
26 minute read
Education and Continuing Professional Development (CPD
from Gamma Gazette Winter Edition 2020 - The Australian and New Zealand Society of Nuclear Medicine
by ANZSNM
Education & CPD Case Study
Author: Stephanie Ruyten, Monash Health
INTRODUCTION
Technetium labelled red blood cell studies are commonly performed in Nuclear Medicine to evaluate red blood cell distribution within the body to image GI bleeds, hepatic haemangiomas, functional splenic tissue and gated cardiac imaging. The radiolabelling of red blood cells involves the reduction of stannous ion converting pertechnetate to the oxidative state Tc 4+ allowing binding to the haemoglobin in red cells.
The three common labelling techniques include in-vivo, where red blood cells are labelled within the body via an injection of non-radioactive stannous pyrophosphate (PYP) 30 minutes prior to pertechnetate administration. In-vitro, performed using of a biohazard cabinet where blood washing occurs, requires spinning the red blood cells to remove free pertechnetate through the supernatant; or alternatively in-vitro labelling without a cabinet using an Ultra-Tag kit (Callahan, 2006). Lastly, in-vivtro, the modified in-vitro method, involves a combination of both in-vitro and in-vivo techniques. This requires PYP to be injected into the patient and after 30 minutes blood is taken in a syringe containing acid citrate dextrose (ACD) and pertechnetate. The blood is then incubated for a further 30 minutes and reinjected into the patient as radiolabelled red blood cells (Ziessman, O'Malley, Thrall, & Fahey, 2014). The three techniques have their advantages and disadvantages including blood handling, the use of a biohazard cabinet, labelling efficiency and cost; these are contributing factors to the following case.
GAMMA GAZETTE CONTENT SUBMISSIONS
Scientific submissions on all aspects of nuclear medicine are encouraged and should be forwarded to the Secretariat - instructions for authors published at https://www.anzsnm.org.au/activities/gamma-gazette-content-submission-and-guidelines/
Letters to the Editor or points of view for discussion are also welcome.
If original or public domain articles are found and considered to be of general interest to the membership, then they should be recommended to the Editor who may seek permission to reprint.
The ANZSNM Gamma Gazette is published three times a year. Deadlines for each issue of the journal can be found on our website anzsnm.org.au gamma GAZETTE 2020 Winter Edition
Education & CPD Case Study
(Continued)
Various drugs and devices can alter the binding of stannous ion to red blood cells, impacting adequate labelling. These drugs and devices include heparin, methyldopa, chemotherapeutic agents, whole blood transfusions, intravenous catheters, propranolol and iodinated contrast (Callahan, 2006). It is important to take note of these to determine a labelling technique that provides the best labelling outcome. This case study provides a comparison between the two in-vitro methods and how the labelling efficiency can alter due to pre-existing factors.
CASE STUDY HISTORY
A 47-year-old Caucasian male presented to the department with previously known cystic lesions in segments 7/8 of the liver; found within the superior portion of the right liver lobe. These lesions were identified on CT and ultrasound, with recent CT revealing changes to the lesions requiring assessment for a potential haemangioma.
INITIAL IMAGING
On the patient’s original presentation, the Ultra-Tag labelling method was used. Dynamic, early (figure 1) and delayed (figure 2) statics were acquired, where delayed images were acquired 3 hours post injection.
The early static (figure 1) identified expected uptake within the liver and spleen. The delayed statics (figure 2) however, demonstrate tracer distribution within the stomach, thyroid and salivary glands indicating the presence of free pertechnetate and therefore confirming a low yield red blood cell label. After reviewing the labelling process and finding no errors; this prompted an investigation into the patients past medical history.
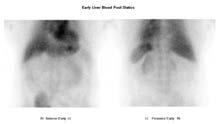
Figure 1: Early static images
Figure 2: Delayed statics at 4 hours post injection
Education & CPD Case Study
(Continued)
REPEAT IMAGING
The patient returned two months later for further investigation of a potential haemangioma. On this instance an in-vitro label was performed within the biohazard cabinet, washing out any remaining free pertechnetate. The invitro label produced a labelling efficiency of 87%, where normal is considered >80% (Callahan, 2006). Dynamic, early (figure 3) and delayed (figure 4) statics were again performed along with a delayed SPECT/CT (figure 5), all demonstrating normal radiolabel distribution. As evident in figure 5, there was no free pertechnetate using the in-vitro labelling method, providing a diagnostic conclusion of a negative scan.
