
13 minute read
DAIRY FOOD
Texture control, stabilisation and xanthan gum
The estimated size of the global ice cream market is around 15 billion litres. Consumption patterns vary significantly between countries and regions.
ICE CREAM IS made up of a complex matrix of frozen foam, emulsified fat globules and suspended ice and lactose crystals. The matrix is highly susceptible to temperature variations. Reducing this sensitivity will help to maintain the quality of the ice cream during storage and improve shelf life. A significant improvement can be achieved by using hydrocolloids to bind water and thereby control the growth of ice crystals. Additionally, hydrocolloids can be used to modify the melting process and mouthfeel.
According to market analysis of hydrocolloids used in ice cream, 13 per cent of approximately 14 000 total new ice creams launched (2012 to 2014) contain xanthan gum, while 75 per cent of the newly launched ice creams were milk-based. Xanthan gum is often used in combination with guar gum, locust bean gum and carrageenan, but pectin carboxymethylcellulose and tara gum are also used as stabilising agents. The hydrocolloids derive from different origins: XG is obtained by bio-fermentation of glucose with Xanthomonas campestris; guar gum is an extract of endosperm from guar beans and LBG or carob is an extract from the seeds of the carob tree. Carrageenan derives from red algae, seaweed or Irish moss. Many hydrocolloids are approved for use in food, registered with the following E-numbers: XG (E415), GG (E412), LBG (E410), CMC (E466), TG (417), pectin (E440) and carrageenan (E407) – often without a defined upper limit, but to quantum satis.
FUNCTIONALITY OF HYDROCOLLOIDS AND THEIR SYNERGISTIC EFFECTS When properly hydrated, hydrocolloids increase the viscosity of aqueous solutions. As with water-binding capabilities, the viscosities vary strongly between different hydrocolloids. The hydrocolloid solutions also show varying degrees of non-Newtonian behaviour and they are more of less shear thinning. In comparison to the galactomannans GG, LBG and TG, XG provides by far the highest viscosity at lower shear rates and the most extreme shear thinning. XG is thus the superior stabiliser, whereas galactomannans provide more thickness and high shear rates.
Combinations of xanthan and one or more the galactomannans are known to display synergistic behaviour. This means that the viscosity of the combination is higher than the sum of the viscosities of the individual components. Theory explains that this is due to a special cross-linking of molecules.
Xanthan gum has a glucose backbone and every second glucose-unit is substituted with a side chain of mannosepyranosyl – glucuronic acid – acetyl mannose. GG has a mannose backbone with galactose roughly every second monomer. LGB has the same backbone with a galactose on every fourth monomer. The cross-linking effects vary depending






