9 minute read
Mind your metals
Biometals – copper, iron and zinc – are crucial to ensure our brains operate smoothly. They make sure brain cells talk to one another, produce the energy they require to function properly, remove toxic freeradical waste products and, of course, deliver the oxygen they need to survive. Up to half of all proteins in our bodies interact with biometals to keep our bodies healthy.
In a Goldilocks dilemma, too much metal can be harmful, but too little can also mean our brains don’t function properly. Normal learning and our capacity to remember critically depend on these metals existing within the ‘healthy’ range. If metal levels stray outside this range the brain can’t function properly, and it may eventually lead to neurodegenerative diseases like Alzheimer’s, motor neurone or Parkinson’s disease. Our research aims to protect brain health by understanding how metals are involved in neurodegeneration.
Zinc: Associate Professor Paul Adlard wants to understand what zinc is doing at the brain’s synapses – the tiny gaps between brain cells across which information is passed from cell to cell. Synaptic zinc is critical for normal learning and memory. Paul’s group had established that low zinc levels in the hippocampus, a key brain region required for learning and memory, result in a decline in cognitive function regardless of whether it’s normal ageing, or due to the onset of Alzheimer’s.
Paul hoped that restoring zinc levels in the hippocampus would result in improved memory in normal aged mice. He used a compound called PBT2, provided by commercial partners Prana Biotechnology, and showed that the aged brain remains capable of performing at a level seen in much younger mice.
Paul’s project has been highlighted by the National Health and Medical Research Council as one of its “Top Ten” in Australia, showcasing the high quality and innovative nature of this work.
Paul’s zinc therapy may also have beneficial effects in seemingly unrelated conditions, like acrodermatitis enteropathica, a congenital disease that results from the body’s inability to absorb zinc. The harmful neurological implications are clear, and Paul’s research offers a glimmer of hope for the families affected by this rare but devastating disease.
Copper: Although discovered decades ago, a particular class of compounds that deliver copper are now showing great promise in treating neurological diseases. Associate Professor Kevin Barnham works closely with Dr Blaine Roberts, using a molecule called Cu(ATSM) that delivers copper to the motor neurones in the brain and spinal cord, to protect those that would die off in MND.
Although the exact cellular mechanisms behind the rapid and devastating death of motor neurones is still unknown, one of the major players is a copper-dependent protein called superoxide dismutase-1 (SOD1). The protein defends against neuronal death by converting damaging free radicals into inert molecules.
Mutated versions of the protein inefficiently bind copper, meaning it performs its job poorly, thereby leaving cells vulnerable to free radicals. It may even cause SOD1 to do the reverse, producing excessive free radicals instead of clearing them away. Giving Cu(ATSM), obtained from Associate Professors Anthony White and Paul Donnelly at The University of Melbourne, to an MND mouse model extended the lifespan of the mice by an impressive 25 per cent, while delaying the onset of their movement symptoms.
In Australia, approximately 15% of the population is aged 65 and older. This figure is estimated to grow to 21% of the population (8.4 million) by 2050, with global numbers in excess of 1.5 billion.
28% of Australians aged over 85 years require assistance with cognitive tasks.
In the USA, more than 16 million people live with a cognitive impairment.
In collaboration with long-time friend and mentor Professor Jeff Beckman from Oregon State University, Blaine extended this work by giving copper to a mouse model of MND that, along with the mutated human SOD protein, also expressed a protein known as human Copper Chaperone (CCS). It helped the copperdependent protein to properly function by inserting copper, and when this extra copper was delivered using Cu(ATSM), the model mice lived a staggering 18 months (500 per cent) longer than non-treated mice.
The results of these experiments were so successful that the new molecule has been fasttracked into MND clinical trials, which have already started in Sydney, and will soon begin in Melbourne.
Iron: What do a baby’s teeth have to do with diagnosing Parkinson’s disease in a 60-year old grandmother? Biometal chemist Dr Dominic Hare plans to find out.
Around 1300 Australians are diagnosed with Parkinson’s every year, and almost 15 per cent of those are aged under 50. Parkinson’s disease kills the cells in the brain that produce dopamine. A major problem with current approaches to treating the disease is that within a year of someone being diagnosed, more than half of the brain’s dopamine producing cells have died, so therapies given after formal diagnosis are likely to be too late.
