
11 minute read
New Filtration Material Could Remove Long-Lasting Chemicals From Water
By David L. Chandler
Water contamination by the chemicals used in today’s technology is a rapidly growing problem globally. A recent study by the U.S. Centers for Disease Control found that 98 percent of people tested had detectable levels of PFAS, a family of particularly long-lasting compounds also known as “forever chemicals,” in their bloodstream.
A new filtration material developed by researchers at MIT might provide a nature-based solution to this stubborn contamination issue. The material, based on natural silk and cellulose, can remove a wide variety of these persistent chemicals as well as heavy metals. And, its antimicrobial properties can help keep the filters from fouling.
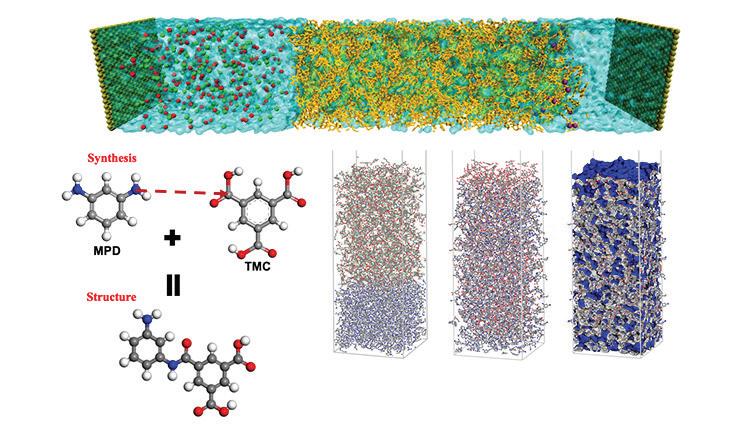
The findings are described in the journal ACS Nano, in a paper by MIT postdoctoral Yilin Zhang, professor of civil and environmental engineering Benedetto Marelli, and four others from MIT (Yilin Zhang, Hui Sun, Yunteng Cao, Maxwell J. Kalinowski, Meng Li, Benedetto Marelli).
PFAS chemicals are present in a wide range of products, including cosmetics, food packaging, water-resistant clothing, firefighting foams, and antistick coating for cookware. A recent study identified 57,000 sites contaminated by these chemicals in the U.S. alone. The U.S. Environmental Protection Agency has estimated that PFAS remediation will cost $1.5 billion per year, in order to meet new regulations that call for limiting the com- pound to less than seven parts per trillion in drinking water.
Contamination by PFAS and similar compounds “is actually a very big deal, and current solutions may only partially resolve this problem very efficiently or economically,” Yilin Zhang says. “That’s why we came up with this protein and cellulose-based, fully natural solution,” he says.
“We came to the project by chance,” Marelli notes. The initial technology that made the filtration material possible was developed by his group for a completely unrelated purpose – as a way to make a labelling system to counter the spread of counterfeit seeds, which are often of inferior quality. His team devised a way of processing silk proteins into uniform nanoscale crystals, or “nanofibrils,” through an environmentally benign, water-based drop-casting method at room temperature.
Zhang suggested that their new nanofibrillar material might be effective at filtering contaminants, but initial attempts with the silk nanofibrils alone didn’t work. The team decided to try adding another material: cellulose, which is abundantly available and can be obtained from agricultural wood pulp waste. The researchers used a self-assembly method in which the silk fibroin protein is suspended in water and then templated into nanofibrils by inserting “seeds” of cellulose nanocrystals. This causes the previously disordered silk molecules to line up together along the seeds, forming the basis of a hybrid material with distinct new properties.
The electrical charge of the cellulose, they found, also gave it strong antimicrobial properties. This is a significant advantage, since one of the primary causes of failure in filtration membranes is fouling by bacteria and fungi. The antimicrobial properties of this material should greatly reduce that fouling issue, the researchers say.
“These materials can really compete with the current standard materials in water filtration when it comes to extracting metal ions and these emerging contaminants, and they can also outperform some of them currently,” Benedetto Marelli says. In lab tests, the materials were able to extract orders of magnitude more of the contaminants from water than the currently used standard materials, activated carbon or granular activated carbon.
