
34 minute read
Chemical substances
5
Chemical reactions
Reading and listening
Modern agriculture
Normally, when hearing the term “pollution” the first thing that comes to our minds is releasing greenhouse gases to the atmosphere or toxic fluids in rivers, seas and oceans. However, we rarely think of soil pollution. Modern agriculture, based on ongoing production models (to produce more in less time and at less cost), has become a vicious cycle. Since the invention and broad use of fertilizers by the end of the 19th century, global agriculture focused on monocrop rather than polycrop farming, and on intensive rather than extensive exploitation. This new agricultural trend has meant that, year after year, higher pollution levels in the soil and in groundwater are measured. The overuse of fertilizers, herbicides, and pesticides has given rise to less productive farming soils, increasing the dependence of the agricultural sector on the industrial chemistry.
1 Why do you think that we give more importance to greenhouse gases or toxic fluids rather than soil pollution? 2 Why do we want to produce more crops in less time? 3 What are fertilizers? 4 Where does the water that you drink come from? 5 What do you think is the difference between herbicides and pesticides?
Speaking Describing an image

Look at the picture and describe thoroughly what you observe. Describe the pipes, the lights and the different levels. What kind of place is it? What else is happening in the picture? In pairs, discuss what are the pros and cons of producing crops in this way. Create questions and answers by using the quantifiers how many and how much. Example: How many levels can you count? How much water do these systems need?

Writing Asking questions Vertical farming seems to be the solution for the current agricultural problem. Vertical farming adapts to the productive needs of our society because it allows us to produce more crops in less time, without using too much space and without the need for pesticides or herbicides. The basic idea behind vertical farming is to grow crops without soil. In countries where light is not a problem, this system can be installed within greenhouses. In countries with a lack of sunlight, LED lights can replace it. Nowadays Europe is moving towards this type of agriculture. Ask LANGUAGE BANKLANGUAGE BANKLANGUAGE BANKLANGUAGE BANKLANGUAGE BANKLANGUAGE BANKLANGUAGE BANK LANGUAGE BANKLANGUAGE BANK LANGUAGE BANKLANGUAGE BANK LANGUAGE BANK questions about vertical farming that can contribute to achieving SDGs targets 15.4 and 15.5. 103
CHANGES IN MATERIAL SYSTEMS
ä 1.1 Changes in matter
A chemical change produces new substances that did not exist before the change.
As you have studied previously, change where new substances do not appear are called physical change. For example: • A phase change is not a chemical change. In a phase change, the substance is the same before and after the process. For example, when water melts, the water molecules become more mobile than in their solid state, but they remain water molecules. • Dissolving one substance in another is not a chemical change. It is a homogeneous mixture of the two substances. No new substance appears.
ä 1.2 How do we identify a chemical change?
To identify whether new substances appear from a chemical change, we must analyse the composition of the matter after the change. This is not always possible, so sometimes we must use other physical evidence (see photos on the next page). • Gas release. Gases may not be visible. Sometimes we identify them via bubbles in a liquid. • Colour change. New substances from a chemical reaction may have a different colour. • Thermal energy exchange. All chemical changes involve energy exchanges. In thermal energy, the surrounding temperature will change. • Energy released as light. Some chemical reactions generate light. One well-known case is combustion, which produces flames. There are also other natural reactions that generate light without combustion.
Physical changes
Dissolution

In the process of salt dissolution, the crystals, consisting of cations and anions, and the water molecules do not form different bonds at the atomic level. Changes of state

In a change of state, the interactions between the elementary entities of the substance may be stronger or weaker, but they do not change, they are still water molecules.
Possible evidence of a chemical change
Release of a gas

Appearance of a bubbling on the surface as a result of the chemical change between vinegar and sodium bicarbonate. Thermal energy exchange
Cold packs are an example of a chemical change that causes a decrease in the temperature of the environment. Colour change

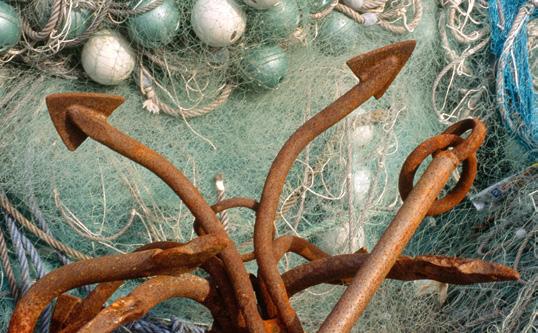
The rust on a piece of iron is a chemical process; iron oxide is formed, which is orange in colour. Light
Fireflies emit light due to a chemical change involving a substance called luciferin.

