1 minute read
Cryptosporidiosis
Cryptosporidiosis remains a threat to the lives of young, malnourished children and immunocompromised people around the world There are currently no vaccines or effective treatments for these patients for cryptosporidiosis Using genetics, proteomics-based techniques, and new culture methods, we are uncovering more about the biology of this important parasite at WCAIR.
Cryptosporidium is a fecal-oral pathogen, spread through contaminated food and water. Its robust shell, called the oocyst wall, protects the parasite from environmental factors including common water treatments like chlorination. Recently, the Pawlowic Lab described the proteome of the oocyst wall Excitingly, this list included for the first time all 9 members of a family of proteins called the Cryptosporidium Oocyst Wall Proteins (COWPs) COWPs are unusual in that every 10–12 amino acid residues is a cysteine. This is fascinating because cysteines can form very strong chemical bonds We think that these bonds could be the critical ingredient that strengthens the parasite’s shell We are using CRISPR/Cas9 to determine which COWPs are required for parasite transmission, and if it is the COWPs cysteines that make the shells so strong.
Advertisement
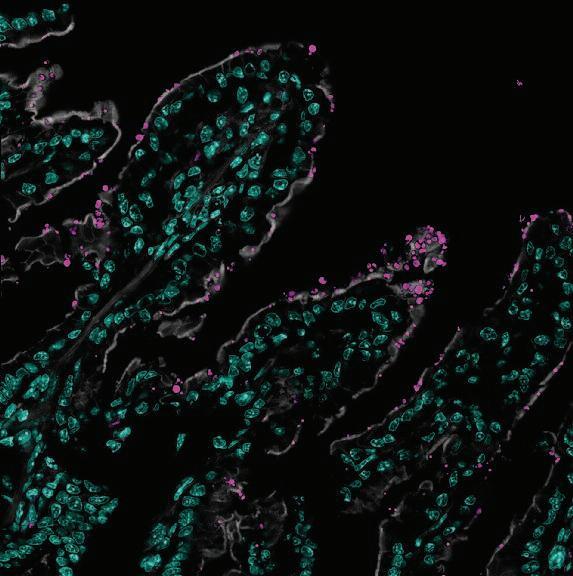
The Pawlowic lab also works closely with the Mode of Action group, led by Dr Susan Wyllie, to adapt chemical proteomics approaches to identify the target of anti-cryptosporidial compounds We have successfully performed thermal proteome profiling in Cryptosporidium for the first time The data from our first experiments provides direct evidence that the Drug Discovery Unit’s lead compound is on-target. Now we are working to apply this approach to compounds whose target is unknown This is really exciting as identifying the target of a drug is very important to understanding how the drug works
We are also developing new tools for studying this parasite We have partnered with Dr Curtis Thorne’s lab the University of Arizona to culture fluorescent Cryptosporidium with human “enteroids”, aka miniature intestines Using microscopy we can create movies that will allow us to investigate the timings of the life cycle in more depth This will enhance our understanding of the basic biology of the parasite, drug mode of action, and improve our understanding of what happens to the intestinal tissue during infection
This year the Pawlowic Lab saw some milestones: 3 PhD students successfully completed their viva, the lead optimization project with the Drug Discovery Unit finished, and we submitted our first manuscript for publication
Our Drug Discovery Unit Apicomplexan Portfolio team, led by Dr Beatriz Baragaña, in collaboration with the Pawlowic lab, Prof. Chris Huston at University of Vermont and Eisai have developed two potent late lead molecules capable of clearing Cryptosporidium infections in mouse and calf models. The drug discovery project started with hit molecules with activity against a protein that is essential for the parasite survival. We optimized the potency and drug-like properties of these compounds using a structure-driven target-based approach