While the new work serves as a proof of principle, Marelli says, the team plans to continue working on improving the material, especially in terms of durability and availability of source materials. While the silk proteins used can be available as a byproduct of the silk textile industry, if this material were to be scaled up to address the global needs for water filtration, the supply might be insufficient.
Study Reveals Membrane Behavior Impacting WaterPurification Performance
Desalination can help provide muchneeded freshwater to communities lacking access, but the process can be difficult at scale. Now, scientists have discovered one of the reasons behind that difficulty.
Roughly 80% of the world’s desalination involves the use of reverse osmosis –a water-purification process utilizing a semi-permeable membrane to separate water molecules from other undrinkable substances. Engineers and scientists around the world have leveraged essential thin-film-composite reverse-osmosis membranes for more than 40 years.

Also, alternative protein materials may turn out to perform the same function at lower cost.
Initially, the material would likely be used as a point-of-use filter, something that could be attached to a kitchen faucet, Zhang says. Eventually, it could be scaled up to provide filtration for municipal water supplies, but only after testing demonstrates that this would not pose any risk of introducing any contamination into the water supply. But one big advantage of the material, he says, is that both the silk and the cellulose constituents are considered food-grade substances, so any contamination is unlikely.
“Most of the normal materials available today are focusing on one class of contaminants or solving single problems,” Zhang
A multi-institutional research team from the UCLA Samueli School of Engineering, the University of WisconsinMadison, Yale University and the University of Connecticut has recently published a study investigating the behavior of the membranes as governed by their viscoelastic properties.
Published in the Journal of Membrane Science, the study looked at the compaction (the process by which the polymer membranes increase in density under pressure) and relaxation (the corresponding decrease in density when pressure is relieved) involved in reverse osmosis.
The researchers found that by increasing the crosslinking-degree, or density of polymers in the polyamide layer, the membrane will exhibit decreased compaction and increased relaxation, thereby improving water permeability and salt says. “I think we are among the first to address all of these simultaneously.”
“What I love about this approach is that it is using only naturally grown materials like silk and cellulose to fight pollution,” says Hannes Schniepp, professor of applied science at the College of William and Mary, who was not associated with this work. “In competing approaches, synthetic materials are used – which usually require only more chemistry to fight some of the adverse outcomes that chemistry has produced. [This work] breaks this cycle! ... If this can be massproduced in an economically viable way, this could really have a major impact.”
The research team included MIT postdoctorals Hui Sun and Meng Li, graduate student Maxwell Kalinowski, and recent graduate Yunteng Cao PhD ’22, now a postdoctoral at Yale University. The work was supported by the U.S. Office of Naval Research, the U.S. National Science Foundation, and the Singapore-MIT Alliance for Research and Technology.
Read: https://news.mit.edu/2024/new-filtrationmaterial-could-remove-long-lasting-waterchemicals-0906
Paper: https://pubs.acs.org/doi/full/ 10.1021/acsnano.4c07409 rejection. Polyamide, known for its use as a versatile material, is a type of synthetic polymer composed of long chains of molecules linked together by specially arranged atoms known as an amide group.
Findings from the research have the potential to alleviate some operational challenges of the desalination industry by highlighting the importance of developing new reverse-osmosis membrane materials with enhanced compaction resistance to improve long-term desalination performance.
Eric Hoek, a professor of civil and environmental engineering at UCLA Samueli, and Ying Li, an associate professor of mechanical engineering at the University of Wisconsin-Madison, are the co-corresponding authors.
“Mechanistic studies like this one can provide practical insights not just about
University Of Missouri
Designing a Better Water Filter
Mizzou researcher Maryam Salehi is developing a fabric-like filter to remove tiny plastics and lead from drinking water.
