11 minute read
EMERGENCE
Compiled by Caryn Smith, IFN Chief Content Officer
International Filtration News Explores Trending Innovation
IFN highlights significant research from universities and institutions around the world. If you are a part of a project you would like to highlight, email csmith@inda.org. Please write “IFN Emerging Research Submission” in your subject line in order to apply. Please send a completed press release and/or summary of the research as you would want it to be printed, a link to the university online story (if applicable), and all high resolution photographs/charts/graphs, short researcher bio(s). All selections could be edited for length.
The University Of Southern California
USC Researchers Discover Method To Fully Recycle Carbon Fibers
The method can upcycle carbon fibers to restore 90% of the strength of original fibers. By
UYashna Dodrajka
SC researchers have developed a study that promises complete upcycling of high-performance composite materials, according to Travis Williams, a professor of chemistry at Dornsife College of Letters, Arts and Sciences and the leader of the study.
Carbon fibers are expensive, highperformance materials largely used in the construction of aircrafts and automobiles. The substance that keeps airplanes together is made up of eight layers of woven carbon fiber held together with a strong type of epoxy resin. Williams said as an aircraft reaches the end of its life, it will turn into waste because there is currently no technology to separate the carbon fibers from the epoxy.
“Trying to un-thermoset a thermoset, un-epoxy an epoxy, is like trying to unboil an egg,” Williams said. “We started inventing reactions to do that for the different kinds of epoxies and different kinds of thermosets that they use in aviation. It worked in a number of cases … you can get it back, still woven as fabric, and nobody else has been able to do that. So this got people’s attention.”
Williams said 99.1% of the composite materials end up in landfills due to the “expensive” process of recycling. He said 1% of the composite that is recycled is subjected to high heat, which damages p Carbon fibers are expensive, high-performance materials largely used in the construction of aircrafts and automobiles. USC research shows promise to fully recycle these fibers to restore 90% of the strength of the original fibers. Caryn Smith the fibers, such as polymer. The study has pioneered a method that transforms polymer, one of the cheapest fibers in the composite space, into high-cost, valuable substances, Williams said.
“Nobody had ever asked that about taking aircraft epoxy apart, so we were the first to report if you do it oxidatively, these are the molecular events that happen,” Williams said. “Now, all sorts of people are making all sorts of creative contributions to this [because] we taught them how to think about it like molecules, not like the material … A lot of molecular people are coming up with great ideas to handle all sorts of composites. It’s really cool to watch.”
Williams said in the study, researchers have focused on breaking down the mechanism behind different chemical reactions. Despite initially being published in lesser-known journals, according to Williams, the study has quickly gained the attention of many.
“I would argue that our method is superior to other methods out there in the literature today,” said Justin Lim, a doctoral student studying chemistry. “Our group is advantageous because we aim to make our methods sustainable.”
Ding-Yuan Lim, a graduate student studying chemistry, said another factor that sets USC’s approach apart is their effort to incorporate common chemical reagents, which helps in cost reduction, ensures safety and makes large-scale application feasible, while most of the studies out there focus on using super critical conditions like high pressure and high temperatures.
The researcher’s process of upcycling carbon fibers from composite material consumes 10.8 to 36 megajoules of energy per kilogram, while the production of new carbon fibers consumes 198 to 594 megajoules per kilogram, said Madison Fette, a graduate student studying chemistry. Fette said the cost of upcycling the carbon fibers is also only a fifth of producing original fibers.
Williams said the carbon fibers upcycled through this method are lighter, stronger and stiffer than aluminum. As a result, they benefit the aviation industry tremendously. Due to their light weight, aircrafts made from recycled carbon fibers require 20% less fuel which also leads to a significant reduction in CO2 emissions, Williams said.
Lim said the study also ensures that carbon fibers recycled through their process would maintain nearly the same mechanical properties as original fibers and are at least within 90% or higher of carbon fibers’ original strength, which has been tested by USC researchers through single fiber tensile tests.
New Filter Captures and Recycles
Aluminum from Manufacturing Waste
MIT engineers designed a nanofiltration process that could make aluminum production more efficient while reducing hazardous waste.
By Jennifer Chu | MIT News
than 99 percent of aluminum ions in these solutions.
