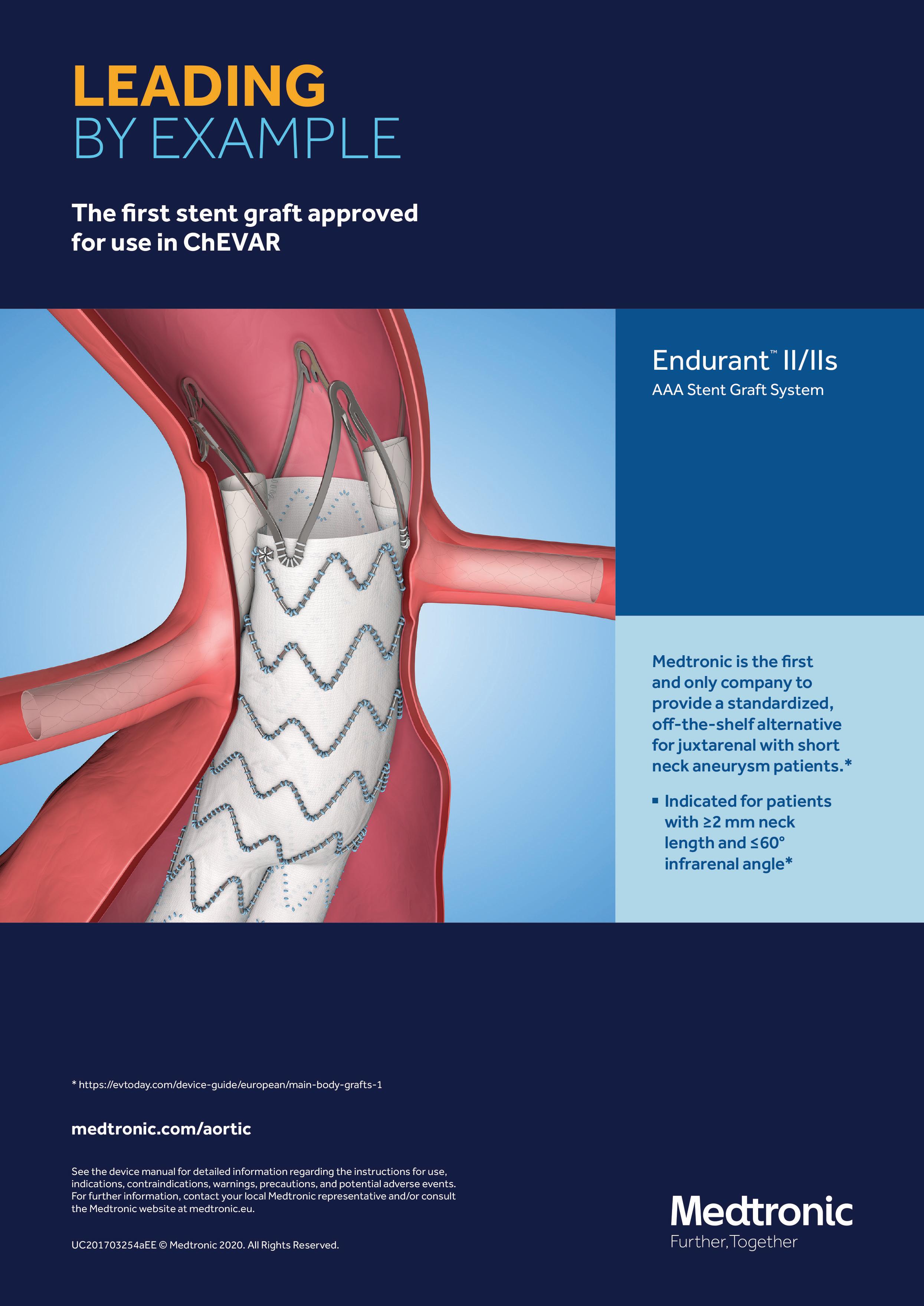
7 minute read
Latest clinical evidence on ChEVAR
Latest clinical evidence on ChEVAR: What is next?
Writing that clinical evidence on chimney endovascular aneurysm repair (ChEVAR) has “flourished” in the last decade, Gergana T Taneva highlights key elements of the most recent research on the technique.
