
1 minute read
Electrochemical Oxidation
persulfate, sulfite) has shown improvement in the decomposition rate in laboratory studies. The destruction mechanism is the formation of active radicals by UV radiation.
Titanium dioxide nanotubes and composites have shown promise in laboratory testing. These catalysts apparently produce hydroxyl radicals (•OH) and other photochemically generated reactive intermediates to more effectively defluorinate PFAS. (doi.org/10.1016/j.nxmate.2023.100077).
Hybrid processes, combining electrochemical oxidation with photocatalysis, for example, have been studied in the laboratory. One such study is described in a May 2024 issue of Nature Water, “NearComplete Destruction of PFAS in Aqueous Film-Forming Foam (AFFF) by Integrated Photo-Electrochemical Processes (doi. org/10.1038/s44221-024-00232-7).
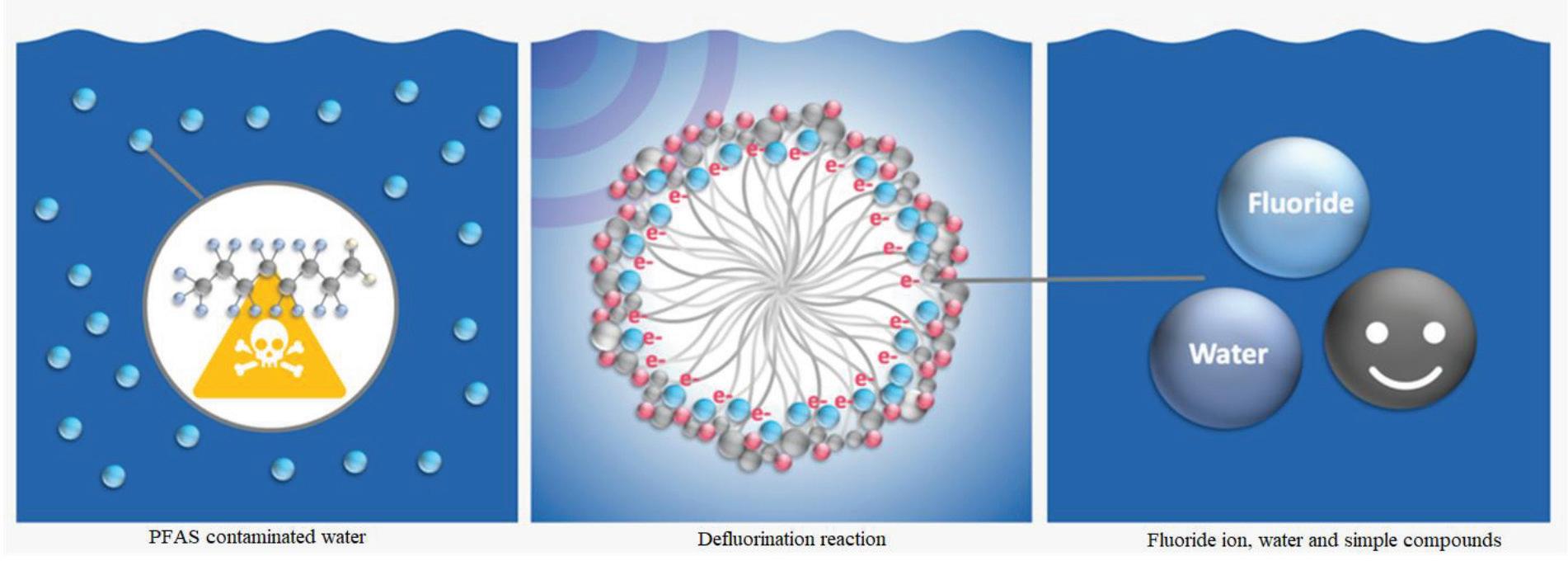
A November 2023 ESTCP (U.S. Department of Defense Environmental Security Technology Certification Pro- gram), project ER21-7569 report titled “Photoactivated Reductive Defluorination PFAS Destruction,” details a new technology. The addition of a chemical produces a surfactant micelle cage that apparently encloses PFAS. Another chemical addition is stimulated by UV radiation to break down PFAS. This technology is depicted in the following illustration in Figure 6.
