
4 minute read
Identical Twin Eye Images Lead to Promising Alzheimer’s Discovery
Loss of Blood Vessels in Retina May Signal Alzheimer’s Disease
Apair of 96-year-old identical twins, one with advanced Alzheimer’s disease (AD), the other cognitively normal and two very different optical coherence tomography angiography (OCT-A) images. Using automated mapping of the superficial capillary plexus, the twin with advanced AD had a significantly reduced vessel density compared to her cognitively normal sister. This finding convinced Sharon Fekrat, MD, professor of ophthalmology, that retinal images could potentially become a
Advertisement
Sharon Fekrat, MD Professor of Ophthalmology
Dilraj Grewal, MD Associate Professor of Ophthalmology
biomarker for AD and she began her mission to recruit patients and test the theory. Nearly 6 million Americans live with Alzheimer’s disease. No viable treatments or noninvasive tools for early diagnosis exist. Fekrat, along with her colleagues at Duke Eye Center, are studying this and related retinal changes, such as the thinning of nerve layers, that could provide early signals of cognitive disease. Fekrat and Duke retina colleague Dilraj Grewal, MD, associate professor of ophthalmology, led a study published in Ophthalmology Retina that found decreased superficial retinal blood vessel density, as well as thinning of the ganglion cell layer of the retina, in individuals with symptomatic Alzheimer’s disease compared to those with mild cognitive impairment and compared to cognitively healthy adult controls, even after controlling for age and sex. Ultimately the use of OCT-A alone, or in combination with multimodal retinal imaging, may be able to diagnose Alzheimer’s disease—perhaps even before a person begins to show signs of memory loss.
Retinal Blood Vessel Scan In people with healthy brains, microscopic blood vessels form a healthy, dense web in the retina located at the back of the eye, as seen in 254 eyes of 133 cognitively healthy participants. But in 72 eyes of 39 individuals with Alzheimer’s disease, that web was less dense and even sparse, researchers say. The study found differences in the retinas of those with Alzheimer’s disease compared to cognitively healthy people and compared to mild cognitive impairment, often a precursor to Alzheimer’s disease. “We’re measuring blood vessels that can’t be seen during a regular eye exam and we’re doing that with noninvasive technology that takes high-resolution images of very small blood vessels within the retina in just a few minutes,” says Fekrat, senior author on the study. “We know that there are changes that occur in the small blood vessels of the brain in people with Alzheimer’s disease, and because the retina is an extension of the brain, we wanted to investigate whether these changes could be detected in the retina using a new technology that is less invasive and easy to obtain,” Fekrat continues. The study used a noninvasive technology called optical coherence tomography angiography (OCTA). OCTA machines use light waves that reveal blood flow in every layer of the retina. An OCTA scan could even reveal changes in tiny capillaries—most less than half the width of a human hair—before blood vessel changes show up on a brain scan such as
Could An Eye Exam Reveal Alzheimer’s Disease?
Study suggests loss of blood vessels in retina reflect changes in brain health
cognitively healthy adult adult with Alzheimer’s disease
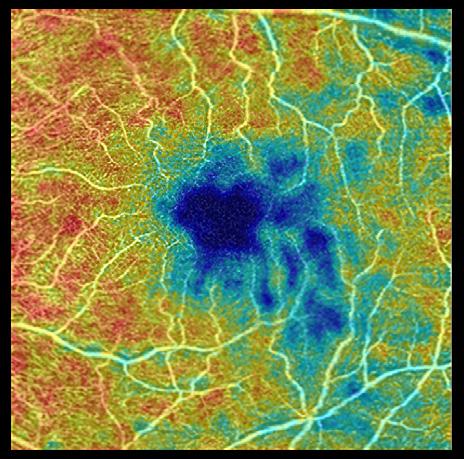
Blood vessels

FEWER MORE
an MRI or cerebral angiogram, which highlight only larger blood vessels. Such techniques to study the brain are invasive and costly. “These changes in blood vessel density in the retina likely mirror what’s going on in the tiny blood vessels in the brain. We are now studying whether these retinal changes can be detected before someone, who has a higher genetic risk for Alzheimer’s, develops signs of memory loss,” Grewal comments.
Promise for the Future Over 400 clinical trials for Alzheimer’s disease have failed and this may be due, in part, to the diagnosis of Alzheimer’s being made too late in the disease course. Alzheimer’s changes in the brain start about 20 years before symptoms of memory loss manifest. “Ultimately, the goal would be to use this technology to detect Alzheimer’s early, before symptoms of memory loss are evident, and enter participants into clinical trials earlier to study new Alzheimer’s treatments,” Fekrat said. Fekrat and Grewal and their team are collaborating with Duke computer engineers to train and test artificial intelligence deep learning models to
predict the presence of Alzheimer’s disease before symptoms start and differentiate Alzheimer’s from other types of dementia. Collaboration across disciplines is key to timely progress. Fekrat and Grewal’s clinical research team includes many individuals, such as:
Duke Ophthalmology Residents and Faculty— Atalie C. Thompson, MD, MPH; C. Ellis Wisely, MD, MBA; Pam Bhullar, MD; Michael Quist, MD; Nora Lad, MD, PhD Duke Medical School Students—Cason Robbins, Srinath Soundararajan, Delaram Mirzania, James Powers, Stephen P. Yoon, Bryce W. Polascik Duke Neurology—Cynthia Dunn, PA; Brenda Plassman, PhD; James R. Burke, MD, PhD Duke Engineering and Biostatistics—Dong Wang, PhD; Ricardo Henao, PhD; and Lawrence Carin, PhD Duke Geriatrics—Heather Whitson, MD
If you are interested in supporting this groundbreaking team and their work, contact Dr. Fekrat at 919-684-4524 to learn more.

