma, and is likely to reduce rates of oesophageal intubation when compared with direct laryngoscopy.
These findings come from 222 randomised controlled trials of 26,149 participants undergoing tracheal intubation in any setting, comparing outcomes of interest across the three broad categories of videolaryngoscopes: Macintosh-style, hyperangulated, and channelled. One of the highlights was the strong signal of benefit in a prespecified subgroup analysis looking at patients with features of airway difficulty, where hyperangulated devices, such as the GlideScope, the C-MAC D-Blade or the McGrath X-blade, outperformed direct laryngoscopes by a wide margin (RR 0.29, 95% CI 0.17 - 0.48). It is worth pointing out, however, that our study was not designed to answer the question which of these device types may perform better than others, a knowledge gap addressed to some extent by de Carvalho et al. in Anaesthesia [3]. The conclusions of their ranking network meta-analysis are broadly in keeping with our findings, yet offer the interested reader a slightly different lens to view the evidence.
Recently the concept of universal videolaryngoscopy, first popularised by Cook et al. [4], came under scrutiny in a somewhat controversial editorial by Lyons and Harte that generated a fair amount of correspondence among interested parties [5]. Ideas for alternative terms were floated on social media, and it is yet to be seen whether the era of universal videolaryngoscopy will be renamed something else entirely, now that the die has been cast and the Rubicon is slowly being traversed. However, more importantly, the editorial and the river it referenced provided anaesthetists with an unlikely mirror, and an opportunity to reflect: what is the evidence threshold that needs to be crossed for us to update our tools? I suspect factors that have little to do with evidence-based medicine and practice are at play. Embarrassingly, I have little in the way of published evidence to support this assertion, so it may present a ripe area for future qualitative research to anyone interested in exploring clinicians' biases and aversion to change.
In closing, I can only hope that readers will take the evidence presented at face value, consider how it may apply to their practice setting, and adjust their approach to securing the airway accordingly; I know with what I would want to be intubated. I will finish off with a very unscientific (n = 1) anecdote. When Professor
18 Anaesthesia News | May 2023 | Issue 430 Back to contents
Andrew Smith first presented me with the opportunity to work on our review, I was what some might call an ardent direct laryngoscopist but was also comfortable with both commonly-used videolaryngoscope designs. I entered my Cochrane journey with clinical equipoise, and having seen the evidence at first from close up, followed later with an overview of the whole forest, I now opt, conditions permitting, for a videolaryngoscope on most occasions.
Jan Hansel
NIHR Academic Clinical Fellow in Intensive Care Medicine
Wythenshawe Hospital, Manchester University NHS Foundation Trust
Twitter: @VirtueOfNothing
References
1. Hansel J, Rogers AM, Lewis SR, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation. Cochrane Database of Systematic Reviews 2022; 4: CD011136.
2. Lewis SR, Butler AR, Parker J, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database of Systematic Reviews 2016; 11: CD011136.
3. de Carvalho CC, da Silva DM, Lemos VM, et al. Videolaryngoscopy vs. direct Macintosh laryngoscopy in tracheal intubation in adults: a ranking systematic review and network meta-analysis. Anaesthesia 2022; 77: 326-38.
4. Cook TM, Boniface NJ, Seller C, et al. Universal videolaryngoscopy: a structured approach to conversion to videolaryngoscopy for all intubations in an anaesthetic and intensive care department. British Journal of Anaesthesia 2018; 120: 173-80.
5. Lyons C, Harte BH. Universal videolaryngoscopy: take care when crossing the Rubicon. Anaesthesia 2023; in press doi.org/10.1111/anae.15977.
Cochrane Review Conclusion
Videolaryngoscopy is likely to provide a safer risk profile compared to direct laryngoscopy for all adults undergoing tracheal intubation.
Videolaryngoscopes of all designs are likely to - reduce rates of failed intubation, - result in higher rates of successful intubation on the first attempt, - improve glottic views.
Macintosh-style and channelled videolaryngoscopes are likely to reduce rates of hypoxaemic events.
Hyperangulated videolaryngoscopes probably reduce rates of oesophageal intubation.
Call for Honours and Awards nominations
Nominations for the various Association honours and awards are now open. The Association gives a number of honours and awards to recognise outstanding achievement in the field of anaesthesia, critical care and pain management.

