
45 minute read
Cardiac causes (< 5 a. Ischemic = angina b. Inflammatory I. Seizures; can be arrhythmic event
Fetal Physiology & Transition A. Fetal circulation; four sites of shunting 1. Placenta a. Receives largest amount of combined ventricular output b. Has lowest vascular resistance in the fetus c. Highest pO2 (~32 mmHg) is found in the umbilical vein d. Umbilical vein empties into IVC; therefore IVC O2 saturation>SVC O2 saturation 2. Ductus venosus; connects umbilical vein to inferior vena cava (bypasses liver) 3. Foramen ovale a. Lower body IVC flow, coronary sinus flow and 95% SVC flow (Eustachian valve) drain into the RV b. IVC flow from L HV and ductus venosus directed to LA through foramen ovale i. Higher saturated blood is delivered to the brain and coronary arteries 4. Ductus arteriosus a. Less oxygenated blood is shunted away from the lungs into the descending aorta to the placenta b. Low O2 content of descending aorta blood increases the efficiency of O2 extraction from the placenta B. Fetal Cardiac Output 1. Fetal heart unable to increase stroke volume when HR falls; CO dependent on HR 2. Decrease in HR an indicator of, or can lead to decreased CO C. Changes in circulation after birth 1. Interruption of umbilical cord a. Immediate increase in systemic vascular resistance b. Closure of ductus venosus as a result of absent placental blood flow 2. Lung expansion a. Decrease in pulmonary vascular resistance (PVR) increases PBF b. Functional closure of foramen ovale secondary to increased LA pressure c. Involved in closure of ductus arteriosus 3. Changes in pulmonary vascular resistance a. PVR normally high in-utero (PBF only 8% total CO) b. Rapid partial fall in PVR with lung expansion immediately post birth c. Slower fall in PVR 4-6 weeks after birth associated with progressive pulmonary vascular wall changes d. Inadequate oxygenation may interfere with normal relaxation causing persistent pulmonary hypertension (PAH) 4. PDA closure a. Functional closure often < 24 hours after birth b. Multiple factors involved in functional and anatomic closure; lower oxygen level delays closure c. Responsiveness decreases with decreasing gestational age d. Decreasing prostaglandin levels mediates closure, thus the pharmacologic use of PGE1 to keep ductus open
CONGENITAL HEART DEFECTS (CHD)
Advertisement
Basic Pathophysiology Of CHD A. Left to right shunt (volume overload) 1. Higher blood flow in pulmonary circulation compared to systemic circulation 2. Oxygenated (red) blood is shunted back to the pulmonary (blue) deoxygenated system 3. Location of shunt determines presentation a. Pre-tricuspid; R- sided overload (e.g. ASD,PAPVC, unobstructed TAPVC) b. Post-tricuspid; L-sided overload (e.g.VSD, complete AV canal (CAVC)/ septal defect (CAVSD), PDA, Truncus Arteriosus) B. Right to left shunt (cyanotic heart disease) 1. Lower blood flow to pulmonary circulation compared to systemic circulation 2. Deoxygenated (blue) blood is shunted into systemic (red) circulation 3. Degree of R-to-L shunt determined by the degree of obstruction to pulmonary blood flow (e.g., TOF, Tricuspid/ Pulmonary Atresia) C. Pressure overload (e.g., PS, AS, PAH) D. Obstruction of systemic circulation
Atrial Septal Defect (ASD) A. Types 1. Secundum a. Most common b. Defect of septum primum is at the site of the fossa ovalis/ PFO 2. Sinus venosus a. Superior; defect in the postero-superior aspect of septum beneath the SVC b. Inferior; defect in the postero-inferior aspect of septum above the IVC c. Associated with Partial Anomalous Pulmonary Venous Connection (PAPVC) 3. Primum ASD = partial AV canal/ AVSD a. Really falls under endocardial cushion defects b. Inferior aspect of atrial septum
c. With “cleft mitral valve”; actually gap between bridging leaflets of common atrioventricular valve which has attachments to crest of ventricular septal separate right and left orifices 4. Coronary sinus a. Very rare b. Defect in floor of LA/roof of coronary sinus just left of atrial septum B. Findings 1. History; usually asymptomatic until late childhood or adulthood a. Small = asymptomatic b. Slow somatic growth, exercise intolerance c. Symptoms of right heart failure, dysrhythmias d. Isolated atrial arrhythmias e. Cyanosis sometimes seen in untreated cases > 3rd-4th decade secondary to pulmonary vascular disease from increased pulmonary blood flow (PBF) 2. Exam a. Small = normal b. Prominent RV precordial impulse ~ mid LSB c. Wide, fixed split S2 (characteristic; secondary to increased RV enddiastolic volume and prolonged ejection time) d. Grade 1-2/6 systolic ejection murmur at ULSB (increased PBF) e. Mid-diastolic rumble at LLSB (increased flow across the tricuspid valve) f. High pitched holosystolic murmur at apex = mitral regurgitation (MR) in ostium primum defect 3. ECG a. RAD, RVH = rSR’ in V1, RAE; secundum defect b. LAD, RVH = rsR’ in V1, RAE; ostium primum defect 4. CXR a. Small L-> R shunt = normal b. Large L-> R shunt i. Cardiomegaly; right heart enlargement ii. Increased pulmonary vascularity
C. Treatment 1. Anti-congestive treatment rarely needed in childhood unless ostium primum defect with MR 2. Small defects (~ <5mm) = no treatment 3. Medium to large defects = transcatheter device occlusion versus surgery (usually performed 3-5 years of age; even large defects can close spontaneously prior) Ventricular Septal Defect (VSD); Except for bicuspid aortic valve (BAV) is most common defect A. Types (multiple classifications); in 10% multiple defects are present 1. Perimembranous (infracristal, membranous) 2. Double-committed sub or juxta arterial (supracristal, subpulmonary) 3. Muscular B. Findings 1. History a. Small (1/3 AoV annulus) = asymptomatic b. Medium (1/2 AoV annulus) to large (= AoV annulus); Qp/Qs > 2 -> left ventricular volume overload and pulmonary venous congestion; occurs ~ 2 weeks-2 months i. CHF = tachypnea, diaphoresis, problems feeding ii. FTT
2. Exam a. Small = murmur only b. Signs of CHF c. Hyperactive precordium with PMI displaced inferolaterally d. S2 is single or narrowly split and accentuated (PA pressure increased) e. Gallop present f. Holosystolic murmur at lower to mid LSB i. Higher pitch=smaller defect ii. Absent with large defects g. Apical mid-diastolic rumble (increased flow across mitral valve);
Qp/Qs > 2 h. 1-2/6 low pitched systolic ejection murmur ULSB (increased PBF) 3. ECG; LAE, LVH or BVH a. BVH in first month indicative of a large shunt with elevated right heart pressures b. RVH only indicates large VSD with pulmonary hypertension 4. CXR a. Small L-> R shunt = normal b. Large L-> R shunt i. Cardiomegaly; left heart enlargement ii. Increased pulmonary vascularity and pulmonary congestion
C. Treatment 1. Majority, up to 75% small defects and up to 20% of larger ones (usually muscular), close spontaneously (typically within first 1 to 2 years of life) 2. Small defects = no intervention usually 3. Anti-congestive measures 4. Nutritional support 5. Surgery a. For large defects before 1 year to prevent pulmonary vascular disease -> Eisenmenger’s syndrome (usually performed 3-4 months of age
b. Any size defect with aortic valve leaflet prolapse and new AR 6. Transcatheter device occlusion for some muscular defects
Complete Atrio-Ventricular Canal/Septal Defect (CAVC/CAVSD) Secondary to abnormal development of endocardial cushions A. Components 1. Ostium primum ASD 2. Inlet VSD 3. Common atrio-ventricular (AV) valve B. Findings 1. High association with Down syndrome (VSD still most common defect) 2. History a. Similar to large VSD b. Age of presentation is related to degree of AV valve regurgitation i. Moderate to severe; 1-2 weeks ii. No or mild; 2 weeks-2 months
3. Exam a. Similar to large VSD b. Holosystolic murmur at apex (AV valve regurgitation) 4. ECG a. Superior QRS axis (0 to -150⁰); LAD i. Very characteristic ii. Secondary to posterior displacement of AV node, His bundle and distal left bundle b. Prolonged PR interval c. Bi-atrial enlargement d. BVH 5. CXR a. Cardiomegaly (disproportionate to the vascular congestion caused by AV valve regurgitation) b. Increased pulmonary vascularity and pulmonary congestion C. Treatment 1. Anti-congestive measures 2. Nutritional support 3. Surgery; usually before 6 months of age
Patent Ductus Arteriosus (PDA) More common with premature infants A. Findings 1. History a. Small = asymptomatic b. Medium-large = CHF, FTT 2. Exam a. Possibly normal = silent PDA; small
