1 minute read
Impact
from E3 Advocacy Issue 5
by healcanada
Figure 4
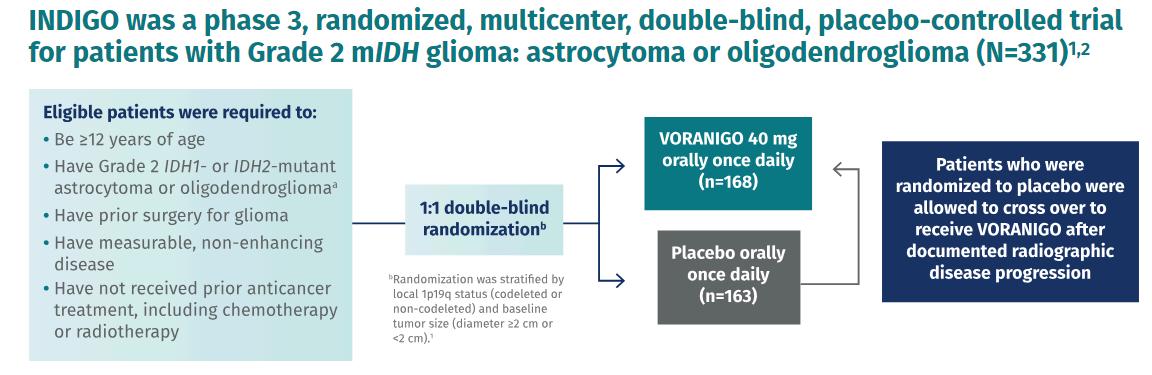
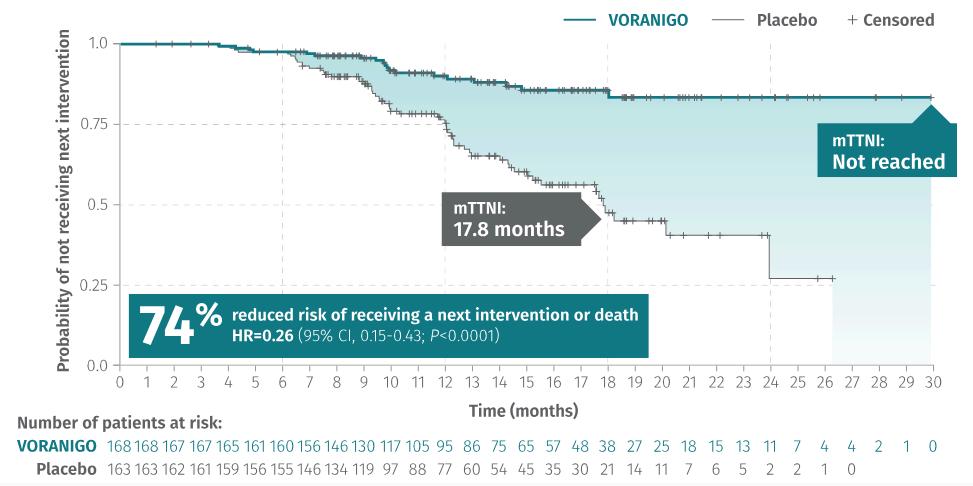
In this clinical trial, Voranigo reduced the risk of disease progression by 61% compared to the watch-and-wait strategy (placebo) (Figure 5). Also, during the study, patients treated with Vorangio didn’t reach the median time to the next treatment (mTTNI). However, patients on the placebo reached mTTNI at 17.8 months(Figure6).
It is an oral therapy, so it is more convenient than radiotherapy. The toxicities observed were mild and comparable between the Voranigo and placebo groups. Taking Voranigo didn’t meaningfully impact the patients’ quality of life. For most patients, it is challenging to be on active observation when you know that the disease is still thereandisgoingtoprogress.
Because of its efficacy and tolerability, Voranigo can replace active observation to delay progression as long as possible. Also, the usage of Voranigo can report and delay the necessity to receiveradioandchemotherapy.
Figure 5
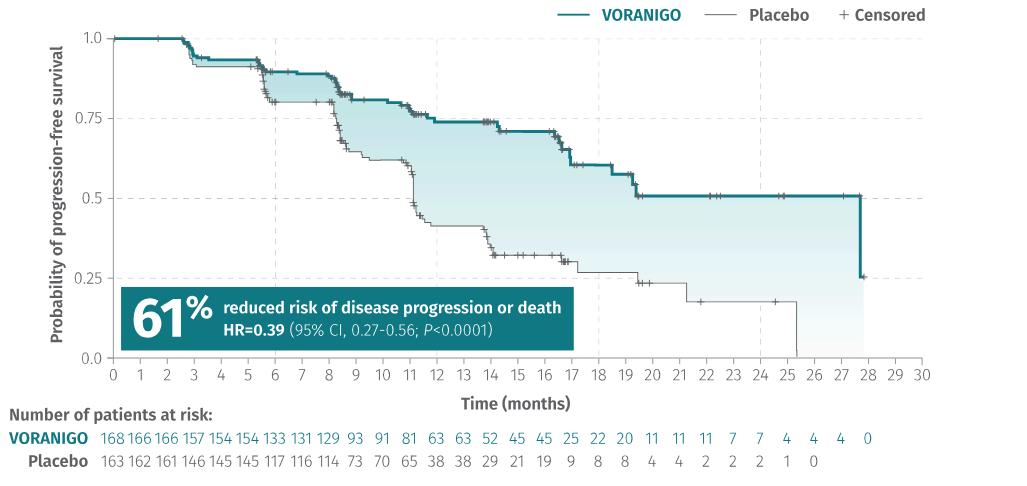
Figure 6
Hopefully, patients will have enough energy to return to work after surgery, care for their loved ones,andlivewithadecentqualityoflifeuntilthenecessitytousemoreaggressivetreatment.

Belonging, Diversity, Inclusion and Equity
Welcome to the Belonging, Diversity, Inclusion and Equity section of Heal Canada’s Digital Magazine. In this section, we provide information on ensuring that BDEI is part of the patient conversation in our Health ecosystem. Our focus is to illuminate the pathways through which individuals grappling with health challenges can not only find their voice but also harness it to drive their own journey.
Healthcare and the patient’s experiences should not be determined by social determinants of health.
We believe that an informed and engaged patient is an empowered one. Through enlightening articles, expert insights, and inspiring stories, we aim to equip our readers with the tools and knowledge necessary to navigate the complex health care landscape.