Full details of the honours and awards available can be found on the website and include the Sir Ivan Magill Gold Medal, John Snow Silver Medal, Anniversary Medal, Honorary Membership, Pask Award and the Association Award.
Nominations using the official form, accompanied by a short citation will be considered by the Association’s Honours and Awards Committee. Dr Matt Davies, President of the Association said “This is an opportunity to recognise individuals who have made outstanding contributions to the profession. Have a look through the award criteria and consider nominating a colleague today for their exceptional achievement.”
Applications are also welcomed for the Featherstone Professorship.
Applications and nominations must be received by 17:00 on Friday 5 May 2023
For more information, visit www.anaesthetists.org/Home/About-us/ Honours-awards.
19 Anaesthesia News | May 2023 | Issue 430 Back to contents
A deeper plane
The Self – a QIP in a different direction
"To know thyself is the beginning of wisdom.” Socrates
There she is again. You'd thought she'd gone hadn't you? No. You never learn; she's always there. Niggling, niggling... That thought; that feeling.
Sit with her. Observe her. You don't need to engage with her. She isn't you. You are not her.
You are not your thoughts.
She's a wave now. A heavy crash over-head. Drowning you; slowly, slowly...
But she isn't. You are not submerged.
Breathe....2....3.....4.....hold.....2.....3......4......out......2.......3…......4….......
Who are you if not her?
No, that’s your name. You’re not an anaesthetist you silly thing! That’s your job. You may be asked to leave work tomorrow – off with your head! – and not return. No, you’re not a daughter or a son. They won’t live forever, you know that. A mother? A father? You might think so, but one day they may not.... Labels. Yes. Roles. Yes. You? No.
Come on! Who are you?! Now, right now!! Don’t refer to the past or future, the stories you tell yourself over and over, those you are pedalling now.
WHO ARE YOU??!!
“I took a deep breath and listened to the old bray of my heart. I am. I am. I am.” Sylvia Plath
Meditation is not relaxing. It can be. It can be uncomfortable. It can be joyous. It can be surreal. It can be terrifying. Bring kind awareness to these thoughts, these feelings, these sensations. And I mean proper kindness. Love. This is it.
Sensations. Perceptions. An ‘isness’ of sensations and perceptions. But go behind these, still further, deeper still. This is it. Being aware of being aware.
Here is where the journey begins. From here a journey of self-understanding, of self-acceptance unfolds. From here, We shine. From here We love.
This is quality improvement. When embarking on your next QIP, take heed of the words of Saint Augustine "Love, and do what you want."
Acknowledgements: when writing these words, Jonny was inspired by the work of Rupert Spira, Bernardo Kastrup and Ram Dass.
Jonny Dauncey ST4 Anaesthetist University Hospital of Wales,
Cardiff
Jonny would welcome direct messages to: jdauncey@nhs.net
20 Anaesthesia News | May 2023 | Issue 430 Back to contents

Get in touch: +44 (0)1494 551200 @MediplusTIVA marketing@mediplusuk.com www.mediplusuk.com Mediplus Peripheral IV Connectors Safer, more efficient drug delivery Designed to ensure optimal drug delivery for enhanced safety in ICU, HDU, orthopaedic and obstetric departments
Trainee Conference 2023, Leeds
6-7 July 2023, Hilton Leeds City, Leeds
Learn, network and socialise at Trainee Conference 2023, Leeds

Booking is now open for Trainee Conference 2023, the Association’s popular annual anaesthetic conference for trainee anaesthetists, foundation year doctors and first year consultants, as well as medical students interested in the specialty. This year’s Trainee Conference will take place on Thursday 6 and Friday 7 July at the Hilton Leeds City. Members receive special booking discounts, including early-booking savings. Book your study leave and register your place today for Trainee Conference 2023, visit anaesthetists.org/TraineeConference.
Unmissable education and CPD
You do not want to miss our first fully in-person Trainee Conference in over four years. We’ve a fantastic programme to deepen your knowledge, get hands on experience, and focus on your wellbeing and professional development:
• Different ways of learning: lectures, debates, Q&As, group sessions and practical workshops.
• Sessions on obstetric anaesthesia from the OAA and peri-operative medicine from TRIPOM, and paediatric interests are also catered for with lectures and workshops.
• Practical workshops on POCUS, regional anaesthesia and airways.
• Exam preparation workshops: Primary and Final FRCA, as well as help in preparing for your first consultant post.
Inspiring keynote speakers
As a first for Trainee Conference, we’re excited to bring together experts in extreme physiology:
Extreme Altitude: Prof Daniel Martin OBE - Professor of Perioperative and Intensive Care Medicine, who holds the record for the lowest PaO2 ever recorded in a living human after performing a femoral stab on Everest, and was named one of the BBCs Five Greatest Daredevils to have advanced science.



Extreme Space: Dr Peter Hodkinson is a Senior Lecturer and the Head of Aerospace Medicine at King’s College London. He was the UK’s first consultant in Aviation and Space Medicine and is a member of the European Space Agency’s Medical Board and a Fellow of the Aerospace Medicine Association.
Extreme Inflammation: Dr Jessica Manson is a consultant in Rheumatology and Associate Professor at University College London. Dr Manson is a world leading expert in haemophagocytic lymphohistiocytosis (HLH) and the founder and Co-chair of the UK’s HLH Across Specialty Collaboration Group.