If scaled up and implemented in existing production facilities, the membrane technology could reduce the amount of wasted aluminum and improve the environmental quality of the waste that plants generate.

U“The thing that’s going to break us are the people who buy these materials philosophically believe that the recycled fiber is inferior,” Williams said. “What’s going to kill us is … the manufacturing community who seem to believe that recycled [fiber] is lower quality.”
Williams said he believes the situation might change as President Donald Trump’s administration shifts national priorities in energy.
“Policy wise, whilst there’s a lot that you can say that’s not real flattering about our current president, I think he might help us as we try to change manufacturing through energy transition, through the transition from traditional metals to composite and modern materials,” Williams said.
© University of Southern California/Daily Trojan. All rights reserved.
Read: https://dailytrojan.com/2025/02/13/ usc-researchers-discover-method-to-fullyrecycle-carbon-fibers/ sed in everything from soda cans and foil wrap to circuit boards and rocket boosters, aluminum is the secondmost-produced metal in the world after steel. By the end of this decade, demand is projected to drive up aluminum production by 40 percent worldwide. This steep rise will magnify aluminum’s environmental impacts, including any pollutants that are released with its manufacturing waste.
MIT engineers have developed a new nanofiltration process to curb the hazardous waste generated from aluminum production. Nanofiltration could potentially be used to process the waste from an aluminum plant and retrieve any aluminum ions that would otherwise have escaped in the effluent stream. The captured aluminum could then be upcycled and added to the bulk of the produced aluminum, increasing yield while simultaneously reducing waste.
The researchers demonstrated the membrane’s performance in lab-scale experiments using a novel membrane to filter various solutions that were similar in content to the waste streams produced by aluminum plants. They found that the membrane selectively captured more
“This membrane technology not only cuts down on hazardous waste but also enables a circular economy for aluminum by reducing the need for new mining,” says John Lienhard, the Abdul Latif Jameel Professor of Water in the Department of Mechanical Engineering, and director of the Abdul Latif Jameel Water and Food Systems Lab (J-WAFS) at MIT. “This offers a promising solution to address environmental concerns while meeting the growing demand for aluminum.”
Trent Lee
Lienhard and his colleagues report their results in a study appearing today in the journal ACS Sustainable Chemistry and Engineering. The study’s co-authors include MIT mechanical engineering undergraduates Trent Lee and Vinn Nguyen, and Zi Hao Foo SM ’21, PhD ’24, who is a postdoc at the University of California at Berkeley.
A Recycling Niche
Lienhard’s group at MIT develops membrane and filtration technologies for desalinating seawater and remediating various sources of wastewater. In looking for new areas to apply their work, the team found an unexplored opportunity in aluminum and, in particular, the wastewater generated from the metal’s production.
As part of aluminum’s production, metal-rich ore, called bauxite, is first mined from open pits, then put through a series of chemical reactions to separate the aluminum from the rest of the mined rock. These reactions ultimately produce aluminum oxide, in a powdery form called alumina. Much of this alumina is then shipped to refineries, where the powder is poured into electrolysis vats containing a molten mineral called cryolite. When a strong electric current is applied, cryolite breaks alumina’s chemical bonds, separating aluminum and oxygen atoms. The pure aluminum then settles in liquid form to the bottom of the vat, where it can be collected and cast into various forms.
Cryolite electrolyte acts as a solvent, facilitating the separation of alumina during the molten salt electrolysis process. Over time, the cryolite accumulates impurities such as sodium, lithium, and potassium ions – gradually reducing its effectiveness in dissolving alumina. At a certain point, the concentration of these impurities reaches a critical level, at which the electrolyte must be replaced with fresh cryolite to main process efficiency. The spent cryolite, a viscous sludge containing residual aluminum ions and impurities, is then transported away for disposal.
“We learned that for a traditional aluminum plant, something like 2,800 tons of aluminum are wasted per year,” says lead author Trent Lee, who carried out the new work as part of the MITEI Energy UROP program. “We were looking at ways that the industry can be more efficient, and we found cryolite waste hadn’t been well-researched in terms of recycling some of its waste products.”
A Charged Kick
In their new work, the researchers aimed to develop a membrane process to filter cryolite waste and recover aluminum ions that inevitably make it into the waste stream. Specifically, the team looked to capture aluminum while letting through all other ions, especially sodium, which builds up significantly in the cryolite over time.
