4 minute read
Focus on anti-infective oxaboroles
Small molecules that contain boron have yielded several anti-infective drugs Indeed, a recent phase IIb/III clinical trial with acoziborole, carried out by DNDi, demonstrated excellent efficacy against sleeping sickness In a New Scientist article, David Horn, Interim WCAIR Director said, “The findings show acoziborole to be a safe, effective, oral therapy for the treatment of human African trypanosomiasis. The target set by the World Health Organisation is to interrupt disease transmission by 2030 and the challenges here must not be under-estimated, but the improvements that acoziborole offers over current alternative therapies could prove pivotal in helping to reach this goal ”
Oxaboroles are now in veterinary trials to treat the cattle disease, nagana, and in phase I trials for the treatment of Chagas disease and leishmaniasis. These compounds also display antiviral, antibacterial, antifungal, and anti-inflammatory activity and are under development for the treatment of malaria and cryptosporidiosis
Advertisement
WCAIR teams have been specifically interested in identifying the target of acoziborole, and in understanding how other oxaboroles are metabolised by specific trypanosome enzymes
Thanks to work carried out by WCAIR teams, including the Horn lab, the Field, lab, the Wyllie Mode-of-Action team, and Drug Discovery Unit teams, we now know how these drugs work, why they are selective for trypanosomatid parasites, and how resistance may emerge in the field
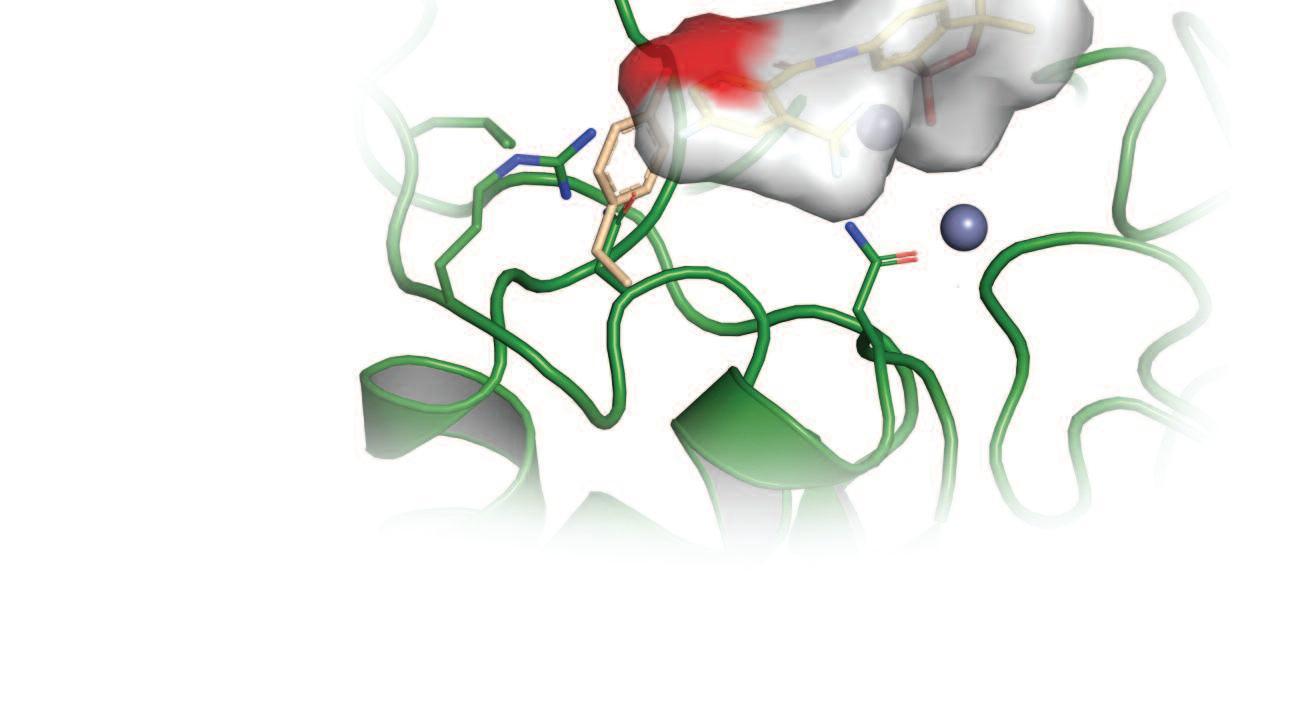
A key collaboration with colleagues at Anacor pharmaceuticals facilitated access to a diverse set of oxaboroles, as well as expertise in boron-based chemistry Initially, these compounds were profiled using genome-scale RNA interference screens, and further assessed using chemical biology, and structural biology approaches, revealing a specific class of pro-drugs that are activated in two steps, involving first host and then parasite enzymes 1 The resulting novel drug activation mechanism, catalysed by a parasite aldehyde dehydrogenase, may now be further exploited to improve therapeutic index
To identify the target of acoziborole, we developed a trypanosome genome-scale over-expression library. Screens carried out using this library showed that acoziborole targets an RNA-processing enzyme called CPSF3 2 Further structural modelling, and gene editing studies, revealed key differences between human and parasite CPSF3, at the site where the drug binds, helping to explain why the drug is safe and non-toxic to humans. Notably, we also found CPSF3 to be the target of the related oxaborole currently in veterinary trials against animal trypanosomiasis.