b. Signs of CHF c. Hyperdynamic precordial activity with PMI displaced inferolaterally d. S2 narrowly split or single and accentuated (increased PA pressure) e. Continuous murmur (higher pitch->smaller size) ULSB f. Apical mid-diastolic flow rumble (increased flow across the mitral valve) g. Increased/ bounding pulses 3. ECG; similar to VSDs 4. CXR a. Small L -> R shunt = normal b. Large L -> R shunt i. Cardiomegaly; left heart enlargement ii. Increased pulmonary vascularity and pulmonary congestion
B. Treatment 1. Anti-congestive measures 2. Nutritional support 3. Transcatheter occlusion (usually > 6 months of age) with coils/plugs versus surgical ligation/ division 4. No intervention for very small PDA
Coarctation Of Aorta (COA) A. Ductal tissue related aortic constriction adjacent to the left subclavian artery opposite the ductus arteriosus (juxta-ductal); often with associated arch hypoplasia B. Findings 1. Presentation variable a. If critical coarctation; circulatory collapse when PDA closes b. With less severe obstruction or when associated anomalies, e.g. VSD; early CHF c. Proximal hypertension; usually in older child/ adult d. High incidence of bicuspid aortic valve; up to 85% 2. Exam; varies with degree of coarctation, presence of associated anomalies a. BP and pulses diminished in lower compared to upper extremities ( > 20 mmHg difference is usually indicative of significant obstruction) b. Upper extremity, at least RUE, hypertension c. Systolic ejection (possibly continuous) murmur at suprasternal notch or upper mid back d. Signs of CHF; usually only young infant 3. ECG a. If early presentation; RAD, RVH in the absence of other anomalies b. LVH
4. CXR; findings variable a. Can be normal b. Cardiomegaly c. Pulmonary vascular congestion d. Post-stenotic dilatation in descending aorta and rib notching appreciable in older children C. Treatment 1. Anti-congestive measures 2. PGE1 infusion 3. Balloon angioplasty +/- stenting 4. Surgical repair; usually excision with end to end reanastomsosis versus patch, left subclavian flap 5. For mild CoA, if normal proximal BP, normal aortic valve function, no LVH, normal LV function = no intervention
Tetralogy Of Fallot (TOF); most common cyanotic defect A. Components 1. Large malalignment VSD; usually perimembranous 2. Right ventricular outflow (RVOT) obstruction/ pulmonary stenosis (PS): accounts for the variability in presentation 3. Right ventricular hypertrophy 4. Aorta overriding the ventricular septum (anterior malalignment of outlet septum) B. Findings 1. Presentation is a function of the degree of PS->R to L shunt a. Severe = severe cyanosis/ hypoxemia in the first days of life as the PDA closes b. Moderate = asymptomatic mild to moderate cyanosis/ hypoxemia c. Mild = behaves like an isolated large VSD; CHF (“pink” Tet) d. Hypercyanotic or “Tet” spells; rare in early infancy 2. Exam; typically a. +/- cyanosis b. Right ventricular tap (prominent impulse) c. Grade 3-5/6 long, systolic ejection murmur at MLSB to ULSB (PS) which can shorten/ soften with dynamically worsening of RVOT obstruction 3. ECG; RAD, RVH (not rSR’ in V1) > 3 months; can be normal 4. CXR a. Boot-shaped heart or Coeur-en-sabot (absent MPA segment and uplifted apex) b. Normal-sized heart because of absence of volume overload c. Decreased pulmonary vascularity in cyanotic/ hypoxemic infants d. 25% R arch C. Treatment 1. PGE1 infusion if severe cyanosis/ hypoxemia 2. Palliation with surgical aorto-pulmonary shunt e.g modified BlalockTaussig (BT), or transcatheter ductal stenting (rarely balloon pulmonary valvuloplasty) 3. Surgical repair (VSD closure, infundibular muscle resection, outflow tract augmentation/ transannular patch vs. pulmonary valve repair/ replacement, patch PA augmentation) 4. Transcatheter balloon pulmonary angioplasties/ stenting common need post surgeries D. TOF with Pulmonary Atresia (PA); also termed PA/ VSD (very different from PA/ intact ventricular septum a. (IVS)->variable RV hypoplasia, well developed pulmonary arteries, CA abnormalities with RV to CA fistula) 1. Most severe form of TOF 2. Development of pulmonary arteries depends on flow through PDA and systemic arteries forming major aorto-pulmonary collaterals (MAPCA’s) 3. Infants can present with decompensation once PDA closes 4. Minimal cyanosis ->late presentation if prominent collaterals 5. Surgical repair/ outocomes primarily depends on anatomy/ adequacy of pulmonary vasculature
Transposition Of Great Arteries (TGA); most common cyanotic defect presenting in newborn, M > F A. Components 1. Aorta arises anteriorly from RV, PA arises posteriorly from LV 2. Normal ventricular looping/ AV relationships 3. Pulmonary and systemic circulations are in parallel not in series
B. Types 1. Simple = PFO/ ASD, +/- small VSD 2. Complex = large VSD +/- LVOTO/ PS C. Findings 1. Survival depends on presence of mixing between the two circulations; severe cyanosis/hypoxia -> shock with inadequate PFO/ASD, PAH; regardless of PDA 2. Exam a. Cyanosis (occasionally > in upper body; O2 sat > upper vs. lower body) with few findings on exam b. Prominent RV precordial impulse c. Single S2 d. Presence of a prominent murmur indicates associated disease (e.g. VSD, PS) 3. ECG; RAD, RVH; can be normal
4. CXR a. Possibly normal b. Egg-shaped (“egg on string”) or oval cardiac silhouette produced by narrow mediastinum secondary to anteroposterior orientation of great arteries c. Increased pulmonary vascular marking and pulmonary congestion All other types of cyanotic congenital heart disease are associated with diminished pulmonary vascular markings on CXR. D. Treatment; if untreated TGA is associated with 30% mortality in the first week, 50% in 1st month, 95% in first year; high incidence of pulmonary vascular obstructive disease if repaired late 1. Supplemental O2, but limited benefit 2. PGE1 infusion 3. Balloon atrial septosomy ASAP!!! (consider NO if not improved by BAS) 4. Surgical repair; arterial switch (ASO/ Jatene)=anatomic correction a. Includes coronary artery translocation b. Usually before 2 weeks of age to avoid LV deconditioning; can delay if large VSD
Truncus Arteriosus A. Characterized by a single great artery (trunk) arising from the base of the heart supplying the pulmonary and systemic arteries, a large VSD, and +/- truncal = aortic valve stenosis/ regurgitation; type determined by pulmonary artery branching (I-III) B. “Classic” findings; mild cyanosis, CHF, to and fro murmurs, bounding pulses 1. Presentation; depends on degree of PS, usually < mild, and truncal valve function a. Early CHF (lack of PS->increased PBF) most common; worsened by presence of truncal valve regurgitation (AR) b. Cyanosis secondary to presence significant PS; uncommon c. Coronary insufficiency, myocardial ischemia possible 2. Exam; typically a. Signs of CHF (unless significant PS) b. Hyperactive precordium c. Bounding pulses (secondary to diastolic run-off to pulmonary arteries +/- AR) d. Single S2 e. Systolic ejection click (truncal valve; quadricusp 20%, bicuspid 10%) f. Grade 2-3/6 systolic ejection murmur +/- early diastolic murmur mid SBs to base (to and fro murmur) g. Mid-diastolic apical rumble (increased flow through mitral valve) 3. ECG a. Normal QRS axis b. BVH> LVH> RVH c. ST-T changes if myocardial ischemia 4. CXR a. Cardiomegaly b. Increased pulmonary vascularity; usual c. Decreased pulmonary vascularity if severe PS d. 30% R arch C. Treatment 1. Anti-congestive measures 2. Nutritional support 3. Early “complete” surgical repair to prevent myocardial ischemia/ pulmonary vascular disease; usually includes pulmonary valve conduit placement
Partial Anomalous Pulmonary Venous Connection (PAPVC) A. Characterized by 1 or 2 pulmonary veins connecting to RA or one of its venous tributaries 1. Often associated with Sinus Venosus ASD 2. Findings similar to that of ASD 3. Treatment is surgical if 2 anomalous veins, +/- for one anomalous vein
Total Anomalous Pulmonary Venous Connection (TAPVC) A. Characterized by the absence of a connection between the pulmonary veins and the LA with pulmonary veins connecting into the RA or, more commonly, to one of its venous tributaries B. Types 1. Supracardiac; common pulmonary v. drains into the SVC (11%) and innominate vein (35%) 2. Cardiac; common pulmonary v. drains into the coronary sinus (19%) or directly into R atrium (4%) 3. Infracardiac; common pulmonary v. drains into the umbilico-vitelline system (ductus venous or portal system (21%), hepatic vein or IVC 4. Mixed C. “Classic” findings = obstruction; severe cyanosis/ hypoxemia unresponsive to O2, small heart and pulmonary venous congestion on CXR 1. Postnatal presentation depends on degree of obstruction to pulmonary venous flow and adequacy of ASD a. Severe obstruction = presents at birth with cyanosis/ hypoxia,respiratory distress, shock b. No or mild obstruction = usually presents by 6 months with CHF (due to increased PBF), FTT c. Restrictive ASD; early CHF with low cardiac output 2. Exam