Don’t miss Dr Daniele Bryden, Consultant in Intensive Care Medicine Sheffield and Dean of the Faculty of Intensive Care Medicine, who will be presenting ‘A personal view of power', and Professor Rupert Pearse - Professor and Consultant in Intensive Care Medicine and Director of Clinical Research, Queen Mary University of London and Barts Health NHS Trust.

22 Anaesthesia News | May 2023 | Issue 430 Back to contents
Networking opportunities

Trainee Conference offers unmissable opportunities to meet with friends, peers, consultants and industry. Connections made at Trainee Conference 2023 can lead to friendships and even potential job opportunities.


Complimentary Trainee Social
Join us for our complimentary Trainee Social event on the Thursday evening at the Cuckoo Bar, Leeds. We’ve booked a private bar with a rooftop terrace and DJ just for Trainee Conference 2023 delegates. Catch up with friends and colleagues while enjoying complimentary drinks, food and entertainment.
Destination Leeds
Trainee Conference 2023 will take place at the Hilton Leeds City, conveniently located in the heart of Leeds and close to Leeds Central Rail Station. Making this year’s Trainee Conference sustainably accessible. Leeds is a friendly and vibrant city, with great nightlife and a wide selection of accommodation options to suit all budgets.
“We are really excited to have the Trainee Conference open for bookings in Leeds. We’ve put together a packed programme of interesting speakers, expert-led workshops, and plenty of opportunities for networking and socialising now we’re back in person. We can’t wait for you to join us. Get that study leave booked and see you there!”
Dr Sarah Marsden
Trainee Conference 2023 local organiser, Trainee Committee, Association of Anaesthetists
Trainee Conference 2023: the lowdown
You can attend both days or choose to attend one day only. There are special discounts for Association members, including exclusive early booking discounts (valid on and before Thursday 25 May 2023). For more information, please visit the Association website anaesthetists.org/TraineeConference
23 Anaesthesia News | May 2023 | Issue 430 Back to contents
Book now!
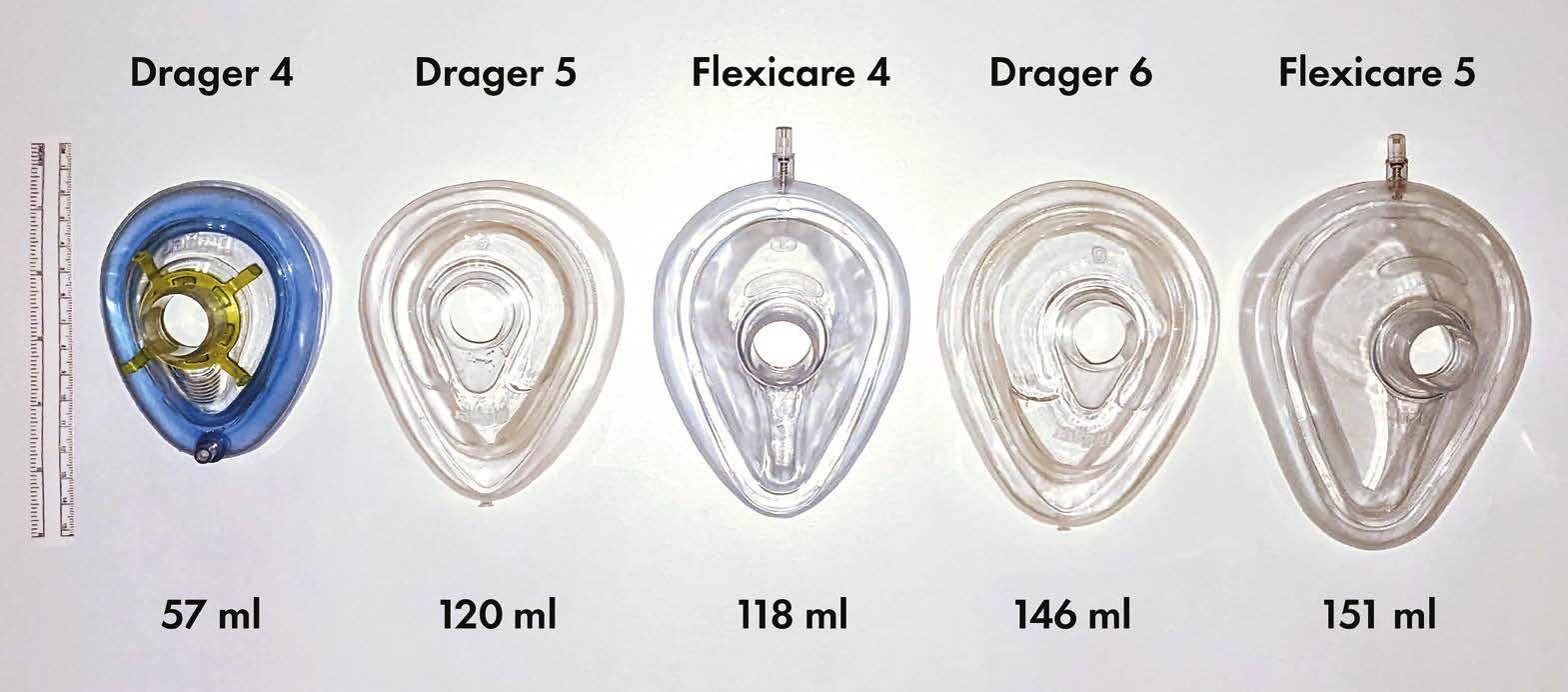
Size matters – with facemask brands
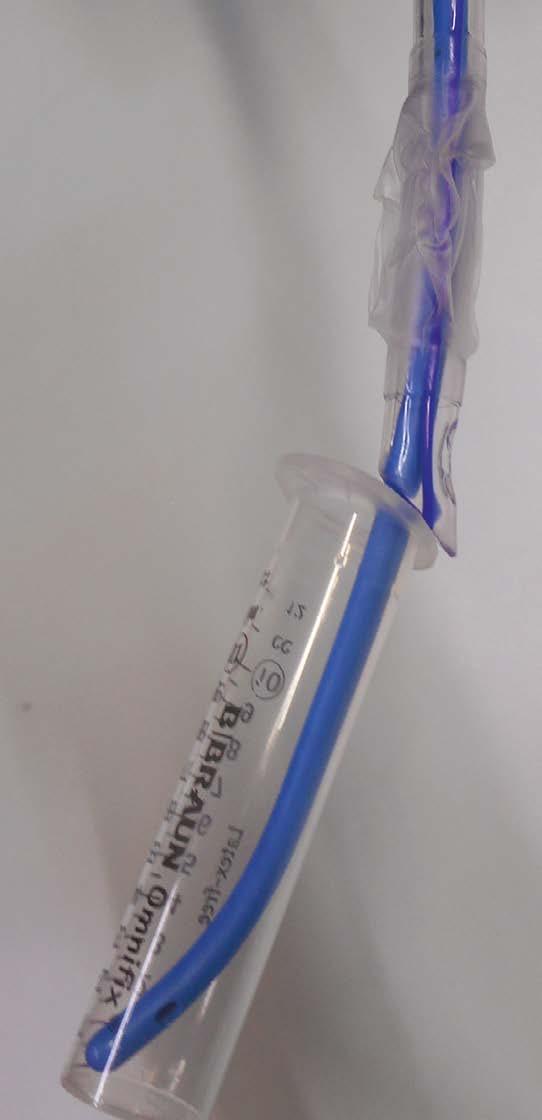
We recently encountered difficulties ventilating the lungs of a patient with a facemask at induction of anaesthesia. Upon checking, it was apparent that this was not the standard Flexicare 5 facemask (Flexicare Group Limited, Mountain Ash, UK) usually used in our department, but rather a Drager 5 (Dräger Medical UK Ltd, Hemel Hempstead, UK) supplied as an alternative. When equivalent sizes of both masks were compared, we noted large discrepancies in volume (Figure 1; volume measured by occluding outlet and measuring to junction of rigid section and soft seal).
Much airway equipment such as tracheal tubes, laryngoscope blades, and oropharyngeal airways have nominal sizes; however arguably the most important piece of airway equipment in our armoury – required for both Plan A and Plan C of the DAS 2015 guidelines for unanticipated difficult intubation in adults [1] – seems to have little standardisation. The size 4 Drager is half the volume of the size 4 Flexicare, and as a consequence a size 6 or even 7 Drager facemask (which we didn’t even know existed) might be required to substitute adequately for a size 5 Flexicare. Perhaps now is the time to highlight this safety issue to manufacturers, and tell them that size matters.
 Arjun Ardeshana ST7 Anaesthetics Registrar
Ned Gilbert-Kawai Consultant Intensivist & Anaesthetist
Tom Irving Consultant Anaesthetist, Airway Lead (RLBUHT) Liverpool University Hospitals
Arjun Ardeshana ST7 Anaesthetics Registrar
Ned Gilbert-Kawai Consultant Intensivist & Anaesthetist
Tom Irving Consultant Anaesthetist, Airway Lead (RLBUHT) Liverpool University Hospitals
References
1. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827-48.
Dear Editor
Tracheal tube impingement demonstrator
Impingement of the tip of a tracheal tube on laryngeal structures when railroading over a flexible introducer or flexible bronchoscope is well described [1, 2], most commonly at the right arytenoid with a left-facing bevel [2]. Once impingement has occurred, increasing the force applied proximally to the tube will increase the gap between the tube tip and the guide, making passage into the trachea impossible. When this happens, the best solution is to withdraw the tube and rotate 90º anticlockwise (for a left-facing bevel) before re-advancing [3].
