31 minute read
Vaccine use for foot and mouth disease control
vials out of the ice box to be protected from intense and direct sunlight during the vaccination operation. Insulated boxes with vaccine vials and coolant should not be left uncovered in sunlight.
Additional equipment
Advertisement
Syringes and sterile needles are carefully selected to correspond to species targeted by vaccination programmes. The use of long sterile needles (21 G ´ 0.5 inch or 0.8 ´ 12.7 mm) is recommended for the withdrawal of vaccine from each vaccine vial, to allow air to enter the vial and avoid the creation of a vacuum. Multi-dose syringes of good quality with accurate measurement of required doses can be deployed and are particularly useful in an area vaccination campaign. Reusable syringes and needles should be regularly sterilised, preferably by heat procedure, but not using chemical methods of sterilisation, because of the risk of chemical residues damaging the FMD antigens. Disposable equipment should be destroyed carefully after use in accordance with the regulations of the country.
Vaccine application
Animals to be vaccinated Special training is given to vaccinators to avoid any mistakes during handling and injection of animals. Precise instructions concerning species, age, pregnant animals, etc. should be given in written form (operating procedures) beforehand. Animals should be in a tranquil condition and, if they have been recently transported on foot or by vehicle, time for resting should be allowed before vaccination. When carrying out vaccination or revaccination programmes under outbreak conditions, considerable care is required so as not to exacerbate the spread of the disease from infected premises. There have been occasions when hastily implemented and badly planned vaccination campaigns in the face of an outbreak have assisted the spread of infection because of poor hygiene practices by the vaccination teams. A vaccination campaign in the face of an outbreak should begin in the known, uninfected populations. Then, the vaccination cover should be extended in such a manner that groups of animals which may be silently incubating the disease are vaccinated only at the end of the campaign. That is why vaccination teams should carry out all the basic decontamination procedures in a disciplined manner at all times, and particularly when moving between villages or farms.
Vaccination of cattle and buffalo As the success of vaccination programmes is in the correct vaccination of all the accessible animals, adequate means of restraint may need to be planned beforehand for difficult animals not used to handling. The ideal facility is that designed in South American countries, where cattle races, capable of holding 10 to 30 cattle (head to tail) per loading, are in common use. The race should be open at the top so that vaccinators can work over a top rail without any vertical obstructions. As all animals should be vaccinated, great care should be exercised with adult bulls, pregnant cows and heifers. In a peasant farming situation, which is frequent in the tropics, animal capture and restraint for vaccine administration are more difficult. For small numbers of free-ranging cattle, the use of a pen to crowd animals in a corner using a metal crush or a spare farm gate is in common use. In all cases, only a sufficient amount of vaccine without excess should be removed from the ice box.
Vaccination of sheep and goats As these animals are difficult to handle, especially when pregnant, it is important to arrange beforehand for sufficient assistance to be available so that the vaccinator can concentrate on the correct administration of the vaccine. It is recommended that a system of pens is selected to separate groups of unvaccinated animals from vaccinated ones, without possible confusion between them.
Vaccination of pigs Pigs are currently vaccinated using a deep intramuscular injection to a cervical site. This objective will be greatly assisted by the proper restraint of pigs to be vaccinated using the help of sufficient assistance. Pregnant sows and gilts should be handled with care; smaller pigs up to two to three months old are caught and held by the upper forelegs.
Injection sites The injection of a vaccine dose should follow strictly the ‘directions for use’ printed by the manufacturer on a leaflet accompanying each box of vials and which are summarised on the label of each vaccine vial. Injection sites are common knowledge; for subcutaneous injections the preferred sites are under the skin of the neck in front of the shoulder or in the dewlap for cattle and zebu. For deep intramuscular injections, the preferred sites are the cervical region and, in pigs, just behind the ear. In pigs, special attention should be paid to avoid the ear canal. For deep intramuscular injection, the needle should be inserted obliquely rather than perpendicularly, so that on withdrawal of the needle, no backtracking of the vaccine dose will be observed. It is not unusual, during incompetent vaccination of piglets, to observe a small quantity of vaccine flowing back out of the animal, the result of which is a decreased vaccine dose injected and, consequently, poor immunisation.