a. Severe obstruction i. Cyanosis/ respiratory distress; signs of shock ii. Quiet precordium vs prominent RV impulse iii. Single or narrowly split S2, accentuated iv. Often no murmurs b. Mild to moderate obstruction i. Signs of CHF ii. Hyperdynamic precordium with prominent RV impulse iii. Widely fixed split S2 iv. Mid-diastolic murmur at LLSB (increased flow across TV) v. 1-2/6 systolic ejection murmur ULSB (increased PBF) c. Restrictive ASD, similar to “b” plus poor perfusion 3. ECG; RAD, RAE, RVH 4. CXR
a. Severe obstruction i. Normal heart size ii. Pulmonary venous congestion b. Mild to moderate obstruction i. Cardiomegaly; right heart enlargement ii. Increased pulmonary vascularity iii. On occasion, dilated accessory venous channels to SVC visualized in AP view D. Treatment: surgical; with obstruction is surgical emergency
Hypoplastic Left Heart Syndrome (HLHS) A. Components; a range of anatomic abnormalities 1. Mitral valve hypoplasia/ stenosis or atresia 2. LV hypoplasia 3. Aortic valve hypoplasia/ stenosis or atresia 4. Hypoplastic ascending aorta/ arch +/- a coarctation B. Findings 1. Presentation; survival depends on PFO/ ASD and PDA a. Respiratory distress with CHF (pulmonary congestion) b. Circulatory collapse and myocardial ischemia with PDA closure c. Severely restrictive ASD/ intact atrial septum->severe respiratory distress/hypoxia, shock at birth 2. Exam a. Signs of circulatory compromise/ shock b. Prominent RV precordial impulse c. Single S2 d. Murmurs unusual; tricuspid regurgitation (TR) if RV failure 3. ECG; can be normal a. RAD, RVH b. Absent Q wave in V6 c. RAE d. Diminished left ventricular forces e. ST-T wave changes secondary to ischemia 4. CXR a. Marked cardiomegaly (mainly right heart) b. Increased pulmonary vascularity and pulmonary congestion C. Treatment; median age of death 4-5 days in untreated infants 1. PGE1 infusion 2. Anti-congestive measures; control of ventilation 3. Possible balloon atrial septostomy/ stenting 4. Staged surgical palliation leading to Fontan procedure (initial surgical vs. hybrid Norwood procedure); 85-90% survival 5. Cardiac transplantation 6. No intervention
“Single” Ventricles; can have two normal ventricles A. Abnormal development of right or left heart structures, valves or/and ventricle, leading to functional inability of right or left side of heart to adequately support either the pulmonary or systemic circulation; e.g. Tricuspid Atresia, Pulmonary Atresia/ Intact Ventricular Septum,Double Inlet Left Ventricle, Unbalanced (usually RV dominant) Complete Atrio-Ventricular Canal or Septal Defect (common with heterotaxy syndromes) B. Presentation, findings, and management primarily determined by presence/ degree of obstruction to pulmonary or systemic blood flow; ? ductal dependency C. Majority not associated with genetic syndromes D. Treatment is staged surgical palliation with ultimate Fontan procedure versus transplantation
CARDIAC SURGERY PROCEDURES
Shunts A. Classic Blalock-Taussig (BT) = division distal left subclavian artery with end to side anastomosis between subclavian and ipsilateral pulmonary arteries B. Modified Blalock-Taussig = tube graft interposed between innominate/ subclavian and ipsilateral pulmonary arteries C. Waterston = anastomosis between ascending aorta and RPA (historical) D. Pott’s = anastomosis between descending aorta and LPA (historical) E. Central = tube graft from ascending aorta to pulmonary artery confluence (variation is Melbourne = Direct connection MPA stump to ascending aorta) F. Classic or unidirectional Glenn = ligation/division of proximal pulmonary artery and distal SVC with end to side anastomosis of SVC to ipsilateral pulmonary artery G. Bidirectional Glenn or superior cavo-pulmonary = end to side anastomosis of SVC to ipsilateral pulmonary artery with ligation/division of distal SVC
H. Hemi-Fontan = side to side anastomosis of SVC to RPA with patch occlusion SVC-RA junction I. Sano = tube graft from RV to pulmonary artery confluence (with Norwood procedure)
Blalock-Hanlon = surgical creation of ASD (does not require CBP and generally historical)
Norwood Procedure; initial palliation of HLHS A. Ligation/division of distal MPA B. Anastomosis of proximal MPA (neo-ascending aorta) to proximal aortic arch (native ascending aorta becomes functional proximal coronary artery) C. Coarctectomy and patch recontruction of aortic arch D. Placement of Sano (most common) or modified BT shunt E. Atrial septectomy
Stage II Palliation (for “single” ventricle) = bidirectional Glenn or superior cavo- pulmonary shunt; initial/ partial separation of systemic venous/pulmonary and systemic circulations
Fontan procedure - bypass of inferior vena caval flow directly into pulmonary arteries; completes separation of systemic venous/pulmonary and systemic circulations in “single” ventricle anatomies A. “Classic” = anastomosis of right atrial appendage to pulmonary arteries with ASD closure (historical) B. Lateral tunnel = patch baffling of IVC flow thru atrium (creates new atrial septum) to ipsilateral pulmonary artery C. Extra-Cardiac Conduit (ECC) = ligation/ division IVC-RA junction with placement of tube graft from IVC around/ over heart to ipsilateral pulmonary artery D. Fenestrated Fontan; communication created between Fontan channel and functional LA (acts as pressure pop-off)
Rastelli - biventricular repair that separates left and right-sided structures at ventricular level (commonly associated with repair of Double Outlet Right Ventricle (DORV) and forms of TGA) A. Baffled patch used to create intra-cardiac tunnel between VSD and aorta so LV communicates with aorta B. Placement of conduit with or without valve between RV and pulmonary arteries
Damus-Kaye-Stansel (DKS) - bypass subaortic stenosis in complex defects A. Division of distal MPA B. Anastomosis of proximal MPA to ascending aorta C. Must have or create alternative source of PBF; modified BT or bidirectional Glenn shunt, RV to PA conduit Mustard - intra-atrial baffle (pericardium and prosthetic material); physiologic repair of TGA
Senning - intra-atrial baffle (native tissue); physiologic repair for TGA
Arterial Switch (ASO/Jatene)-anatomic repair of TGA A. Pulmonary artery and aorta transected above valves and switched B. Coronary arteries transferred from old to new aortic root (coronary translocation) C. LeCompte = splay of central branch pulmonary arteries across front of ascending aorta
Coarctation Repairs A. End-to-end; resection of coarcted segment and direct anastomosis of aortic ends B. Patch type; doesn’t include coarctectomy C. Subclavian flap: ligation/ division of distal left subclavian artery with use of proximal left subclavian artery as lay-on patch D. Combination; coarctectomy, end to end re-anastomosis, patch augmentation (for associated arch hypoplasia)
POSTPERICARDIOTOMY SYNDROME
Definition - inflammatory syndrome which consists of fever beyond first postoperative week with systemic symptoms, and signs of pericardial (often also pleural) inflammation
Pathogenesis A. Unclear etiology B. Immunologically mediated process C. Self-limited
Clinical features A. History 1. Febrile illness one to several weeks after surgery, up to 40⁰C 2. In infants/ young children a. Irritability b. Malaise c. Decreased appetite d. Nonspecific chest pain 3. In older children a. Malaise b. Chest pain often described as feeling of “tightness” i. Pain intensified by activity, deep breathing, lying flat ii. Pain radiates to neck, shoulder and back
B. Exam 1. Relative tachycardia
2. Pericardial friction rub 3. Evidence of fluid retention (weight gain, edema, hepatomegaly) 4. Pleural rubs or decreased breath sounds C. Laboratory studies 1. Leukocytosis 2. Elevated ESR, CRP D. ECG 1. Generalized ST elevation 2. Low voltage QRS = large effusion E. CXR 1. Cardiomegaly; absence doesn’t exclude diagnosis 2. Pleural effusions F. Echocardiogram 1. Pericardial effusion 2. Presence/absence of pericardial effusion does not equal/ rule out diagnosis
