2 minute read
Cancer Cells Modify Extracellular Matrix to Induce Carcinoma-Associated Fibroblasts
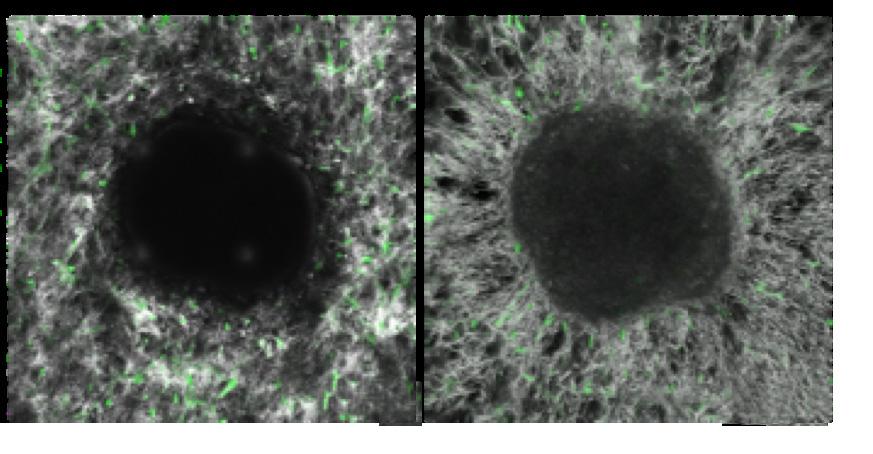
ECM fibrils were radially aligned by the cancer cells (right), while the fibrils remained network-like in the culture containing normal cells (left).
Cancer Cells Modify Extracellular Matrix to Induce CarcinomaAssociated Fibroblasts
Advertisement
By Catherine Graham During cancer development, cancer cells sometimes recruit healthy cells surrounding the tumor to become their accomplices. These accomplices are known as carcinoma-associated fibroblasts (CAFs). CAFs promote tumor growth and metastasis. Therefore, cancer patients with a higher number of CAFs face a worse prognosis.
CAFs have been studied in terms of how they promote tumor progression, but their origin remains a mystery. Yun Chen, associate researcher in the Department of Mechanical Engineering and associate faculty member at the INBT, and her team, are studying the underlying mechanisms by which breast cancer cells turn healthy cells into CAFs during early stages of breast cancer. Cancer cells induce CAFs by delivering chemicals to healthy cells. In early stages, however, cancer cells don’t migrate far enough to reach healthy cells because the tumor’s surrounding tissue is made up of dense fibers, called the extracellular matrix (ECM). The question remains, how do CAF-promot-
ing chemicals pass through the dense ECM barrier to reach healthy cells? “Healthy cells are sometimes very far from the tumor, and cancer cells diffuse too slowly to reach them. The goal of our study was to understand how cancer cells modify the surrounding ECM and induce CAF activation at the early stage,” said Chen. The team constructed a 3D in vitro model to mimic the in vivo tumor microenvironment of early-stage breast cancer. They observed that early-stage breast cancer cells generate strong forces to reorganize the ECM fibrils. In fact, cancer cells grab the fibrils using their finger-like protrusions and realign them. This remodeling turns the ECM fibrils into an aqueduct-like conduit, carrying CAF-promoting chemicals even further to reach more healthy cells, create more CAFs, and kickstart metastasis.
“Research has shown that biochemical factors contribute to cancer progression, and that’s true,” said Wei-Hung Jung, a member of the team and a PhD student in Chen’s lab. “We show here that biophysical factors also play a critical role in remodeling the ECM, and facilitate the delivery of cancer-secreted biochemical factors to healthy cells, turning them into cancer’s accomplices.” Most importantly, these insights could inspire new cancer treatment strategies to suppress CAF induction and subsequent metastasis. Future research will focus on testing their hypothesis in vivo with a new cell therapy invented by the team. They will implant genetically engineered cells to counteract the ECM remodeling by cancer cells. “ECM remodeling is important to establish the connection between cancer cells and the surrounding healthy cells. Essentially, we need to cut that connection to prevent the spread of cancer. The hope is that, eventually, we can implement this knowledge clinically to stall this ECM remodeling and stop metastasis,” adds Jung.


Further Highlights
Yun Chen was awarded a National Institutes of Health Trailblazer Award. The two-year, $400,000 award, administered through the National Institute of Biomedical Imaging and Bioengineering, supports innovative early-career researchers tackling high-risk, potentially high-impact projects. The ultimate goal is to foster the invention of methods, instruments, and materials that will open new avenues of research.