Organisation of vaccination operations
Organisation of vaccination operations in susceptible animals against FMD is no more a matter of protection at individual or herd level but at a large population level in an economic sector, region, country or group of countries within a continent. Owing to the presence of virus carriers after clinical disease in cattle, FMD vaccination is targeted more to prevent infection circulating than to protect individuals against signs of the disease. FMD vaccination should be organised by campaigns in order to synchronise the level of immunity of the largest possible targeted population. A time period of three months is considered the maximum for a campaign. FMD control in any country implies that vaccination should be used and judged in a population context. Evaluation of the effects of a vaccination campaign should be finalised and discussed before starting a revaccination campaign in order to correct eventual defects. Containment of FMD epidemics is the most modest goal. It requires only a priming dose and the natural exposure acts as a booster with the negative consequences of, first, FMD virus carriage on a large scale in cattle and buffalo for several years and, second, the creation of mutations in the virus population. This approach to FMD control is to be prohibited. Elimination of FMD from an entire population or only an animal sector is more difficult to achieve. The vaccine efficacy is continuously challenged by the continued presence of the virus in pockets of infection and in carrier animals. High standards of management of vaccination programme include repeated use of high-potency vaccines and continuous vaccine-matching tests to keep the FMD virus evolution under surveillance. Eradication is the highest level of FMD control. It requires highly efficacious vaccines administered intensively and widely and, at the same time, the continuous search for traces of remaining infection in susceptible and vaccinated animals over several years. This task is now greatly facilitated by the existence of serological methods which detect antibodies against the markers of FMD infection, virus enzymes called non-structural proteins (NSPs). When FMD vaccines do not contain such proteins, thanks to in-depth purification (see later), differentiation becomes possible between infected herds and vaccinated herds. Detecting Infection in Vaccinated Animals (DIVA) is the purpose of the policy. This policy is the ultimate step towards the status of ‘free from FMD without vaccination’. Of course, stringent measures at the borders should prevent the reintroduction of FMD virus from neighbouring countries. In order to get FMD under control or eliminate or eradicate the virus, the proportion of the targeted population to be vaccinated is based on the highly contagious characteristics of the virus and aptitude to circulate unknown in carrier animals. Vaccination coverage above 90% to 95% is the standard universally admitted to fully control FMD in a targeted population. Below these figures, vaccination programmes are considered at risk and, below 70% coverage, vaccination against FMD is considered a waste of money because it contributes to hiding the virus presence and perpetuating infection with the consequence of feeding many vaccination programmes for the future. One of the main issues concerning the calculation of vaccination coverage is the accuracy and reliability of available figures for animal inventory. A variation of 10% or more in the detailed account of individuals to be vaccinated has an immediate consequence on vaccination coverage, vaccine consumption and the future success of the vaccine use.
The confidence of farmers in the potential of vaccination to protect all their animals should not be jeopardised by the use of a precise animal inventory by vaccinators for the benefit of tax administration. If vaccination operations are perceived by farmers as a tool for making an inventory of their property for tax usage, they will hide as many animals as they can. If repeated on the country scale, these farmer reactions can reach a very important proportion of animal population and turn a well-organised vaccination programme into a failure. Dissimulating animals and excluding them from the vaccination programme is also the obvious sign that the awareness programmes for the public were not successful. That could also be an indication that vaccinator visits are perceived as harmful for the livestock well-being and for livestock productions; this should lead to re-examining the training of vaccination teams with a view to improving their professionalism.
Pharmaceutical presentations and vaccine use
Foot and mouth disease vaccines available on the market are under different pharmaceutical presentations, according to the nature of adjuvant (aqueous or oil) and purification or absence of purification of the antigens. They should have received a valid marketing authorisation by the authorities of the country where they are used. Table I gives the characteristics of the different adjuvants used for FMD vaccine presentations; each of them can be made either with only filtrated FMD antigens or with highly purified FMD antigens. The route for injection, the volume of the dose according to species and age and the precautions for use are specific to each vaccine presentation. Consequently, before starting vaccination, it is highly recommended for persons in charge of vaccine teams to publicly comment on the ‘directions for use’ put on each vaccine box by the manufacturer.
