1 minute read
Considerations for Bioequivalence studies for Oncologic treatments
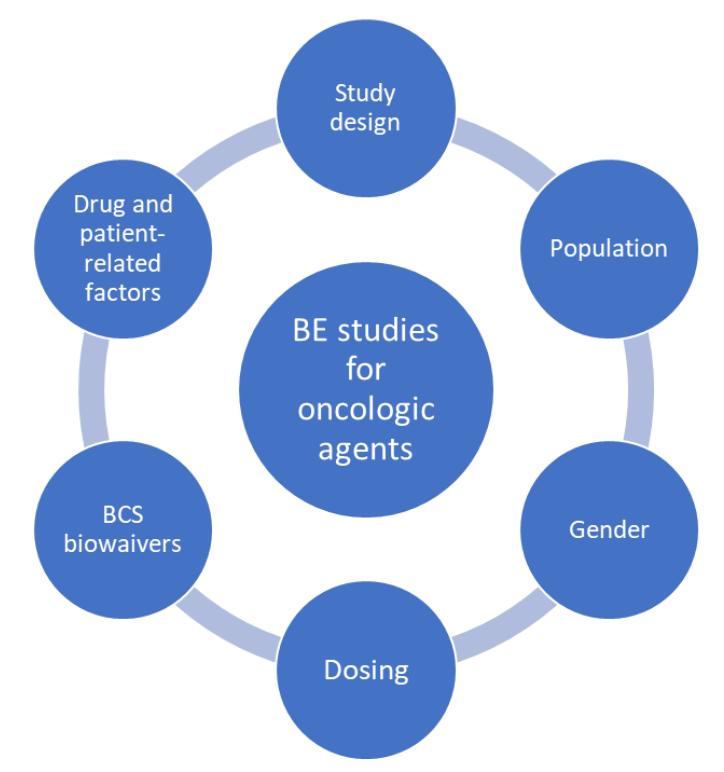
The following factors need to be considered when designing a BE study for oncologic safety keeping patient safety the utmost priority
● Study design
Advertisement
Most BE studies are designed to be crossover studies in which the RLD and proposed generic product are administered to the same participant in a two-period, two-sequence pattern following randomization. Although the crossover design helps eliminate intra-subject variability as the subjects serve as their own control and reduces the number of subjects required for the study, crossover designs are not suitable for drugs that have long half-lives due to carryover effects that can affect the next treatment. In these cases, parallel designs should be considered in which subjects in the RLD and generic treatment groups are matched for various demographic characteristics.
● Population
Unlike BE studies for most drugs that include healthy participants, cancer patients need to be included in BE studies for oncologic agents. This is because of safety concerns and the cytotoxicity of cancer drugs. Finding enough patients with similar cancer staging or diagnosis may be difficult for BE studies because of strict inclusion/exclusion criteria. Recruitment of patients using registries is recommended to meet target patient enrolment goals. In cases where the oncologic agent is non-cytotoxic healthy volunteers may be considered.
● Gender
In cases where BE studies are performed in healthy volunteers, it is necessary to ensure that the anticancer agent does not cause other undesirable side effects such as birth defects or teratogenicity In certain cases, it may be recommended to include only male participants in the BE studies.
● Dosing
In BE studies done on cancer patients, it is important to ensure that the regular treatment schedule of the patient is unaffected. The anticancer agent should be administered at the same dose and schedule as done routinely so as not to affect the patient’s treatment response. Although the highest dose level of the drug is recommended for BE studies this may not be feasible for oncologic agents as the patient’s normal dose may not be the highest dose strength.
●