Dominic believes that iron fortification of staple foods like cereals, flour and infant formula, may be responsible for this dramatic rise in iron levels in high-income countries. Iron fortification is a huge public health success story, but with sufficient dietary iron now available we may actually be getting too much of a good thing.
As Dominic says, “The brain especially isn't very good at getting rid of excess iron. If the brain is given a head start with too much iron and its 'warehouse' is spilling over as it gets older, there may be a risk that the excess iron will interact with dopamine and end up damaging brain cells.“
To test this idea, Dominic plans to examine whether ‘growth lines’ of iron deposits in teeth, much like a tree’s growth rings, correlate with dietary iron intake, mapping the source and timing of infant nutrition.
Adult teeth begin to calcify at birth, finishing by about age 10. This means they contain a record of dietary iron exposure, which people will carry right into their mid- and old age. These teeth will act as our own ‘iron passport’, documenting the iron environment that a person has been exposed to throughout his or her life.
Using teeth, in conjunction with iron imaging in Parkinson’s patients’ brains, Dominic is seeking a connection between iron exposure and the risk of developing Parkinson’s disease. “We need a way to identify those at risk of the disease before it happens. A test that can detect early exposure to excessive iron may be a way of doing that.”
Finally, the group will recruit a cohort of middle-aged people deemed to have varying risk levels for developing Parkinson’s disease, then follow them for a number of years while administering an experimental drug called deferiprone, an iron binder which ‘mops up’ excess brain iron. Deferiprone is currently being trialled for Parkinson’s in an end-stage study by a French team. Dominic hopes that by identifying at-risk people early in life, they can be given the drug in time to prevent the neuronal death, and subsequently, the onset of Parkinson’s disease.
Identifying those at risk: Dominic Hare believes that all life, and all our brain activity, is a chemical reaction. Therefore, it stands to reason that all disease is a chemical reaction – gone wrong.
Many of the reactions happening in our brains right now depend on the right mix of copper, iron and zinc, and it is important to remember that these reactions do not occur in isolation. Iron-dependent proteins like ferritin, for example, rely on a copper-dependent enzyme to deliver the iron. Disruptions in one or the other could be the underlying cause of several neurodegenerative diseases.
Copper and zinc work together to function properly – meaning that Paul Adlard’s work with zinc therapeutics could have a major impact on motor neurone disease.
Paul says: “Teasing out these interactions between metal-dependent proteins is really going to be a major job in the coming decades. By working out these relationships we will be able to develop and deliver therapeutic agents that target the underlying cause, not just treat a by-product of some aspect of the disease we’re working on.”
Professor Ashley Bush, head of the Oxidation Biology unit, says: “We have been working hard over the last couple of years to bring together a multidisciplinary team with a vast array of experience in areas like metal chemistry, advanced mass spectrometry, animal behaviour, the Australian synchrotron, PET and MRI imaging and, of course, patient trials.”
“I foresee major advances being made in predicting who will come down with Alzheimer’s or Parkinson’s many years before symptoms emerge. We will identify those who need treatment to stave-off these diseases. We are developing technologies, especially MRI, to measure these metals in the brain. At the same time, treatments that correct these metals are being tested, and could complement diagnosis.”
Tapping into the future
When Jess Nithianantharajah was 10 she looked at her siblings and wondered how they could share the same parents yet be so different. Today, Jess’s curiosity about behaviour, environmental influences and genes, and her pioneering work with mice and touchscreens, has taken her to the forefront of behavioural neuroscience and the search for improved medications for psychiatric illnesses.

Iron build up in neurodegenerative diseases can be visualized in the living brain using advanced magnetic resonance imaging techniques. This image shows high iron concentrations especially in areas deep within the brain.
Credit: Dr Jon Cleary, Dr Scott Kolbe, Camille Shanahan, Prof Trevor Kilpatrick, Prof Roger Ordidge.
In 2001, while Jess was working on her PhD, she read a scientific paper published a few years previously in the journal Nature. It was to define her whole research career. She remembers the excitement as she read about the work of Professor Seth Grant, a leader in the field of synaptic genes and brain function. Synapses are the junctions between neurones where sensory information from the environment is ultimately processed. Disruptions to synapse-regulating genes are linked to more than 130 brain diseases.
Seth had worked with Nobel Laureate Dr Eric Kandel in the US, pioneering mouse models with gene mutations. They wanted to understand the impact of genes on learning and memory. He had generated the first mouse model lacking the same synaptic protein that Jess was investigating for her PhD.