on the structure of the galactomannan. XG and GG combinations provide higher viscosity when dissolved in cold water, whereas XG and LBG only react when solutions are heated.
The synergistic effect between XG and GG is quite remarkable as visible in picture 1. It shows the shear curves of 0.5 per cent of the pure products and a combination of both. At a shear rate of around 40/s, the viscosities of XG and GG are approximately equal. The viscosity of GG increases only slowly to around one Pa.s at lower shear rates, whereas the viscosity of XG increases to 40 Pa.s. When using 0.5 per cent of a blend containing 25 per cent xanthan and 75 per cent GG (X1 G3), the resulting shear curve is very comparable to xanthan gum, however with a significantly higher viscosity over the whole shear range.
The synergistic effects vary depending on the hydrocolloids used and the medium production processes (e.g. heating steps). They offer food developers the opportunity to tailor the stability, thickness and mouthfeel of products.
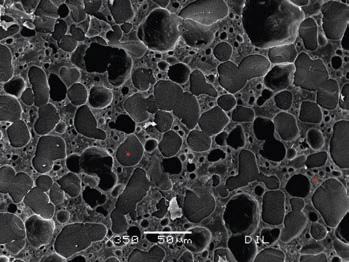
For ice cream production, manufacturers often use a stabiliser system, which is specifically tailored blends of different hydrocolloids, sometimes in combination with some emulsifiers. These combinations will obviously vary depending on whether the final products are dairy-based ice cream, sorbet or lollies. Specifically, for dairy-based ice creams, the blends may also contain a small fraction of carrageenan. This ingredient is very compatible with milk proteins and can develop a gel structure, even in low concentrations. Stabiliser blends are typically used in the range of 0.1 to 0.3 per cent of the total ice cream formulation. Consumers will not store the ice cream continuously at -20°C. It must be taken from the freezer to be consumed and before any left-overs are returned to the freezer the temperature of the ice cream may Pictures 2 and 3 show the microstructure of an ice cream sample by SEM. This sample was stabilised with a standard compound containing LBG, GG and carrageenan at a concentration of 0.16%. Pictures 2: First day sample stored at -40°C test Pictures 3: 14 th day sample after heat shock black spots: small air bubbles greyish spots: ice crystals liquid concentrate Pictures 4 and 5 show the microstructure of an ice cream sample by SEM. This sample was stabilised with a compound containing XG, LBG in a ratio of 25:75 and a concentration of 0.16%. Pictures 2 and 3 show the microstructure of an ice cream sample by SEM. This sample was stabilised with a standard compound containing LBG, GG and carrageenan at a concentration of 0.16%. Pictures 2: First day sample stored at -40°C test Pictures 3: 14 th day sample after heat shock black spots: small air bubbles greyish spots: ice crystals liquid concentrate Pictures 4: First day sample stored at -40°C test Pictures 5: 14 th day sample after heat shock Table 1: A typical ice cream composition INGREDIENT % Semi-skimmed milk 46.59 Cream 31.00 Sucrose 13.00 Skimmed milk powder 4.60 Dry glucose syrup 4.00 Emulsifier 0.40 Flavour 0.25 Stabiliser 0.16 Dry matter content 38.70 When properly hydrated, hydrocolloids increase the viscosity of aqueous solutions. As with the water binding capacities, the viscosities vary strongly between different hydrocolloids. The hydrocolloid solutions also show varying degrees of non-Newtonian behaviour and they are more or less shear thinning. In comparison to the galactomannans GG, LBG and TG, XG provides by far the highest viscosity at lower shear rates and the most extreme shear thinning. XG is thus the superior stabiliser, whereas galactomannans provide more thickness at high shear rates. Combinations of Xanthan and one or more of the galactomannans are known to display synergistic behaviour, which means that the viscosity of the combination is higher than the sum of viscosities of the individual components. Theory explains that this is due to a special cross-linking of the molecules. Xanthan gum has a glucose backbone and every second glucose-unit is substituted with a side chain of mannosepyranosyl – glucuronic acid – acetyl-mannose. GG has a mannose backbone with a galactose roughly on every second monomer. LBG has the same backbone with a galactose on every fourth monomer. The cross-linking effects vary depending on the structure of the galactomannan. XG and GG combinations already provide higher viscosity when dissolved in cold water, whereas XG and LBG only react when the solutions are heated. The synergistic effect between XG and GG is quite remarkable as it is visible in picture 1, which shows the shear curves for 0.5% of the pure products and a combination of both. At a shear rate of around 40/s, the viscosities of XG and GG are approximately equal. The viscosity of GG increases only slowly to around 1 Pa.s at low shear rates, whereas the viscosity of XG increases to 40 Pa.s. When using 0.5% of a blend containing 25% xanthan and 75% GG (X1 G3), the resulting shear curve is very comparable to xanthan gum, however with a significantly higher viscosity over the whole shear range.
3 0.5% XG 0.5% GG 0.5% X1 G3 0.01 0.1 1 10 100 100 10 1 0.1 0.01 Viscosity (Pa·s) Shear Rate (’/s) XG, GG and Blend in 1% NaCl after heating Picture 1: The viscosity of xanthan, guar and a blend
Savannah Fine Chemicals – www.savannah.co.za Picture 1: The viscosity of xanthan, guar and a blend have risen significantly. Even producers and retailers face problems continuously keeping ice cream at ideal storage conditions.
Hydrocolloids are being used to limit the negative effects of temperature swings on the quality of the ice cream. These effects can be measured by determining the change in size and frequency of the ice crystals and air bubbles in samples of ice creams using scanning electron microscopy (SEM). Different ice creams were prepared following one recipe where they only the type and dosages of hydrocolloid was varied. Samples of the ice creams were stored at varying, but tightly controlled temperatures according to the protocol for the heat shock test to mimic a prolonged storage period with temperature swings. • *This is an abbreviated version of a white paper by Jungbunzlauer. For more information, please contact Savannah Fine Chemicals. Table 1
Table 1 shows a typical ice cream composition. The individual ingredients can vary widely. The total dry matter is mostly maintained in a rather narrow range as its variation can easily make the texture hard, crumbly or too cold. Pictures 4 and 5 show the microstructure of an ice cream sample by SEM. This sample was stabilised with a compound containing XG, LBG in a ratio of 25:75 and a concentration of 0.16%.