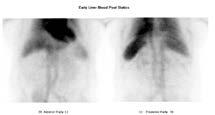
Figure 3: Early statics.
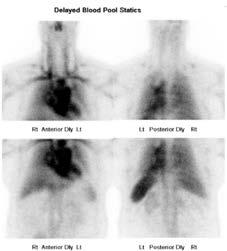
Figure 4: Delayed Statics 4 hours post injection.
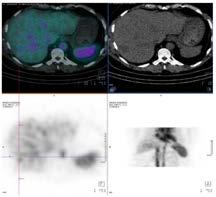
Figure 5: Delayed SPECT/CT 4 hours post injection.
DISCUSSION
When assessing the Ultra-Tag labelling technique, it was confirmed to have been performed to manufacturer specifications, therefore why was the in-vitro method successful and not Ultra-Tag?
On further investigation of the patient’s medical history it was identified that he had undergone a renal transplant in January of 2016 and was therefore taking immunosuppressive medication mycophenolate and tacrolimus post-transplant.
Education & CPD Case Study
(Continued)
Tacrolimus is a calcineurin inhibitor immunosuppressant prescribed to patients post organ transplant to prevent and treat allograft rejection; it has a half-life between 3.5 and 40.5 hours where most of the drug metabolises in the liver (Prescriber, 1998). Tacrolimus is administered post organ transplants to inhibit calcium dependent events such as gene transcription and cell degradation (Barbarino, Staatz, Venkataramanan, Klein, & Altman, 2013). It is highly bound to red blood cells and 99% bound to plasma proteins with a half-life of 3.5 to 40.5 hours (Australian Prescriber, 1998). The pharmacokinetics of this drug alters depending on the transplanted organ (Marfo, Altshuler, & Lu, 2010).
Tacrolimus elicits immunosuppressant effects via binding to immunophilins known as FK-binding proteins. These proteins interfere with the activity of calcineurin preventing dephosphorylation to occur, thereby stopping T-cell proliferation (Barbarino J. M., Staatz, Venkataramanan, Klein, & Altman, 2013). Tacrolimus is approximately 99% bound to plasma proteins; its concentration is dependent on erythrocyte distribution with a blood to plasma ratio of 15:1 (Venkataramanan, et al., 1995). The red blood cell distribution of tacrolimus is influenced by factors such as hematocrit, temperature and protein concentration (Marfo, Altshuler, & Lu, Tacrolimus Pharmacokinetic and Pharmacogenomic Differences between Adults and Pediatric Solid Organ Transplant Recipients, 2010). Tacrolimus is administered orally in capsule form with a dose of 75-300 mcg/ kg daily in 2 doses, the medication is adjusted according to patient response and whole blood concentration (Australian Medicines Handbook Pty Ltd, 2020).
The high affinity of Tacrolimus in red blood cells resulted in competitive binding between the drug and radiolabel, thereby increasing overall percentage of unbound free pertechnetate (Zahir, McCaughan, Gleeson, Nand, & McLachlan, 2003). Unlike the extensive washing process involved in the in-vitro method, blood labelling using the Ultra-Tag kit doesn’t allow the removal of unbound pertechnetate, increasing competitive binding (Spicer, Hladik, & Mulberry, 1999). It is not possible for a patient on immunosuppression therapy to cease their treatment, therefore methods need to be adapted to best suit the situation.
CONCLUSION
This case study demonstrates that it is important to tailor the labelling technique for each individual patient; in particular a comprehensive drug history and questionnaire to eliminate causes for poor red cell labelling is crucial, especially in patients on immunosuppressive medications. In this instance, in-vitro labelling with a biohazard cabinet was the most effective label method, allowing the removal of free pertechnetate to provide a diagnostic outcome. As such, patients on Tacrolimus should ideally have their scan performed in a department that has the equipment and ability to perform blood labelling in a biohazard cabinet.