Working closely with DNDi, the Wyllie lab established that the oxaborole DNDI-6148, a pre-clinical candidate in development for visceral leishmaniasis, also targets CPSF3 3 The team demonstrated that overexpression of CPSF3 in L. donovani, as well as specific mutation of this enzyme, was sufficient to modulate the potency of DNDI-6148. WCAIR computational chemists were able to confirm the precise binding site of this oxaborole at its molecular target and identify new vectors for exploration, should a back-up series for visceral leishmaniasis be required
Further studies on the veterinary oxaborole, carried out in collaboration with our colleagues at the Wellcome Centre for Integrative Parasitology at the University of Glasgow, revealed another distinct and novel pro-drug activation mechanism, involving a peptidase-like enzyme 4 In this case, the drug activation mechanism was characterised in Trypanosoma brucei and in Trypanosoma congolense, a major causative agent of nagana Mutations that prevent drug activation also dramatically reduce drug accumulation in parasites.
We’ve now identified several drug transporters that allow drugs to enter parasites and now also several enzymes that modify drugs, converting them to their active form, often also a form that remains ’trapped’ and concentrated inside the parasite cell The oxaboroles are having major impacts on clinical and veterinary practice against the trypanosomatids and we now have a clearer view as to how these drugs work. WCAIR teams will continue to use cutting-edge technologies to further characterise these drugs and to develop understanding in this area
References
1 Host-parasite co-metabolic activation of antitrypanosomal aminomethyl-benzoxaboroles Zhang N, Zoltner M, Leung KF, , Horn D, Field MC PLoS Pathog 2018 Feb 9;14(2):e1006850
2 Clinical and veterinary trypanocidal benzoxaboroles target CPSF3 Wall RJ, Rico E, Lukac I, Zuccotto F, Elg S, Gilbert IH, Freund Y, Alley MRK, Field MC, Wyllie S, Horn D Proc Natl Acad Sci U S A 2018 Sep 18;115(38):9616-9621
3 DNDI-6148: A novel benzoxaborole preclinical candidate for the treatment of visceral leishmaniasis Mowbray, C, Braillard, S, Glossop, PA, and Wyllie, S J Med Chem 2021 Nov 11; 64(21):16159-16176
4 Veterinary trypanocidal benzoxaboroles are peptidase-activated prodrugs Giordani F, Paape D, Vincent IM, Pountain AW, Fernandez-Cortes F, Rico E, Zhang N, Morrison LJ, Freund Y, Witty MJ, Peter R, Edwards DY, Wilkes J, Van der Hooft J, Regnault C, Read KD, Horn D, Field MC, Barrett MP PLoS Pathogens 2020 Nov 3;16(11):e1008932










