2 minute read
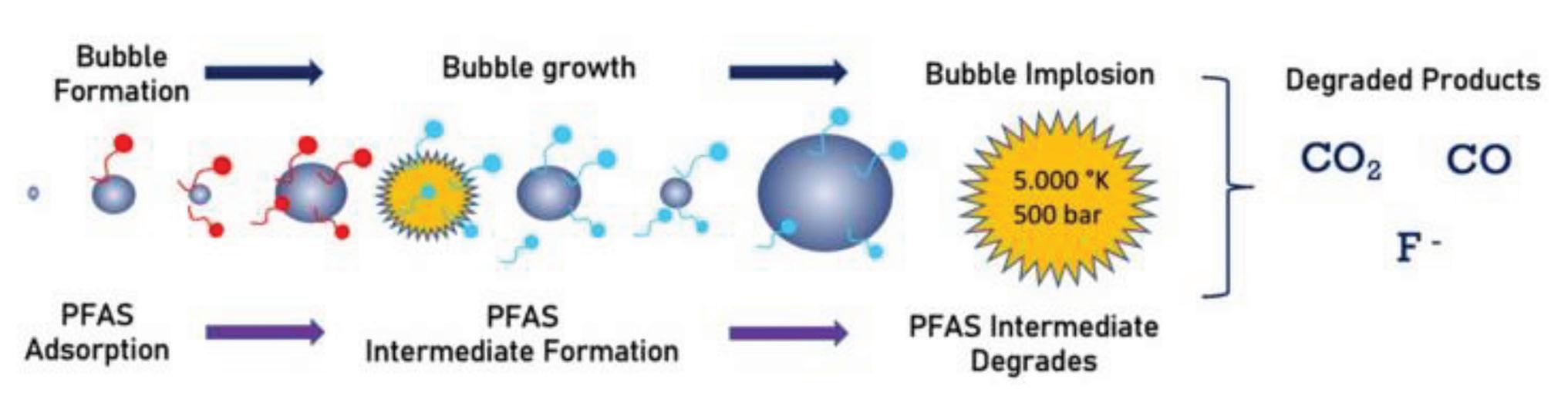
Sonolysis
A recent study of municipal wastewater microorganisms found that two bacterial species can cleave the bonds between carbon and chlorine atoms, which destabilizes the molecule. The reactive oxygen and hydrogen atoms replacing the chlorine atoms contribute to the cleavage of the carbon-fluorine bonds.
Bioremediation
There are very few naturally occurring organic chemicals containing fluoride, and none has more than one fluorine atom in the molecule. Additionally, the fluoride anion is known to cause toxic effects at low concentrations. Therefore, there are almost no known microorganisms capable of consuming these chemicals. As a result, investigations into the destruction of microorganisms by PFAS are very limited.
A recent study of municipal wastewater microorganisms found that two bacterial species can cleave the bonds between carbon and chlorine atoms, which destabilizes the molecule. The reactive oxygen and hydrogen atoms replacing the chlorine atoms contribute to the cleavage of the carbon-fluorine bonds. This approach only works on those PFAS that also contain chlorine (www.newscientist. com/article/2373513).
Another study evaluated the defluorination of carboxylic acid-based PFAS and determined that it is possible to destroy certain PFAS by the combination of anaerobic and aerobic treatments (pubs. acs.org/10.1021/acs.est.1c05509).
Yet another study has shown that material fabricated from renewable lignocellulosic sources adsorbs PFAS and promotes bacterial and fungal growth to degrade these compounds. This technology utilizes low-cost sustainable materials; however, long reaction times (30 – 45 hours) are required and incomplete mineralization may result (https:// doi.org/10.1038/s41467-022-31881-5).
Others
Several other technologies referenced in the literature appear to be still in the very early stages of laboratory investigation. Some investigators claim PFAS destruction but withhold the specific scientific process(es) employed. This lack of detail makes it difficult to place a particular technology in a specific category.
One provider offers “nanoporous” and “piezoelectric” catalysts, which, under turbulent conditions, generate “highly reactive, oxidative and reductive radicals” that break chemical bonds. Specific technical details are considered proprietary.
Thermomechanochemical treatment refers to a milling technology that utilizes stainless steel balls to crush soils and other solids containing PFAS. The collisions of the balls create high temperatures and the addition of an alkaline reagent promotes PFAS destruction. EPA/ORD has filed a patent application.
There are numerous hybrid treatment technologies under development, combining more than one of the above categories in an attempt to optimize performance.
The categories listed herein are as inclusive as possible, and as developments continue, more will undoubtedly appear. The key to complete defluorination is to produce sufficient energy to break the carbon-fluorine chemical bond.
The factors that influence performance are many and varied and include: particular PFAS chemistry (short-chain PFAS, containing less than six carbon atoms, are generally more difficult to break down), water volume to be treated, chemical makeup, pH, temperature, interference from co-contaminants, quality requirement of treated water, economic considerations, etc. (doi:10.1111/ gwmr.12657).
Determinations
The world is entering a whole new era of drinking water contaminant identification and measurement. Analytical chemists can now measure contaminants in the nanogram per liter (ppt) range, which is at least 1000 times smaller than most all previous measurement capabilities.
There is every reason to believe that soon it will be possible to routinely measure concentrations in the part per quadrillion (ppq) or Picogram per liter range. This is 1000 times smaller yet and equivalent to 1 second in 32 million years!
As the bulk of the estimated 85,000 chemicals in our drinking water are presumed to be in tiny concentrations, this capability will undoubtedly allow us to identify new contaminants we are not now aware of, and the bottom line is that a “Pandora’s Box” of water-borne contamination issues will be opened.
Do these tiny concentrations of hereto-fore unknown chemicals represent a health hazard? We have no idea, yet. On the other hand, it’s hard to believe that consuming all these chemicals is good for you!
The good news is that every time we identify new contamination issues, humans soon find a solution to make our drinking water more safe.
More viable solutions certainly will come forward for PFAS removal from our water sources as research and development continue.
This information is as of August, 2024.