The team reasoned that if they could selectively capture aluminum from cryolite waste, the aluminum could be poured back into the electrolysis vat without adding excessive sodium that would further slow the electrolysis process.
The researchers’ new design is an adaptation of membranes used in conventional water treatment plants. These membranes are typically made from a thin sheet of polymer material that is perforated by tiny, nanometer-scale pores, the size of which is tuned to let through specific ions and molecules.
The surface of conventional membranes carries a natural, negative charge. As a result, the membranes repel any ions that carry the same negative charge, while they attract positively charged ions to flow through.
In collaboration with the Japanese membrane company Nitto Denko, the MIT team sought to examine the efficacy of commercially available membranes that could filter through most positively charged ions in cryolite wastewater while repelling and capturing aluminum ions. However, aluminum ions also carry a positive charge, of +3, where sodium and the other cations carry a lesser positive charge of +1.
Motivated by the group’s recent work investigating membranes for recovering lithium from salt lakes and spent batteries, the team tested a novel Nitto Denko membrane with a thin, positively charged coating covering the membrane. The coating’s charge is just positive enough to strongly repel and retain aluminum while allowing less positively charged ions to flow through.
“The aluminum is the most positively charged of the ions, so most of it is kicked away from the membrane,” Foo explains.
The team tested the membrane’s performance by passing through solutions with various balances of ions, similar to what can be found in cryolite waste. They observed that the membrane consistently captured 99.5 percent of aluminum ions while allowing through sodium and the other cations. They also varied the pH of the solutions, and found the membrane maintained its performance even after sitting in highly acidic solution for several weeks.
“A lot of this cryolite waste stream comes at different levels of acidity,” Foo says. “And we found the membrane works really well, even within the harsh conditions that we would expect.”
The new experimental membrane is about the size of a playing card. To treat cryolite waste in an industrialscale aluminum production plant, the researchers envision a scaled-up version of the membrane, similar to what is used in many desalination plants, where a long membrane is rolled up in a spiral configuration, through which water flows.

“This paper shows the viability of membranes for innovations in circular economies,” Lee says. “This membrane provides the dual benefit of upcycling aluminum while reducing hazardous waste.”
© MIT News Office, part of the Institute Office of Communications. Read: https://news.mit.edu/2025/new-filter-capturesand-recycles-manufacturing-waste-aluminum-0107
UNIVERSITY OF DUISBURG-ESSEN
I-based Predictions: Early Warning System for Drinking Water Suppliers
By Juliana Fischer
About 12% of drinking water in Germany comes from lakes and reservoirs. Their water quality is significantly influenced by the organisms living within them. However, climate change, environmental pollution, and invasive species such as blue-green algae threaten biodiversity –and thus the quality of drinking water. In the research project IQ Wasser,* an interdisciplinary team at the University of Duisburg-Essen is examining microbial biodiversity using environmental DNA analyses. The goal is to develop an AIbased early warning system that detects changes in water quality.


“Many organisms contribute to water quality in drinking water reservoirs,” explains Dr. Julia Nuy from Environmental Metagenomics at the Research Centre
One Health. “Mussels filter particles out of the water, freshwater amphipods break down organic matter, and certain bacteria metabolize nitrogen or carbon.” A key principle is: the greater the diversity of species, the more stable ecosystem services such as water filtration remain. However, the role of biodiversity – particularly microbial diversity – has so far been largely overlooked when assessing water quality. Microorganisms like bacteria play essential roles in the ecosystem but also pose risks, search as cyanobacteria (blue-green algae), which proliferate as temperatures rise.
Over the next three years, the interdisciplinary team will collect samples four times a year from the Wahnbach Reservoir and the Kleine Kinzig Reservoir. “After filtration, we extract and fully sequence the DNA,” says Dr. Julia Nuy, who leads the subproject on microbial ecology and biodiversity. “By working genome- resolved, we can reconstruct nearly complete genomes from small fragments, providing precise insights into microbial diversity and the ecosystem’s services,” she explains. “From the genomes, we can identify whether bacteria metabolize nitrogen or carbon – a core function for the ecosystem.”

