
9 minute read
A2 or not A2? A cautionary tale in dairy (goat) science
Words by Dr Martin Palmer, Dr Xu Li, Garrick Spencer, Dr Lydia Ong and Dr Sally Gras
Over the last 20 years, following the formation of the a2 Milk Company in New Zealand, the publication of “Devil in the Milk” and some very successful marketing campaigns, foods with compositional and/or nutritional claims relating to “A2 protein” or “A2 beta-casein protein”, now account for a significant share of the global market for bovine milk and dairy products (including infant formulae).1,2
Although there has been some controversy around the validity of some biochemical and nutritional studies that purport to indicate beneficial health effects of such products and/or undesirable effects of milk containing A1 beta-casein (A1 β-casein), there remains strong consumer interest in this category.
More recently, the dairy goat industry has taken an interest in the β-casein content of caprine milk and milk products to better understand some apparently beneficial nutritional characteristics of goat’s milk and potentially to provide a stronger basis for health claims. Although there is some inconsistency in the scientific literature, it is generally accepted that the main protein in most commercial types of caprine milk is caprine β-casein, which has often been characterised as “A2” or “A2-like”, with reference to bovine β-casein.6
This, together with the reported absence of A1 β-casein in goat’s milk, has led many to equate caprine milk directly with A2 bovine milk biochemically and in relation to some nutritional and health claims. A Google search on “goat milk A2” reveals numerous examples of commercial, research and consumer advisory sites that make these assumptions.
Our interest in this topic started with some analytical work on locally sourced goat’s milk. This gave results on protein composition that were inconsistent with some scientific and commercial publications, in that the major proteins corresponded to caprine β-casein A and C, which differ in amino acid sequence to both bovine β-casein A1 and bovine β-casein A2.6,7
This led us to undertake a thorough review of recent advances in the science of both bovine and caprine β-caseins, to try and understand reasons for inconsistency in the scientific literature and possible implications for physiological functionality and health claims.6 The following is a summary of the main findings - please refer to the original review for a detailed analysis and discussion of this issue.6
The complexity of these protein families is shown in Figure 1, based on a compilation of data from both
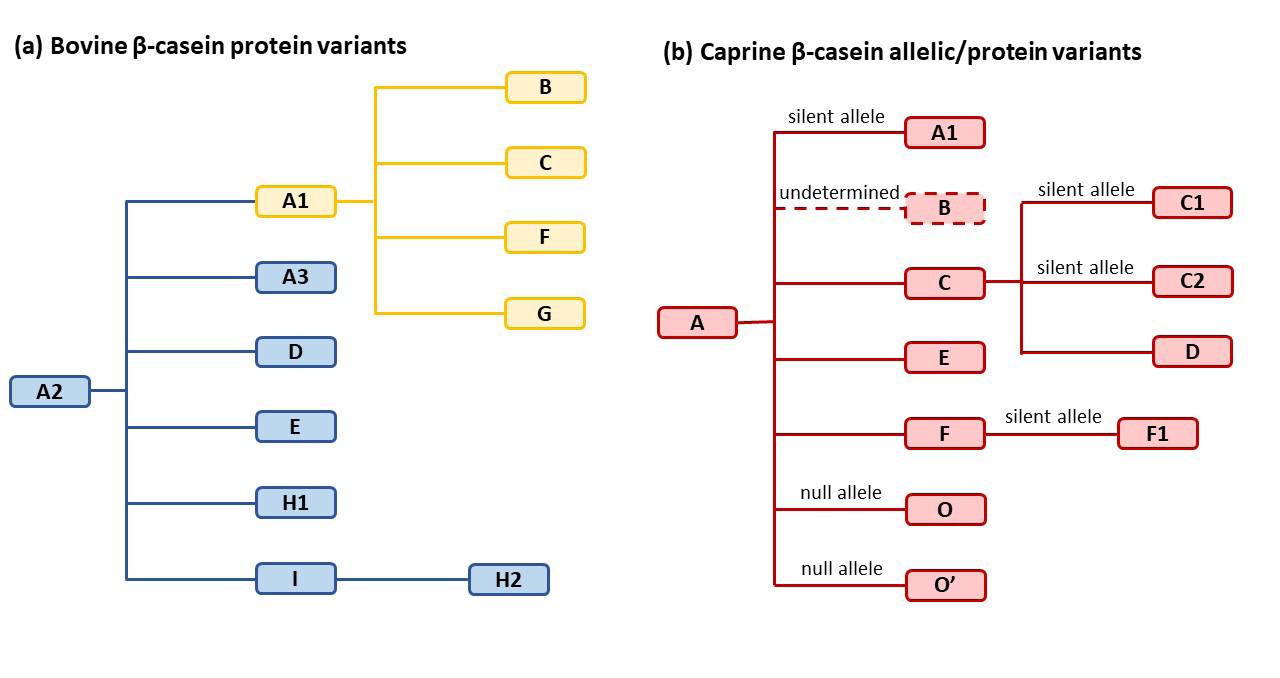
Figure 1. Relationship between (a) the 12 reported bovine β-casein protein variants and (b) the 12 reported caprine β-casein alleles/protein variants, based on amino acid sequence. Variants shown in yellow are bovine β-CN A1 or A1like proteins with histidine (H) at position 67, while the variants in blue feature proline (P) at position 67 and are bovine β-CN A2 or A2-like proteins. The caprine β-CN variants (in red) also feature (P) at position 67 but are structurally distinct in other respects. A2 and A are considered to be the ‘ancestral’ types of bovine and caprine β-caseins, respectively. Adapted from Li et al.6
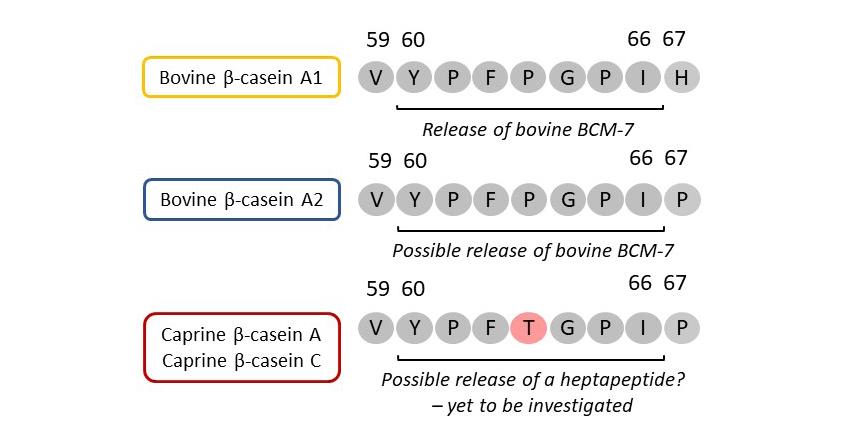
