9 minute read
Chemistry
ChemiSTEAM 2021
Andres Tretiakov – Science technician and recipient of an honourable mention at ChemiSTEAM 2021 run by the Chemical Institute of Canada
We may not have fancy equipment or specialised techniques in secondary school science labs but we do try to expose students to the beauty of science and in particular chemistry. Science technicians like myself play a fundamental role in this area. We are willing to try new things and share our enthusiasm with students and teachers. So, in the midst of a worldwide pandemic, technicians stepped up to the challenge and offered a more practical way to study and appreciate science.
Most practical work in the lab was out of bounds, inaccessible, or otherwise completely closed for the majority of people. So, armed with smartphones, USB microscopes, and visualizers, technicians all over the world including myself started filming chemistry practicals, demos, and science experiments for the benefit of teachers and students. An article reflecting on our experiences was published in Chemistry World magazine in September 2021 and in last year’s issue of The Pauline.
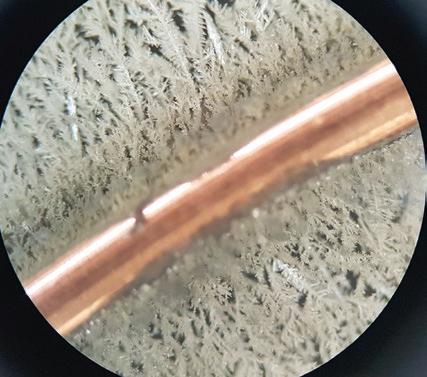
Microscale chemistry is slowly but surely gaining traction in UK schools. After decades of repeating the same experiments over and over again with the same equipment following the same recipe, microscale has opened a door of wonder, curiosity, and understanding – sparking a new interest in learning. We knew microscale was the way forward so we included quite a range in our videos: precipitation reactions “in a puddle’’, gas formation and halogen displacement reactions contained in a petri dish, acid-base neutralisation reactions, etc. All were ready to be performed by students once they returned to the school lab and it was safe to carry out their investigations. The benefits far outweigh the preparation and time taken to build new apparatus, buy new plastic dropping bottles and laminate a few instructions sheets. For starters, the amount of chemicals used is vastly reduced as are potential hazards, complying with the 12 Principles of Green Chemistry. Students can work individually and focus on the reaction taking place with spectacular detail and finish in a surprisingly short period of time leaving time for questions and discussions at the end. The cleaning up is straightforward. Suddenly, metal displacement reactions as the one shown in the photo come alive with exquisite definition and artistic flamboyance when viewed under the microscope at its lowest magnification (40×). A single piece of copper wire about 1cm in length was placed in a cavity glass slide and three drops of 0.1 M silver nitrate solution were added. A few seconds later fragile metal ferns or dendrites started growing on the copper metal surface. Copper is more reactive than silver so it displaces the less reactive silver from the solution forming a copper nitrate solution. The beauty of those fern looking metal crystals is mesmerising. You can tell by the silence and awe experienced by children when they observe this reaction for the first time. They see them growing in real time and at a much faster rate than in the more familiar ‘’silver tree’’ demonstration usually reserved for the period approaching the Christmas holidays. Afterwards, questions kept pouring in, can we grow other metal ferns? Is it possible to form metal crystals out of solutions using other methods e.g. electricity?
Fractal patterns such as these are very common in nature. So immediately, comparisons are drawn to lightning strikes, electrical discharges on powder coated insulators, snowflake crystals, etc. Science has become art and art has become science. Both are interchangeable and both are inspiring and beautiful.

Images of silver dendrite crystals produced as copper displaces silver from a solution of silver nitrate
Thank you to the organisers of ChemiSTEAM, Louise Dawe, Vance Williams for his stunning liquid crystal photographs, and in particular Brian Wagner for being a continual source of inspiration and for sharing my undying love of all glowy fluorescent things. I am honoured to have been selected to be a contributor to their
series of posts. ❚
Chemistry Competitions
MJPS
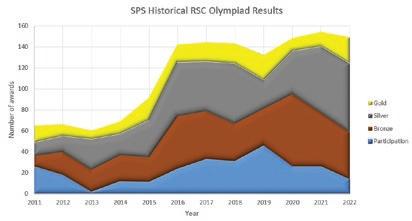
In recent years, St Paul’s has had fantastic success in two national problem solving competitions for senior pupils: the Royal Society of Chemistry’s Olympiad and the Cambridge Chemistry Challenge, organised by the university. The Pauline cohorts of 2022, however, have surpassed all previous triumphs.
In January, 149 Paulines drawn from the lower and upper eighths sat the Chemistry Olympiad and they managed to set three new school records, enjoying, unambiguously, the school’s best-ever year in the event. More awards than ever before were won (135 this year beats 128 in 2021), more silver awards than ever before were won (65 beats 63 in 2021); and, most excitingly, and more gold awards than ever before were won (25 beats 24 in 2019).