Treatment A. Supportive measures B. Diuretics for fluid retention, effusions (unless potential tamponade) C. Anti-inflammatory agents 1. Aspirin 80-100mg/kg/d divided q 6 hr for 2-6 weeks 2. Ibuprofen 10mg/kg/dose up to 600 mg q 6-8 hr for 2-6 weeks 3. Steroids in severe cases or if non-responsive to NSAIDs; prednisone 2 mg/kg/d up to 60 mg/d taper over 3-4 weeks 4. Pericardiocentesis with echo guidance vs. open pericardial window in patients with large or rapidly enlarging effusions, evidence of tamponade
ELECTROPHYSIOLOGY
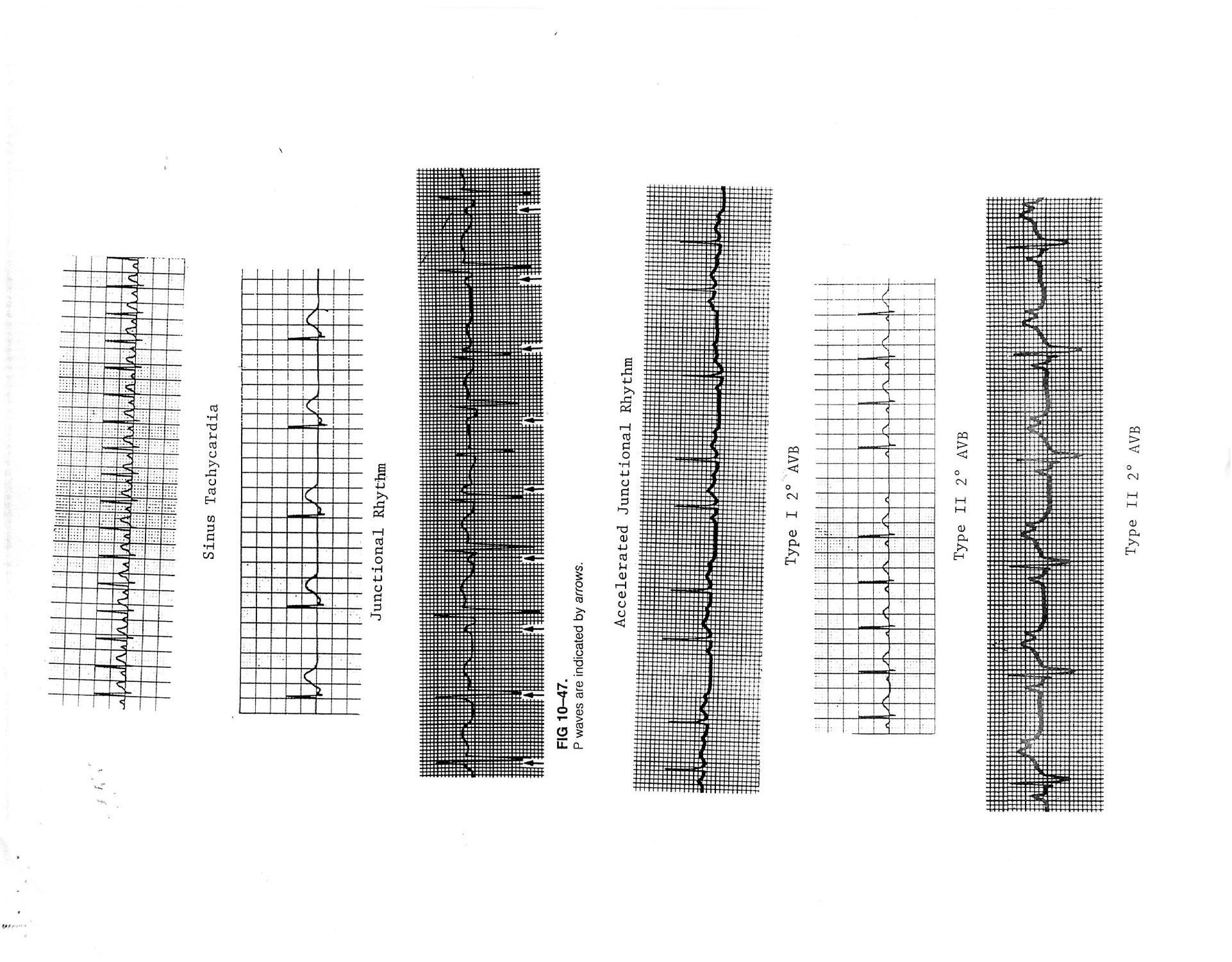
Nonpathologic Rhythms A. Sinus bradycardia; sinus rhythm, but rate < lower limit of normal for age B. Sinus tachycardia; sinus rhythm, but rate > upper limit of normal for age C. Sinus arrhythmia (see appendix) 1. Phasic variation in HR with respiration 2. HR increases with inspiration and decreases with expiration D. Sinus pause; momentary cessation of sinus node activity resulting in absence of P wave and QRS complex (see appendix) E. Wandering atrial pacemaker 1. Alternative atrial pacemaker at rest/ sleep, increase in vagal tone 2. Physiologic rates F. Junctional escape 1. Alternative pacemaker at rest/ sleep, increase in vagal tone 2. Physiologic rates Sick Sinus Syndrome (SSS) A. Inadequate sinus node activity->non-physiologic bradycardia B. Rare except post cardiac surgery; particularly post Fontan C. Can be inherited “ion channelopathy”
Premature Atrial Contractions (PACs) - generally benign and not associated with CHD A. P wave, non-sinus, occurs prematurely (closely inspect T wave) B. P wave morphology depends on location of ectopic focus C. Often incomplete compensatory pause = length of two cycles including premature beat less than two normal cycles D. Blocked; no QRS complex post premature P wave (see appendix) E. With aberrancy; early ventricular activation results in intra-ventricular conduction delay -> QRS prolongation, different QRS morphology (see appendix) F. Treatment 1. None usually 2. Correct metabolic abnormalities
Supra-Ventricular Tachycardia (SVT); narrow complex QRS tachycardia (aberrancy is possible) A. Atrio-ventricular re-entry via accessory pathway; regular with retrograde P wave in SVT 1. Wolff-Parkinson-White (WPW/delta wave) a. Retrograde pathway conduction in SVT most common (orthodromic) b. Prograde pathway conduction in SVT (antidromic) -> wide complex tachycardia with QRS morphology equal to baseline 2. Concealed pathway a. Atrio-Ventricular Reentry Tachycardia (AVRT), Atrio-Ventricular Node Reentry Tachycardia (AVNRT); P wave usually not seen in SVT b. Permanent Form of Junctional Reciprocating Tachycardia (PJRT); often incessant > CHF and dilated cardiomyopathy (DCM) c. Retrograde pathway conduction during SVT -> QRS morphology equal to baseline B. Ectopic foci; automatic tachycardia (warm up/ cool down), often incessant -> CHF and DCM 1. Atrial a. Atrial Ectopic Tachycardia (AET); if LA usually single focus, if RA often multiple foci (see appendix) i. Usually see prolonged PR interval ii. Can have variable A-V conduction
b. Chaotic Atrial Tachycardia (CAT) i. Multiple, at least 3, P wave morphologies ii. Associated with pulmonary disease iii. Often spontaneously resolves 2. Junctional Ectopic Tachycardia (JET) a. Post-operative or congenital (possibly familial) b. Retrograde P wave with 1:1 V-A association or V-A dissociation c. Often incessant -> CHF and DCM C. Intra-atrial re-entry tachycardia 1. Sinus node; extremely rare 2. Intra-atrial muscle (IART); common post extensive atrial surgery, e.g. post Fontan a. Similar to atrial flutter, but slower with 1:1 A-V conduction 3. Atrial flutter (see appendix) a. Presence of “sawtooth” flutter waves (best seen in leads II, III, aVF and V1) b. Atrial rate approximately 300-400 bpm with variable A-V node block/ventricular response (2:1, 3:1, 4:1) 4. Atrial fibrillation (see appendix) a. Rapid atrial rate > 400 bpm b. Irregularly irregular ventricular response with normal QRS complexes c. Infrequent in children; 10-15% of WPW D. Treatment 1. Acute for atrio-ventricular re-entry tachycardia a. Ice bag to face (smother); for infants b. Facial immersion ice water (diving reflex) c. Vagal maneuvers (Valsalva, carotid massage) d. Adenosine 0.1-0.3 mg/kg (up to 12 mg) rapid IV push e. Trans-esophageal overdrive atrial pacing f. Synchronized DC cardioversion i. Initial application 0.5 J/kg ii. Increase to 1-2 J/kg g. IV amiodarone or esmolol, procainamide +/- “d”, “e” or “f” 2. Chronic for atrio-ventricular re-entry tachycardias a. Anti-arrhythmics (digoxin (not WPW), B-blockers, Class IC or 3 drugs) b. Radio-frequency catheter ablation (RFCA) or cryoablation 3. Acute and chronic for automatic tachycardias a. Anti-arrhythmics; as above; usually combination therapy b. Catheter ablation c. Not responsive to vagal maneuvers, adenosine, pacing, cardioversion 4. Acute for IART/ atrial flutter a. Trans-esophageal overdrive atrial pacing
b. Synchronized DC cardioversion 5. Chronic for IART/ atrial flutter a. Anti-arrhythmics; as above b. Catheter ablation c. Surgery; MAZE procedure 6. Acute and chronic for atrial fibrillation a. Anti-arrhythmics; conversion/ suppression versus rate control b. Synchronized DC cardioversion (pre TEE?) c. Anti-coagulation (pre and/ or post cardioversion?) d. Catheter ablation; about mouth of RUPV
AV Node Block A. First-degree AV Block; prolongation of PR interval (in isolation generally not pathologic) B. Second-degree AV Block (see appendix) 1. Mobitz Type I (Wenckebach); PR interval becomes progressively longer (progressive conduction delay) until one QRS is dropped completely (can be normal at rest/ asleep) 2. Mobitz Type II; complete conduction block with intermittent normal conduction-> QRS complex follows every 2nd, 3rd, 4th, etc. P wave C. Third-degree or complete AV block; atrial and ventricular activities are entirely independent of each other (A-V dissociation) (see appendix) D. Treatment of symptomatic bradycardia 1. Atropine 0.02mg/kg IV/ ET; repeat x 1 2. Epinephrine 0.01mg/kg SQ/IV, 0.1 mg/kg ET vs. 0.01-1 mcg/kg/min IV continuous infusion 3. Isoproterenol .01-0.5 mcg/kg/min IV continuous infusion 4. Temporary or permanent pacing
Premature Ventricular Contractions (PVCs) - generally benign and not associated with CHD A. QRS complex is premature B. QRS morphology different from normal QRS 1. Monomorphic/ unifocal 2. Polymorphic/ multifocal C. QRS usually prolonged D. Often full compensatory pause = length of two cycles including premature beat equals two normal cycles E. Patterns e.g. bigeminy/ trigeminy and couplets; considered similar to isolated PVCs F. If suppress with exercise usually benign G. Treatment 1. None usually if isolated finding 2. Correct metabolic abnormalities
Ventricular Tachycardia (VT) - wide complex QRS tachycardia (can be SVT with aberrancy) (see Appendix) A. General 1. 4 or more repetitive premature ventricular beats 2. Monomorphic or polymorphic (includes Torsades de Pointes = Long QT Syndrome (LQTS) and bidirectional = Catecholoaminergic Polymorphic Ventricular Tachycardia (CPVT)) 3. 1:1 V-A association versus V-A dissociation (diagnostic) B. Causes 1. Functional a. Inheritable arrhythmia syndromes = “ion channopathies” i. LQTS ii.CPVT iii. Brugada Syndrome (BS) iv. Short QT Syndrome b. Dilated cardiomyopathy (DCM) c. Myocarditis d. Myocardial injury; contusion e. Myocardial ischemia f. Pulmonary Hypertension (PAH) 2. Structural a. Aortic stenosis (AS) b. Hypertrophic cardiomyopathy (HCM) c. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) d. Repaired CHD; TOF e. Tumors 3. Idiopathic a. RVOT (LBBB pattern, inferior QRS axis) b. Left posterior fascicular (RBBB pattern, LAD) 4. Metabolic/ Pharmacologic a. Hypoxia b. Acidosis c. Hypoglycemia d. Hypo kalemia/ calcemia/ magnesemia e. Hyperkalemia f. Drug induced/ acquired LQTS g. Cocaine/ Amphetamines h. Anti-arrhythmics; particularly class IC and 3) C. Treatment 1. Acute stable VT (consider SVT with aberrancy) a. Lidocaine 1 mg/kg IV, may repeat q 5 min x 2, then continuous infusion 20-50 mcg/kg/min b. Amiodarone 1-5 mg/kg IV load, 1 mg/kg over 5 min, then 10 mg/ kg/d PO vs. continuous IV infusion c. Procainamide 5-15 mg/kg IV load at 0.5-1 mg/kg/min, then continuous infusion 30-80mcg/kg/min d. If suspect RVOT/ Fascicular VT, IV Verapamil 0.1-0.3 mg/kg not to exceed 5 mg over > 2 min, repeat x 1 in 30 min e. Synchronized DC cardioversion 0.5-2.0 J/kg 2. Acute unstable VT a. Synchronized DC cardioversion 2-4 J/kg b. Lidocaine, IV amiodarone, IV procainamide 3. Torsades de Pointes; usually self-limited (see appendix) a. Defibrillation (unsynchronized); 2-4 J/kg b. MgSO4 10-50 mg/kg IV c. Isoproterenol 0.01-0.5 mcg/kg/min d. Lidocaine D. Chronic treatment 1. Treat underlying disorder 2. Anti-arrhythmics (B-blockers, Class 1 or 3, Verapamil for RVOT/ Fascicular VT) 3. Implantable Cardiac Defibrillator (ICD) 4. Surgery; cervical-thoracic sympathectomy 5. Catheter ablation; RVOT/ Fascicular VT