It may be difficult to explain the mechanism of impingement and the solution to trainees. This might be seen clearly in real time during videolaryngoscopy; alternatively a detailed demonstration would be possible using a parttask airway trainer. However, for an immediate ‘debriefing’ explanation we have found that using the barrel of a 10 ml syringe (Figure 1) is quick, cheap, and worth more than a thousand words.
Andrew J. Coe Consultant Anaesthetist
James P. Coe Trainee Operating Department Practitioner
Jennifer A. Coe Operating Department Practitioner Hull University Teaching Hospitals, Kingston-Upon-Hull
References
1. Asai T, Shingu K. Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: incidence, causes and solutions. British Journal of Anaesthesia 2004; 92: 870-81.
2. Jackson AH, Orr B, Yeo C, Parker C, Craven R, Greenberg SL. Multiple sites of impingement of a tracheal tube as it is advanced over a fibreoptic bronchoscope or tracheal tube introducer in anaesthetized, paralysed patients. Anaesthesia and Intensive Care 2006; 34: 444-9.
3. Dogra S, Falconer R, Latto IP. Successful difficult intubation. Tracheal tube placement over a gumelastic bougie. Anaesthesia 1990; 45: 774-6.
24 Anaesthesia News | May 2023 | Issue 430 Back to contents Dear Editor
Figure 1
Congratulations to Arjun Ardeshana for winning May's Letter of the Month prize.
Figure 1
Your letters
Dear Editor
National total intravenous anaesthesia survey: establishing current practices
TIVA is considered to be more environmentally friendly than inhalational anaesthesia, but there is still an environmental impact from manufacture, transport, and plastic disposables required for administration. The Association of Anaesthetists guidelines for the safe practice of TIVA [1] recommend that: new sterile syringes are used each time (no reuse); preparation of propofol should ensure no risk of contamination (most formulations support bacterial growth); propofol and remifentanil should not be mixed in the same syringe. Despite these guidelines, we are aware of significant variability in practice; to explore this we surveyed members of the Association of Anaesthetists in January 2022. We received 1660 responses: consultants 71%, SAS doctors 6%, trainees 22%, Anaesthesia Associates 1%; teaching hospital 47%, DGH 39%, specialist tertiary hospital 10%; adult practice only 40%, paediatric only 3%, mixed 57%. Table 1 summarises the responses. The main points to highlight are that: 68% reuse TIVA syringes; 20% mix opioids and propofol in the same syringe [2]; 79% are not aware of guidelines on safe TIVA practice.
We suggest that improved scientific evidence [3] might help establish practice consistency, with research focused on: whether pre-filled syringes have overall cost and environmental benefits; the role of drug mixing in paediatric practice [2]; and best practice in drawing up medication to reduce the risk of microbial growth.
Yumna Haroon-Mowahed
Consultant in Anaesthesia and Perioperative Medicine
Laura Elgie
Consultant in Anaesthesia and Perioperative Medicine
University College Hospitals, London
Twitter: @uclh; @haroonmowahedy; @elgie_laura
References
1. Nimmo AF, Absalom AR, Bagshaw O, et al. Guidelines for the safe practice of total intravenous anaesthesia (TIVA). Anaesthesia 2019; 74: 211-4.
2. Goh A-CN, Bagshaw O, Courtman S. A follow-up survey of total intravenous anaesthesia usage in children in the UK and Ireland. Pediatric Anaesthesia 2019; 29: 180-5.
3. White S, Fang L, Shelton C. Propofol waste and the aggregation of marginal gains in green anaesthesia. Anaesthesia 2023; 78: 282-7.
Please see instructions for authors on the Association's website www.anaesthetists.org
How often do you use TIVA?
How many times would you reuse a TIVA syringe per patient?
Does whether or not you reuse a TIVA syringe depend on which drug is in the syringe?
How do you aseptically reuse your syringes?
If you do not reuse your TIVA syringes, why is this?
Do you mix opioid and propofol in the same syringe?
Are you aware of any guidelines about safe TIVA practices including reusing syringes?
25 Anaesthesia News | May 2023 | Issue 430 Back to contents
Mostly 21% > 50% 43% Infrequently 27% Rarely/ never 9%
Never 28% 1-2 23% 3-5 13% As many times as required 32% Other 4%
Yes 22% No 50% N/A 28%
New sterile drawing up needle 51% Disinfect rubber bung 17% Filter needle 45% Good hand hygiene 74% Drawing up close to time of administration 61%
Concerns about bacterial contamination 20% Concerns about syringe sticking 12% Other reason 12%
Yes 20% No 80%
Yes 18% No 79% Other 3% Table 1.
Send your letters to: The Editor, Anaesthesia News at anaenews.editor@anaesthetists.org