Figure 2. Schematic showing the possible enzymatic release of peptides from the “BCM-7 region” of the most commonly occurring bovine and caprine β-casein variants. Both A1 and A2 forms of bovine β-casein are susceptible to enzymatic cleavage between residues 59 and 60 (valine-V and tyrosine-Y) but A1 is more prone to cleavage between residues 66 and 67 (isoleucine-I and histidine-H) than A2, which has proline (P) at position 67. With caprine β-caseins A and C, there are some sequence similarities with bovine β-caseins in this region but heptapeptide release from caprine β-caseins has yet to be investigated. Other amino acid residues are: phenylalanine (F), threonine (T) and glycine (G). Adapted from Li et al.6
genetic/nucleotide-level studies, as well as studies of milk protein. Bovine A2 and caprine A and C have been identified as the most commonly occurring β-casein alleles in their respective species.8,9
The caprine family is further complicated by the identification of at least four ‘silent’ alleles and two null alleles in some goat types – gene mutations that code for β-casein but are either not expressed in the phenotype or produce shorter proteins. Thus, caprine β-casein A1, which is a silent allele, produces a protein the same as caprine β-casein A that differs in sequence to bovine β-casein A1. The nomenclature itself may well be a source of some confusion, in that it was developed independently for bovine and caprine β-caseins and reflects the historical order of discovery as much as any biochemical relationship.
Much of the original interest around bovine β-casein polymorphism centred around the susceptibility of the molecular variants to enzymatic cleavage between amino acid residues 66 and 67, and 59 and 60, during digestion, to yield β-casomorphin 7 (BCM-7) and possibly other bioactive peptides.1,6
In this respect, bovine β-caseins can be grouped into an A2 and “A2-like” (A3, D, E, H1, I and H2) family, some of which show less susceptibility to BCM-7 generation by this mechanism, and an A1 and “A1-like” (B, C, F and G) family, some of which show greater susceptibility to enzymatic cleavage, as shown in Figure 1.
There are very few published studies of the release of bioactive peptides in general from caprine β-casein and apparently none on the potential or actual release of BCM-7 during digestion.6 As shown in Figure 2, the equivalent of the ‘BCM-7’ region in caprine β-casein A or C is similar to the BCM-7 region of bovine β-casein A2, in that it is bounded by proline at residue 67, but has a clear sequence difference – threonine at residue 63, compared to proline at the same position in both bovine A2 and A1 β-caseins. It is not known how this might affect enzymic hydrolysis which, if it occurred, would release a heptapeptide of different amino acid sequence and unknown functionality, compared to BCM-7.6
Quite apart from the focus on the BCM-7 region, it should also be noted that there are at least 19 other sequence differences between these bovine and caprine proteins that may also give rise to different protein properties.6 The devil may or may not be in the milk but is certainly in the detail! On this basis, there is very little justification for describing goat’s milk as “A2” or “A2-like”, as is commonly seen in the literature and associated with some food products.
