
8 minute read
Emerging Mycotoxins – A Threat beyond Regulations?
By Lilian KUSTER, Product Manager at Romer Labs®
The number of detectable mycotoxins has increased in recent years. Spot On investigates what is being done to measure and monitor them.
Advertisement
Mycotoxins are naturally occurring, secondary metabolites produced by various molds. These compounds are toxic to humans and animals. Toxigenic molds contaminate a wide range of crops and produce mycotoxins as a result of the infection of plant tissues in the field. Unfortunately, the formation of these toxins can continue even after harvest and the level of mycotoxins in grains continues to increase during storage. Contaminated crops represent a major health risk to humans and animals. The most prominent mycotoxin-producing field-fungi are represented by fungi of the species Fusarium and Aspergillus. Beyond these, there are over 300 different fungi that are known to produce over 400 different mycotoxins.
In recent years, more and more mycotoxins have been considered as relevant as they contribute to the risk posed to humans and animals. Risk assessment studies have been performed for various important mycotoxin groups including ergot alkaloids (see Box 1), Alternaria toxins (see Box 2) and modified or masked mycotoxins.
Modifying mycotoxins as plant defense
Typically, mycotoxins are explicitly produced by fungi and their parent structure is often modified by the fungus itself which releases a cocktail of structurally related compounds. During infection, these substances are then often further modified by the host plant of the fungus. The living plant might change the chemical structure of toxins and produce so-called masked mycotoxins.
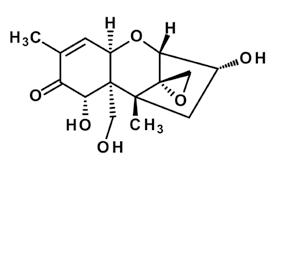
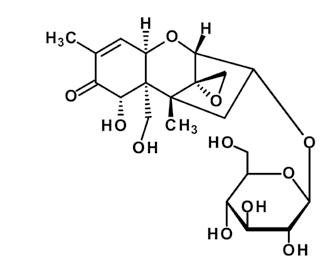
The formation of these masked toxins is a major detoxification strategy of crops, as they are less toxic for the plant. Usually, a glucose molecule or a sulfate is involved in the conjugation and detoxification. Although these masked toxins do not further harm the plant, their toxicity to humans and animals might reemerge when the added masking molecule is cleaved in the gastrointestinal tract of mammals during digestion (Figure 1). In plant breeding, the increasing occurrence and production of some masked mycotoxins might be linked to novel resistant breeds. Deoxynivalenol-3-glucoside, for example, has been reportedly linked to resistance against Fusarium head blight. This means that Fusarium resistant plants have been proven to show higher deoxynivalenol-3-glucoside to deoxynivalenol ratios, but these are accompanied by lower levels of total deoxynivalenol and the modified form due to higher Fusarium resistance.
Ergot Alkaloids – an ancient story
Ergot alkaloids are secondary metabolites usually produced by fungi belonging to the genus Claviceps . The most commonly occurring species producing ergot alkaloids is Claviceps purpurea. “Ergot” is a french word meaning “spur”, and was chosen as the name since grains, when infected, present so-called sclerotia and often resemble the spurs on the legs of a rooster. Many different cereal plants and grasses, including rye, wheat and triticale among others, can become infected by these fungi during cool, wet weather conditions. These fungi then produce structures called sclerotia. These sclerotia contain different classes of ergot alkaloids, the most prominent being ergometrine, ergotamine, ergosine, ergocristine, ergocryptine and ergocornine together with their epimeric –inine forms. If grains containing sclerotia are processed by grinding into flour, high contamination levels of ergot alkaloids typically follow.