ICE CREAM HEAT SHOCK TEST AND STRUCTURE ANALYSIS Elevated temperatures may inflict irreversible damage to the delicate gas-liquid-solid structure. black spots: small air bubbles greyish spots: ice crystals liquid concentrate
Dairy foods’ inline insurance policy

Contamination of a product with foreign objects is a permanent threat to the dairy sector. Its implications can be deeply unpleasant for consumers and manufacturers.
FOOD SAFETY IS enforced through a raft of regulations and legislation. Responsibility begins in the field and ends long after the point of sale. Contamination can occur at any of these points: from processing machinery, factories, transportation or through the addition of non-dairy ingredients. In the dairy industry, legal and reputational risk extends to processors, manufacturers and retailers who have a vested interest in keeping dairy products contaminant-free.
For dairy producers that supply major retailers, the risk is particularly acute. Retailers may impose substantial fines or other penalties when contamination with a foreign object occurs.
OPTIONS IN CONTAMINANT DETECTION There are a wide variety of options available, but two main technologies stand out: • Metal detection – used since the 1950s • X-ray technology – a more recent innovation and an excellent way to enhance technical performance to prevent foreign body contamination.
Metal detecting machines have several advantages. They are established, reliable, easy to maintain, require low initial investment and are simple to install.
For those smaller independent businesses, metal detection offers an ideal method of quality control. If a dairy product is contaminated with stones, glass, plastics or rubber, a metal detector will let the product go through. Detectors may also have problems finding metals according to its ferrous or non-ferrous nature. It is easier for these units to pick up a ferrous metal than a non-magnetic metal such as stainless steel. While a metal detector may find a ferrous metal particle of 1.5mm, a foreign body made of stainless steel may have to be three millimetres or greater to be detected by the same machine.
Metal detectors can also detect problems with items packaged in metallised packs, such as foils for yoghurt pots or packages insulated with metallic materials.
These limitations are significant in the dairy sector, where foreign objects often reach foodstuffs from factory processes i.e. rubber seals falling from machines, or in products such as yoghurt with added fruit sections. Protective and foil packaging are also likely to increase as demand for long-shelf life products continues to grow. The question to ask then: how can the dairy industry improve on quality control offered by traditional metal detection systems?
X-RAY TECHNOLOGY These systems measure the density of a product as it crosses the inspection area. It creates an image that is analysed to identify a foreign object. Like metal detection, X-ray technology can be applied during production; in bulk flow materials prior to packaging and to packaged retail goods before shipping.
While X-ray won’t necessarily detect every possible contaminant, the technology does provide a wider detection spectrum than metal detectors and will pick up stone, glass, dense plastic/rubber and metal. It can pick up contaminants that have a greater density than water (1g/cm3). The technology is versatile and can assist in counting, weight estimation, detect fill levels, identify product flaws and ensure packaging integrity.

PRIMARY PROCESSING OF CHEESE Many processes are required to produce cheese and each of these has a risk of adding foreign bodies. This normally occurs during processing i.e. rubber sealing ring failure from mixing equipment, pipework or metal from poorly adjusted cutting or blending processes. The high level of operator intervention during processing also carries foreign body risk. While rubber may be present in the block at this point, detection may not be possible due to the relatively low-density differential between the depth of the cheese block and the material requiring detection.
At the primary phase, cheese must be checked for any metal contamination to protect the integrity of the cutting blades. Metal detectors are commonly used in this instance because of the large block size. A larger aperture metal detector is required, which reduces potential sensitivity.
This, coupled to the salt and moisture level in cheese, means detection limits are high - typically around 5.0mm or greater for stainless steel. Even a piece of metal of three to four millimetres will do significant damage if in contact with cutting blades. An X-ray, on the other hand, can provide detection down to 1.5 to two millimetres on the largest blocks and down to one millimetre in most cases. This provides a much higher level of protection.
For operations producing grated cheese, the challenge is probably the greatest. The final product offers a high variable density as the strands overlap and combine with each other. Here the differential with metals is still large. Due to the lower height and overall density of the metal, detection levels achievable with X-ray are impressive and consistently below 1.0mm - below 0.6mm level in most cases. The varying nature of the product does mean that lower density contaminants are more difficult to detect. Rubber seals, having passed through the grating process are unlikely to be detected.

SHOULD YOU INVEST IN TECHNOLOGY? Suppliers seeking to invest in quality control equipment must consider factors such as the nature and volume of their products, the nature of foreign body contamination, customer demand and contractual obligations. This should be offset against the perceived nature and impact of a contamination incident and the company’s tolerance of risk.
Using X-ray technology to detect foreign bodies in dairy products can assure the quality of food produced, acting as an insurance policy against prosecution and brand damage. It can also give suppliers a real competitive edge. By using X-ray to exceed the standard demanded by customers, companies can demonstrate their commitment to quality while assuring potential clients they can cope with demanding specifications. •
Ishida’s range of X-ray systems