Table I Commercial presentations of foot and mouth disease vaccines according to the adjuvants used
Vaccine Alhydrogel saponine Single-oil emulsion Oil in water Single-oil emulsion Water in oil Double-oil emulsion Water in oil in water
Recommended route Strictly subcutaneous Intramuscular or subcutaneous Strictly intramuscular Intramuscular or subcutaneous
Indicated species All ruminants All ruminants and pigs All ruminants All ruminants and pigs
Regions where used Europe (formerly) Southern Africa Middle East South East Asia South America Europe South East Asia
Aqueous vaccine: the historical standard
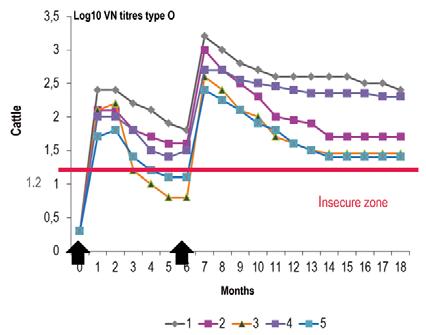
This pharmaceutical presentation appeared during the first half of the 20th Century and was gradually improved. Aqueous vaccines are made from FMD antigens adsorbed onto aluminium hydroxide gel in the presence of saponine, a mixture of heterosides extracted from the bark of a South American tree. In aqueous vaccines, FMD antigens could be filtrated or highly purified, monovalent or plurivalent up to seven valencies if highly purified. Aqueous vaccines are thermosensitive and destroyed by freezing, and therefore the quality of the cold chain is of prime importance for their correct use. Once injected, they induce an exceptionally early protection only four days after injection, as demonstrated by Doel et al. (5) using a virulent challenge in cattle. Saponine is an adjuvant well tolerated by ruminants and is very efficient in all their species. On the contrary, saponine is not active in pigs, which means that aqueous vaccines are not used in this species and are not often used when vaccination campaigns involve vaccinating pigs and ruminants on the same farms. Figure 1 shows the typical kinetics of antibodies observed in a group of cattle after two injections of aqueous vaccine. The onset of antibody presence is very early and levels rocket fast, but start to decline after 30 to 35 days post vaccination if a second injection is not performed. Aqueous vaccines are ideally the vaccines used to create very rapidly an immunological barrier with two injections one or two months apart. During the 1960s, Europe eradicated FMD using annual campaigns of vaccination with this kind of vaccine injected once a year, when calves and naive animals were vaccinated twice. Compulsory mass vaccination policy in Europe took advantage of winter stalling to reach more than 95% coverage of cattle population. During the 1990s, several countries in southern Africa reached the same results following the same policy with the same kind of vaccine. Aqueous vaccines prepared with highly purified antigens are eligible for use in a DIVA policy. Aqueous vaccines are easy to produce; they use inexpensive ingredients and so are affordable.
Oil vaccines: vaccines of the next generation for pigs and cattle extensively bred
This pharmaceutical presentation of the next generation appeared a long time after the published works of Cunliffe et al. (3) for vaccination in cattle in 1963 and of McKercher et al. (10) for vaccination of pigs in 1967. Since this historic formula was presented, much progress has been made in the preparation of emulsions to make oil vaccines very sophisticated products. Oil vaccines are made from FMD antigens emulsified once or twice in a mixture of paraffin oil and surface-active agents making water and oil compatible. In oil vaccines, FMD antigens could be filtrated or highly purified, monovalent or plurivalent. Oil vaccines are thermosensitive but some can accept
Fig. 1 Aqueous vaccine: antibody kinetic in naive cattle
Source: (7)
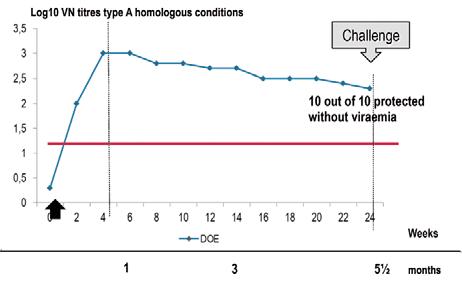
Fig. 2 Double-oil emulsion vaccine: antibody kinetic in naive cattle
Source: (4)
freezing; nevertheless, the quality of the cold chain is of prime importance for their correct use. Depending on whether the aqueous phase with FMD antigens receives droplets of oil or, on the contrary, the oil phase receives droplets of aqueous phase, the immunological characteristics of the emulsion are completely changed, which means different indications for use. Single emulsions are either oil-in-water emulsion (O/W) or water-in-oil emulsion (W/O). When water is the external phase, the product is well tolerated locally but the adjuvant power is weak. On the contrary, when the oil phase is external, oil triggers a reaction, inducing a granuloma locally and a lymph node reaction regionally both responsible for a strong adjuvant power and a slow release of the immunogens. To conciliate the opposing properties of these single emulsions, some years ago the so called ‘double emulsions’ appeared on the market, the only kind of which is a W/O emulsion emulsified again in an aqueous buffer to make a ‘water in oil in water’ emulsion. The strong adjuvant power and the good tolerance at the injection site make double emulsion the tool of choice for the vaccination of pigs and increasingly for ruminants. Once injected, oil
