9 minute read
International and regional reference laboratory network
S. Metwally*
Advertisement
Food and Agriculture Organization of the United Nations (FAO), Viale delle Terme di Caracalla, 00153 Rome, Italy Correspondence: Samia.Metwally@fao.org
* The views expressed in this publication are those of the authors and do not necessarily reflect the views of the Food and Agriculture Organization of the United Nations. In addition, the designations employed and the presentation of material in this information product do not imply the expression of opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Content and errors are exclusively the responsibility of the authors.
Summary
The global strategy for control of foot and mouth disease (FMD), developed by the Food and Agriculture Organization (FAO) and the World Organisation for Animal Health (OIE) in 2012, proposes establishing networks for FMD diagnostic services which consist of an integrated international, regional and national network of laboratories that can collectively respond quickly to needs for rapid and accurate testing and timely notification. Such a structure provides real-time knowledge on FMD virus strains circulating globally, improves vaccine selection, supporting both endemic and free countries – essential to progressive control – and enhances diagnostic capability for other priority diseases. The global strategy also proposes establishing and strengthening an epidemiology network to link with the laboratory networks. The epidemiology network would consist of national units for epidemiology and regional epidemiology centres for improving infrastructure related to achieving core capacities in countries’ surveillance, response, preparedness, risk communication and human resources. A global database housing epidemiological and diagnostic data will be developed. It is envisaged that the communication between the laboratory and epidemiology networks can be a key component for a systematic coordinated programme towards establishing global and regional progressive control pathways.
Keywords
Diagnostic – Food and Agriculture Organization of the United Nations – Foot and mouth disease – Global strategy – Laboratory network – Progressive Control Pathway – World Organisation for Animal Health.
Introduction
Foot and mouth disease (FMD) is a highly contagious disease of cloven-hoofed animals. The disease is endemic in many low-income countries including most parts of Asia, Africa and the Middle East and a few countries in South America. An FMD outbreak causes devastating impacts on farmers with adverse effects on livestock assets, production income and consumption. FMD may spread to FMD-free countries through animal movement and international trade, as seen during outbreaks in the United Kingdom (2001) (2) and Japan and the Republic of Korea (2010) (3). Foot and mouth disease virus (FMDV) is not randomly dispersed throughout the world but is associated with particular ecological niches. Studies on FMDV occurrence over many years have provided information to suggest the clustering or grouping of FMDV serotypes and subtypes into seven virus pools, with three pools covering West Eurasia, the Middle East and Asia, three pools covering Africa and one pool covering South America. The concept of ‘regional virus pools’ provides an organising principle for coordinating laboratory and epidemiology activities towards diagnostics and disease surveillance.
The Food and Agriculture Organization of the United Nations (FAO)/World Organisation for Animal Health (OIE) global FMD control strategy is a 15-year programme with five-year increments (1). The strategy includes three components: − improving global FMD control; − strengthening Veterinary Services; and − improving the prevention and control of other major diseases of livestock.
Food and Agriculture Organization of the United Nations–World Organisation for Animal Health foot and mouth disease reference laboratory network
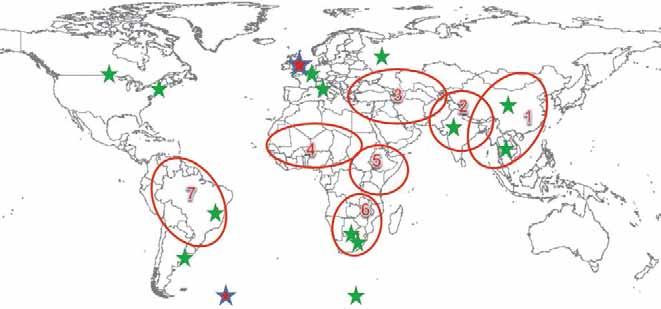
Currently, there are 13 FAO–OIE reference centres (Fig. 1) of which five fall outside of the regional virus pools, three in Pool 1, one in Pool 2, two in Pool 6 and two in Pool 7. Reference centres are lacking in East and West Africa and West Eurasia, which in turn contributes in part to the vast gap in knowledge on FMDV circulation and disease control in these regions. Building on the reference laboratory network, the global strategy would strengthen their regional network.
WRL-FMD Reference lab/centers
Fig. 1 Composition of Food and Agriculture Organization/World Organisation for Animal Health reference laboratory network (stars) in relation to the seven virus pools (red outlines)
Global and regional networks
Effective and reliable diagnostics are crucial in disease intelligence and control. This is particularly challenging for developing countries, where the capabilities of national veterinary laboratories are often weak. The FMD global strategy attempts to rectify this situation by assisting countries in need, mainly those in the lower stages of the Progressive Control Pathway (PCP), by establishing and/or supporting global and regional FMD laboratory networks. The global FMD laboratory network was depicted in the global strategy to have at least one reference laboratory physically located within each of the virus pools and to establish regional leading laboratories where reference centres do not exist. For the global network, the world reference laboratory of FMD (WRL-FMD) will provide the technical leadership and forum for the global coordination. The reference laboratories will serve as WRL-FMD surge–capacity in supporting technical and diagnostic services to their designated region.
The main responsibilities of the global FMD laboratory network will be to conduct high-definition diagnostics and laboratory training at the global level and to ensure that all the FAO/OIE reference centres are fully participating in, contributing to and benefiting from the network cooperation. With a coordination mechanism within the global and regional framework, the WRL-FMD and the regional reference laboratories will support training, technology transfer and providing diagnostic reagents and proficiency testing to national laboratories. The regional FMD laboratory network will have similar responsibilities to the global laboratory network in coordinating activities at the national level in their respective region, with specific responsibilities in supporting training to national laboratories in the areas of good laboratory practice, including biosafety and biosecurity, diagnostic method platform, specific FMD test and sequence analysis. The regional network will ensure harmonisation of laboratory results by providing proficiency tests, standard controls and diagnostic reagents and kits to the national laboratories. The regional network will maintain steady communication with the national laboratories and conduct confirmatory testing on submissions from countries of their region.