References
Australian Prescriber. (1998, September 01). Tacrolimus. NPS Medicine Wise, 80(3), 21. doi:DOI: 10.18773/austprescr.1998.076; Barbarino, J., Staatz, C. E., Venkataramanan, R., Klein, T. E., & Altman,
R. B. (2013, October). PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics, 23(10), 563-585. doi:10.1097/FPC.0b013e328364db84
Callahan, R. J. (2006). Radiolabeled Red Blood Cells: Methods and Mechanisms. (J. Norenberg, Ed.) The University of New Mexico Health Sciences Center College of Pharmacy, 12(1), 24.
Retrieved from https://pharmacyce.unm.edu/nuclear_program/freelessonfiles/Vol12Lesson1.pdf; Marfo, K., Altshuler, J., & Lu, A. (2010, September 09). Tacrolimus Pharmacokinetic and
Pharmacogenomic Differences between Adults and Pediatric Solid Organ Transplant Recipients. Pharmaceutics, 2(3), 291-299. doi:10.3390/pharmaceutics2030291; Spicer, J. A., Hladik, W. B., &
Mulberry, W. E. (1999). The Effects of Selected Antineoplastic Agents on the Labeling of Erythrocytes with Technetium-99m Using the UltraTag RBC Kit. Journal of Nuclear Medicine Technology,
27(2), 132-135.
Venkataramanan, R., Swaminathan, A., Prasad, T., Jain, A., Zuckerman, S., Warty, V., . . . Starzl, T. (2012, October 19). Clinical Pharmacokinetics of Tacrolimus. Clinical Pharmacokinetics, 29, 404-430.
doi:10.2165/00003088-199529060-00003; Zahir, H., McCaughan, G., Gleeson, M., Nand, R. A., & McLachlan, A. J. (2003, March 21). Factors affecting variability in distribution of tacrolimus in liver
transplant recipients. British Journal of Clinical Pharmacology, 57(3), 298-309. doi:10.1111/j.1365-2125.2003.02008.x; Ziessman, H. A., O'Malley, J. P., Thrall, J. H., & Fahey, F. H. (2014). Nuclear
Medicine: The Requisits. Philadelphia: Saunders, Elsevier.
Education & CPD Case Study
Two’s a crowd - Duplex Kidney Case Study
Author: Courtney King, Royal Melbourne Institute of Technology
BACKGROUND AND PATIENT PRESENTATION
A 53-year-old male presented to his GP with right flank pain. He was referred to get a CT scan to investigate this further.
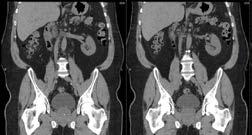
The CT images helped doctors to discover that the patient had a duplex kidney, which can be seen in figure 1. He was born with two pelvicalyceal systems in his right kidney 1 . This condition occurs due to two ureteral buds arising and developing into ureters during foetal development 2 . Duplex kidneys are often asymptomatic 2 ; however, this was clearly not this case for this patient.
Vesicoureteral reflux (VUR) is a common condition associated with duplex kidneys 2 . It involves urine flowing from the bladder into the ureter and pelvis of the kidney. It was important to rule out this potential diagnosis as it can lead to renal scarring, hypertension and renal failure 2 .
The patient was further referred to get a nuclear medicine renal scan. This was to determine whether this patient had an obstruction or VUR, as well as provide accurate information about the cortical function of each moiety.
INVESTIGATION
99m Tc-MAG3 was the best radiopharmaceutical to be used due to its excellent extraction rate of 55%, more than double that of 99m Tc-DTPA 1 . This characteristic is important in ensuring a high target-to-background ratio 4 .
Figure 1. CT of patient’s abdomen and pelvis
The patient was administered 384MBq of 99m Tc-MAG3. A dynamic acquisition of one frame per three seconds was acquired to show initial blood flow to the kidneys. This
was followed by a dynamic; one frame per minute for twenty minutes. This demonstrated the rate at which his kidneys were taking up and excreting the radiopharmaceutical, which is directly proportional to the rate at which urine is excreted. A one-minute pre micturition and one-minute post micturition images were then acquired. Lasix was not required due to the radiopharmaceutical clearance in the kidney.
RESULTS
The radiopharmaceutical distribution demonstrated no evidence of obstruction. The uptake was prompt and showed asymmetrical reduced cortical uptake in the right kidney compared to the left, particularly involving the lower region of the right kidney.