CHEVAR HAS SHOWN comparable results to fenestrated EVAR (FEVAR) for the treatment of juxtarenal abdominal aortic aneurysms (jAAA).1–3 The clinical evidence on ChEVAR has flourished in the last decade, with an increasing number of key publications released within recent years.4–8 ChEVAR has gained popularity to the point that its role within the AAA treatment algorithm is considered complementary depending on a patient’s characteristics and aortoiliac anatomy.9 However, costeffectiveness issues, long-term outcomes, and procedural tips such as the deal with angulated renal arteries remain unknown. In this context, we present an overview of the latest evidence and publications on these appealing topics (Table 1). 1. Widely spoken of and much needed, a cost analysis and comparison of both techniques, ChEVAR and FEVAR, was performed, evaluating all elective and symptomatic patients treated at
St Franziskus Hospital in Münster,
Germany, for jAAA by single or double chimney (n=111) or by FEVAR with three fenestrations (n=37) between 2013 and
January 2017.10 The cost-effectiveness analysis was defined as the summary of material costs, in-hospital costs, and additional costs due to procedurerelated reinterventions. Index procedure and hospitalisation median costs were higher for FEVAR (€42,116 vs. €22,171, p<0.001). The median overall costs, including costs after reinterventions during follow-up, remain higher for FEVAR (€42,128 vs. €22,872, p<0.001) for a follow-up period of almost four years.10
Six patients (5.4%) in the ChEVAR group required readmission compared to three patients (8.1%) who required readmission for reinterventions in the FEVAR group (p=0.69). Both FEVAR and ChEVAR proved to be expensive and technicallydemanding interventions for the treatment of juxtarenal aortic pathologies.
However, ChEVAR was significantly more cost-effective compared to FEVAR at comparable readmission rates for reinterventions.11 2. Also highly-anticipated, and extension of the follow-up and long-term evaluation of the multicentric PERICLES Registry was performed analysing clinical and radiographic data from patients treated with ChEVAR between 2008 and 2014.12 A subgroup of 244 patients with 387 chimney grafts placed and follow-up of at least 30 months was used to analyse specific anatomic and device predictors of adverse events. In the subgroup, the technical success was 88.9%, while primary patency was 94%, 92.8%, 92%, and 90.5% at two-and-a-half years, three years, four years, and five years, respectively. Mean aneurysm sac regression was 7.8±11.4mm, p<0.0001. Chimney graft occlusion occurred in 24 target vessels (6.2%).
Late open conversion was required in
five patients for endograft To investigate the outcomes, we infection (n=2), persistent evaluated all elective patients type 1a endoleak (n=2), and treated at St Franziskus Hospital endotension (n=1). This in Münster, Germany, over nine analysis of the PERICLES years (January 2009–December
Registry provided the missing 2017) with placement of Advanta long-term experience on the V12 (Getinge) as chimney
ChEVAR technique. It showed graft in combination with the favourable results with over Endurant stent graft (Medtronic) half of the patients surviving as abdominal endograft.13 A total for more than five years. Up Gergana T Taneva of 116 patients were included, to 48 months’ follow-up, the with lining performed in 43 stented vessels remained patent in over vessels for 32 patients. Lining was not 92% of the cases. The absence of infrarenal performed to increase the radial force of neck and a proximal sealing zone diameter the covered stents. The subgroup analysis >30mm were significantly associated with revealed significantly higher primary long-term device-related complications and patency within the non-lined group with poorer outcomes in terms of persistent (96.9%) at one year versus the lined group type 1a endoleak. The evidence advocated (77.1%; p=0.001).13 Lining represented the anatomical limits of the technique, a risk factor for chimney graft occlusion demanding adequate preoperative planning (odds ratio 9.9; p=0.006).13 This singleand indication. centre nine-year ChEVAR experience 3. The cause of much speculation, chimney with more than 110 Advanta V12 chimney graft lining in the case of highly angulated stents showed durable results. However, renal arteries was evaluated as a risk factor lining in angulated renal arteries showed for occlusion. Lining for deployment of a significantly higher risk for chimney an additional stent and smoothening the graft occlusion.13 These data highlight the transition in a branched vessel are normally importance of finding new ways to achieve performed when the distal part of the better conformability of the stent grafts chimney graft is seen within an angulated within the target vessel. segment of the target vessel. Typically, The presented clinical evidence contributes an additional bare metal nitinol stent is to broaden the global knowledge on the placed to improve the flexibility and even chimney technique, clarifying several major the transition. In order to minimise the issues such as cost comparison with FEVAR, reduction of the patent lumen by deploying long-term performance evidence of the an additional device, we preferred the use largest related registry, and the issue of lining of bare metal instead of covered stents. contributing to stent graft occlusion. Further
Topic Cost-effectiveness analysis of chimney/snorkel versus fenestrated endovascular repair
Journal
Year of publication
Main findings J Cardiovasc Surg (Torino)
2020
ChEVAR was significantly more cost-effective at comparable reintervention rates Long-term chimney EVAR experience within the PERICLES registry
J Vasc Surg
2020
ChEVAR showed favourable longterm patency and patient survival rates Use of balloonexpandable chimney grafts is durable, though caution is required when lining angulated renal arteries
J Endovasc Ther
2020
Lining represented a risk factor for chimney graft occlusion
Table 1. Overview of the latest evidence and publications on cost-effectiveness issues, long-term outcomes, and lining of chimney stent grafts. research is paramount to expand and confirm the cited findings.