Water filters on the market today can remove some contaminants, but they’re not designed to capture microplastics. In fact, some of them may actually add small plastics into drinking water during the filtration process. What’s worse is these microplastics can cause inflammation, increase the risk of cancer and act as carriers for harmful chemicals that trigger other health problems.
how a reverse-osmosis membrane can be damaged at high applied pressure, but also how new membrane materials might be designed to withstand such harsh operating conditions,” says Hoek, who directs UCLA’s Nanomaterials & Membrane Technology Research Laboratory, which explores membrane formation and application for water treatment.
To examine the polyamide layer’s role in compaction, the team created five reverse-osmosis membranes with varying degrees of crosslinking in the polyamide film. These membranes, all supported by identical base layers, demonstrated that higher crosslinking resulted in lower water permeability, higher salt rejection, less compaction and better recovery of permeability when pressure was reduced.
The study’s primary authors are Jishan Wu – postdoctoral researcher in Hoek’s lab – and Jinlong He of the University of Wisconsin-Madison. Other paper authors are members of Hoek’s lab – postdoctoral researchers Derrick Dlamini and Javier Alan Quezada, graduate students Xinyi Wang and Minhao Xiao, as well as undergraduate students Kay Au, Kevin Guo and Jason Le. They are joined by Hanqing Fan, Kevin Pataroque and Menachem Elimelech from Yale University, as well as Yara Suleiman and Sina Shahbazmohamadi from the University of Connecticut.
Xiao recently received a fellowship from the American Membrane Technology
Association and the U.S. Bureau of Reclamation for his membrane-related research in innovations for water treatment.
The study was funded by the National Alliance for Water Innovation through the U.S. Department of Energy’s Industrial Efficiency and Decarbonization Office, the UCLA Samueli School of Engineering and the UCLA Sustainable LA Grand Challenge.
An announcement of this study was first published by Water Desalination Report and adapted for this story.
Read: https://samueli.ucla.edu/study-revealsmembrane-behavior-impacting-water-purificationperformance/ © Copyright 2024 UCLA Samueli School Of Engineering
University of Missouri researcher Maryam Salehi and collaborators are coming up with a new way to trap those tiny invaders – and protect the public –through a fabric-like filter.
“The idea is to design a filter that can be attached to a faucet so it can remove microplastic and lead at the same time from tap water,” says Salehi, an assistant professor of civil and environmental engineering in Mizzou’s College of Engineering. “We’re envisioning an inexpensive point-of-use filter that could connect to any faucet.”
The filter membrane is made from polyvinyl alcohol fibers, which are polymers currently used in biomedical applications. The team chose the material because it’s low-cost and biocompatible, meaning it’s not toxic to humans, animals or plants.

It’s also proving to be effective. In lab tests, the membrane was able to remove nearly 100% of larger microplastics and nearly 80% of the smallest microplastics, while at the same time removing about 70% of lead contamination.
“We still need to test the filter to see how it tolerates other conditions – such as disinfectant use in water – but the idea is to hopefully have something that can be commercialized and used to easily purify tap water,” Salehi says.
The team outlined their findings in a cover article published in Applied Polymer Science. Co-authors were Anandu Gopakumar Nair, a doctoral student at Mizzou, Alexander Ccanccapa, a postdoctoral researcher, and Kati Bell, an industry partner. The work is supported by Brown and Caldwell, as well as the National Science Foundation.
Salehi is a core researcher at the Missouri Water Center, which brings together stakeholders from academia, industry and government to advance water research.
Read: https://showme.missouri.edu/2024/designinga-better-water-filter/
JOHNS HOPKINS BLOOMBERG SCHOOL OF PUBLIC HEALTH
Filters and Digital Health Program
Reduced Participants’ Arsenic Levels by Nearly Half in American Indian Households Relying on Well Water
Acommunity-led water-testing project made up of households that rely on private well water with high arsenic levels saw on average a 47 percent drop in participants’ urinary arsenic levels after filters were installed and a digital health program was implemented, according to a new study led by researchers at the Johns Hopkins Bloomberg School of Public Health. Over the two-year study period, participating households were contacted to encourage use of the filter and remind users to replace the filter cartridge.

