3 minute read
Drug Resistance in People With Viremia on Dolutegravir-based ARVs
By: Dr. Richard Lessells
This month we published a report that helps to build our understanding of HIV resistance to dolutegravir, the antiretroviral drug that is the cornerstone of HIV treatment across the world.
Over 25 million people living with HIV in low- and middle-income countries currently receive treatment with a dolutegravir-containing antiretroviral therapy regimen. Dolutegravir is in the class of drugs known as integrase-strand transfer inhibitors, and it is highly effective when used in combination with other antiretrovirals. Although HIV resistance to dolutegravir does occur, it is rare - this means we currently have quite a limited understanding of the situations in which resistance emerges, which people are at highest risk of resistance, and how the resistance will affect future treatment options. Our research is designed to plug some of these knowledge gaps.
In this open access report published in Clinical Infectious Diseases, we present some initial results from the DTG RESIST study, our NIHfunded international collaborative study nested within the IeDEA network (a global network of HIV clinical cohorts). In this study, we enroll adults and adolescents (10 years and older) living with HIV who have at least one elevated viral load (≥1000 copies/mL) whilst receiving dolutegravir-based ART. If the elevated viral load is confirmed on the study sample, we perform HIV drug resistance testing - most of that laboratory work for the study happens at our sequencing laboratories at KRISP at the University of KwaZulu-Natal.
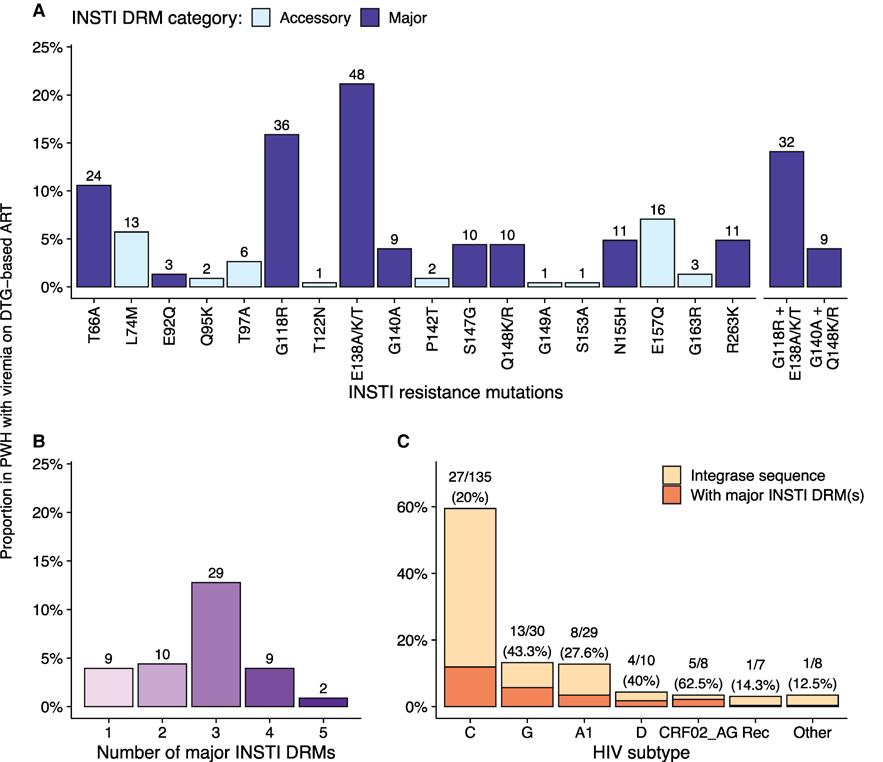
For this report, we included data from 16 clinical sites in seven African countries (Cameroon, Côte d’Ivoire, Malawi, Republic of Congo, Uganda, Zambia, and Zimbabwe). Most participants were receiving TLD (the combination of dolutegravir with two other antiretrovirals, tenofovir and lamivudine); although importantly most had received at least one other antiretroviral regimen prior to this. We detected dolutegravir resistance in approximately one in four of the participants with confirmed elevated viral load and with successful sequencing (59/227). Most of those with resistance had multiple drug resistance mutations giving rise to high-level dolutegravir resistance (which in simple terms means that dolutegravir will no longer be effective for treatment of that individual)[see figure]. As we enrolled participants from southern, western and central Africa the virus sequences represented a range of HIV subtypes, and we observed a wide range of different mutation patterns.
One of the most important findings, which is consistent with other studies, was that all the observed cases of dolutegravir resistance were participants who had received at least one other antiretroviral regimen prior to starting dolutegravir. If the risk of resistance is truly concentrated in this group, it will make it easier for HIV programmes to develop clinical algorithms for people with virological failure.
As part of the project, we continually feedback results to the clinical sites and to policy makers and programme managers in participating countries, as well as to the HIV drug resistance team at the World Health Organization. The study recently surpassed the mark of 1000 participants enrolled across all the international study sites, and the KRISP laboratory is busy completing the sequencing work on this project. All the sequence data are publicly accessible at the NCBI BioProject database (accession number PRJNA1197182). We are now busy preparing presentations for upcoming conferences (IAS 2025 and HIV drug resistance workshop) and further manuscripts that will provide additional insights into dolutegravir resistance.
Access full publication:
Loosli et al. Drug Resistance in People With Viremia on Dolutegravirbased Antiretroviral Therapy in SubSaharan Africa: The DTG RESIST Study. Clinical Infectious Diseases, ciaf204, https://doi.org/10.1093/cid/ciaf204
https://academic.oup.com/cid/ advancearticle/doi/10.1093/cid/ ciaf204/8131783