Jess knew she had to meet Seth. She flew to the UK, exchanged ideas and flagged the idea that they might work together in the future. She went on to conduct postdoctoral work at the Florey Institute, investigating how gene-environment interactions affected synaptic plasticity (the ability of synapses to change). Soon the call came and she was recruited as a postdoctoral fellow by Seth in 2008 to work at the Wellcome Trust Sanger Institute in Cambridge.
At this time, Seth had developed more than 100 lines of geneticallyengineered mice with synaptic mutations relevant to various disorders. His team was looking at the way these mutations impacted brain function at the molecular signalling level.
But he wasn’t doing much research on behaviour so Jess stepped in, asking, “How do these mutations impact on complex behaviour and cognition?”
Jess forged a collaboration with Professors Tim Bussey and Lisa Saksida at Cambridge University. They were developing a novel behavioural tool – the rodent touchscreen cognitive tests. These touchscreen tests, based on the tests that are routinely used in the clinic with humans, allowed scientists to look more comprehensively at cognitive profiles in mice and rats. Using this technology, the team made some novel insights into the evolution of cognition in a leading paper published in 2013 in the prestigious journal Nature Neuroscience
Extending this work, Jess was first author in another paper published in 2015 that showed – for the first time – that identical touchscreen-based cognitive tests could be used in both mice and humans carrying diseaserelated genetic mutations to assess complex learning and problem-solving. This created an important tool and approach to improve the way scientists measure and model cognitive dysfunction in human disorders in animals, and offered a potential way of standardising results between labs around the world.
Moreover, it meant that new psychiatric drugs could be tested and validated in mice in the hope they would then also have the same positive effects on cognitive problems in humans – currently a big obstacle for developing effective therapies.
In 2014, after a stint at the University of Edinburgh, Jess returned to Melbourne and the Florey to head the Synapse Biology and Cognition laboratory on a coveted Australian Research Council Future Fellowship. Her lab continues to seek the genetic basis of brain disorders, expanding the number of disorders it investigates and creating new tests to tap into complex cognitive processes. The Florey is now the largest site in Australia using the touchscreen technology.
Jess has created clinical collaborations in Edinburgh, Netherlands, Adelaide and Melbourne, studying patients with genetic mutations in synaptic genes that increase the risk of psychiatric diseases such as schizophrenia, autism and depression, using genetically engineered mice with the same mutations. She is on the brink of the next phase of this research, and her career, working with the Drug Discovery Biology group at Monash University testing drug compounds for schizophrenia.
Now a mother of two-year-old Charlotte, Jess is still fascinated by the play of genes on personality. “She’s my biggest project!”
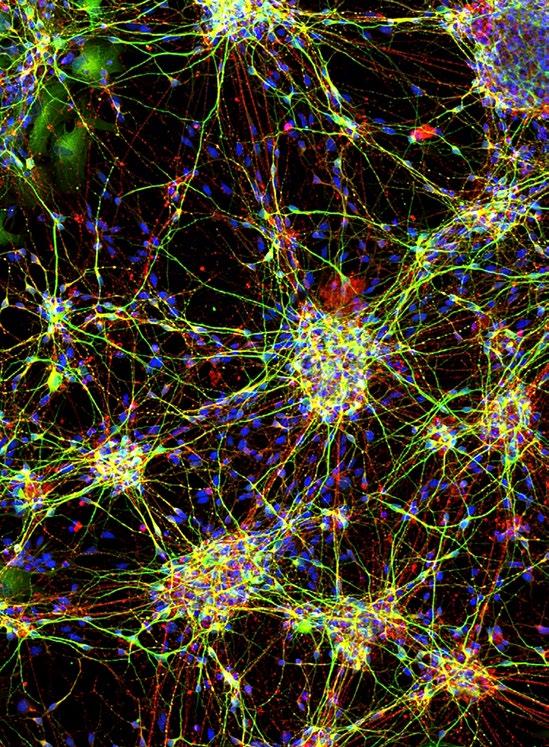
This is an image of neurones grown from stem cells taken from children with epilepsy. We are growing these neurones to understand how changes in normal cellular mechanisms can lead to this severe form of epilepsy. Importantly, by growing these neurones in a dish we are able to treat them with next-generation drugs without exposing the children to any potential risks or adverse effects. In this image the cytoskeletal structures of neurones are shown by staining with fluorescent probes. Here green fluorescence represents dendritic structures and red, axons.