Plasma Technology
A plasma is an electrically charged gas. The preferred design for PFAS destruction, Non-Thermal Plasma (NTP), can be generated using electrodes in several ways: spark discharge, glow discharge, dielectric barrier discharge, and gliding arc discharge. This discharge energy produces electrons that react with air or a specific gas such as helium, neon, argon, oxygen, or nitrogen in the water to generate reactive species, including •OH radicals, atomic oxygen, singlet oxygen, hydrogen peroxide, and solvated electrons. Ultraviolet radiation, shockwaves and localized high temperatures are also produced in situ. Argon has been reported as the gas most appropriate for the treatment of PFAS (www.enviro.wiki/ index.php?title=PFAS). PFAS molecules adsorbed onto the bubble interface are impacted by the reactive species. The advantages of this technology are short treatment time, minimal interference by co-contaminants and more complete PFAS destruction. On the other hand, this process makes the treated water acidic, although the exact destruction process is not fully understood (doi.org/10.3390/ ijerph192416397). Plasma technology is illustrated in Figure 7.
Sonolysis
Acoustic cavitation involves the generation and implosion of vapor bubbles. Ultrasonic irradiation creates pressure waves that generate localized areas of low and high pressure, forming vapor bubbles that continue to grow and, ultimately, collapse, causing instantaneous high temperatures (4000–10,000 K) and pressures (580,000–870,000 psi). This results in the pyrolysis of water, producing hydroxyl radicals and atomic hydrogen and oxygen at the cavity-bulk interfaces. Testing has revealed that PFAS can be broken down into carbon monoxide, carbon dioxide and fluoride ion (10.1016/j. ceja.2022.100421). This is illustrated in Figure 8.
Plasma Discharge
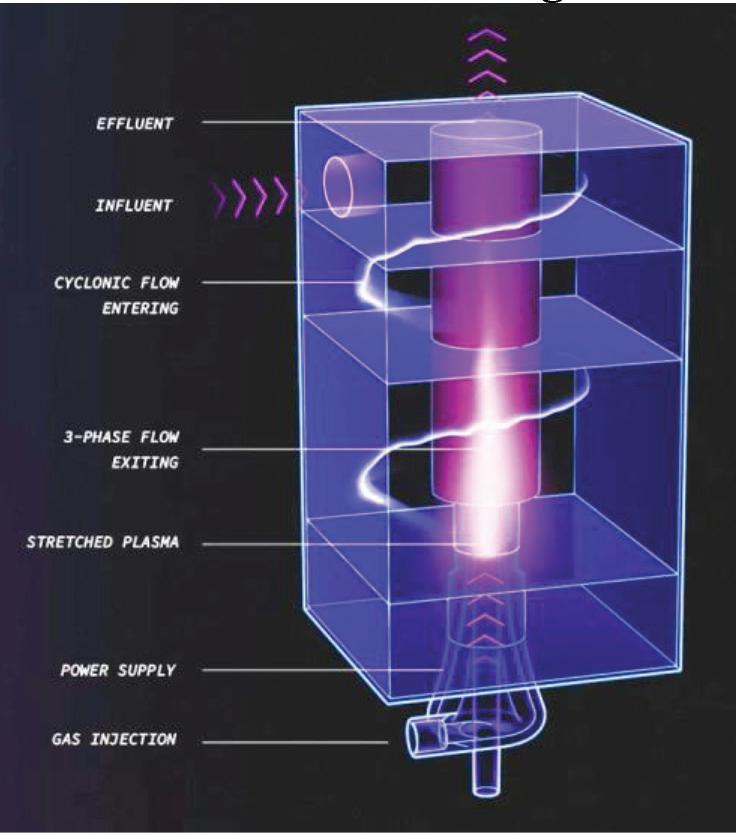
Figure 8.