Understand, think, investigate…
1 Indicate whether a chemical change occurs in the following everyday phenomena. Justify your answer according to whether new substances appear or not: a) Clouds forming. b) Dissolving sugar in water. c) Rust forming on a piece of iron. d) Caramelisation of sugar during cooking. e) Blending vegetables to make a cream or puree. f) Fruit oxidizing by air. 2 Statues made of bronze (an alloy of copper and tin) that adorn some town squares acquire a greenish hue over time, which is not the original colour of the bronze. Find information on the chemical change they undergo. 3 When food is digested, it is transformed into nutrients.
Is this change physical or chemical, and do you know of any other such changes that occur in our bodies?
Explain your answers. 4 Explain what elementary entities are shown in the image on the previous page. 5 Make an attribute wheel with the evidence that allows us to identify a chemical change. Add examples other than those shown on this page and explain why you have chosen them as evidence of a chemical change. 6 Research the Maillard reaction. What are the reactants and what evidence of a chemical change is observed?
CHEMICAL REACTIONS ä 2.1 Chemical reactions
Most chemical changes in our environment are complex, as physical changes also occur at the same time. In addition, many chemical changes are interrelated. However, it is possible to isolate a simple chemical change and analyse it; in this case, we can study and describe a chemical reaction.
A chemical reaction is the transformation of starting substances, which we call reactants, into new substances known as products.
Favourable conditions are necessary for the reactants to react, as two or more substances come into contact with each other will not always react. For example, in the combination of iron with oxygen to form iron oxide, contact with water is necessary, or in the combination of oxygen with a fuel there is only a combustion reaction under certain energy circumstances (presence of a spark, high temperature, etc.).
Stoichiometric coefficients
These are the numbers preceding each chemical formula in a chemical reaction. They indicate the number of elementary entities of each substance involved in that particular reaction. If the value of this number is one, it is not written.
ä 2.2 Chemical equations
To represent a chemical reaction, we use the chemical equation. In it, the chemical formulae of the reactants are written on the left, and those of the products on the right, separated by an arrow, which marks the direction of the reaction. If there is more than one reactant or product, the “+” sign is written between them. In addition, we write the number of elementary entities of reactants and products involved in the reaction, choosing the values of the stoichiometric coefficients.
A chemical equation contains all the relevant information about a chemical reaction.
Determining the value of the stoichiometric coefficients is called balancing the chemical equation, as shown below.
A popular chemical reaction
Vinegar (contains acetic acid) Carbon dioxide

