6 minute read
Protecting STEM Cells and Letting Them Free
PROTECTING STEM CELLS
AND SETTING THEM FREE
Advertisement
Megan Butler
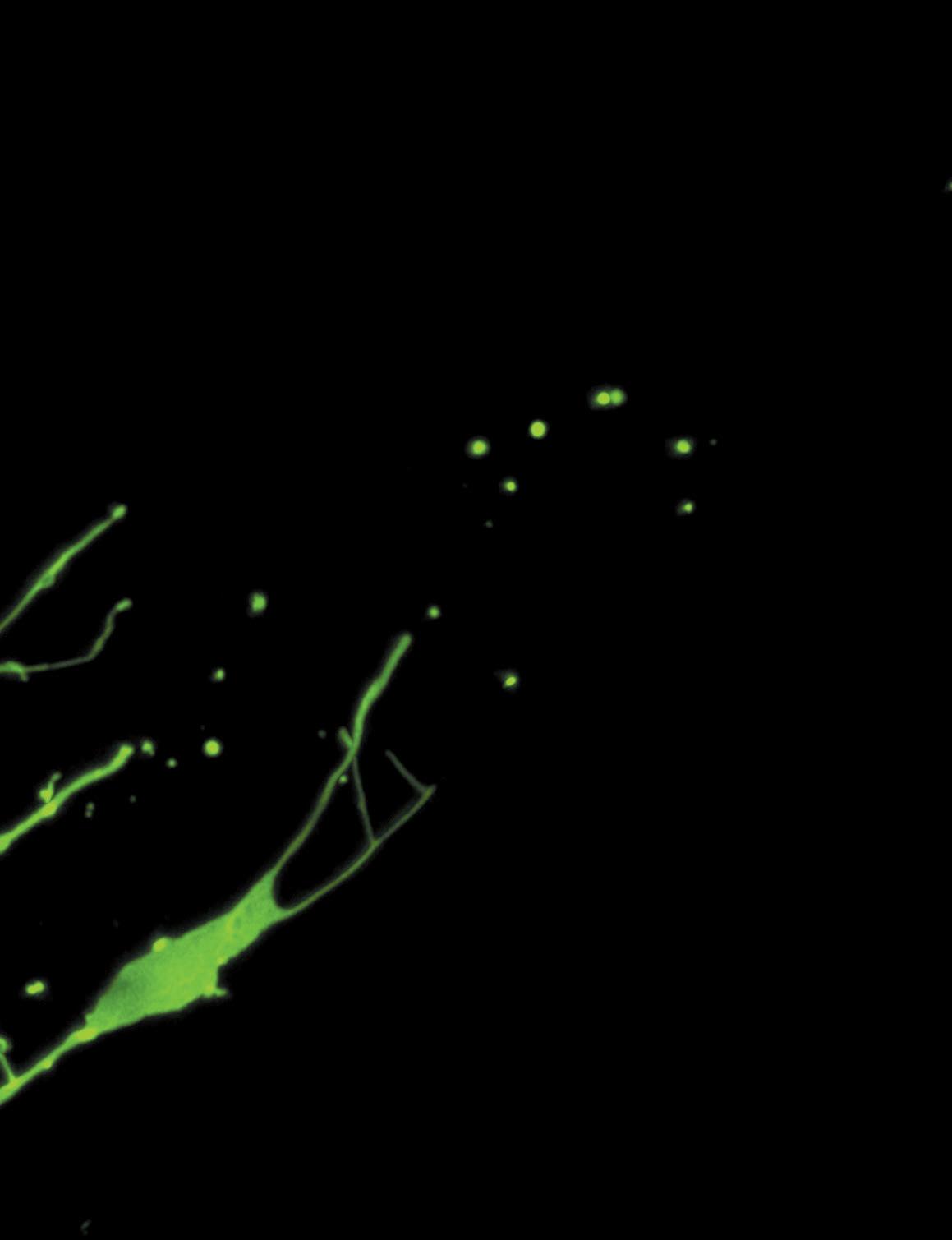
Fluorescent image of the distal tip cell in the gonad of C. elegans. Image courtesy of Kacy Gordon.
