2 minute read
PHYSICS HELPS
YOU STAY COZY AND COOL IN COLD WEATHER —- WHY DO PUFFY JACKETS WORK?
One of the less pleasant aspects that usually accompanies winter is perhaps the chilling coldness, which was often followed by the necessity of wearing thick clothes. Recall: how many gentle chidings from Ms. Keddy or a more serious admonition from Mr. Belcher have been about the importance of wearing jackets? You would probably say too many! But allow a stage for science to speak its reason and your answer would probably change into: never too many!
Advertisement
A question naturally follows the prior situation where a human being is simply trying to stay warm: how do we preserve heat? Well, in order to answer this question, let me first explain what “heat” is. According to thermodynamics, the study of different forms of energy and their relationship, “heat” is the transfer of thermal energy that is due to a difference in temperature. So in order for a transfer of energy to occur from your body into the lonely big world, you need to be warmer than the environment, or, as how the universe likes to operate —- the other way around. But then how does heat get transferred exactly?
There are three ways:
1). Conduction
2). Convection
3). Radiation
And the reason why jackets can keep us warm has mostly to do with the first way of heat transfer. First thing first, conduction mostly happens through molecular collisions. As some of you may already know, the higher the temperature, the faster the movements of the molecules. As the molecules bump into each other as they mindlessly meander in the air, a motion almost like the domino effect spreads out and the originally slower molecules would now have gained more speed while the faster ones would slow down due to the collisions. A poorly conjured analogy is offered: imagine a roomful of kindergarteners.
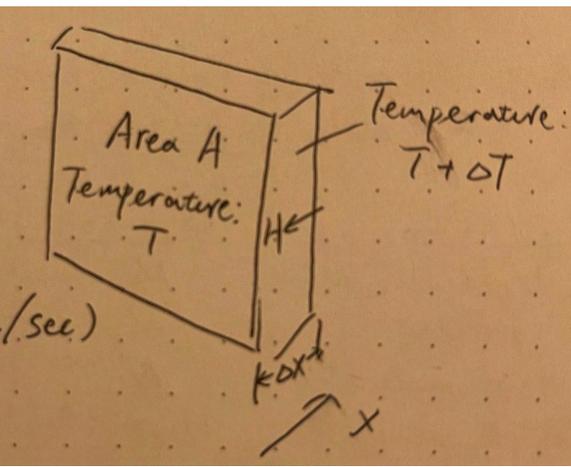
Given their energetic nature, they humorously bump shoulders and fist with each other, sometimes mischievously stepping on the feet of others. While outside the room are the solemn ninth graders, with their advantage of age and a mystified air of maturity they are cool and composed. Let’s then assume the kindergarteners are released. Now they not only just messed with their peers, but the bolder ones began to approach the ninth graders. Slowly but surely, this jolly and active energy is being spread even to the older students and everyone began to interact with each other physically. The rate of how fast is this energy/heat is being spread out is captured by this formula:
H is the heat flow rate, k is a constant that is determined by the kind of material in which the activity happens, also called “thermal conductivity,” dT is the change in temperature and dx the thickness.
A noteworthy fact is that the thermal conductivity of air is remarkably small, making the flow of heat rather difficult (see chart).

Before you read on, I recommend you to grab your puffy jacket and try to squeeze it. The jacket would get smaller … then after you remove the force it would expand right back again, right? The air pockets are doing most of the restoring work. Your jacket does not keep your warm by being thick and dense to prevent cold air from going in through the fabric, but instead it traps air inside to reduce the heat flow rate.
The next time you are heading to DA for sports or sprinting to the dining hall only to find you have to wait in the freezing weather for some time, remember to grab your jacket, remember that the thermal conductivity of air is less than one, remember that all jackets exist for a reason and most importantly, remember that PHYSICS keeps you warm!
-Cleo X. '23










