1 minute read
Health Canada recent approvals.
by Brigitte Leonard, Ph.D
AccordingtotheHealthCanadadatabase,theagencyhasevaluatedanddecidedonthefaithof 53 products in 2024. Of these 53, only 44 were authorized for commercialization in Canada (83%).Ofthese44,only26werenewactivesubstances.
SincethelastpublicationofE3Advocacy,onlytwonewproductshavebeenapproved:Casgevry fromVertexPharmaceuticalandOjjaarafromGSK.
Newhopeforpeoplewithsicklecelldiseaseorbeta-thalassemia
Health Canada approved Casgevry for commercialization in September 2024 to treat two geneticblooddisorders:sicklecelldiseaseandbeta-thalassemia.
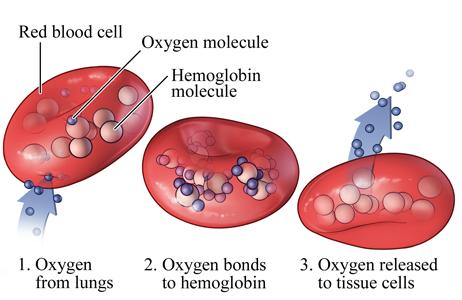
Inthesetwoinheriteddiseases,theproductionofhemoglobin is affected. Hemoglobin binds and carries oxygen from the lungstootherpartsofthebody(Figure1).Eachredbloodcell circulating in blood vessels contains several hundred million hemoglobinmolecules.
Hemoglobinalwayscontainsfourunitscalledchains,whichall contain a heme linked with iron (Figure 2) Hemoglobin chain types are different before and after birth The predominant form of hemoglobin (Hemoglobin F) in the fetus will contain four chains: two alpha and two gamma The body will stop producing this variant around 12 weeks after birth At that time, the Hemoglobin A variant, which contains four chains (two alpha and two beta), will become the most predominant and stay during the rest of our lives (Figure 2) Both types of chains and their structure are essential for the function of hemoglobin
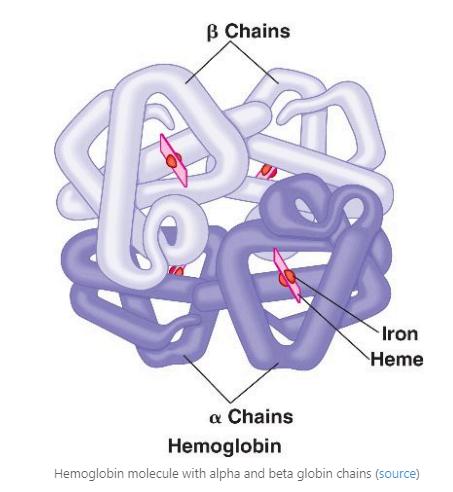
In sickle-cell disease and beta-thalassemia, a mutation occurs in the gene that produces the beta chain on chromosome 11. This mutation causes the production of abnormal beta chains and disrupts red blood cell production and oxygen transport.
Casgevy (exa-cel: exagamglogene autotemcel) is a gene therapy developed by Vertex Pharmaceuticals and CRISPR Therapeutics The FDA approved it in the United States for treating sickle cell disease in December 2023 and beta-thalassemia in January 2024 EMA approved it for the European market in February 2024
Casgevy is the 1st gene therapy approved that does not use a viral vector. Casgevy uses CRISPR/Cas9 technology to edit the patient's DNA cells that produce red blood cells. Casgevy does not repair the mutated gene on chromosome 11. It stimulates the production of the fetal form of hemoglobin silenced after birth. The fetal form (gamma chains) replaces the defective beta chains in the composition of hemoglobin to eliminate issues with the abnormal beta chain.