Additionally, Eamon Coates (Upper Eighth), qualified for the second round by scoring more marks than any Pauline has ever done before in the first round: he was ranked 4th of the 8,668 competitors, only 34 of whom went on to contest round 2, aiming to gain national selection for the International Olympiad, which was held in China in July.
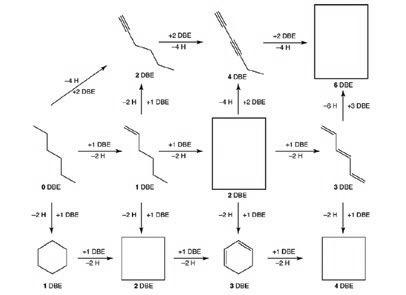
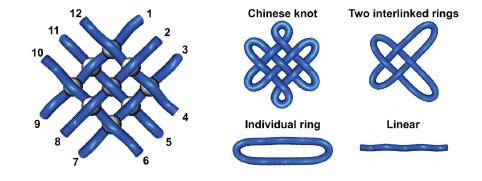
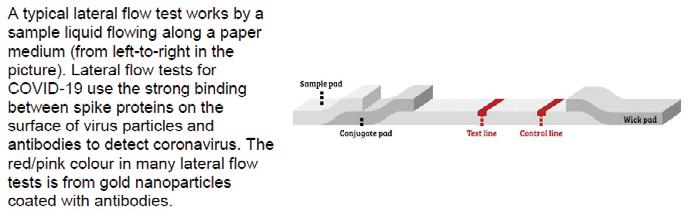
In the paper, competitors tackled some topical questions, including analysing the mechanics of lateral flow tests and the thermodynamics of vaccine storage, as well as more novel chemistry, such as methods of synthesising molecules with the same structures as Chinese knots.
In June, the Cambridge Chemistry Challenge rolled around, but this time, it was the job of the lower eighth chemists – with a little help from a few Sixth Formers who had stayed in school after a morning GCSE chemistry exam – to put themselves to the test, counting up double bond equivalents and contending with some poor humour on the part of the question setters. The results bore out the promise of the Olympiad earlier in the year: more awards than ever before were won (89 beating 87 in 2021), including a spectacular 42 gold awards (smashing the previous record of 37 in 2021). Jash Jhaveri, who announced himself last year by earning a gold award in this event as a Sixth former, finished in the top 15 of the 8,400 entrants in this year’s national competition, earning himself an award of the most rare of the coinage metals, roentgenium, plus an invitation to a residential camp in Cambridge during the summer. ❚
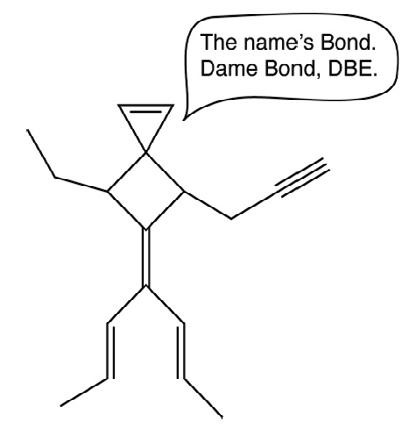
An example of academic humour that fell planar, despite the character’s multiple sp3 centres
The abundance of precious metals continues to increase A reaction scheme through which pupils had to navigate, in which every molecule has six carbons, but where the number of hydrogen atoms dictates the structure and the number of double bond equivalents (DBEs)
Junior Chemistry Projects Club
Dermot Christmas
Simple Experimental Techniques
We spent the beginning of the year mastering simple experimental techniques, such as measuring volumes using a burette, with a focus on precision and technique, as we competed to make the most accurate measurements. We then practised extractions: first of limonene from orange peel and then clove oil from cloves. This made use of several pieces of equipment we had not already encountered in our Chemistry lessons, and we also had to set up a far more complex arrangement of the equipment than we were used to. We then applied other techniques of mixing and filtration to make pure silver from a silver compound through reaction with ammonia, which produced beautiful, silver coated test-tubes.
Esters
In the second half of the autumn term, we produced esters, which are used in perfumes and as sweeteners, from carboxylic acids, with different amounts of carbon, and alcohols, which also had different amounts of carbon.
The general trend was that as the number of carbons within each reactant decreased, the more pungent the resulting smell was.
Our smells were Ethereal (which was so strong smelling that it clung to our nostrils for several days) in addition to PVA Glue, Pears and Bananas (which was our preferred smell).