Another area of focus is the potential for pathogenicity. “We are examining how antibiotic resistance develops over time, whether specific resistance genes are only found in certain bacteria or across a wide range of microorganisms. Additionally, we analyze whether current trends in antibiotic use are detectable in the bacteria we study,” says Dr. Nuy.
The data collected feeds into AI models that predict environmental changes and their impact on biodiversity. “Our aim is to create an early warning system for drinking water suppliers,” emphasizes Nuy. “This will enable the early detection


• Rigid plastic cores
• Flexible tubular sleeves
• Flow channel spacers
• Media, pleat support
• Welded tube overwraps of potential hazards, such as algal blooms or antibiotic-resistant pathogens, allowing targeted countermeasures to be implemented.”
• You design it, we create it!
Juliana Fischer, Editor, University of DuisburgEssen, can be reached at +49 203/37 9-1488, juliana.fischer-uni-due.de.
*The IQ Wasser project is funded by the Federal Ministry of Education and Research with approximately two million euros and coordinated by the TZW: DVGW Water Technology Center under Prof. Dr. Andreas Tiehm. At the University of Duisburg-Essen (UDE), Prof. Dr. Alexander Probst is a consortium partner. Other partners include the Fraunhofer Institute IOSB (Dr. Christian Kühnert), the Museum für Naturkunde Berlin (Dr. Sabrina Kirschke), Moldaenke GmbH (Christian Moldaenke), and Ident Me GmbH (Anne Findeisen).
More information: https://tzw.de/projekte/ project details/projects/iq-water-ki-bioiversitate
Contact: Dr. Julia Nuy, Environmental Department, Research Centre One Health, Tel. +49 201/18 3-4109, mail julia.nuy.uni-due.de
Read: https://www.uni-due.de/2025-01-14-iqwasser-ai-based-early-warning-system-for-drinkingwater-suppliers
UNIVERSITY OF DUISBURG-ESSEN
Fighting Chemical Pollution: Water Purification Using Algae
By Juliana Fischer
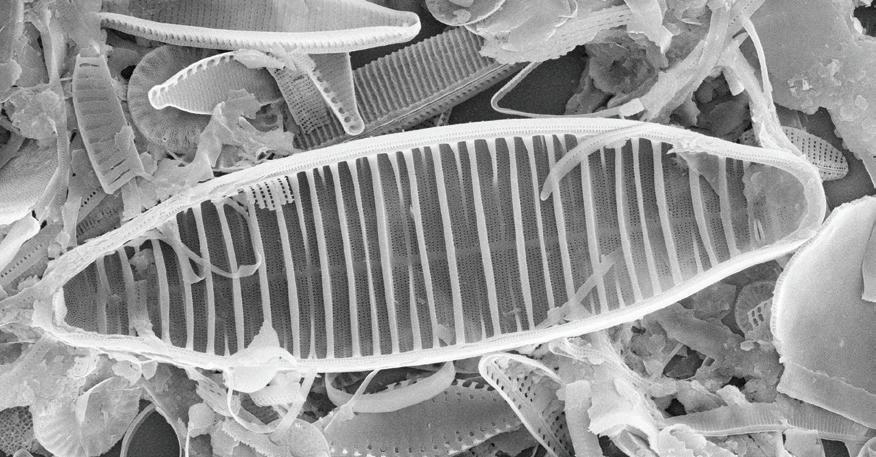
Europe’s water bodies are in poor condition: more than half of them are heavily polluted with chemicals. This is hardly surprising – every day, up to 70,000 different chemicals are used in Europe’s industries and agriculture. Researchers at the University of Duisburg-Essen have now developed a new method for purifying polluted water. Their recent study demonstrates* that the fossil remains of diatoms (a type of algae) can efficiently remove contaminants from water after being chemically modified.
Researchers have identified more than 500 chemicals in Europe’s rivers, originating from industrial and agricultural sources, threatening aquatic habitats. The team led by Junior Professor Dr. Anzhela Galstyan aims to remove these chemicals using algae. “Diatoms are microscopic single-celled organisms that live in water and possess a cell wall made of silica (silicon dioxide). Thanks to its porous structure, it can absorb a wide variety of pollutants,” Galstyan explains.