Sodium bicarbonate Sodium acetate

Acetic acid
is combined with
sodium bicarbonate resulting in sodium acetate and
CH3COOH + NaHCO3 8 CH3COONa carbon dioxide and water
CO2 H2O The image shows the chemical reaction that takes place when vinegar (a mixture in which one of its components is acetic acid, which is the one that reacts) is put in contact with sodium bicarbonate, to obtain sodium acetate, carbon dioxide and water. The form in which this chemical reaction is expressed is its chemical equation.
ä 2.3 Interpretation of a chemical reaction
In a chemical reaction, the appearance of new substances is the result of the rearrangement of the atoms that make up the reactants to form the products. As the chemical formulae representing the substances cannot change, the number of crystals or molecules of reactants and products involved in the reaction must be such that the number of atoms of each chemical element is the same on the reactant side as on the product side. Consider for example the combustion of methane. The reaction is represented molecularly as follows:
O2
CH4 (methane)
O2 Reaction
(combustion)
CO2 (carbon dioxide) H2O
H2O
The dashed arrows show the rearrangement of the atoms. Notice that each molecule of methane, CH4, reacts with two molecules of oxygen, O2, to form two molecules of water, H2O, and one molecule of carbon dioxide, CO2. The balanced chemical equation is CH4 + 2 O2 8 CO2 + 2 H2O Check that the number of atoms of each chemical element does not change:
No. of atoms in reagents 1 · 1 6 Carbon, C 8 1 · 1 2 · 2 6 Oxygen, O 8 1 · 2 + 2 · 1 1 · 4 6 Hydrogen, H 8 2 · 2
No. of atoms in products
The number of atoms of each chemical element before and after the chemical change, that is before and after the substances are rearranged, is the same, since matter is neither created nor destroyed in a chemical reaction.
CHEMICAL FORMULAE OF SOME COMMON SUBSTANCES
Water
Oxygen Hydrogen Methane
Ammonia Sodium chloride Hydrochloric acid Nitrogen Carbon dioxide Nitrogen monoxide
H2O
O2
H2
CH4
NH3
NaCl
HCl
N2
CO2
NO
Understand, think, investigate…
7 Using the formulae in the table on this page, write the chemical equation for these reactions: a) Three hydrogen molecules combine with one nitrogen molecule to form two ammonia molecules. b) Two hydrogen molecules combine with one oxygen molecule to form two water molecules. c) Two sodium atoms combine with two molecules of hydrochloric acid to form two molecules of NaCl and one molecule of hydrogen. 8 Make a diagram like the one on this page with all the information contained in this chemical equation: 4 NH3 + 5 O2 8 4 NO + 6 H2O 9 Indicate which are the reactants and which are the products in the following chemical reactions. With the help of your teacher and in pairs, find the chemical formulae of the substances named and write the chemical equation for each reaction: a) Iron combines with oxygen in the atmosphere to produce iron oxide. b) Limestone breaks down under the action of heat into calcium oxide and carbon dioxide. c) Water, under the effect of an electric current, breaks down into molecular hydrogen and molecular oxygen.
CHARACTERISTICS OF CHEMICAL REACTIONS
Chemical reactions have some important properties. This year we will focus on two laws: one related to the masses of the reactants and products, and the other to the rate of a chemical reaction.
ä 3.1 Law of conservation of mass
If we mix sand and salt, the mass of the mixture is equal to the sum of the masses of its components. That is, the mass is conserved. What if, instead of a mixture, two substances undergo a chemical change and produce new substances? Will the mass be conserved? French chemist A. Lavoisier answered this question in 1789, when he discovered and stated the law of conservation of mass.
In a chemical reaction, matter is neither created nor destroyed, only transformed. Therefore, the sum of the masses of the reactants is equal to the sum of the masses of the products of the reaction.
We highlight two aspects and the circumstances in which it was stated: • The statement of this law is based on multiple experiments in which the mass of the reactants and the products of different chemical reactions were measured. It is therefore a law drawn from experience; it is an empirical law. • Experimental verification of this law in reactions involving a reactant or a product in the gaseous state was technically complicated at the time, and represented an advance in the knowledge of the transformations of matter. This empirical law was later explained by the atomic theory proposed by Dalton between 1803 and 1808.