Colours
In the Spring Term, we explored how the properties of chemicals affected the colours they produced in reactions. We started by designing ‘chemical clocks’, aiming to produce a solution of reactants which would start colourless before changing colour after thirty seconds. This required very accurate measuring, building on the skills we learnt in the autumn term, to ensure the reaction took place at exactly the right time. This proved to be very challenging and it was soon clear that our chemical clocks were not the most reliable. We then applied this method to the acid and base reactions we covered in the Fourth Form, aiming to produce a variety of colours by varying the levels of the acid and base across multiple attempts. To complete our colour-based investigation, we constructed a full rainbow, ranging from crimson to lilac by lighting various metals, including copper (blue), calcium (orange) and our favourite, strontium (crimson).
Dyes
At the beginning of the summer term, we experimented with mordants for the Fourth Form Get Creative Week. We used mordants such as alum and alazarine with the hope that these would bind the dyes onto the fabrics and cause a colour change. The dyes that we used included malachite green and beetroot. Our success with these dyes and mordants was limited but we still very much enjoyed trying to make the Fourth Form creative.
Appreciation to AXJ
Those of us who have attended Junior Chemistry Projects Club have really enjoyed the opportunity to improve our experimental skills and have always relished the interesting, mentally stimulating and engaging aspects of developing our practical understanding of the subject. Ms Jeffery has been remarkably dedicated to the running of the society every week and never fails to propose novel ideas of what to explore and investigate. ❚
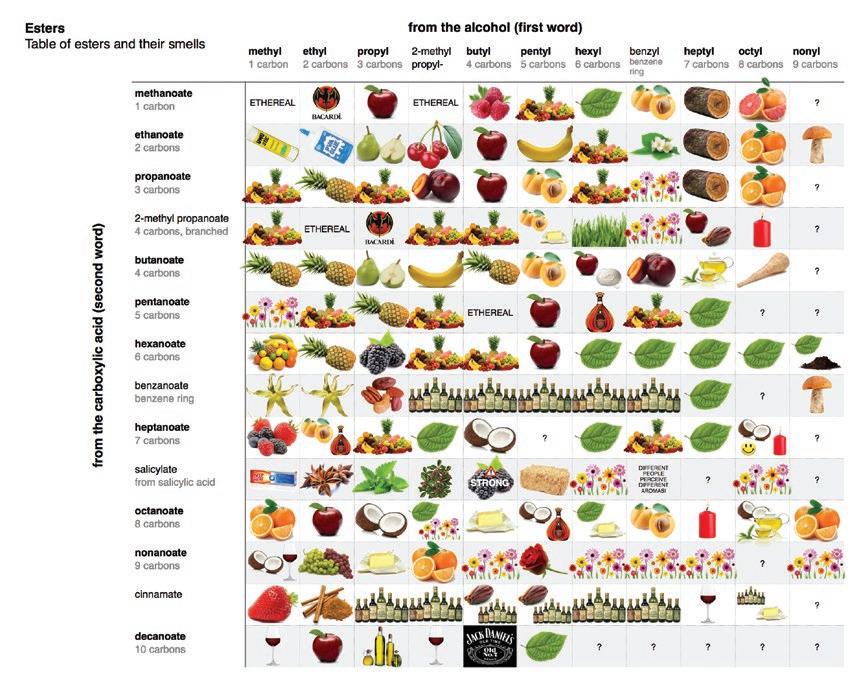
The ester odour matrix Source: https://jameskennedymonash.wordpress.com/2013/12/13/infographic-table-of-esters-and-their-smells/ (NB permission to reproduce granted)
Techognition
MJPS
Friday 4 March was Techognition day – a day to celebrate the work of science technicians and the enormous contribution they make to science education.
The technicians’ prep rooms at the school are particularly busy places with over 400 A Level pupils and nearly 600 GCSE pupils to cater for. In the lead up to the day, the chemistry department asked A Level chemistry pupils to write about how practical work had contributed to their experience of learning chemistry. The department’s teachers then posted these comments all over the chemistry corridor for the technicians to see. A large cake was also involved. Thank you to all of our superb technicians! ❚



Get Creative
MJPS
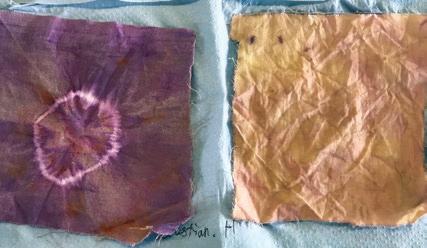
As part of Get Creative week, Ms Jeffery and the Fourth Form experimented with dyeing using mordants, which are compounds that change how dyes stick to fabrics at the molecular level.
The patterns below were created using a single dye, alizarin, and several different treatment conditions using the mordant tannic acid. Alizarin on its own is a rich purple colour, but does not necessarily stick fast to cotton. When treated with tannic acid, however, it sticks well, but its colour changes to a gentle pink. Different orders of treating with the mordant, treating with the dye and tie-dyeing were explored. ❚