13-15 September 2023, EICC, Edinburgh Let’s meet in Scotland Annual Congress 2023 anaesthetists.org/AnnualCongress Join us this September in Edinburgh for Annual Congress 2023. • New innovative ways to gain your CPD • Enhanced focus on interactivity and active learning, with live demos and workshops • Access first-class scientific content, covering clinical and non clinical subjects • Join in live symposia and meet representatives from industry at the exhibition Book now!
Your membership –Maximise your benefits
Make sure you are getting the most out of your Association membership.
New - your exclusive member app – easier to connect
We’re excited to announce that our new member app has launched. On the app you can access guidelines, book events, read our monthly publications, and connect with other members on our online forum Community. You can also keep up to date with all the news from your Association. The app makes information much more accessible so make sure you download the latest version.
Log in using your Association login details. If you have any queries, please email members@anaesthetists.org
Your dedicated platform for CPD - Learn@
Learn@ is an extremely valuable CPD resource exclusively available to members, containing more than 1000 videos of talks from Association events. These are categorised and searchable by date, speaker, event, title, keyword or any combination of those. The system will allow you to create and store a CPD record of your reflections on these videos, and to record any other CPD activities you undertake. You can export your CPD records for use in your appraisal.
To log on to the Learn@ platform use your Association login details https://learnat.anaesthetists.org
If you have any queries, please email learn@anaesthetists.org.
Learn@ app