The mass of the reagents and the laboratory material is equal to the mass of the products obtained and the same material. In order to carry out the experiment, the container must have an adequate volume so that the CO2 produced does not cause the stopper to burst.
Acetic acid CH3COOH Sodium bicarbonate NaHCO3 Verification of the law of conservation of mass
Sodium bicarbonate
Vinegar (contains acetic acid) Carbon dioxide
Aqueous sodium acetate solution
We bring the reagents into contact with each other
Sodium acetate CH3COONa Water H2O
Carbon dioxide CO2 At the microscopic level, we observe that the atoms that form the reactants rearrange themselves to form the new substances, the products, which is why the mass does not change during a chemical reaction.
ä 3.2 Law of definite proportions
With regard to mass, another law that a chemical reaction follows is the law of definite proportions.
In a chemical reaction, the masses of the reactants and the masses of the products are in constant proportion to each other.
This law is related to the fact that the elementary entities of each substance involved in a reaction have different masses, and always combine in the same numerical proportion. We will study this law in detail next year.
ä 3.3 Rate of a chemical reaction
Some chemical reactions are extremely slow, such as the formation of minerals in the Earth’s crust, while others are very fast, such as an explosion or the combustion of methane that we have already studied. There are several factors on which the rate of a chemical change depends. The following are the most important ones: • Temperature. Generally speaking, it can be seen that as temperature increases, the rate of a chemical reaction increases. • Concentration of the reactants. The higher the concentration of the reactants, the faster the rate of the reaction.
Problem solved
1 When 30 g of nitrogen react with hydrogen, 170 g of ammonia are obtained. What mass of hydrogen has reacted? What mass of ammonia would be obtained if 90 g of nitrogen react?
To calculate the mass of hydrogen that has reacted, we apply the law of conservation of mass: mass of reactants = mass of products
Thus, the mass of hydrogen will be the difference between the mass of ammonia and the mass of nitrogen: mass of hydrogen = 170 g – 30 g = 140 g
We can calculate the mass of ammonia obtained from the law of definite proportions; thus, from 90 g of nitrogen we obtain: 30 nitrogen 9 g 0 g =
170gammonia nitrogen x
x 30 90 170$ = = 510gammonia
We can represent this reaction as:
3 H2
N2 2 NH3 Understand, think, investigate…
10 Find information about the chemical reaction between vinegar and sodium bicarbonate described above to test the law of conservation of mass, and answer these questions: a) What are the reactants and products of this reaction?
b) Is vinegar a pure substance? c) Why is the result of the reaction an aqueous solution?
d) Think and share in pairs. Explain why this experience proves that gases have mass. e) Explain, using the KTM, what would happen if we did the experiment at a temperature much higher than room temperature.
11 If 224 g of iron reacts with oxygen to form 320 g of iron oxide, how much iron oxide will be formed from 112 g of iron?
12 Why do you think most cooking techniques involve raising the food’s temperature?
13 Explain why storing food in refrigerators conserves it.
CHEMICALS OF NATURAL AND ARTIFICIAL ORIGIN
ä 4.1 Development of chemical industry
Since the discovery of fire, mankind has carried out chemical transformations to improve quality of life. Knowledge of these reactions is often limited to necessity and experience. Examples include obtaining quicklime from limestone, synthesising soap from fats and fermenting milk to make cheese, among many others. Since the Industrial Revolution, and with the development of modern chemistry, we have increased our knowledge about the factors that influence the reactions that aim to improve our quality of life, obtaining products through what is known as synthesis reactions. This has led to the development of the chemical industry.
A synthesis reaction is a reaction aimed at obtaining, or manufacturing, a substance.
These reactions range from those that take place in our bodies to those that occur on a large industrial scale.
ä 4.2 Products of natural and artificial origin
Synthesis reactions exist in nature and can also be performed in the laboratory. They are distinguished by the products obtained, which are natural in the first case and man-made in the second.
We sometimes hear the claim that something, because it is natural, is less harmful to health. But it is not always the case that the natural or artificial (also called synthetic) origin of a product is related to its benefit to society and the environment.
Acetylsalicylic acid