vaccines work as a slow-release system for antigens, as the droplets of oil with FMD antigens migrate in the lymphatic system from the injection site to lymph nodes and spleen, where antibodies are produced. Thanks to this mechanism, oil vaccines induce an exceptionally long-lasting protection. The start is slower than for aqueous vaccines, as the peak of antibodies is observed between two and four months, depending to the oil formula. Figure 2 shows the typical kinetics of antibodies observed in a group of cattle injected once with an oil vaccine manufactured with a high antigenic payload. The rising antibodies continue slowly to reach a peak at three to four months post injection and afterwards maintain a good level as a plateau up to 5.5 months post vaccination where all the animals have resisted to a virulent challenge even if vaccinated only once. Oil vaccines are typically the vaccines for mass vaccination campaigns of cattle populations bred extensively in large countries and not easily rounded up. On the contrary, these vaccines are not appropriate to rapidly create an immune barrier in a cattle population in order to stop a threatening epidemic. In pigs, only oil vaccines are used and they are double emulsion vaccines, even if use of a single oil emulsion was reported in Taiwan. The slow-release system of oil vaccine is well designed for the protection of pigs, the economic life of which does not exceed six to seven months. In order to cover this period of time using the smallest number of vaccine injections, vaccination programmes make use of maternally derived antibodies for the initial phase or protection from birth to 2.5 months, then vaccination brings active protection up to slaughtering period, using one or two injections depending on the virus pressure around the pig farm. To enhance the production of maternally derived antibodies, pregnant sows are boostered one month before farrowing.
Non-purified versus purified antigens and vaccine use
The traditional manufacturing process for FMD vaccine consists of clarifying industrial virus harvests and inactivated antigens to remove debris and precipitates at a microscopic scale. Antigens are then concentrated to reach the selected payload before adding adjuvants. This simple technology has produced billions of doses of potent and inexpensive vaccines. In the middle of the 1990s, new studies in FMD research shed light on the role of FMD virus NSPs in the immune response against FMD infection and their potential use in serology for identifying convalescent and carrier animals by serology, an important breakthrough (5). The other side of the coin is that pluri-vaccinated animals, mainly cattle, with traditional FMD vaccines developed the same kind of antibodies as NSPs were also present, even in small amounts, in the vaccine doses. These findings were a revolution in FMD vaccine use, as a vaccine cleared from NSPs could be used in vaccination programmes without hampering the serological diagnosis of virus-infected/carrier animals. That was the wish of all the FMD vaccine manufacturers, which were blamed for decades because their products were hiding potential infection behind the protection conferred by vaccination. Finally, FMD control strategies could benefit from the DIVA strategy already used for some other disease controls. A new kind of FMD vaccine was born, pure (almost without NSPs) and potent, but more costly than the traditional one because purification technology is expensive to carry out even if its yield remains high. The selection of one of these two kinds of vaccine (not purified or purified) is a matter of progression in the different stages for controlling the disease. Non-purified vaccines are recommended for affordable FMD control in countries heavily infected for decades, corresponding to Stages 2 and 3 of the Food and Agriculture Organisation of the United Nations (FAO) Progressive Control Pathway (PCP). However, progressing further in the full control of the disease, thanks to regular mass vaccination campaigns with no outbreaks observed, which corresponds to Stages 4 and 5 of the PCP, the use of purified vaccines is compulsory in association with the serological methods of the DIVA strategy. This means that FMD control strategies associating purified vaccine use and serological studies make FMD control more and more costly when approaching the ultimate objective of FMD elimination and later FMD eradication.
Factors influencing results of vaccine use
Correspondence between field virus and vaccine strain
The correspondence between the vaccine strain(s) and the virus(es) to be controlled in the field is the first of two key factors for success against an outbreak or an endemic situation. Vaccine matching is a recommended activity for vaccine manufacturers, which should offer the most appropriate vaccine strain(s) to their customers. Figure 3 demonstrates the loss of expected protection after vaccination in a heterologous condition (vaccine against a non-related field virus) compared with a homologous situation (vaccine against a related virus). Before ordering vaccine doses for FMD control, the first question to ask is about the appropriateness of vaccine strain(s). The World Organisation for Animal Health (OIE) reference laboratories are able to give all the pertinent information on this.