You can also access Learn@ on your phone or tablet via the Association app. The app also lets you browse, filter and search for lectures by keywords, categories, GMC evaluation areas, events and find content specific to trainees. You can bookmark lectures of interest to view later and download audio or video versions of lectures (both with slides) so you can view and reflect offline. Your activity will be synced with your Learn@ account when your device is connected to the internet.



27 Anaesthesia News | May 2023 | Issue 430 Back to contents
Anaesthesia and Anaesthesia Reports Pilot LMIC-based Editorial Fellowship: September 2023 – September 2024
Getting a paper published is hard work and so too is gaining the skills, knowledge, and experience to become an Editor. Following publication of a consensus statement on measures to promote equitable partnership within international partnerships, and recognising a need to increase representation and diversity within our editorial board, the Anaesthesia journal is piloting an extension of our established editorial fellowship scheme aimed specifically to support an exceptional trainee anaesthetic doctor based in sub Saharan Africa to develop editorial skills. Three of our current Editors completed this journal fellowship scheme, demonstrating the value we place on our Fellows as well as the programme more generally. We invite you to join the team to gain training and experience in every area of journal editorial work. This will be a 12-month post, potentially extendable for a further 12-months after interim review and mutual consent.
Objectives:
• Review and handle one to two manuscripts a week submitted to Anaesthesia and Anaesthesia Reports under direct supervision. You will spend four months with three separate Editors and learn how to construct decision letters and subedit accepted manuscripts for style before publication;
• Undertake a project with supervision from one or more Editors to be presented at a major Association of Anaesthetists international meeting with an aim to submit for publication;
• Contribute to an editorial published in one of the journals;
• Contribute at least one ‘Anaesthesia Digested’ for publication in Anaesthesia News;
• Attend editorial meetings held throughout the year (usually four) remotely
• Be involved with areas of special clinical interest as well as contributing to #TheAnaesthesiaBlog, live broadcasts and podcasts;
• Compile a report at the end of the Fellowship, to be submitted to the Editorial Board and the Fellow’s School of Anaesthesia/Deanery as appropriate (journal appraisal).
Successful applicants will gain experience of working across both journals. There will be an honorarium of £2500 for the year with additional support for data costs and travel (for one in-person meeting per year) according to usual Association of Anaesthetists policy. The successful applicant will also receive membership of the Association of Anaesthetists during this year with associated benefits and training opportunities. There is an option to extend the fellowship for a further 12 months if all parties are in agreement.
You must:
• Be a qualified medical doctor with a M.MED degree or equivalent in Anaesthesia
• Be working in a training position or be within 3 years of starting a substantive (fully trained) position
Applications should:
• Include a one-page CV and copies of medical and anaesthetic qualifications
• Include a 300-word outline of a project that you wish to undertake with an Editor which should be relevant to the journals, publishing or editorial work more generally [1–3].
• Be formatted in accordance with Anaesthesia journal style, which can be found in the ‘instructions to authors’ section of the journal website
Examples of previous Fellowship projects published with the journal:
1. Docherty AB, Klein AA. The fate of manuscripts rejected from Anaesthesia Anaesthesia 2017; 72: 427–30.
2. El-Boghdadly K, Docherty AB, Klein AA. Analysis of the distribution and scholarly output from National Institute of Academic Anaesthesia (NIAA) research grants. Anaesthesia 2018; 73: 679–91.
3. Charlesworth M, Klein AA, White SM. A bibliometric analysis of the conversion and reporting of pilot studies published in six anaesthesia journals. Anaesthesia 2020; 75: 247–53.
Applications must be received via email by midnight on Wednesday 31 May 2023 to anaesthesia@anaesthetists.org. Shortlisted applicants may be asked to undertake an editorial task or interviewed remotely. The Fellowship will start in September 2023.