Salicylic acid is obtained from willow bark. It was traditionally used as an analgesic and antipyretic, but caused stomach irritation. Its artificial modification to acetylsalicylic acid is the active ingredient in aspirin. Products of natural and artificial origin
Recyclable bags

Plastic bags are a problem for the environment. Nowadays bags that can decompose can be made from potato starch. Their origin is natural, but they are processed industrially. Artificial flavours

The flavour of vanilla is due to a substance called vanillin, which is present in vanilla pods. It is expensive to extract, so it is synthesised from lignin, which is a substance found in wood.
ä 4.3 Sectors of the modern chemical industry
The main objective of the chemical industry is to obtain substances that are necessary for society. These substances are very varied and have many different applications. The most important of these are:
Chemical industry
Pharmaceuticals

Drugs can be classified according to their action (antipyretics, analgesics, antibiotics, etc.). This industry invests heavily in research to formulate and develop processes for the synthesis of new medicines. Petrochemicals

In addition to its use as a fuel source, oil is a raw material for the manufacture of other products. These include synthetic polymers (plastics), some synthetic fibres (nylon or polyester), glycerine, and some solvents. Other products

In addition to the two previous groups, there are a myriad of products obtained in the chemical industry, all of which are necessary in our daily lives, such as fertilisers, acids, additives for construction, and food additives.
Understand, think, investigate…
14 Copy the composition of some clothes you have at home and classify the fibres they are made of as natural or synthetic. What type of fibres are there more of?
Why do you think this is? 15 Look up information on formica and answer: a) Is it a natural or synthetic product? b) What are its main applications?
16 I think, I’m interested, I investigate. Look for information on the types of plastic bags that exist on the market and on the meaning of: biodegradable, compostable, reusable and recyclable. From the above information, indicate what use should be made of each type of plastic bag in order to minimise the pollution caused by these products. 17 Find the formula for vanillin and explain whether it matters if this substance is obtained from the vanilla pod or it has a synthetic origin from lignin. 18 Search the Internet for the formula of salicylic acid and acetylsalicylic acid. In view of the properties of each of these substances:
a) Discuss the contribution of chemistry in this case. b) Was the modification of salicylic acid beneficial?
Explain your answer. c) Is the geographical location of the white willow tree relevant to the artificial development of salicylic acid? Explain your answer.
19 Why are artificial products also called synthetic products?
20 Make a list of chemical products that help to improve our lives. Classify them into categories according to their use and the industry they come from.
21 Are there synthesis reactions in the natural world?
Justify your answer.
SUSTAINABLE CHEMISTRY AND COMMITMENTS
What is sustainable development?
According to the 1987 Brundtland Report, sustainable development is “development that meets the needs of the present without compromising the ability of future generations to meet their own needs”.
The chemical industry has evolved from the economic optimisation of manufacturing a product to sustainable chemistry. Here, the emission of pollutants is minimised and future consequences are foreseen in order to avoid them.
ä 5.1 Pollution by the chemical industry
Since the Industrial Revolution, and with the development of the chemical industry, we have caused alterations to the material systems that surround us (the atmosphere, soil and water). As a result, in the last few decades problems have arisen such as: • Atmospheric pollution, due to the emission of substances that affect the composition of the atmosphere and the reactions that take place in it, as we will see in the following section; • Pollution of inland waters, affecting the quality of drinking water, which will contain dissolved substances harmful to health; • Soil pollution, by releasing toxic substances or an excessive use of fertilisers, leading to problems related to soil impoverishment and excess organic matter, thus modifying ecosystems.
ä 5.2 Sustainable chemistry and environmental
chemistry
These and other problems have had consequences on the health of the planet and all the species that inhabit it. This has led to the emergence of sustainable development as a response to this situation. This concept has been taken into account in both industry and research, resulting in the development of sustainable chemistry and environmental chemistry: • Environmental chemistry is mainly concerned with identifying what pollutants are in the environment, where they were emitted, in what chemical reactions they have been produced, and how they have reached the place where they were detected. • Sustainable chemistry is concerned with the design of environmentally friendly processes and products in order to reduce the production of pollutants. Industries that emitted pollutants without control have been progressively replaced by others that try to avoid waste production (see figures below), analyse the consequences on the environment, study the life cycle of the products they manufacture, and use energy efficiently.
Developments in the chemical industry
ä 5.3 Sustainable Development Goals
Chemical knowledge contributes significantly to the achievement of several of the targets of the Sustainable Development Goals. The most relevant is related to chemical transformations that occur in the environment as a result of products that have been released over decades and have caused pollution problems. The diagram shows the relationship between chemistry and the SDGs.
Chemistry and the SDG targets
SUSTAINABLE CHEMISTRY
acts on
HARMFUL CHEMICAL CHANGES
AIR
TARGET 11.6