Ventricular Fibrillation (VF); sustained bizarre QRS complexes of varying size and configuration with rapid irregular rate and ineffective circulation->terminal rhythm (see Appendix) A. Causes 1. Degeneration of VT most common 2. Atrial Fibrillation with WPW 3. Commotio Cordis (discrete blunt force chest trauma within 15-30 ms of peak of T wave) B. Treatment 1. Defibrillation; 2-4 J/kg 2. Lidocaine, IV amiodarone, IV procainamide
PROCEDURES A. Electrophysiology study (EPS) 1. Specialized catheterization procedure for recording of intra-cardiac electrograms and evaluating mechanisms of and potential for arrhythmias 2. General areas: a. Assess baseline rhythm b. Pacing maneuvers to evaluate conduction characteristics c. Programmed stimulation with premature beats to initiate tachycardia
B. Catheter Ablation 1. Intra-cardiac electrical mapping first 2. Radio-frequency catheter ablation (RFCA); use of radio-frequency energy to create thermal injury 3. Cryoablation; cooling of intra-cardiac catheter tip to freeze tissue C. Transvenous pacemaker/ ICD placement
PACEMAKERS
Terminology A. Lower rate; programmed rate at which pacing initiated in the absence of intrinsic activity B. Upper rate 1. Fastest atrial rate at which consecutively paced ventricular complexes maintain a 1:1 synchrony with sensed atrial events 2. Upper rate limit or maximum tracking rate C. AV Delay (AV Interval) 1. Length of time in dual chamber pacing between sensed or paced atrial event and the delivery of a consecutive ventricular output pulse 2. Analogous to the physiologic PR interval D. Output 1. Electrical impulse generated by pacemaker delivered to the heart to cause depolarization 2. Composed of: a. Pulse amplitude i. Peak of the pacemaker’s electrical signal ii. Expressed in volts (V) or milliamperes (mA) b. Pulse width (pulse duration) i. Length of time during which an electrical stimulus is applied to the heart by the pacemaker ii. Expressed in milliseconds (ms)
E. Sensitivity 1. Minimum intra-cardiac signal amplitude that will be sensed by the pacemaker to initiate the response (inhibited or triggered) 2. Expressed in millivolts (mV) F. Threshold 1. Minimum level of a stimulus that will evoke a response in excitable tissue 2. Pacing threshold; minimum electrical stimulation required to consistently elicit a cardiac depolarization 3. Sensing threshold; minimum spontaneous intra-cardiac intrinsic signal required to be sensed consistently by pacemaker Code Position
Chamber Paced Chamber Sensed Response to Sensing Programmable Function/ Rate Modulation Anti-tachycardia
V (Ventricle) V (Ventricle) T (Triggers pacing)
A (Atrium) A (Atrium) I (Inhibits pacing) D (Double) D (Double) D (Triggers and inhibits) P (Programmable rate and/or output) M (Multiprogrammable) C (Communication function) P (Anti- tach pacing)
S (Shock)
D (Dual P+S)
O (None) O (None) O (None) R (Rate modulation) O (None) O (None)
CARDIAC MEDICAL CONDITIONS
HYPERCYANOTIC SPELLS (“Tetralogy Or Tet Spells”)
Definition - abrupt severe increase in cyanosis due to an acute increase in right to left shunting leading to hypoxia and shock
Common etiologies A. Agitation B. Fever C. Dehydration D. Anemia
Pathophysiology A. Dynamic worsening of muscular RVOT obstruction= infundibular/ sub PS -> decreased PBF -> increased R to L shunting -> hypoxia B. Hypoxia induced hyperpnea leads to decreased systemic vascular resistance -> further R to L shunting/ decrease in PBF C. Primary and secondary agitation increase pulmonary vascular resistance, decrease PBF, by increasing intra-thoracic pressure D. Hypoxia produced by prolonged spell leads to metabolic acidosis/ shock
Evaluation -> disappearance or decreased intensity of murmur indicates significantly decreased PBF
Treatment A. Attempt to calm patient; use parent, remove bothersome stimuli B. Knee-chest position (increases systemic resistance and decreases venous return)
C. Morphine; 0.1-0.2 mg/kg/dose SC or IV q 1 hr 1. Decreases venous return 2. Relaxes infundibulum 3. Depresses respiratory center and tendency to hyperventilate 4. Sedates agitated patient D. Ketamine; 1-2mg/kg IM, 1mg/kg IV E. For severe spells may need to deeply sedate/paralyze and mechanically ventilate F. Oxygen (of limited value) and delivery may cause agitation G. Fluid bolus: 10ml/kg NS H. Phenylephrine (increases systemic vascular resistance); 0.1mg/kg/dose SC, IM q1-2 hr vs. infusion 0.1-0.5 mcg/kg/min I. Propranolol: 0.15-0.25 mg/kg/dose IV over 2-5 min, may repeat x 1 after 15 min
1. Negative inotropic effect on infundibular myocardium 2. May stabilize peripheral vascular reactivity J. Sodium bicarbonate (correct acidosis): 1-2 mEq/kg IV K. Avoid positive inotropic agents (digoxin, epinephrine) L. Treat fever and anemia (increase O2 delivery to tissues) M. Emergent surgical intervention sometimes required
Management of Tetralogy patients in the hospital to avoid a spell A. Calm environment; minimize bothersome/ painful stimuli B. Aggressive management of fever C. Maintain adequate hydration D. Give morphine prior to any invasive or painful procedure E. Avoid medications with stimulant or vasodilatory effect F. Avoid anemia
CONGESTIVE HEART FAILURE Definition - clinical syndrome in which the heart is unable to meet the demands of the body or adequately dispose of venous return or both
Etiologies A. Congenital Heart Defects (CHD) 1. Volume overhead; VSD, PDA, AVSD, single ventricle without PS, valvar insufficiency 2. Pressure overload; Coarctation, AS B. Dilated Cardiomyopathy (DCM) 1. Familial 2. Post myocarditis/ HIV 3. Muscular dystrophies 4. Metabolic; inborn errors, nutritional deficiencies, endocrine disorders 5. Toxic; anthracyclines, radiation, sepsis 6. Ischemic; post Kawasaki’s, post cardiac surgery 7. Hypertensive 8. Sustained/ incessant tachycardias; SVT, AET, JET, VT C. Hypertrophic Cardiomyopathy (HCM) D. Restrictive Cardiomyopathy (RCM)
Clinical findings A. History 1. Fatigue 2. Respiratory symptoms; tachypnea, grunting, cough, shortness of breath 3. Poor feeding; infants 4. Decreased exercise tolerance; children 5. Abdominal complaints; anorexia, nausea, vomiting 6. Poor weight gain/ weight loss vs. acute excessive weight gain 7. Cold sweats, cool extremities 8. Orthopnea 9. Edema, abdominal swelling B. Exam 1. Vitals a. RR/ HR b. BP 2. Inspection a. Irritable, lethargic b. Pallor c. Tachypnea, grunting, retractions d. Jugular venous distention 3. Palpation a. Tachycardia b. Cool hands/feet; delayed capillary refill c. Decreased pulses d. Edema/ ascites e. Hepatomegaly; RUQ tenderness 4. Auscultation a. Distant heart sounds b. Gallop rhythm c. +/- murmur d. Wheezes or crackles e. Decreased bowel sounds
C. CXR 1. Cardiomegaly 2. Pulmonary vascular congestion/ edema 3. Pleural effusions
Treatment A. Treat underlying disorder if possible
B. Fluid management, dietary changes/ supplement C. Reduce preload (diuretics e.g furosemide/ aldactone, nitroglycerin) D. Inotropic support (dobutamine, milrinone, digoxin?) E. Afterload reduction (Nipride, milrinone, ACE inhibitors, Angiotensin Receptor Blockers (ARB) F. Supplemental oxygen G. Reverse deleterious compensatory mechanisms (B-blockers e.g. metoprolol, carvedilol) H. Ventricular assist devices/ ECMO I. Transplantation
INFECTIVE ENDOCARDITIS Definition = microbial infection of endocardial surface of heart
Pathogenesis A. High velocity, turbulent flow damages endothelium B. Platelets and fibrin adhere to damaged tissue and form sterile thrombus C. Circulating bacteria adhere to thrombus
Epidemiology A. Pre-existing congenital or acquired structural defect B. Predisposing factors with normal hearts 1. Indwelling central venous catheters 2. Intravenous drug use
Etiology A. Hemolytic streptococci e.g. Streptococcus viridans #1 B. Staphylococcus aureus + epidermidis #2 C. Others include Enterococci sp., gram-negative organisms e.g. Haemophilus and Pseudomonas sp., fungus e.g. Candida sp.