28 Anaesthesia News | May 2023 | Issue 430 Back to contents
Anaesthesia Digested
Performance and usability of pre-operative prediction models for 30-day peri-operative mortality risk: a systematic review
Vernooij J, Koning N, Geurts J, Holewijn S, Preckel B, Kalkman C, Vernooij L.
Novel predictors of mortality in emergency bowel surgery: a single centre cohort study
When (not) to apply clinical risk prediction models to improve patient care
Lee A, Moonesinghe SR.
“Yes, I remember Aldershot” – no, that’s not right – “Yes. I remember Adlestrop”. Knowing which train you are on is important. Hampshire would not have afforded Edward Thomas’ poem the misty distance of Oxfordshire and Gloucestershire.
Lee and Moonesinghe comment on the paper by Vernooij et al., whilst Derbyshire et al. describe the association of variables with death 30 days after emergency laparotomy. I – like the authors of these papers – am interested in mortality, particularly my own survival trajectory and health. And the thousands of patients I’ve seen in clinic over the past three decades have similarly been interested in discussions about their own survival trajectories. Edward Thomas’ poetic train can illustrate two metrics often used to measure the performance of a prediction model: calibration is when my train arrives at the station; discrimination is which carriage I arrive on. We want to know when we’ll get to our destination, not whether we’ll be there before or after someone else. Discrimination is no more than a side effect of calibration. Mr Brown wants to know his survival trajectory; he does not want to know whether his is better or worse than Mrs Smith’s trajectory. Informed discussions depend upon calibration and its derivatives, such as decision curve analysis.
But what use is any metric if we get on the wrong train to the wrong destination? Thirty-day postoperative mortality is Aldershot. Patients don’t want to go to Aldershot. Being dead in 30 days time is always more likely after scheduled surgery than if you just stay at home. No discussion is required to inform the conclusion that scheduled surgery makes survival to 30 days less likely: do not get on the train. The same criticism cannot be levelled at discussing whether emergency surgery increases or decreases survival to 30 days. But, nevertheless, why 30 days? Why not the wholeness of survival trajectories?
Florence Nightingale railed against the stupidity of 30-day mortality as an outcome, yet here we remain, 150 years later, stuck on the wrong train. It makes you want to look out of the window and dream the meadowsweet and haycocks dry.
N.B. the articles referred to can be found in either the latest issue of Anaesthesia or on Early View (ePub ahead of print)
John Carlisle, Editor, Anaesthesia
29 Anaesthesia News | May 2023 | Issue 430 Back to contents
May 2023
Darbyshire AR, Kostakis I, Meredith P, Toh SKC, Prytherch D, Briggs J.
Poetry corner
Having worked on the Haiku and Limerick as poetic forms within which to explore, or even joke about, details of anaesthesia and science [1, 2], I recently heard on the quiz show Only Connect of a format called a ‘Clerihew’. It’s a four-line poem typically about an individual, is supposed to be funny and it emphasises their biographical achievements.
Part of the humour involves having irregular numbers of syllables in lines and also in rhyming words that are hard to match, such as the person’s name. The structure takes its name (Wikipedia tells me) from the impressively named Edmund Clerihew Bentley. One of his better-known works is about a significant figure in the history of anaesthesia, revealing a little-known aspect of his dietary preferences:
Sir Humphry Davy Abominated gravy. He lived in the odium
Of having discovered Sodium.
Taking this as my inspiration, I thought it might be worth commemorating other major contributors to our speciality in the same way, all of which leads up to:
Before Mallampati
You’d have thought I was batty, Or, at least, behaving most unusually When inspecting patients’ uvulae.
Perhaps our readers might wish to immortalise leading anaesthesiologists in a similar way?
Brian O’Brien Consultant
Department of Anaesthesia
Cork University Hospital, Cork
References
1. O’Brien B. Gas laws stated as haiku – poem. Irish Medical Journal 2022: 115; 568.
2. O’Brien B. Limerick corner. Anaesthesia News 2023: Issue 428; 16.
Notices
Dr Ralph Vaughan, retired member of the Association has sadly died.
Honorary Secretary to the Association Junior Anaesthetists Group 1969-72; Honorary Secretary to the Council of the Association 1992-94; Vice President of the Association 1995-96; Vice President of the Royal College of Anaesthetists; Founder member and first Chairman of the Difficult Airway Society.
We thank Dr Vaughan for his dedication and contribution to the Association over the years and send deepest sympathy to his family at this difficult time.
Letter of the Month prize
It's your Anaesthesia News… and we’d love to encourage more of our readers to share their opinions and experiences.
A Letter of the Month prize will be awarded to the best letter each month. The winner will receive a £50 voucher to use against the cost of one of the Association of Anaesthetists educational events. The award will be made at the discretion of the Editor, and his opinion will be final. No cash alternative will be available. The voucher will remain valid for 12 months.
To increase your chances of winning:
• Keep it short (Max 300 words)
• Be clear and accurate
• Use humour where appropriate
• Keep it topical
Send your letters to: The Editor, Anaesthesia News at anaenews.editor@anaesthetists.org
30 Anaesthesia News | May 2023 | Issue 430 Back to contents