Currently, available data on ergot alkaloids show that the intake of contaminated food or feed can severely affect animals and humans. Ergot poisioning is called ergotism, a severe pathological syndrome. Symptoms include hallucinations, itchy and burning skin, nausea, dizziness and even abortion. Ergotism is one of the oldest known diseases caused by mycotoxins and was first described in the Middle Ages as so-called St. Anthony’s fire. Furthermore, ergot alkaloids are not only known as mycotoxins. Ergotamine, for example, is one of the components of the psychoactive drug lysergic acid diethylamide (LSD). Ergot alkaloids are also used for medicinal purposes, including the treatment of migraines and the induction of birth process among others.
Up to now, the amount of ergot alkaloids present in food and feed is not regulated, but regulations are under strong discussion in the European Union.
Currently, the most widely used detection method for ergot alkaloids is based on HPLCFLD (High Performance Liquid Chromatography with fluorescence detection) and can be performed using calibrants and a one-step cleanup provided by Romer Labs. An official CEN LC-MS/MS (liquid chromatography – mass spectrometry) method is currently under development.

The term “modified mycotoxin” includes both the modification of a parent toxin molecule by the fungus itself, and the masking of the toxin which only occurs in the plant tissue. Another type of modification takes place in mammals when aflatoxin B1 is consumed through contaminated feed and converted to aflatoxin M1. This aflatoxin M1 migrates into the milk of lactating animals and is excreted with it. In addition, modifications of toxins can also occur during food processing, in particular heating and fermentation, increasing their prevalence. These modified mycotoxins might occur in relevant amounts in food and feed.
The phenomenon of modifying mycotoxins is particularly related to Fusarium toxins (trichothecenes, zearalenone and fumonisins) but modified forms have also been reported for other mycotoxins like aflatoxins, ochratoxin A or patulin.
Altered and masked forms of deoxynivalenol – an example
Deoxynivalenol is the mycotoxin with the most studies conducted on the different versions of frequently observed modifications. The modified forms of deoxynivalenol can be divided into two main groups: altered and masked forms. There are two main altered forms of deoxynivalenol secreted by the fungus itself: 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol, as found in Fusarium-contaminated cereals. Plants are able to mask the deoxynivalenol to deoxynivalenol-3-glucoside and as recent studies show, this may take on two sulfonated forms: deoxynivalenol-3-sulfate and deoxynivalenol-15-sulfate (Table 1).
How harmful are modified and emerging mycotoxins?

Alternaria toxins
Alternaria toxins represent a possible health-endangering group of mycotoxins produced mainly by the Alternaria species. These are a widespread group of fungi contaminating mainly fruits and vegetables, but also other crop plants, during growth as well as storage. The most important mycotoxin-producing species is Alternaria alternata which occurs mainly on cereals and seeds but also on olives, various fruits and tomatoes. A vast number of Alternaria mycotoxins are known to occur naturally on infected crops, fruits and vegetables, including tenuazonic acid, alternariol, alternariol monomethyl ether, altenuene and altertoxin I. Structurally, these toxins are related to fumonisins. Even though Alternaria toxins are normally associated with fruits and vegetables that are visibly infected by Alternaria rot, they have also been found in cereals, such as wheat, rye, sorghum, rice and even tobacco.
Alternaria toxins have been shown to exhibit both acute and chronic effects and therefore represent a threat to animal and human health. The most studied mycotoxin in the group of toxins produced by the species Alternaria is tenuazonic acid. Its main function is the inhibition of protein synthesis and results in antitumor, antiviral and antibacterial activity. Most of the other Alternaria toxins show cytotoxic activity in mammals, some of them are mutagenic like the altertoxins, while others are toxic to the unborn like alternariol and alternariol monomethyl ether.
Currently no guidelines or legislative limits are set for Alternaria toxins. So far it has been of general belief that their occurrence in food is very low and therefore the risk of human exposure is very limited. Nevertheless, data for their risk assessment is currently collected and methods for the detection of Alternaria toxins based on liquid chromatography – mass spectrometry (LC-MS) are under development.