Education & CPD Case Study
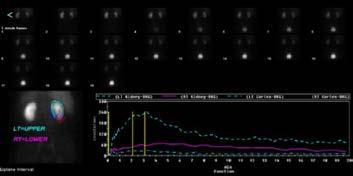
Figure 2. Renogram of upper and lower moieties in the right kidney.
The maximum uptake peak occurred at three minutes for both the left kidney and upper moiety of the right kidney, which is considered normal 4 . However, the lower moiety of the right kidney uptake was about a quarter of the uptake in the upper moiety. The excretion was also significantly slower, and an uptake peak was not seen.
The regions of interest indicated that the left kidney function is much greater, being 63% compared to 37% for the right, of which the upper pole contributes 80%.
DISCUSSION
This case highlights the usefulness of functional imaging. The diagnosis of differential function of the left kidney and right kidney and upper and lower moieties would not have occurred without having a nuclear medicine renal scan. The processing of this scan was different from routine renal scans as regions of interest were drawn around the upper and lower regions of the right kidney to determine the contribution of each moiety.
The reduced function of the right kidney may be the cause of the patients flank pain 2 . Flank pain along with incontinence, urinary tract infections and haematuria are common indications for a duplex kidney 2 .
Understanding that the patient’s lower moiety functions significantly less than the upper moiety can hopefully help the patient and doctors to determine a treatment plan. Recommended treatment for this patient may be a urethrectomy or lower pole heminephrectomy 2 . Surgical approaches can either be laparoscopic or open 2 . This would decrease his risk of developing urinary tract infections and VUR 2 .
References
REF 1. Mendichovszky, I., Solar, B., Smeulders, N., Easty, M., & Biassoni, L. (2012). Nuclear Medicine in Paediatric Nephro-Urology: An Overview. Seminars in Nuclear Medicine. 47(3):201-228. Doi:
10.1053/2016.12.002 ; REF 2.Davda, S. & Vohra, A. (2013). Adult duplex kidneys: An important differential diagnosis in patients with abnormal cysts. Journal of the royal society of medicine
short reports. 4(2):13. Doi: 10.1177/2042533312472126 ; REF 3. Liu, G., Ma, H., & Li, Y. (2012). Multilocular Renal Cell Carcinoma in Lower Pole Moiety of a Duplex kidney. International Journal
of Surgical Pathology. 20(6): 613-17. Doi: 10.1177/1066896912438098 ; REF 4. Ziessman, H., O’Malley, J., & Thrall, J 2015). The Requisites- Nuclear Medicine (4th ed). Boston, MA: Elsevier ; REF 5.
Ponto, J. (2012). Mechanisms of radiopharmaceutical Localization. University of New Mexico Health Sciences Centre. 16(4):21
Education & CPD Case Study
Pre-Lung and Heart Transplant V/Q Scan
Author: Courtney King, Royal Melbourne Institute of Technology.
A 15-year-old boy presented to the nuclear medicine department for a V/Q scan. This scan allowed doctors to assess the patient’s ventilation and perfusion. Lung transplants are associated with a significant risk, therefor the patient must undergo many tests to ensure that the transplant is a feasible option and to make sure that the patient’s condition is serve enough to require a transplant 1 . The tests not only look at the heart and lungs but also determine whether the patient has other conditions that could exclude them from being suitable for a transplant 2 .
The patient was born with an intraventricular septal defect, measuring 39mm. This created a bi-directional cardiac shunt. He was also diagnosed with cyanotic congenital heart disease, pulmonary hypertension and severe cardiomegaly, as shown in figures 1 and 2.
Figure 2: Transverse CT scan Retrieved from: The Alfred Hospital, Melbourne
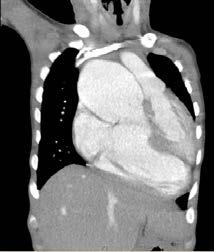
Figure 1: Coronal CT scan
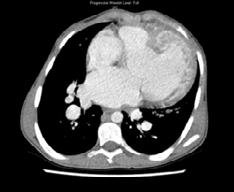
Retrieved from: The Alfred Hospital, Melbourne
INVESTIGATION
The patient was administered with 0.5k counts of technegas while laying supine under the gamma camera. This administered dose was below the standard ventilation dose due to his age and pulmonary hypertension. A SPECT image of the lungs was then acquired on a Siemens Symbia Evo gamma camera.