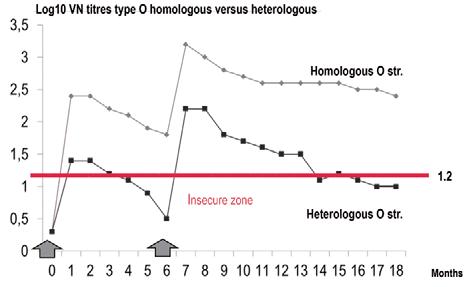
Fig. 3 Cattle antibody kinetics in homologous/heterologous systems
Source: (7)
Vaccine potency and antigen payload
The second key factor for obtaining good results with vaccine use is the potency/efficacy of supplied vaccine. Too many vaccines used in some parts of the world are still of inadequate efficacy to maintain a durable immunity (6 to 12 months). FMD vaccines present on the market should comply at least with the minimum efficacy/potency standard of the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Terrestrial Manual) (13), which corresponds to 75% of observed protection in tests using cattle. If not of certified potency, vaccine batches should not be used for controlling FMD. With the development of physical measure of virus mass expressed
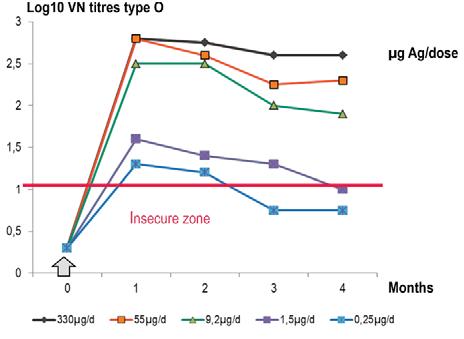
Fig. 4 Effect of five antigen payloads in cattle
Source: (12)
in micrograms per millilitre of virus harvest, standardisation of antigen payload in each vaccine dose became easier and of universal use by manufacturers. Figure 4 shows the effect on antibody secretion by use of several experimental vaccines prepared with increasing antigen payloads. Vaccines with antigen payloads that are too low do not induce a high and durable immunity and should not be used. Commercial vaccines by renowned manufacturers are twice or more the OIE minimum standard for potency, which is a guarantee for establishing a strong and long-lasting immunity after vaccination. Vaccine potency is an important criterion for vaccine selection and, for example in the European Union (EU), for antigens of the EU-FMD bank and for antigen banks of some countries with a no-vaccination policy against FMD, a potency more than twice the minimum standard of the OIE is required; that is more than six protective doses of 50% (PD50) per commercial dose instead of three PD50 per commercial dose, one of the two OIE standards for 75% protection. The rationale behind such a requirement for antigen banks is the naive immunological status of the European or other countries’ livestock facing a possible, very threatening, FMD outbreak.
Frequency of re-vaccinations
Foot and mouth disease vaccines are inactivated and not live. Consequently, immunity should be maintained by regular re-vaccinations, the frequency of which influences the results of vaccine use. The standard vaccination scheme in naive cattle is two injections one to four months apart, depending on the disease threat in the area. Before a progressing epidemic or to extinguish an outbreak (8), the second vaccination is usually made one month after the first (Fig. 5). On the contrary, in a mandatory vaccination scheme and in the absence of an immediate threat, naive animals could receive their second injection four months after the first one, the booster effect being much more effective than the booster effect observed after a one-month interval. Post-vaccination immunity cannot reach the level observed with post-infection immunity, and this fact has led to the need to vaccinate annually or bi-annually, and even tri-annually in areas with a high risk of exposure to field virus, especially if different from the vaccine strain. More than two routine vaccinations a year are observed in the intensive dairy units in Middle East. When initiated, regular FMD vaccinations cannot be stopped precociously without jeopardising the previous efforts by the return of the disease. Once launched, FMD vaccination campaigns should continue year after year until the status of free from FMD without vaccination can be granted.
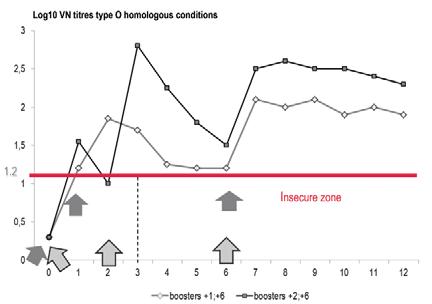
Fig. 5 Effects of boosters at one and two months post vaccination in calves, France
Source: (7)
Maternally derived antibodies and vaccine use
Dam vaccination schemes are especially beneficial for the protection of neonates in a heavily contaminated environment. The strategy is to enhance neonatal immunity by augmenting antibody titres in colostrum (2). This is, for example, the vaccination programme for sows using an FMD oil vaccine. One injection one month before farrowing maximises antibody titres in the colostrum for the benefit of suckling piglets, which receive an increased
passive immune protection effective for two to three months. Once this period of time has elapsed in piglets, and also in lambs or calves, the level in maternally derived antibodies (MDAs) is too low to protect against infection, but the transition to active immunity, thanks to vaccination, is critical because the residual levels of MDA neutralise partially or completely the immunogens present in the vaccine. These variable residual antibody levels depend on each individual, which demonstrates poor to absent serological reaction after a single vaccine injection. That is why a second vaccine injection (Fig. 6) is recommended to trigger an active immune response fully protective, as demonstrated in calves in Brazil (1, 11). In this context, oil vaccines with slow release of antigen(s) were recommended. After this second vaccination, calves entered into the six-month vaccination cycle, which was recommended for cattle under two years of age in Brazil at this time.