National laboratory network
The majority of developing countries where FMD is still endemic are self-assessed to be at PCP Stage 0. In order to move to a higher stage, their diagnostic capability has to be advanced gradually and steadily to accommodate the need for surveillance and preliminary diagnostic screening. The national laboratories should take the responsibilities on performing screening tests for seromonitoring when countries at PCP Stages 1 and 2 are expected to advance in their diagnostic capabilities as they move to PCP Stage 3 or higher to perform confirmation testing, virus characterisation and sequence analysis. It is also important for the national laboratories to take part in annual proficiency testing, communicate with reference and regional laboratories on non-conforming results and forwarding specimens to the appropriate laboratories for further analysis. It is an important responsibility for the national laboratories to participate in diagnostic assay development and validation.
Challenges in establishing and sustaining laboratory network
The complexity and challenges of multi-country laboratory networks reflect tensions that create barriers to performance and sustainability – they impede the development, strengthening and maintaining of core capacity as required for the implementation of the global control strategy. Some of these challenges include, but are not limited to, financial resources, laboratory infrastructures (facility, biosecurity and biosafety), the ability of countries to ship and receive biologicals, equipment maintenance, the application of quality control and quality assurance, a limited number of sample submissions for analysis, political engagement of countries at the national and regional levels and commitment and support from all involved.
References
1. Food and Agriculture Organization of the United Nations and World Organisation for Animal Health (OIE) (2012). –
The global foot and mouth disease control strategy – strengthening animal health system through improved control of major diseases. Available at: www.fao.org/docrep/015/an390e/an390e.pdf (accessed October 2012). 2. Knowles N.J., Samuel A.R., Davies P.R., Kitching R.P. & Donaldson A.I. (2001). – Outbreak of foot-and-mouth disease virus serotype O in the UK caused by a pandemic strain. Vet. Rec., 148 (9), 258–259. 3. Yoon H., Yoon S.S., Wee S.H., Kim Y.J. & Kim B. (2012). – Clinical manifestations of foot-and-mouth disease during the 2010/2011 epidemic in the Republic of Korea. Transbound. Emerg. Dis., 59, 517–525.
Vaccines: types, quality control, matching and supply
A.I. Donaldson
290 London Road, Guildford, Surrey GU4 7LB, United Kingdom Correspondence: alex.donaldson3@virginmedia.com
Summary
Vaccines play a crucial role in the control and prevention of foot and mouth disease (FMD). They had their beginnings in the late 1930s in Germany, when the source of viral antigen was epithelial tissue from vesicles on the tongues of infected cattle. Later, Dutch workers showed that virus could be grown on slices of tongue epithelium from freshly slaughtered cattle, and this enabled the large-scale production of vaccine and the implementation of successful mass cattle vaccination campaigns in Europe. The next major advance was in the United Kingdom, where it was shown that FMD virus could be grown in baby hamster kidney (BHK)-21 cells in deep suspension culture. Subsequent developments have been mainly in the areas of virus inactivation, antigen concentration, product purification and improved adjuvants. Although research has led to the development of some promising candidate novel vaccines, their large-scale evaluation has been constrained by the high cost and limited availability of biosecure animal accommodation, coupled with a lack of financial support. The Global Strategy intends to give high priority to the control of FMD in the seven ‘virus pool’ regions. Since FMD is endemic in these regions, the amount of vaccine required for campaigns will be very high. The global production of FMD vaccine will have to be significantly boosted if this demand is to be met. The Global Strategy will strive to promote the use of vaccines that are safe, potent and of a high quality. Selecting the most immunologically appropriate vaccine strain(s) will be important and will require the collection and typing of isolates from outbreaks and their analysis in vaccine-matching tests. A massive investment in human and physical resources in the regions will be needed to enable them to perform those tasks. Training at all levels will be essential to strengthen state Veterinary Services and improve the capability of laboratories.
Keywords
Cattle – Foot and mouth disease – Global Strategy – Vaccine – Vaccine matching – Vaccine strain – Viral antigen.
Introduction
Early workers recognised that animals recovered from foot and mouth disease (FMD) caused by one serotype were solidly immune to that type for many months afterwards, and this prompted attempts to develop a vaccine.
Types of foot and mouth disease vaccine
In the late 1930s German workers developed an effective vaccine by collecting epithelial tissue from vesicles on the tongues of cattle that had been deliberately infected with virulent virus, inactivating the virus by dilute formalin and then absorbing the antigen onto aluminium hydroxide gel as an adjuvant. The product, known as the Vallée–Schmidt–Waldmann vaccine, was used extensively in Europe and elsewhere, but had the major disadvantages of being expensive and risking the spread of disease (24). A major advance came in the early 1950s, when Frenkel and co-workers in the Netherlands devised a largescale production procedure in which virus was grown on slices of tongue epithelial tissue collected from freshly slaughtered cattle in a vigorously stirred, oxygenated culture medium at 37°C for 20 h to 24 h. After clarification and filtration, the virus was adsorbed onto aluminium hydroxide gel and inactivated by formalin (7, 8). This production method enabled the mass annual vaccination of the cattle population of the Netherlands and, later, as other laboratories adopted the technique, the annual vaccination of cattle elsewhere. Vaccination campaigns in the Netherlands, France and West Germany, combined with zoo-sanitary methods, resulted in a dramatic reduction in the incidence of disease.









