2 minute read
Health Canada recent approvals cont'd
Casgevy in sickle cell disease
In the clinical study CLIMB SCD-121, patients who experienced more than three crises yearly became free from crises after Casgevy treatment. For a year, 97% of patients with sickle cell disease were free from crises. Also, participants didn't need hospitalization due to crises during the year The clinical benefits are due to participants' capability to have early and sustained increases in total and fetal hemoglobin levels, reaching near-normal to normal levels at 6 months. Patients were able to maintain better blood count over time.
How does it work?
1) In the hospital, patients' stem progenitor cells from bone marrow that produce mature blood cells are harvested.
2) Patient cells are sent to a central facility to be processed:
Enrichment of progenitor cells
Modification of progenitor cells with Casgevy (called editing)
Proliferation of modified progenitor cells
Cells will be tested, frozen, and shipped back to the hospital.
3) Upon confirmation of viable modified cells, patients will receive intensive chemotherapy (busulfan) to destroy all stem progenitor cells in their bone marrow.
4) After the chemotherapy, modified stem progenitor cells will be infused back into the patients. Patients will need a month to recuperate and get a normal - near normal blood cell production.
The most common side effects in the CLIMB SCD-121 study can primarily be attributable to Busulfan treatment. They include low platelets and white blood cell levels, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, febrile neutropenia (fever and low white blood cell count), headache, and itching.
Health
Canada recent approvals cont'd
Casgevyinbeta-thalassemia
In the CLIMB THAL-111 clinical study, patients with a heavy need for transfusion have been included. They received, on average, 34 blood units per year, representing one transfusion every week to every two weeks. Despite the level of transfusion received, they had a hemoglobin level belownormal.Also,thesepatientshadahighexcessofironintheirblood,liverandheart,putting them at risk for complications due to organ damage. Treatment with Casgevy resulted in transfusionindependencein91%ofpatientsenrolledinthetrial.Thesafetyprofilewasgenerally consistentwithintensivechemotherapybusulfan.Nodeathsorcancersoccurred.
Inconclusion
The data from these two clinical trials show that a one-time infusion of Casgevy provides early andsustainedincreasesintotalandfetalhemoglobinlevels,resultingindurableimprovementof patients'conditionsandqualityoflife
Finally, a treatment option for anemic patients with myelofibrosis!
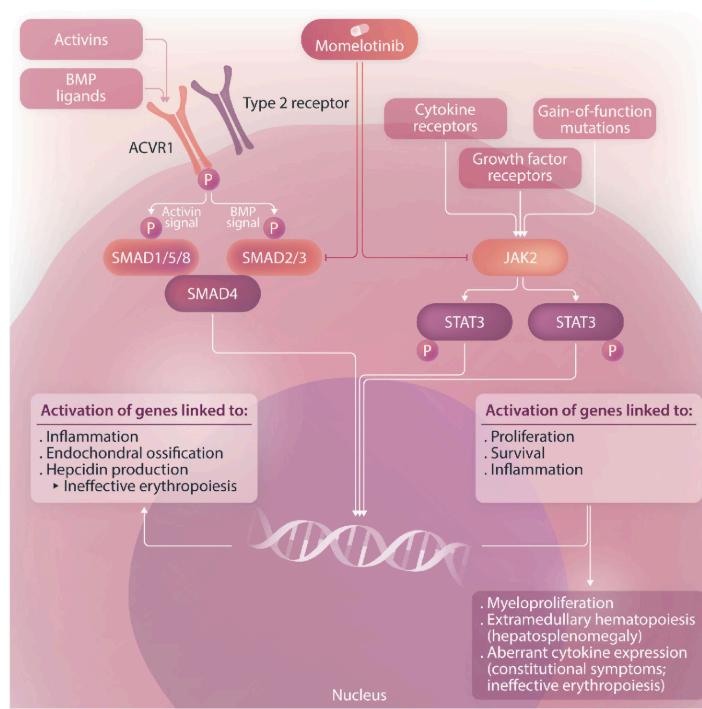
Myelofibrosis is a rare cancer characterized by an overproduction of abnormal blood cells and inflammation markers called cytokines. Treatment of myelofibrosis has improved significantly sincethe1stJAKinhibitor,Jakavi,wasapprovedin2011.JAKinhibitorsaretargetedtherapiesfor cancer, like myelofibrosis, because the disease is caused by mutations that overactivate the JAK/STATpathway.
Currently,theFDAhasapprovedatotaloffourJAKinhibitors:
Jakafi/Jakavi(Ruxolitinib)2011
Inrebic(Fedratinib)in2019
Vonjo(Pacritinib)in2022
Ojjaara(Momelotinib)in2023
Untillastmonth,HealthCanadaonlyapprovedtwoJAKinhibitors:
Jakafi/Jakavi(Ruxolitinib)2012
Inrebic(Fedratinib)in2023
InNovember2024,HealthCanadaapprovedOjjaaraproducedbyGSK.
Why is Ojjaara's addition to the Canadian treatment options good newsforpatients?
Anemia is often present at diagnosis and eventually develops in nearly all patients due to cancer progression. Anemia and blood transfusion are risk factors linked with mortality. Most available treatments for myelofibrosis exacerbated disease-related anemia. Toavoidcomplicationsrelatedtoanemia,physicianswillprescribea smalldoseofJAKinhibitor,whichlimitsitsefficacy.
Ojjaara is as efficient as other JAK inhibitors. However, unlike other therapies, it can improve anemia by facilitating the production of blood cells. SIMPLIFY trials have demonstrated that patients can reach healthier blood levels and become transfusion-independent. Some patients could even delay complex procedures such as transplantation due to this treatment.
Ojjaara's mechanism of action differs from the other JAK inhibitors; it has the unique capacity to target a molecule called ACVR1. By inhibiting ACVR1, Ojjaara reduces hepcidin production. Hepcidin levels are often higher than usual in myelofibrosis patients. The reduction of hepcidin allows better iron management and stimulates red blood cell production in patients with myelofibrosis.
Conclusion
Now, patients with myelofibrosis who are anemic will be able to have access to a JAK inhibitor that can improve their symptoms, reduce their enlarged spleen, and prolong their life while increasing their quality of life (QoL).