Dr. Kacy Gordon distinctly remembers taking developmental biology as an undergraduate at Dartmouth College and looking at images from microscopes for the first time. “It just felt like we were seeing the secrets of the universe.” 1 She pursued those secrets, obtaining a Ph.D. from the University of Chicago and then working as a postdoctoral researcher at the other blue school down the road. It was Dr. Gordon’s time at Duke that led her to study the roundworm Caenorhabditis elegans and its germ stem cell niche – a cellular system that protects and maintains the population of germ cells. She and her colleagues identified many genes that are involved in the regulation of germ stem cells in the C. elegans niche. In the summer of 2019, Dr. Gordon arrived at UNC-Chapel Hill to set up her own lab, and the germ stem cell niche that had infatuated her so much it became one of her main research focuses. C. elegans are very small organisms. Their bodies are made up of only 905 cells, yet their sexual organs arms can each carry 1000 germ stem cells at any given time. One single hermaphrodite, a worm that can self-fertilize, can have 300 to 350 babies or up to 1000 babies if fertilized by a male. 1 For such small creatures to have so many
Dr. Kacy Gordon

offspring, they need to maintain a large germ stem cell population to be differentiated into gametes when necessary, which means there must be some regulation of the stem cells to keep certain portions regenerating differentiating others, in a controlled or sporadic manner. The system of protecting and maintaining stem cells is called a stem cell niche. The C. elegans germ stem cell niche has been studied for over 40 years. As the first niche to be studied in-depth, it has yielded a great deal of information regarding stem cell biology. In worms, the niche cell is called the distal tip cell. If the distal tip cell is terminated, all of the worm’s germ cells differentiate and no longer have the invade the muscle cells when they came in contact. As it turned out, neither of those events occurred. The muscle cells actually extended protrusions, enwrapping the escaped germ cells. However, this niche-like environment was found to be unable to support the proliferation of the germ stem cell population, as this was deemed reliant on the distal tip cell signals. 4 While the specificities of the germ stem cell niche in small worms may seem unrelated to human health and disease, Dr. Gordon’s research has large implications for stem cell biology. “Sometimes a weird little lab critter like a fluorescent worm gonad or a genetically modified
ability to replicate themselves – they are either used up to produce baby worms, or die off. Thus, the distal tip cell uses a set of extended protrusions to enwrap germ stem cells to regulate their differentiation and keep them replicating through mitosis. 2, 3 In her recent paper entitled “Ectopic Germ Cells Can Induce Niche-like Enwrapment by Neighboring Body Wall Muscle,” Dr. Gordon and her colleagues investigated whether or not germ cells can embed into tissues other than the gonad and potentially create a stem cell niche, called an ectopic niche, in those tissues. 4 This research could help to clarify the existence of ectopic niches in C. elegans as well as the mechanism of the gonad germ cell niche. For humans, this research could further elucidate how tumors metastasize in cancers and how stem cells are maintained and released for regenerating and engineering tissues. Dr. Gordon and her colleagues discovered that germ stem cells in C. elegans can induce a niche-like environment when in contact with a distinct tissue – in this case, muscle cells. 4 Like the distal tip cell, the cells of the body wall muscle enwrapped the germ stem cells, indicating that stem cells can create niches outside of their endogenous niche. The researchers used genetic techniques to knock down genes, disrupt the gonad membrane, and then release some germ cells into the body cavity. Then, they used live imaging of the worms through a microscope to see whether the germ cells would fuse with or actively
fly lets us push the boundaries of what we can see about biology and development of all of these living creatures.” 1 In pushing those boundaries, these particular results suggest that even when placed in unnatural environments, stem cells can induce the tissues around them to form nichelike environments. A similar interaction is likely important for metastasizing tumors in human tissues, and such results could be used clinically for tissue engineering and regeneration if niche-like environments can be created even without a gonad. Dr. Gordon hopes to grapple more with a different question of how germ stem cells in their endogenous gonad niche interact with the somatic cells around them. It has long been thought that the distal tip cell would sit in the gonad of the worm and stem cells would sporadically detach from the niche, and then another somatic cell called the sheath would cover the newly differentiating germ cells. Interestingly, this model is very different from other known stem cell niches, such as that of Drosophila melanogaster, the fruit fly. In flies, the germ cells are released from the niche in a controlled way. Recent research indicates that the sheath cell in worms that enwraps differentiating germ cells seems to reach farther towards the distal tip cell than initially anticipated. As such, Dr. Gordon wants to probe for a potential interaction between these somatic cells and re-visit the question of the mechanism of the C. elegans germ stem cell niche. Considering whether C. elegans could be hiding the same old control and release story, she hopes that a better understanding of the structure of this specific niche will reveal more about general requirements for stem cell systems. There is still a lot of work to be done; as Dr. Gordon says, “The endeavor of science is really fun, but it is a story that takes a long time to write.” 1 Dr. Gordon is just getting started.
References
1. Interview with Kacy L. Gordon, Ph.D., 1/21/20. 2. Byrd, D. T.; Knobel, K.; Affeldt, K.; Crittenden, S. L.; Kimble, J. A DTC Niche Plexus Surrounds the Germline Stem Cell Pool in Caenorhabditis elegans. PLoS ONE 2014, 9, e88372. 3. Linden, L. M.; Gordon, K. L.; Pani, A. M.; Payne, S. G.; Garde, A.; Burkholder, D.; Chi, Q.; Goldstein, B.; Sherwood, D. Identification of regulators of germ stem cell enwrapment by its niche in C. elegans. Developmental Biology 2017, 429, 271-284. 4. Gordon, K. L.; Payne S. G.; Linden-High, L. M.; Pani, A. M.; Goldstein, B.; Hubbard, E. J. A.; Sherwood, D. R. Ectopic Germ Cells Can Induce Niche-like Enwrapment by Neighboring Body Wall Muscle. Current Biology 2019, 29, 823-833.