SUSTAINABLE CITIES AND COMMUNITIES SOIL
TARGET 3.9 TARGET 12.4
HEALTH AND WELL-BEING RESPONSIBLE PRODUCTION AND CONSUMPTION UNDERWATER LIFE TARGET 14.3
CLEAN WATER AND SANITATION TARGET 6.3
WATER
Several of the SDG targets refer to problems caused by the release of substances into the natural environment. This alters the chemical reactions that take place in the environment and affects the balance of the ecosystem and the health of the beings that inhabit it, potentially causing irreparable damage.
Understand, think, investigate…
22 Analyse the pictures on the previous page. Do you think that factories like the one on the left still exist? 23 Conduct an analysis of your household waste and draw up an action plan. You can use these guidelines to help you: a) Make a list of the types of domestic waste in your household for one month. b) Classify each waste according to whether it has been separated and deposited in the appropriate container, whether it is a waste that can be reduced, or whether it is hazardous waste (cleaning product or medicine). c) Calculate your household’s water consumption from your utility bill. d) With the above information, draw up an action plan based on specific objectives and share it with the members of your household. 24 Find information about the targets referenced in the diagram above and relate them to the following situations:
a) Development of air quality monitors. b) Development of packaging made of materials other than plastic and that are biodegradable. c) Reuse of plastics in the manufacture of outdoor furniture.
d) Studies of how pH affects underwater life. e) Collection of garbage and other hazardous household waste for treatment.
f) Raising public awareness through an information plan on how our actions have consequences for the environment.
CHEMICAL REACTIONS AND THE ENVIRONMENT
Knowing the causes of environmental problems allows us to start solving them, as we have seen in the previous section. Here we look at three environmental problems that affect our planet’s atmosphere globally.
ä 6.1 Anomalous greenhouse effect
The temperature of our planet is kept within limits that are suitable for life thanks to the atmosphere, which behaves like a greenhouse: it retains part of the thermal energy emitted by the Earth and lets the rest pass through. This phenomenon is called the greenhouse effect. One of the main gases responsible for this effect is carbon dioxide. As its amount in the atmosphere has increased in recent decades due to the use of fossil fuels, an unwanted increase in the amount of energy retained is occurring. This is known as the anomalous greenhouse effect. If CO2 emissions are not reduced, the increase in the Earth’s average temperature will continue to rise, and the global warming our planet is currently experiencing will have catastrophic consequences.
ä 6.2 Acid rain
This is the name given to the polluting phenomenon whereby rainwater carries dissolved highly corrosive acids, such as sulphuric acid, H2SO4, and nitric acid, HNO3. These acids are formed when atmospheric emissions of sulphur dioxide, SO2, and nitrogen oxides, NO and NO2, react with water vapour. When this water precipitates as rain, it is called acid rain. Its consequences on the environment are the destruction of flora and fauna.
Consequences of the greenhouse effect and acid rain
Greenhouse effect

The melting of continental ice, a consequence of global warming, affects not only sea levels but also the global climate, altering the humidity of the atmosphere. Acid rain

Fossil fuels often contain sulphur impurities. When burned in air, which contains oxygen and nitrogen, they produce sulphur dioxide and nitrogen oxides.
ä 6.3 The destruction of the ozone layer