Modified mycotoxins can be either more or less toxic than their parent compounds. For example, they may be more bioavailable due to modifications. Toxicological data on modified mycotoxins are scarce, and current results and knowledge on the real risks and effects of these compounds are insufficient. This lack of knowledge makes it difficult to conduct a proper risk assessment. Nevertheless, there have been studies describing their potential threat to food safety. Furthermore, it has to be highlighted that masked mycotoxins can be “unmasked” again in the digestive tract of animals and humans, releasing the parent compound with its toxicological effects again. A similar situation exists with emerging mycotoxins: toxicological data are scarce which makes it difficult to set up regulations and maximum tolerated limits to protect humans and animals from potential health risks.
Do regulations cover all mycotoxin risks?
To ensure food and feed safety, many countries have established regulatory limits for mycotoxins in crops. Currently, in most developed countries, there are regulations on maximum levels or at least guidance levels for mycotoxins in food and feed. These regulations only cover some of the known mycotoxins such as aflatoxins B1, B2, G1, G2 and M1; fumonisins B1, B2 and B3; ochratoxin A, deoxynivalenol, zearalenone, HT-2 toxin and T-2 toxin.
As modified mycotoxins behave differently in their chemical reactions to parent mycotoxins, they can be easily missed in routine analysis. Current detection methods for regulated mycotoxins in food and feed do not include routine screening for these modified mycotoxins as they are not covered by legislation. Such standard methods may show up contamination levels below legislative limits, while contaminations from modified mycotoxins go undetected. This represents a correct result, but from a toxicological point of view the integration of modified toxins (e.g. as a sum parameter) would provide more sound data for risk assessment. Together, all these facts point to the possible hazards posed by modified mycotoxins to human health.
Regulations on the maximum levels of modified mycotoxins as well as other emerging mycotoxins are currently under discussion in the European Union.
Analytical methods for mycotoxin quantification
Mycotoxins are commonly analyzed by chromatographic methods like liquid chromatography–mass spectrometry (LC-MS) and immunochemical methods like enzyme-linked immunosorbent assay (ELISA). Immunochemical methods can, depending on the cross-reactivity of the antibody, respond to more than one compound (e.g. native mycotoxins and their modified forms) leading to a single result. In contrast LCbased separation methods might underestimate the total toxin levels as those methods resolve each compound as a single parameter and are usually only developed for the parent mycotoxins.
Limits of analytical methods
There are two ways to detect and quantify modified mycotoxins: A “direct” approach that measures the whole modified compound, and an “indirect” approach that measures the parent compound after chemical or enzymatic treatments that lead to the cleavage of modified mycotoxins, mainly by hydrolysis. Among the advantages of the indirect method are that reference materials for modified mycotoxins are not needed for correct quantification, and that all modified forms are included in the final result. The main disadvantages are that the efficiency of the hydrolysis process cannot be verified easily, and that there is no access to the quantities of the different forms of a toxin. Therefore, it is important to develop direct methods to obtain further insight into the occurrence of modified mycotoxins. All chromatographic technologies for parent mycotoxins are also potentially suitable for their modified forms as long as they are soluble and directly available for analysis.
One major constraint of the direct determination and quantification of modified mycotoxins is the limited availability of reference materials (pure substances or calibrants in addition to isotope-labeled internal standards).
Another drawback is that most methods require an adequate cleanup prior to the analysis procedure. Commercially available purification devices are currently designed for native mycotoxins and might not be necessarily suitable for modified forms.
Work is currently underway to develop new reference standards as well as innovative cleanup devices to determine modified mycotoxins directly.
Emerging mycotoxinsA threat beyond regulations?
With the current gaps in routine mycotoxin analysis driven by missing regulations for emerging mycotoxins, many of these compounds can go undetected and pose a threat to both human and animal health.
The extent of this threat, thought to be considerable, is however hard to estimate since toxicological data is still scarce, despite increasing research efforts in this direction.