Fig. 6 Maternally derived antibodies in effect of 0, 1 and 2 vaccinations, Brazil
Source: (11)
All factors affecting the good health of individuals to be vaccinated have an influence on the outcome of FMD vaccination. Fever due to concurrent diseases, acute and chronic parasitic disease and stress are the most well-known indications to delay vaccination. When herds have recently been transported by foot or by vehicle, time for resting should be allowed before vaccination. Vaccination during glucocorticoid therapy has been also traditionally discouraged. All these considerations are not of a nature to prevent individual FMD vaccination in the scope of compulsory mass vaccination campaigns where a poorly vaccinated individual has less chance of becoming infected by virtue of being a member of a well-vaccinated group.
Risks and side-effects associated with vaccine use are generally of three orders: 1. related to the vaccine itself; 2. related to mishandling of animals; and 3. related to each vaccinee health status. Modern vaccines are harmless, with no toxic materials (the inactivant is often neutralised after the inactivation step) but when they are not purified vaccines can keep an allergenic capacity, the power of which increases with the repetition of vaccination campaigns. Allergy is usually of type 1 (anaphylaxis) and should be treated immediately with corticoids, especially if it occurs in high-value animals (insemination centres, intensive dairy farm, etc.). With regard to oil vaccines with an external oil phase (in South America), it is common knowledge that they induce granuloma at the injection site where oil emulsion residues can be observed several months after the injection as well as in the satellite lymph node. Such granulomas and lymph nodes are trimmed later from carcasses in
slaughterhouses. Paraffin oil residues can be detected several months after vaccination in liver, kidneys and, of course, around the injected site (personal observation), and the potential human health risk from ingestion has not yet been determined. Violent handling of animals could also result in trouble, mainly with small ruminants, especially when they are pregnant, with abortions as consequences (goats), or when animals are very young (piglets, lambs). Bringing together livestock populations on large premises practising extensive breeding is always the occasion for accidents such as broken legs or horns, which makes farmers reluctant to vaccinate too often throughout the year. Associating FMD vaccination with blackleg vaccination and anti-parasite dip is the best way to make sure that the local cattle population is vaccinated. Another risk of vaccine use is the contamination of multi-dose vials if an additional long needle is not used for air entrance and if a stopper is punctured many times with the needle used for vaccinating animals. In instructions to vaccinators, strict guidance should be given regarding the proper handling of vaccine vials. It should also stipulate that part-used vials of vaccine should not be reused after 36 h from the first use and must be kept refrigerated throughout the intervening period.
Conclusion
Vaccines against FMD, as with all veterinary vaccines, are medicinal products, carefully prepared by manufacturers following the dossier agreed for registration/licensing/marketing authorisation by national/multinational authorities and complying with the requirements of the OIE Terrestrial Manual. These vaccines are expensive to buy and much more expensive to apply respecting the directions for use, the good practice for logistics and the retained vaccination strategy. When well selected and well applied, vaccines against FMD have always led to successfully controlling threatening FMD outbreaks or eliminating FMD where it has been endemic for decades. However, these combined efforts come at a huge cost and necessitate thorough attention over a period of many years. Thanks to strict FMD vaccination programmes, continents or sub-continents have reached the eradication degree, which allows them to end completely the vaccination policy and to adopt a policy of strict control of the potential sources of re-infection. Thus, they can benefit from a free trade for animal products with countries having reached the same degree for disease status.
Acknowledgements
The author thanks Dr Chris Schermbrucker for his friendly help with the section on general vaccine use.