The ozone (O3) layer is in the stratosphere and shields everything below from harmful ultraviolet radiation from the sun. For the shield effect to work, there must be enough ozone to absorb the radiation. Substances such as nitrogen oxides and compounds known as CFC (which includes chlorine) in the atmosphere cause chemical reactions that destroy ozone. The reactions release chlorine when they reach the stratosphere, where ozone normally forms and degrades in an equilibrium. The chlorine disrupts this balance, reducing the amount of ozone.
Formation and destruction of the ozone layer
Natural process of formation of O3
O2
1 +
UV radiation
4
O2
O3
O 2
O
3 2
O
3
O2
O
O3 4
O3
O2 O2 O2 O2
In the stratosphere, UV radiation separates oxygen molecules into atoms. Each atom combines with separate oxygen molecules, forming a molecule of ozone. This reaction requires a lot of energy and high temperatures.
Destruction of O3 by chlorine
CFC
Rayos UV 1
Cl 2
O3
4
O ClO 3
O2
5
O2 Cl
CFCs are compounds that remain in the atmosphere for a long time. When they release chlorine due to UV radiation, they break down ozone. Chlorine is one of the products of the reaction, so it continues destroying ozone. What global solutions have been proposed to reduce the destruction of the ozone layer? In which products was CFC present? Which products have replaced CFCs? Discuss with the rest of the class the need for global agreements.
1 UV rays attack the O2 molecule and split it into two oxygen atoms. 2 Each of these oxygen atoms combines with an O2 molecule to produce ozone, O3. 3 Each ozone molecule combines with another oxygen atom, giving two O2 molecules as products. 4 The cycle is closed by the reaction, again, of O2 with UV or as a reactant of the O2 reaction.
Working with images
1 UV rays attack CFCs and cause a chlorine atom to be released. 2 The chlorine atom attacks the ozone molecule. 3 Two products are formed: the O2 molecule and the ClO molecule. 4 The ClO molecule reacts with monoatomic oxygen. 5 As a result, one oxygen molecule and one chlorine atom are released into the atmosphere.
homemade glue
YOU NEED • Milk. • Vinegar. • Chemical yeast (baking soda). • A frying pan. • A tablespoon. • A mixing container. • A wooden spoon. • A cup. • A sieve. • A source of heat.
research project
INTRODUCTION As we have studied, if mixing several substances causes the appearance of new substances, we say that a chemical change has occurred. In the experiment proposed, a series of chemical reactions take place which cause the appearance of new substances. Among these is the appearance of a solid substance and the release of a gas. To speed up the process, we will use a heat source.
EXPERIMENT • Pour one and a half cups of milk and three tablespoons of vinegar into a frying pan. With the help of an adult, heat the mixture a little while stirring with a wooden spoon. You will notice some lumps forming; this is the rennet in the milk. • Separate the rennet obtained from the remaining liquid by straining your mixture. • Pour back into the pan, add a tablespoon of baking powder and a little water. Heat and stir the mixture. You will notice some bubbles at first.
Continue heating and stirring until the mixture boils. • Remove the mixture from the heat source and continue stirring. • Finally, transfer it to a container and cover it. You can use it as a glue when it is cold. It will last up to two days before it spoils.
THINK When you add the vinegar to the milk, you separated the casein, the rennet, from the whey, the rest of the milk. When the rennet, proteins and fats are formed, they solidify and agglomerate under the action of an acid (in this case, vinegar), resulting in lumps that are separated with a strainer. The properties of casein as a glue have been known since ancient times. Nowadays, it is used in the manufacture of glues and adhesives. When chemical yeast (sodium bicarbonate) and water are added to the rennet, a gas is released. This is carbon dioxide, which is the result of the chemical reaction between vinegar and bicarbonate; in this way, we eliminate the excess acid (vinegar), since it is used in the chemical reaction. In this reaction, other products are obtained, such as sodium acetate, which has multiple industrial applications.