“A2 milk” is an unusual case of a very specific food constituent term, “bovine A2 β-casein”, being abbreviated and appropriated commercially to name and brand milk and dairy products that contain

this protein. However, with the global growth and diversification of this category over the last 20 years, unlike the standard biochemical nomenclature for the protein itself, there now seems to be no universally accepted, scientific definition of “A2” milk and dairy products.
For example, the current Wikipedia definition is: “A2 milk is a variety of cows’ milk that mostly lacks a form of β-casein proteins called A1, and instead has mostly the A2 form”.11 This definition is so loose that it could arguably include many ‘standard’ milk types, not widely marketed as A2, that commonly contain a mixture of A1 and A2 β-casein, but mostly in the A2 form! This type of inconsistency has no doubt contributed to the overall confusion and misunderstanding in this field, which is now extending to nonbovine milk.
Some of the confusion with this topic may also be related to the original marketing strategies and resultant consumer perceptions associated with A2 bovine milk products, where the key value proposition has essentially been around the absence of A1 rather than the presence of A2 β-casein.
Does this provide an entry point for “A2” or “A2-like” goat’s milk, which also contains no (bovine) A1 β-casein? Perhaps so, from a marketing perspective, but from a food science perspective, caprine milk does not contain protein that resembles bovine A2 β-casein and the use of such terminology is inconsistent with established caprine β-casein nomenclature.
Although apparently not in the scientific literature, it’s also concerning that a similar error is creeping into sources of commercial information and consumer advice about sheep’s milk (try a Google search on “sheep milk A2”). Five distinct ovine β-caseins (A, B, C, X and Y) have been reported to-date but, from a scientific perspective, sheep’s milk, like goat’s milk, does not contain protein that resembles bovine A2 or A2-like β-caseins.6
In future research and product development, there is clearly a need for the food industry and scientific community to pay more attention to the detail around the terminology associated with β-caseins and other milk proteins, to avoid misleading consumers and researchers alike.
The review also highlighted the need for more research on the structure and function relationships of β-casein in food systems and in human nutrition.6
There have been many studies on bovine β-casein in relation to peptide generation, but relatively few in other aspects of physical and physiological functionality. Even less is known about caprine β-caseins, although it is likely that some of the beneficial effects observed on goat milk digestibility, for example, could be related to the properties of its most abundant protein.
References
1. Woodford (2009). Devil in the Milk: Illness, Health & the Politics of A1 and A2 milk. Chelsea Green
Publishing. 240pp. ISBN10: 1603581022 2. Imarc (2022). A2 Milk Market: Global Industry
Trends, Share, Size, Growth, Opportunity and
Forecast 2022-2027. Imarc Market Report. 3. Truswell (2005). The A2 milk case: A critical review. European Journal of Clinical Nutrition 59, 623-631. 4. Ul Haq, Kapila, Shandilya & Kapila (2014). Impact of Milk Derived β-Casomorphins on Physiological
Functions and Trends in Research. International
Journal of Food Properties 17, 1726-1741. 5. Kay, Delgado, Mittal, Eshragi, Mittal & Eshragi (2021). Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? Journal of
Nutrition 151, 1061-1072. 6. Li, Spencer, Ong & Gras (2022). β-casein proteins – A comparison between caprine and bovine milk. Trends in Food Science & Technology 121, 30-43. 7. Li, Spencer, Ong & Gras (2021). Unpublished data 8. Caroli, Chessa & Erhardt (2009). Milk protein polymorphism in cattle. Journal of Dairy Science 92, 5335-5352. 9. Selvaggi, Laudadio, Dario & Tufarelli (2014). Major proteins in goat milk. Molecular Biology Reports 41, 1035-1048. 10. Daniloski, Cunha, McCarthy, O’Callaghan,
McParland & Vasiljevic (2021). Bovine β-casomorphins: friends or foes? Trends in Food
Science & Technology 116, 681-700. 11. Wikipedia (2022). A2 milk entry at https:// en.wikipedia.org/wiki/A2_milk
Dr Xu Li, Garrick Spencer, Dr Lydia Ong, Professor Sally Gras and Associate Professor Martin Palmer are with the Department of Chemical Engineering and the Bio21 Molecular Science and Biotechnology Institute at The University of Melbourne, Parkville.. f