References
1. Taneva GT, Criado FJ, Torsello G, Veith F. Results of chimney endovascular aneurysm repair as used in the PERICLES
Registry to treat patients with suprarenal aortic pathologies 2014: 1–8. Doi: 10.1016/j.jvs.2019.08.228. 2. Donas KP, Lee JT, Lachat M, et al. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies:
The PERICLES registry. Ann Surg 2015; 262(3): 546–52. Doi: 10.1097/SLA.0000000000001405. 3. Ronchey S, Fazzini S, Scali S, et al. Collected transatlantic experience from the PERICLES Registry: Use of chimney grafts to treat post-EVAR type Ia endoleaks shows good midterm results 2018. Doi: 10.1177/1526602818782941. 4. Ballesteros-Pomar M, Taneva GT, Austermann M, et al.
Successful management of a type B gutter related endoleak after chimney EVAR by coil assisted onyx embolisation.
EJVES Short Reports 2019; 42: 38–42. Doi: 10.1016/j. ejvssr.2018.12.002. 5. Bosiers MJ, Tran K, Lee JT, et al. Incidence and prognostic factors related to major adverse cerebrovascular events in patients with complex aortic diseases treated by the chimney technique. J Vasc Surg 2018; 67(5): 1372–9. Doi: 10.1016/j. jvs.2017.08.079. 6. Donas KP, Criado FJ, Torsello G, et al. Classification of chimney EVAR-related endoleaks: Insights from the
PERICLES Registry. J Endovasc Ther 2017; 24(1): 72–4. Doi: 10.1177/1526602816678994. 7. Torsello G, Usai MV, Scali S, et al. Gender-related outcomes of chimney EVAR within the PERICLES Registry. Vascular 2018; 26(6): 641–6. Doi: 10.1177/1708538118797448. 8. Donas KP, Usai MV, Taneva GT, et al. Impact of aortic stent-graft oversizing on outcomes of the chimney endovascular technique based on a new analysis of the
PERICLES Registry. Vascular 2019; 27(2): 175–80. Doi: 10.1177/1708538118811212. 9. Donas KP, Eisenack M, Panuccio G, et al. The role of open and endovascular treatment with fenestrated and chimney endografts for patients with juxtarenal aortic aneurysms. J Vasc Surg 2012; 56(2): 285–90. Doi: 10.1016/j. jvs.2012.01.043. 10. Taneva GT, Donas KP, Pitoulias GA, et al. Cost-effectiveness analysis of chimney/snorkel versus fenestrated endovascular repair for high-risk patients with complex abdominal aortic pathologies. J Cardiovasc Surg 2019. Doi: 10.23736/S00219509.19.11146-9. 11. Taneva GT, Donas KP, Pitoulias GA, et al. Cost-effectiveness analysis of chimney/snorkel versus fenestrated endovascular repair for high-risk patients with complex abdominal aortic pathologies. J Cardiovasc Surg (Torino) 2019; 60(0): 1–6.
Doi: 10.23736/S0021-9509.19.11146-9. 12. Taneva GT, Lee JT, Tran K, et al. Long-term chimney/snorkel
EVAR experience for complex abdominal aortic pathologies within the PERICLES Registry. J Vasc Surg 2020;(S07415214(20)32496-4). Doi: 10.1016/j.jvs.2020.10.086. 13. Taneva GT, Fazzini S, Pipitone MD, et al. Use of stainlesssteel , balloon-expandable chimney grafts is durable though caution is required when lining angulated renal arteries. J Endovasc Ther 2020; 27(6): 902–9. Doi: 10.1177/1526602820948260.
Gergana T Taneva is a vascular surgeon at the University Hospital Puerta de Hierro in Madrid, Spain, and research leader of the Research Centre at Asklepios Clinic Langen, Göthe-University Frankfurt, Langen, Germany.
The content of these articles is meant for general information purposes only and should not be construed as a promotion or solicitation for any product or for an indication for any product which is not authorized by the laws and regulations of the country where the reader resides. The views and opinions expressed therein should be interpreted as personal views. They are completely independent and do not necessarily reflect the opinions of Medtronic. As a health care provider, you should use your own professional judgment in evaluating the information provided and rendering any medical opinion or advice. Your use of and any reliance on such information is solely at your own risk and responsibility. Medtronic makes no representation or warranty, express or implied, including any warranty of accuracy, completeness, or usefulness of any information described in these articles and Medtronic assumes no liability for the use of the information in any manner whatsoever. See the device manual for information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events.