For the study – a randomized controlled trial in American Indian communities in the Northern Great Plains – socalled “point of use” arsenic filters were installed in households whose private wells had arsenic concentrations greater than the Environmental Protection Agency’s recommended limit of 10µg/L. Each of the study’s 50 participating household had two faucets on their kitchen sink: an arsenic filter faucet for drinking and cooking water installed by a community plumber for the study and the main kitchen faucet used for handwashing and washing dishes.
Study participants also reported a significant increase in their household’s exclusive use of arsenic-filtered water for drinking and cooking.
The researchers partnered closely with community members, tribal organizations, and the Indian Health Service throughout the program’s design and implementation to ensure the program suited the needs of the community and to address potential barriers to using the filters. The findings were published online March 27 in Environmental Health Perspectives.
“This trial shows the potential of digital health intervention programs like this to reduce environmental exposures, and may help alleviate costs associated with home visits for intervention delivery,” says
Christine Marie George, PhD, a professor in the Bloomberg School’s Department of International Health and the study’s cofirst author.
Around two million people in the U.S. use private wells with elevated arsenic levels, according to the Environmental Protection Agency. Rural and American Indian communities that rely on well water are disproportionately affected. Prior research has shown an association between arsenic exposure and the development of cardiovascular disease, diabetes, kidney disease, impaired lung function, and various cancers among American Indian communities.
For their randomized controlled trial, the researchers recruited households through a community-led water arsenictesting project that found arsenic levels above the EPA guideline in 29 percent of the 440 tested wells. Of these, 114 households met eligibility criteria to be enrolled in the study. Eighty-four participants from 50 of these 114 households participated in the study.
Participating households were divided into two study arms, with 51 participants in Arm 1 (27 households) and 33 participants in Arm 2 (23 households). Arm 1 consisted of the installation of a point of use filter with follow-up phone messages to encourage use of the filter. Arm 2 consisted of a point of use filter and follow-up phone calls and texts, plus home visits from community members, supporting print materials, and videos of community testimonials in support of the filters.
The study was conducted between July 2018 and May 2021. Tracy Zacher, RN, field office director of the American Indian-owned Missouri Breaks Industries Research, Inc., and the study’s co-first author, led the study recruitment and evaluation activities and the arsenic testing.
Study participants’ arsenic levels were checked via urine samples collected at the beginning of the study and again after two years. Overall, there was a reduction in urinary arsenic levels on average by 47 percent during the two-year follow-up period, with no significant difference between the two study arms. Households reported exclusive use of arsenic-safe water to prepare food and drinks increased significantly from 11 percent at the start of the study to 42 percent of households by the study’s end. There also was no significant difference between study arms for this outcome.
Study participants’ arsenic levels were checked via urine samples collected at the beginning of the study and again after two years.
“It is very exciting that the less intensive arm of the study that only provided phone calls and a filter worked just as well,” says George. “Additional studies are needed to evaluate this digital health approach in other arsenic-impacted settings in the U.S. and globally.”
The authors believe this is the first randomized controlled trial study of an arsenic mitigation program in the Americas. George credits much of the program’s success to the researchers’ collaboration with the impacted communities.
“Effect of an Arsenic Mitigation Program on Arsenic Exposure in American Indian Communities: A Cluster Randomized Controlled Trial of the CommunityLed Strong Heart Water Study Program” was written by Christine Marie George, Tracy Zacher, Kelly Endres, Francine Richards, Lisa Bear Robe, David Harvey, Lyle G Best, Reno Red Cloud, Annabelle Black Bear, Leslie Skinner, Christa Cuny, Ana Rule, Kellogg J. Schwab, Joel Gittelsohn, Ronald Glabonjat, Kathrin Schilling, Marcia O’Leary, Elizabeth D. Thomas, Jason Umans, Jianhui Zhu, Lawrence H. Moulton, and Ana Navas-Acien.
The research was supported by the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH R01ES025135).
Read: https://publichealth.jhu.edu/2024/filters-anddigital-health-program-reduced-participants-arseniclevels-by-nearly-half-in-american-indian-householdsrelying-on-well-water