Clinical features A. History 1. History of associated epidemiologic factors 2. History of invasive procedure e.g. dental, respiratory tract, GI, GU 3. Insidious onset of fever, malaise, anorexia, myalgia, arthralgia B. Exam 1. Fever 2. Changed or new murmur 3. Splenomegaly 4. Skin manifestations a. Petechiae b. Splinter hemorrhages (linear hemorrhagic streaks beneath nails); uncommon c. Roth spots (retinal hemorrhages with central clearing); uncommon d. Osler’s nodes (tender red nodes at ends of fingers); uncommon e. Janeway’s lesions (small, painless hemorrhagic areas on palms and soles); uncommon 5. Embolic phenomena a. Pulmonary emboli; potentially septic b. Brain; seizures, stroke, abscess C. Laboratory studies 1. Blood cultures!!! a. Positive culture is the mainstay of diagnosis b. Obtain 3 cultures in 24 hours (preferably before antibiotics) c. Not necessary to obtain cultures with temperature spikes since bacteremia is continuous 2. CBC a. Leukocytosis b. Hemolytic anemia 3. Elevated ESR, CRP 4. Microscopic hematuria, proteinuria (embolic infarct or glomerulonephritis) 5. Echocardiogram a. Negative echo does not rule out endocarditis b. Vegetation usually only visualized if greater that 2-3 mm (consider TEE) c. Low yield when no physical findings or negative cultures
Diagnosis = modified Duke criteria
Treatment A. Intravenous antimicrobial therapy usually 2 to 6 weeks; depends on organism B. Surgical management 1. Significant embolic events 2. Persistent infection 3. Progressive cardiac failure
Endocarditis (SBE) prophylaxis (AHA 2007); A. Cardiac conditions 1. Prosthetic valves 2. Previous infective endocarditis 3. Unrepaired cyanotic CHD, including shunts and conduits 4. 6 months post transcatheter or surgical repair of CHD with prosthetic material or device 5. Repaired CHD with residual defects at site or adjacent to site of prosthetic material or device (may inhibit endothelialization) 6. Post cardiac transplantation with cardiac valvulopathy B. Procedures 1. Dental procedures that involve manipulation of gingival tissue or periapical region of teeth, or perforation of oral mucosa
2. Procedures of the respiratory tract, or on infected skin, subcutaneous or musculoskeletal tissue 3. Not GI or GU procedures C. Antibiotics; see 2007 AHA guidelines 1. Amoxicillin still primary medication
MYOCARDITIS Definition = inflammatory infiltrate of myocardium with necrosis and/ or degeneration of adjacent myocytes not typical of ischemic damage secondary to infective agents, inflammatory diseases, autoimmune diseases, drugs, or toxins
Etiology A. Viral; predominantly 1. Adenovirus 2. Enterovirus e.g. Coxsackie B 3. Parvovirus B19 4. Others; influenza, RSV, varicella, CMV B. Non-viral 1. Bacterial, rickettsial, fungal 2. Autoimmune disorders 3. Drugs, toxins
Clinical features A. History 1. Infants a. +/- fever b. Lethargy c. Anorexia/ vomiting d. Diaphoresis e. Respiratory distress f. Sudden death 2. Older children a. Recent viral illness b. Low grade fever c. Malaise d. Anorexia e. Nausea, vomiting, abdominal pain f. Decreased exercise tolerance g. Palpitations h. Shortness of breath, persistent cough i. Sudden cardiac death (a cause in young athletes)
B. Exam 1. Signs of CHF 2. Tachycardia 3. Irregular rhythm 4. New murmur
Laboratory studies A. Leukocytosis, elevated ESR, CRP B. Viral studies and evaluation for inflammatory/autoimmune diseases C. ECG 1. Sinus tachycardia 2. Low QRS voltages 3. ST-T changes; pattern of myocardial infarction 4. Inverted T waves 5. Conduction delays 6. Atrial or ventricular ectopy/ tachycardia 7. Complete AV block D. CXR 1. Cardiomegaly; usually mild 2. Pulmonary edema 3. Pleural effusions E. Echocardiogram 1. Dilated cardiac chambers; usually mild of ventricles 2. Decreased LV +/- RV function; global or segmental 3. AV valve regurgitation 4. Pericardial effusion F. Endomyocardial biopsy 1. “Gold standard” for diagnosis; not always performed 2. Patchy mononuclear/ lymphocytic infiltrates and/or myocyte necrosis
Treatment A. Supportive 1. Bed rest 2. Diuretics (e.g. furosemide) 3. Inotropes (e.g. dobutamine, milrinone) 4. Afterload reduction (e.g. milrinone) 5. Anti-arrhythmics 6. +/- IVIG
ACUTE PERICARDITIS Definition = acute infectious or inflammatory process involving the pericardium
Etiology A. Primarily viral; similar to myocarditis B. Bacterial = purulent 1. S. aureus most common 2. High mortality 3. Risk of constrictive pericarditis
C. Others; similar to myocarditis, uremia Clinical features A. History 1. Fever 2. In infants/ young children a. Irritability b. Malaise c. Decreased appetite d. Nonspecific chest pain 3. In older children a. Malaise b. Chest pain often described as feeling of “tightness” i. Pain intensified by activity, deep breathing, lying flat ii. Pain radiates to neck, shoulder and back
B. Exam 1. Relative tachycardia 2. Pericardial friction rub (diagnostic) C. Laboratory studies 1. Leukocytosis 2. Elevated ESR, CRP 3. For bacterial; positive blood cultures (dissemination) D. ECG 1. Early generalized ST elevation 2. Evolution of ST and then T wave changes 3. Low voltage QRS = large effusion E. CXR = +/- cardiomegaly; absence doesn’t exclude diagnosis F. Echocardiogram 1. Pericardial effusion 2. Absence of pericardial effusion does not rule out diagnosis from non-bacterial cause
Treatment A. Supportive measures B. Activity restriction C. For viral 1. Anti-inflammatory agents a. Aspirin 80-100mg/kg/d divided q 6 hr for 2-6 weeks b. Ibuprofen 10mg/kg/dose q 6-8 hr for 2-6 weeks c. Steroids if non-responsive to NSAIDs and bacterial infection ruled out; prednisone 2 mg/kg/d up to 60 mg/d taper over 3-4 weeks d. Pericardiocentesis with echo guidance vs. open pericardial window in patients with large or rapidly enlarging effusions, evidence of tamponade D. For bacterial 1. “A” and “B” above 2. Pericardial drainage 3. Intravenous antibiotics for 3-4 weeks 4. NSAIDs