References
1. Augé de Mello P., Gomes I. & Bahnemann H.G. (1989). – The vaccination of young cattle with an oil adjuvant foot-and-mouth disease vaccine. Bol. Centr. Panam. Fiebre Aftosa, 55, 9–14. 2. Clark M.A. (1993). – Bovine coronavirus. Br. Vet. J., 149, 51–70. 3. Cunliffe H.R. & Graves J.H. (1963). – Formalin-treated foot-and-mouth disease virus. Comparison of two adjuvants in cattle. Can. J. Comp. Med. Vet. Sci., 27,193. 4. Cox S., Parida S., Hamblin P., Bankowski B., Carr V., Paton D. & Barnett P. (2008). – Longevity of protection in cattle following vaccination with emergency FMD vaccines from the UK strategic reserve. In Report of the Open
Session of the Research Group of the Standing Technical Committee of the European Commission for the Control of Foot-and-Mouth Disease, Erice, Italy, 14–17th October. Food and Agriculture Organization (FAO), Rome. 5. Doel T.R., Williams L. & Barnett P.V. (1994). – Emergency vaccination against foot-and-mouth disease. The rate of development of immunity and its implications for the carrier state. Vaccine, 12, 592–600. 6. Food and Agriculture Organization of the United Nations (FAO) (1998). – Report of the Open Session of the
Research Group of the Standing Technical Committee of the European Commission for the Control of Foot-and-
Mouth Disease, Aldershot, United Kingdom, 14–18th September. FAO, Rome. 7. Joubert L. & Mackowiak C. (1968). – La fièvre aphteuse, Vol. III, La lutte anti-aphteuse. Fondation Mérieux-
Expansion Scientifique, Lyon, France. 8. Kitching P. & Salt J.S. (1995). – The interference by maternally-derived antibody with active immunization of farm animals against foot-and-mouth disease. Br. Vet. J., 151 (4), 379–389.
9. Leforban Y. & Gerbier G. (2003). – Recent history and epidemiology of foot-and-mouth disease in Europe. In Footand-mouth disease: control strategies (B. Dodet & M. Vicari, eds). Editions Scientifiques Elsevier SAS, 153–171. 10. McKercher P.D. & Farris H.A. Jr (1967). – Foot-and-mouth disease in swine: response to inactivated vaccines.
Arch. Ges. Virusforsch., 22, 451. 11. Nicholls M.J., Black L., Rweyemamu M., Genovese J., Ferrari R., Hammant A., de Silva E. & Umehara O. (1984). – The effect of maternally derived antibodies on the response of calves to vaccination against foot and mouth disease. J. Hyg. (Camb.), 92, 105–116. 12. World Organisation for Animal Health (OIE) (2004). – Chapter 2.1.1. Foot and mouth disease. In Manual of diagnostic tests and vaccines for terrestrial animals. 5th Ed., OIE, Paris, 111–128. 13. Rweyemamu M.M., Black L, Boge A., Thorne A.C. & Terry G.M. (1984). – The relationship between the 140S antigen dose in aqueous foot-and-mouth disease vaccines and the serum antibody response of cattle. J. Biol.
Standard., 12, 111-120. 14. World Organisation for Animal Health (OIE) (2004). – Chapter 2.1.1. Foot and mouth disease. In Manual of diagnostic tests and vaccines for terrestrial animals. 5th Ed., OIE, Paris, 111–128.
K. De Clercq
Unit of Vesicular and Exotic Diseases, Department of Virology, Veterinary and Agrochemical Research Centre (CODA-CERVA), Ukkel, Belgium; Vice-President Scientific Commission for Animal Diseases (SCAD), World Organisation for Animal Health (OIE) Correspondence: krdec@coda-cerva.be
Summary
Foot and mouth disease (FMD) is one of the most infectious diseases of livestock and continues to pose a significant threat to endemic and free regions. The impact of FMD on society and international trade is high, thereby demanding stringent prevention, surveillance and control plans. On the other hand, there is a global increased demand for animal welfare and ethical considerations necessitating a decreased reliance on eradication of animals to control FMD virus (FMDV) spread, and on the use of animals for the regulatory testing of veterinary products. The FMD research community seeks to balance these apparently contrasting viewpoints by addressing specific gaps in our knowledge on all aspects of FMD control to enable implementation of enhanced animal-sparing vaccine-based control strategies tailored to the needs of free and endemic settings. The actual ongoing FMD research and its application in the FMD control policy is described for the following items: a) methods to reduce and refine in vivo vaccine quality tests by in vitro method, b) high-quality vaccines to implement the vaccinate-to-live policy, c) new-generation FMD vaccines and antiviral products, d) new-generation FMD diagnostics, e) FMDV spread, transmission and persistence following the use of high-potency monovalent or multivalent vaccines, f) FMDV early pathogenesis and immune responses, g) computerised FMD spread models to optimise vaccination schemes, h) economic factors influencing the spread and control of FMD, i) FMDV transmission via animal products and FMDV stability and inactivation in animal products.