PRACTICAL WORK
chemical reactions


PRECAUTIONS Hydrochloric acid is a very corrosive liquid. Follow the safety rules to avoid accidents. STATEMENT OF THE PROBLEM. OBJECTIVE The objective of this practice is to have evidence of a chemical change, and to observe how the temperature and concentration of the reactants affect the rate of reaction.
PRELIMINARY STEPS Make a plan to detect chemical changes, and to observe how the temperature and concentration of the reactants affect the rate of chemical change. OUR PROPOSAL We will carry out two experiments: • Experiment A: we will test the chemical reaction that takes place between metals and hydrochloric acid. • Experiment B: we will use a glow stick to test how temperature affects the reaction rate.
MATERIALS • Glow sticks • Beaker • Ice • Water • Salt • Metal filings • Dilute hydrochloric acid solution • Slightly more concentrated hydrochloric acid solution.
GUIDELINES FOR CARRYING OUT THE EXPERIMENT
EXPERIMENT A • Pour some water into a beaker and add the metal filings. • Repeat the previous experiment, now using the dilute hydrochloric acid solution instead of the water. • Again, repeat the previous experiment, this time using the slightly more concentrated hydrochloric acid solution. • Observe and note what happens in the three cases. EXPERIMENT B • Prepare one beaker with hot water, and another with a mixture of water, ice and salt. • Bend two glow sticks to trigger the chemical reaction inside them. • Put one of them in the glass with hot water, and the other in the glass with the mixture of ice, water and salt. • Write down the differences you observe in each case.
Draw conclusions…
1 How do you explain the differences observed in the first step and the third step of experiment A? 2 What chemical reaction occurs when a metal is mixed with hydrochloric acid? Why does it generate bubbles? 3 How do you explain the differences observed between the last two steps of experiment A? What factor is influencing the reaction rate? 4 Find information on how glow sticks work and their applications. 5 Explain the differences observed between the stick placed in hot water and the stick placed in the beaker with ice. Which factor influences the reaction rate? 6 Find information on why salt is added to ice water. What applications does it have in everyday life?
ORGANISing MY IDEAS Hierarchical concept map
1 Copy the diagram in your notebook and include: a) The name of the scientist who stated the law of conservation of mass. b) A new branch with the law of definite proportions. c) An example of a chemical equation indicating the formulae of the reactants, the formulae of the products, and the stoichiometric coefficients.
Chemical reactions
are
chemical changes
in which occurs a are identified by
physical evidence
which can be
rearrangement of atoms
causing a transformation of change of colour light bubbling
reactants into products energy in the form of heat are represented by
chemical equations
composed of
chemical formulae stoichiometric coefficients comply with
the law of conservation of mass
Changes in material systems
1 Write down three examples of chemical changes and three examples of non-chemical changes. What type are the latter?
2 Are there any chemical changes in the distillation of a mixture of alcohol and water? Explain your answer.
3 Classify the changes as physical or chemical: a) Piece of fruit rotting. b) Boiling water. c) Melting a piece of butter in a hot frying pan. d) Cooking a grilled steak. e) Effervescence of a medicine when it is placed into water. Chemical reactions
4 What makes you say that? Indicate whether the following statements are true or false, and explain why: a) The products of a chemical reaction are always artificial in origin. b) The number of substances in the reactants is always equal to the number of substances in the products. c) Chemical changes are represented by an arrow. d) The number of atoms of each chemical element present in the reactants is equal to the number of these atoms in the products. e) In a chemical reaction, different atoms are obtained in the products. f) In a chemical reaction, there is always a product obtained in gaseous state.
5 State the names of the reactants and products of these chemical reactions, and draw a picture representing them on an atomic scale: a) N2 + O2 8 2 NO. b) C + 2 H2 8 CH4.
Characteristics of chemical reactions
6 Indicate whether the following statements are true or false, and justify your answer: a) The mass of the reactants is destroyed in a chemical reaction. b) In a chemical reaction, atoms are rearranged; therefore, the sum of the masses of the reactants and the masses of the products is equal. c) The atoms that form the products of the reaction are generated from the atoms present in the reactants. 7 If 306 g of one substance reacts completely with 72 g of another, what will be the sum of the masses of the products of the reaction? Which law have you applied? 8 We have a 224 g piece of iron that combines completely with oxygen to form iron oxide. Are the following statements true or false? Justify your answer. a) The mass of iron oxide formed will be the sum of 224 g plus the oxygen that has combined with the iron. b) The mass of the iron oxide will be less than that of the iron at the start. c) The mass of the iron oxide will be the same as the mass of iron. 9 Let’s check. 8 g of hydrogen react with 64 g of oxygen to form 72 g of water. a) Check that the law of conservation of mass is fulfilled. b) Calculate the mass of water that will be formed if 20 g of hydrogen react completely.
Products of artificial and natural origin
10 Classify the following products according to their origin, natural or artificial: a) Unrefined sugar e) Flax b) Saccharin f) Cotton c) Wood g) Nylon d) Formica h) Acrylic Sustainable chemistry and SDG commitments
11 Search the Internet for the twelve principles of green or sustainable chemistry and make a presentation about them. Which SDG targets are they related to?
12 C&R. In pairs, look for information about the
SDG targets in anayaeducacion.es and on the
Internet. Then, make a poster explaining the target you have chosen, why you think it is important, and how you could implement it in your classroom.
Chemical reactions and the environment
13 Shared interpretation. Indicate whether the following statement is true or false, and explain your answer:
‘Carbon dioxide emissions from the use of fossil fuels are creating a hole in the ozone layer, which is having an impact on global climate.‘
14 Mirror. How are the anomalous greenhouse effect and ozone depletion similar and different?
15 Find information on substances that cause the destruction of the ozone layer.
16 Gases that do not destroy the ozone layer are currently used in pressure vessels, but can cause harm to the user if certain precautionary measures are not taken. Which gases are involved? What is the risk to be taken into account when handling these vessels?
17 Climate change, caused by the anomalous greenhouse effect, is an undeniable fact. Find information on the climate summits that have taken place in recent years, and on the measures that have been agreed upon.
18 The effects of acid rain are felt at a great distance from the place where the emission of corrosive gases occurs. During the nineteen-seventies and -eighties, this effect was observed in European forests. What solutions have been proposed to mitigate these effects and to repopulate these affected areas?
19 Roundtable. Massive logging of forests and jungles, as in the case of the Amazon. How can it harm us in the short and long term?
© GRUPO ANAYA, S.A., 2021 - C/ Juan Ignacio Luca de Tena, 15 - 28027 Madrid.