CARDIAC TAMPONADE Definition = a medical emergency in which ventricular filling (diastolic function) is impaired as a result of pericardial fluid accumulation leading to poor cardiac output A. Acute pericarditis B. Postpericardiotomy syndrome C. Post central venous line placement; UVC
Signs of cardiac tamponade A. Tachpynea B. Pallor C. Cool extremities D. Decreased BP/ Pulsus Paradoxus E. Tachycardia F. Jugular venous distention G. Hepatomegaly H. Distant heart sounds
Echocardiographic findings A. Presence of pericardial fluid B. Collapse of right atrium in diastole C. Collapse or indentation of RV free wall in diastole D. Inspiratory decrease in mitral valve inflow; E wave decrease by > 30% E. Inspiratory increase in tricuspid valve inflow; E wave increase by > 70%
Treatment A. Intravenous fluids B. Pericardiocentesis (echo guidance) vs. open pericardial window C. Diuretics are contraindicated; decrease preload/ CVP increases compromise
KAWASAKI’S DISEASE (Mucocutaneous Lymph Node Syndrome) Definition = self-limited systemic vasculitis of unknown etiology affecting the skin, mucous membranes and cardiovascular system
Diagnosis: based on clinical criteria; no pathognomonic symptom/sign or test finding other than coronary artery abnormalities A. Diagnosis 1. Fever, generally high, persisting for > 5 days; not absolute rule 2. Presence of 4 out of 5 principal features a. Bilateral painless non-exudative conjunctivitis (early) b. Changes in lips and oral cavity; strawberry tongue, fissured lips (early); no ulcers
c. Polymorphous exanthema (early) d. Changes in extremities; palmar/plantar erythema, edema (early) desquamation (late) e. Cervical lymphadenopathy; at least one node > 1.5 cm (least common) 3. Exclusion of other causes 4. Can have atypical/ uncommon presentation particularly young infants; < 6 months 5. Can diagnose without meeting criteria if coronary disease present B. Non-cardiac clinical findings 1. Irritability (!!!) 2. Arthralgia or arthritis 3. Abdominal pain, vomiting, diarrhea 4. Hepatitis, hydrops of gallbladder 5. Urethritis 6. Meningitis C. Cardiac findings (pancarditis) 1. Signs of CHF 2. Sinus tachycardia 3. Irregular rhythm 4. Gallop/ new murmur 4. Shock
III. Laboratory findings A. Leukocytosis; blood and CSF B. Elevated ESR, CRP C. Thrombocytosis (late in acute phase) D. Elevated serum transaminases E. Sterile pyuria F. ECG 1. Sinus tachycardia 2. Prolonged PR interval, other conduction abnormalities 3. Low QRS voltages 4. Nonspecific ST-T changes 5. Atrial or ventricular ectopy/ tachycardia 6. Myocardial infarction G. Echocardiogram 1. LV dysfunction +/- MR 2. Pericardial effusion 3. Coronary artery dilatation/ectasia/aneurysms (only potential long term sequelae) a. Up to 25 % if untreated b. Main LCA, proximal LAD CA, and RCA most commonly involved c. Increased risk if i. Male ii.. < 1 year of age iii. Fever > 10 days (aneurysms rarely develop < 10 days) iv. Recurrent fever post initial treatment v. Other cardiac involvement
Clinical course A. Acute phase 1. ~ First 10 days 2. Most clinical criteria manifest 3. Pancarditis B. Subacute phase 1. ~ 11-28 days from onset 2. Fever usually resolves 3. Continued pancarditis 4. Peak development of coronary changes; can develop aneurysms of other systemic arteries C. Convalescent phase; protracted 1. Inflammation resolves 2. Coronary changes resolve or mature a. 50-60 % aneurysms resolve over 1-2 years
Initial Treatment A. IVIG 2 gm/kg as single infusion (preferably in 1st week; primary prevention of CA aneurysms) 1. Repeat x 1 if persistent fever > 36 hours post initial dose B. Aspirin 1. High dose, 80-100 mg/kg/day divided q 4-6 hours till 48-72 hours post resolution of fever 2. Anti-platelet dose, 4-6 mg/kg q day, for 6-8 weeks/ till all inflammatory markers normal if normal CAs C. Initiate anticoagulation, warfarin/ Coumadin or low molecular weight heparin e.g. Lovenox if giant aneurysms present
Chronic treatment dictated by extent and persistence of coronary artery abnormalities
RHEUMATIC FEVER (RF) Definition = inflammatory disorder following a group A streptococcal (GAS) infection that affects the heart, joints, central nervous system and subcutaneous tissue
General features A. Genetic susceptibility
B. GAS infection; pharyngitis or skin infection C. Delayed autoimmune response triggered by exposure to streptococcal antigens that mimic normal human tissue antigens; onset 10 days-5 weeks post infection D. Inflammation of connective tissues; perivascular and interstitial injury without cellular necrosis E. Age 5-15 years; “never” < 2 years F. No single pathognomonic symptom/ sign or test finding (? chorea)
Diagnosis = Jones criteria A. Evidence of preceding/ recent GAS infection 1. Positive throat culture or rapid streptococcal test 2. Elevated or rising anti-streptolysin O (ASO) or anti-deoxyribonuclease B (anti-D Nase - B) titers B. Two major criteria or one major and two minor criteria C. Presence of chorea possible exception to above
Jones Major Criteria A. Carditis 1. Up to 50% of cases 2. Develops within first 2 weeks 3. Mitral regurgitation (MR) most common (can lead to late mitral stenosis); 90-95% of cases of carditis 4. Aortic Regurgitation (AR); 20- 25% of cases of acute carditis, usually in association with MR, 5% alone 5. Pericarditis; 10-15% 6. Potential cause of acute mortality 7. Can lead to chronic heart disease = Rheumatic Heart Disease (RHD) B. Polyarthritis 1. Up to 70% of cases 2. Large joints 3. Migratory 4. Self-limited; resolves in 3-4 weeks 5. Very responsive to aspirin (ASA); within 48-72 hours C. Chorea (Sydenham chorea/ St. Vitus Dance) 1. Up to 30% of cases 2. Inflammation of basal ganglia, cerebellum, cerebral cortex 3. Develops from 1-6 months post GAS infection (inflammatory markers and antibody titers may be normal) 4. Involuntary/ purposeless movements, weakness, incoordination, emotional lability 5. Self-limited, but resolves slowly, over months