Community, our new online member network and forum. A space for members to share ideas, knowledge, advice, solve problems, collaborate, ask and answer questions and, above all, connect with each other. https://community.anaesthetists.org The username and password that you use to login to Community are the same as your username and password on the Association's website.
For your professional development

Forthcoming seminars at our London headquarters, 21 Portland Place W1B 1PY
Peri-operative neurocognitive dysfunction
Thursday 11 May 2023
Peri-operative Allergy Network – Inaugural multidisciplinary seminar
Thursday 25 May 2023
Monitoring the triad of anaesthesia
Wednesday 7 June 2023
Advanced Ultrasound Guided Regional Anaesthesia (AURA) – Workshops
Thursday 8 June 2023
An introduction to ophthalmic anaesthesia (Joint with BOAS)
Tuesday 13 and Wednesday 14 June 2023
CPD around the UK
Core Topics Exeter
Friday 28 April 2023
Sandy Park, Exeter
Core Topics Manchester
Friday 26 May 2023
Manchester Conference Centre, Manchester
Core Topics Nottingham
Friday 16 June 2023
Crowne Plaza, Nottingham
Core Topics York
Friday 6 October 2023
The Principal York, York City Centre
anaesthetists.org/education
 Special discounts for Association members
Special discounts for Association members

25th Anaesthesia, Critical Care and Pain Forum Da Balaia, The Algarve 25-28 September 2023 www.doctorsupdates.com education in a perfect location®


www.anaesthetists.org Your Will is a way for you to set out your own last wishes – to show those you love what you value and how you want to be remembered. By including a gift to the Association, you'll be making sure your memory lives on. For more information, visit our website, email secretariat@anaesthetists.org or call 0207 631 1650, option 3. Make a gift in your memory
you leave a gift in your Will to the Association, you help ensure the life-changing, life-saving profession of
and that our global community of over 10,000
and
Association of Anaesthetists is the brand name used to refer to both the Association of Anaesthetists of Great Britain & Ireland and its related charity, AAGBI Foundation (England & Wales no. 293575 and in Scotland no. SC040697).
When
anaesthesia is represented
is supported, informed,
inspired.
Going Viral: Contagion, Pestilence
What do cholera, polio and COVID-19 have in common…? Anaesthetists.

Discover anaesthetists’ leading role in improvements to patient care and ground-breaking discoveries during the epidemics and pandemics of the last 180 years.
If you are curious about disease and medical interventions this exhibition will help to make sense of it all. Hear first-hand accounts from anaesthetists on the COVID-19 frontline, see a flu vaccine manufactured in 1918 during the Influenza pandemic, and experience seventeenth-century PPE by trying on our replica plague doctor mask.
Exhibition opens 3 April 2023


and
Association of Anae sthetists Heritage Centre www.anaesthetists.org/heritage Association of Anaesthetists Heritage Centre, 21 Portland Place, London, W1B 1PY Open Monday to Friday 10:00 – 16:00 (closed bank holidays). Last admission is 15:30
Pandemics
Image © Punch a Court for King Cholera, Wiki Commons Image © Behind closed doors, Dr Alexander Scott, South Tees Hospitals
Quality, innovation and choice lnteract with us www.intersurgical.co.uk Quality, innovation and choice lnteract with us www.intersurgical.co.uk The USB™ is a hybrid bougie & stylet with enhanced performance characteristics. Find out more about the benefits that the USB can provide by visiting our website: www.intersurgical.co.uk/info/USB The USB™. It just works better. Difficult intubation? USB™ it.


































































































































 Arjun Ardeshana ST7 Anaesthetics Registrar
Ned Gilbert-Kawai Consultant Intensivist & Anaesthetist
Tom Irving Consultant Anaesthetist, Airway Lead (RLBUHT) Liverpool University Hospitals
Arjun Ardeshana ST7 Anaesthetics Registrar
Ned Gilbert-Kawai Consultant Intensivist & Anaesthetist
Tom Irving Consultant Anaesthetist, Airway Lead (RLBUHT) Liverpool University Hospitals















 Special discounts for Association members
Special discounts for Association members