Keywords
Animal products – Antivirals – Control policy – Diagnostics – Foot and mouth disease – Immune responses – Research – Spread models – Transmission – Vaccine quality.
Introduction
Foot and mouth disease (FMD) is one of the most infectious diseases of livestock and continues to pose a significant threat to endemic and free regions alike. The impact of FMD on society (35, 43) and international trade (31, 51) is high, thereby demanding stringent prevention, surveillance and control plans taken up in crisis preparedness plans. On the other hand, there is a global increased demand for animal welfare and ethical considerations, necessitating a decreased reliance on eradication of animals to control FMD virus (FMDV) spread, and on the use of animals for the regulatory testing of veterinary products. The FMD research community seeks to balance these apparently contrasting viewpoints by addressing specific gaps in our knowledge on all aspects of FMD control to enable the implementation of enhanced animal-sparing vaccine-based control strategies tailored to the needs of free and endemic settings.
Methods to reduce and refine in vivo vaccine quality tests by in vitro methods The aim of this research is to replace the current in vivo ‘gold standard’ tests for vaccine efficacy (potency), purity and safety, in light of the 3Rs (Refinement, Reduction, Replacement) principle (28), by validated in vitro laboratory tests. More specifically, by: − the determination and validation of correlation models between in vitro laboratory tests and in vivo protection based on experimental and field data (24, 32, 45, 52); − the development of in vitro immunoassays to monitor vaccine purity by the reduction of FMDV non-structural proteins content during vaccine purification and in the final vaccine (6, 12); and − the development of alternative methods to check the antigen quality (54) and to quantify the antigen payload content in the final vaccine (49). The reduction and refinement of in vivo vaccine quality tests by in vitro methods will guarantee the overall quality of the vaccine batch in a verifiable form to end-users and other stakeholders, strengthening the position of the decision makers when it comes to the implementation of the ‘vaccinate-to-live’ policy (17, 53). Consequently, reliance on animals for regulatory testing of vaccine batch release control will be decreased and animal welfare increased.
The availability of high-quality vaccines to implement the vaccinate-to-live policy The aim is to predict how well a vaccine will protect against a challenge virus of another strain within the same serotype (cross-protection) (9, 34) avoiding in vivo cross-protection studies. Therefore, r-value determination between vaccine strains and FMDV field isolates (46) will be improved by harmonising test methodologies (41) and drafting guidelines (33) for the reliable selection of reagents to include in in vitro vaccine-matching studies. The depth of our knowledge and expertise regarding vaccine spectrum coverage will be increased. The assessment and improvement of heterologous protection by FMD vaccines will help decision-makers in their difficult choice on which vaccine to use in future outbreaks and in their responsibility in updating and reinforcing FMD vaccine/ antigen banks (4).
The development of new-generation foot and mouth disease vaccines and antiviral products The aim is to increase our knowledge by investigating approaches for reinforcing the mucosal immune response (48) in order to prevent FMDV infection at the primary portal for virus entry (2). Methods to elicit and measure (39) mucosal immunity against FMDV in cattle are being evaluated. Ways to stimulate innate (rapid) (50) and adaptive (lasting) mucosal immune responses are being investigated, using novel delivery systems, adjuvants and viral vectors (21, 26, 38, 40, 44). Other new-generation vaccines are being developed and efficacy tested, avoiding the need for virus culture, thereby making the production of FMD vaccines environmentally safer. Moreover, the use of potent and selective antiviral compounds against FMDV, which rapidly and completely prevent FMDV replication, is being investigated in order to decrease the post-vaccination immunity gap (22). The development of new-generation vaccines (19, 42) and antiviral compounds (20, 30), based on safe production methods and specifically aimed at reducing the immunity gap shortly after vaccination, will (a) supplement the existing control tools to combat FMD and (b) allow the enhancement of emergency contingency plans enabling a better, quicker and animal-sparing response to FMD outbreaks.
The development of new-generation foot and mouth disease diagnostics The aim is to:
a) increase the availability of FMD diagnostics; b) improve standardisation and harmonisation of FMD diagnostic results; and c) develop new, and possibly better, diagnostic tools for confirmatory tests and/or test systems for non-structural protein (NSP) serology.