D. Erythema marginatum 1. < 5% of cases 2. Bright pink macule or papule which spreads with serpiginous borders and central clearing; painless and not pruritic and blanches E. Subcutaneous nodules 1. < 10% of cases 2. 0.5-2 cm round, firm, freely movable, non-tender 3. Occur over extensor surfaces or bony prominences 4. Resolve in few days to 2 weeks
Jones Minor Criteria A. Fever B. Arthralgia (can’t be used if arthritis present) C. Elevated ESR, CRP D. Prolonged PR interval on ECG
General Treatment A. Benzathine penicillin G 600,000 units IM (< 27 kg), 1.2 million units IM x 1 (> 27 kg), or oral penicillin V x 10 days (alternatives if allergic) B. Restricted activity for 4-6 weeks C. Initiate secondary prophylaxis (see below) D. Initiate SBE prophylaxis
Carditis Treatment A. Supportive measures; anti-congestive therapy B. Mild-moderate 1. ASA 80-100 mg/kg/day divided q 6 hours for children 2. ASA 4-8 gm/day for adolescents 3. Treat for 4-6 weeks; until inflammatory markers normal C. Severe 1. Prednisone 2 mg/kg/day up to 60 mg/d for ~ 2 weeks then taper 2. Initiate ASA one week prior to discontinuation of steroid and continue as above D. No long term outcome differences between aspirin and steroid therapy E. Surgery if intractable CHF
Secondary prophylaxis (primary = acute treatment of GAS infections) A. Prevent recurrent RF 1. Up to 60% chance if h/o carditis 2. Recurrent RF generally more severe carditis 3. Recurrence of RF increases risk of chronic RHD B. Options 1. Benzathine penicillin G 1.2 million units IM q 3-4 weeks; preferred 2. Penicillin V 250 mg po BID 3. Alternatives if allergic C. Duration 1. If RHD, > 10 years since last episode until at least 40 years of age (RF rare > 35 years of age) 2. If RF with carditis, but no RHD, at least 10 years 3. No carditis, 5 years or until 21 years of age APPENDICES COMMON CARDIAC MEDICATIONS
DRUG CONCENTRATION DOSE ROUTE COMMENTS
Adenosine
(Adenocard)
Acetazolamide
(Diamox)
Amiodarone
(Cordarone) 3mg/ml 0.1-0.3 mg/kg/dose Max single dose: 12mg IVP emergency drug for SVT
125.250 mg tabs 5mg/kg/dose q6-24h PO/IV promotes HCO3 loss
100,200 mg tabs Load: 10mg/kg/d q 12-24h for 7-10 days Maint: 5mg/kg/d q24h
1.5mg/ml Load: 1-5 mg/kg over 5-25 min Maint: 5-10mg/kg/d as continuous Infusion PO check thyroid/liver, lungs decrease digoxin
IV hypotension
Milrinone 1 mg/ml Load: 50 mcg/kg over 10 min Maint: 0.1-0.8 mcg/kg/min
Aspirin 65, 81, 325, 500 3-6 mg/kg/day qd
650 mg tabs 80-100 mg/kg/d q4-6h IV
PO anti-platelet dose
anti-inflammatory dose
Atenolol
(Tenormin)
Captopril (Capoten) 50,100 mg tabs 1-2 mg/kg/d q 12-24h PO
12.5, 25, 50 mg tabs Initial: 0.3-0.5 mg/kg/d q8h Maint: 0.5-3.0 mg/kg/d q8h PO give q12h in renal impairment
Digoxin 50 mcg/mg elixir Digitalizing dose: <2Kg: 20 mcg/kg 0-2 mos: 30 mcg/kg 2-24 mos: 40 mcg/kg >2 yr:max 1 mg Give as three doses
First ½ STAT. second ¼in
6 hr, third ¼ in 6 hr Maint: 10 mcg/kg/d q12h PO level: 0.8-2 ng/ml reduce IV dose to
80% of PO
Dobutamine 12.5 mg/ml 5-20 mcg/kg/min
Dopamine 40 mg/ml 3-20 mcg/kg/min
Enalapril (Vasotec) 2.5, 5, 10, 20 mg tabs 0.05-0.3 mg/kg/d q 12-24h 10-80 mcg/kg/dose q6-24h
Epinephrine 0.1-1 mcg/kg/min
FLecanide
(Tambacor) 50, 100, 150 mg tabs 100 mg/m2/d q8-12h or 3-6mg/kg/d q8-12h
Furosemide 10mg/ml suspension 20,40,80 mg tabs Bolus: 1-2mg/kg/dose q6-24hr Max: 6mg/kg/dose Cont infusion: load 1 mg/kg Maint: 0.1 mg/kg/hr Max: 0.4 mg/kg/hr
Lidocaine
(Xylocaine)
Mexilitene
(Mexitil) 1 or 2% Bolus: 1 mg/kg/dose q5min Max: 5mg/kg Infusion 10-50 mcg/kg/min
150, 200, 250mg tabs 4-15 mg/kg/d q8h IV
IV
PO
IV
IV
PO level: 0.2-1.0 mcg/ ml
PO, IV oral bioavailability poor monitor lytes (q4h) for at least
first 24th of infusion titrate
infusion to urine output
IV antiarrhythmic level: 1.5-5.0 mcg/ml
PO GI upset
Nitroglycerin 5mg/mg
Nitroprusside (Nipride) 50 mg/ml
Phenoxybenzamine 0.5-10.0 mcg/kg/min IV
Initial: 0.1-1.0 mcg/kg/min 0.5-10.0 mcg/kg/min IV methanoglobinemia
cyanide toxicity
Load: 1mg/kg/over 1hr Then 0.5-2.0 mg/kg/d Q6-12h IV
PO/IV
Phenylephrine 0.1-0.5 mcg/kg/min IV Procainamide
(Pronestyl)
Propranolol (Inderal)
Prostaglandin E1 100mg/ml Load (<1y): 7-10 mg/kg (>1yr):10-15 mg/kg over 1 h Maint: 20-80 mcg/kg/min IV may cause SLE level: 4-8mcg/ml (NAPA + PRO= 8-20 mcg/mg)
10, 20, 40 mg tabs 20 mg/5ml suspension 2-4 mg/kg/day q6-8h 0.1-0.25 mg/kg/over 3 min Max 1mg/dose PO
IV multiple drug interactions
500 mcg/vial 0.01-0.1 mcg/kg/min IV apnea, fever, rash, seizures
(Prostin)
Sotolol
(Betapace)
Spirinolactone (Aldactone) 80,160,240 mg tabs 2-8 mg/kg/d q12
25,50 mg tabs 1-4mg/kg/d q12h usually q12h PO
PO onset of action 24-36h
Warfarin 2,2.5,5,7.5,10mg tabs 0.05-0.4 mg/kg/d qd PO infants require higher doses
CHEST ROENTGENOGRAPHY
CARDIOTHORACIC RATIO
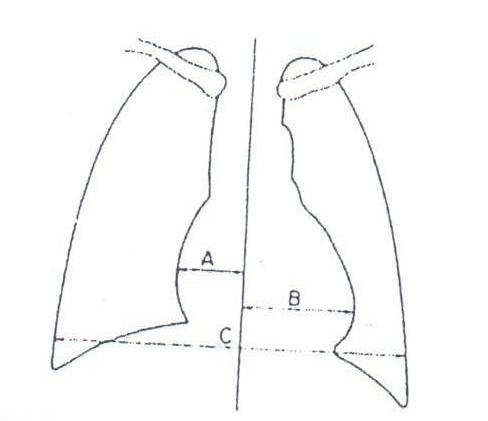
CARDIAC CHAMBERS

VARIOUS SHUNTS

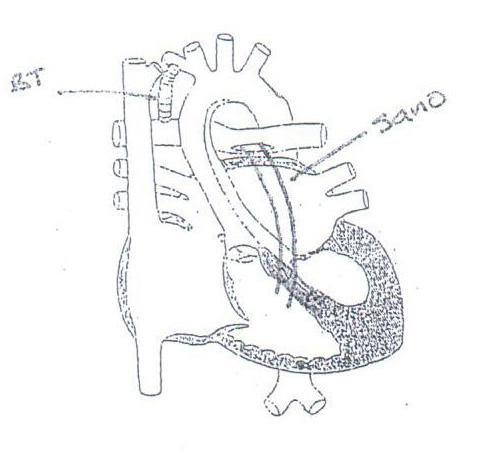
DAMUS-KAYE-STANSEL RASTELLI HLHS NORWOOD PROCEDURE


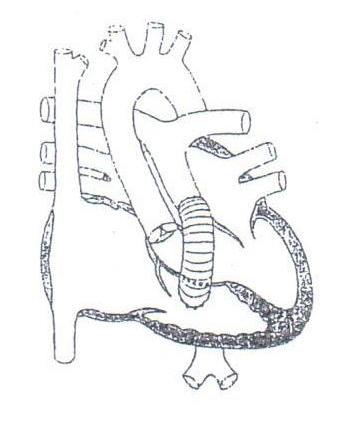

BT
Sano
BIDIRECTIONAL GLENN SHUNT FONTAN PROCEDURES

Lateral Tunnel
ECC
Classic
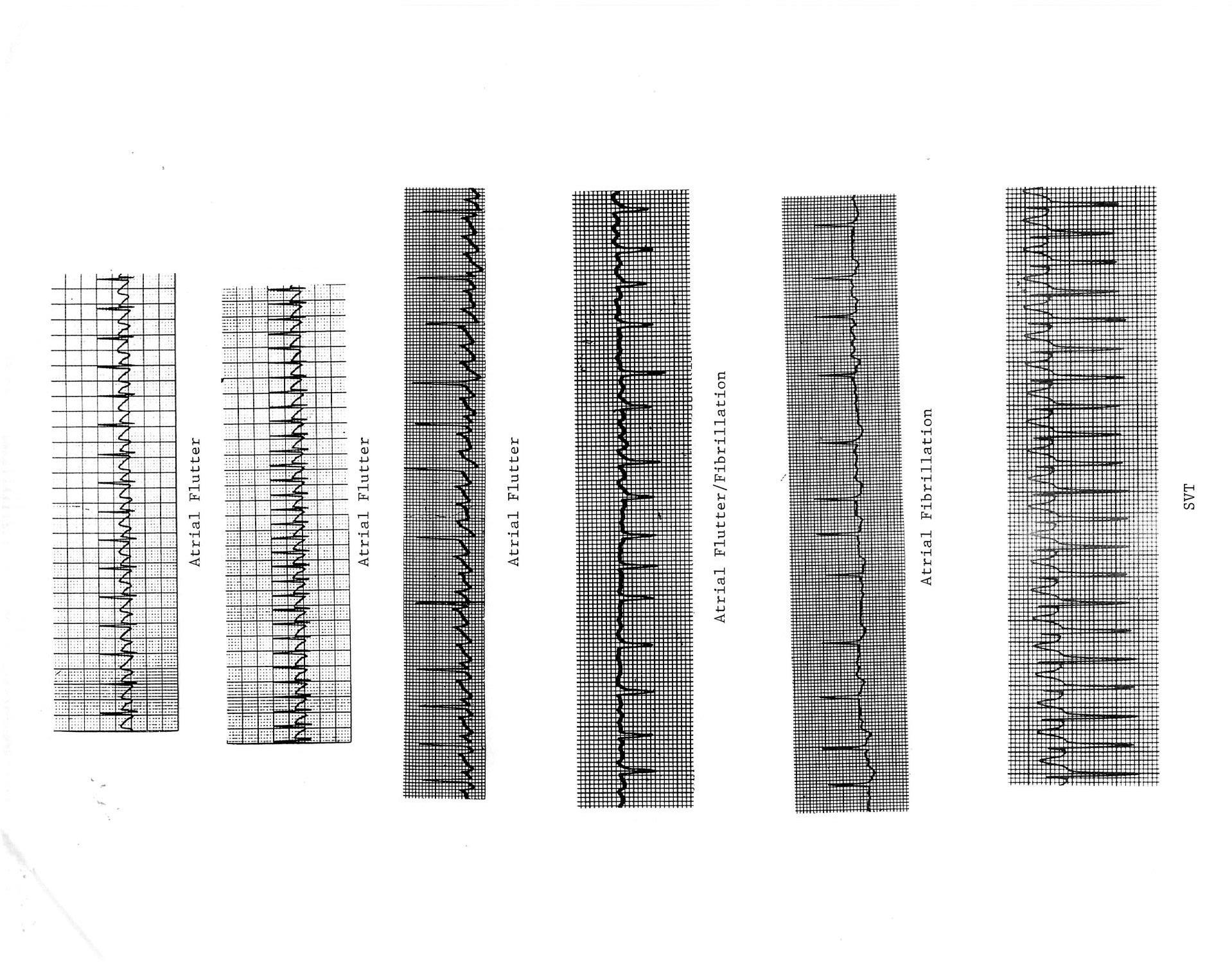



Atrial Ectopic Tachycardia

SCL Health
ST. VINCENT
SCL Health Medical Group - Billings Pediatric Specialty Pediatric Cardiology 1232 North 30th St., Suite 300, Billings, MT 59101 406-237-5300 | svh.org
If you have any questions or concerns, please contact: Andrew G. Lashus, MD, FACC