Education & CPD Case Study
This was followed by the administration of 86MBq of 99m Tc-MAA and another SPECT image of the lungs.
RESULTS
The ventilation and perfusion images reveal matched heterogeneity throughout both lungs. This is indicative
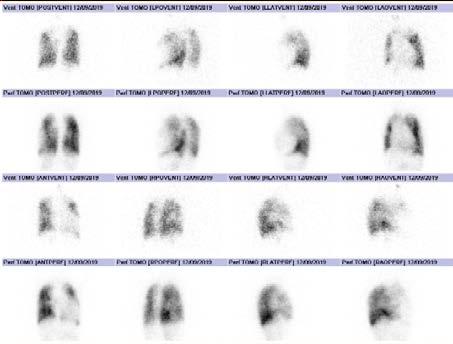
Figure 3: 3D Ventilation and Perfusion images Retrieved from: The Alfred Hospital, Melbourne
of pulmonary disorders such as pneumonia, chronic obstructive airway disease, infarction and atelectasis 3 . The matched pattern indicated that the patient does not have a pulmonary embolism 3 . Sub-diaphragmic radiopharmaceutical activity is seen in the perfusion images, which is expected in patients with a right-to-left cardiac shunt 2 . There is also an abnormally large cardiac shadow due to cardiomegaly. The contribution of each lung is different. After performing a quantitative analysis it was revealed that the right lobe of the lung contributes approximately 61% and the left lobe of the lung contributes 39%.
Education & CPD Case Study
The image quality is relatively low due to the low radiation dose used; however, the images are still diagnostic.
DISCUSSION
The journey that patients go through in order to be considered for a lung and heart transplant is lengthy and involves many health assessments and interactions with multiple healthcare professionals. Nuclear medicine plays an important role as patients must undergo both a V/Q scan and gated blood pool scan 1 .
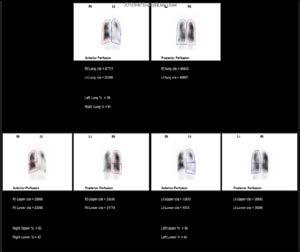
A V/Q scan is non-invasive and successfully identifies ventilation and perfusion defects within lungs, allowing doctors to definitively determine functional tissue 3 .
In addition to the ventilation and perfusion SPECT performed, a SPECT/ Figure 4: Perfusion Quantification Analysis, Retrieved from The Alfred Hospital, Melbourne CT would have given more accurate quantitative information in regard to the function contribution of each lobe of the lung and increased specificity by characterizing the causes of underlying perfusion defects 5
References 1. The Alfred. (2013) Heart Transplant: Patient information. Retrieved from: https://www.alfredhealth.org.au/contents/resources/patient-resources/HEART_ TRANSPLANT_BOOKLET.pdf 2. Heart & Lung Transplant Trust. (2014). Retrieved from: http://www.hlttv.org.au/transplant-journey/pre-transplant 3. Ziessman, H., O’Malley, J., & Thrall, J (2015). The Requisites- Nuclear Medicine (4th ed). Boston, MA: Elsevier 4. Moore, A., Wachsmann,J., Chamarthy, M., Panjikaran, L., Tanabe, Y., & Rajiah, P. (2018). Imagining of acute pulmonary embolism: an update. Cardiovascular diagnosis and therapy; 8(3): 225-243. Doi: 10.21037/cdt.2017.12.01 5. Roach, P., Schembri, G., & Bailey, D. (2013). V?Q Scanning Using SPECT and SPECT?CT. The Journal of Nuclear Medicine, 54(9): 1588-1596. Doi: 10.2967/ jnumed.113.12460
Education & CPD Case Study
Acetazolamide Challenge Cerebral Perfusion Scans: A Case Study Authors: Mia Bono and Wesley NG, Department of Molecular Imaging and Therapy, Austin Health
BACKGROUND
Moya Moya disease is a rare disease affecting <1 per 100000 patients, and involves the cerebral vasculature of the central nervous system, therefore increasing the risk of Transient Ischaemic Attack(TIA) 1 . Moya Moya can present as unilateral or bilateral with a range of symptoms including headache, vision changes, vertigo, altered gait, muscle weakness and lack of coordination that vary with age. Without treatment, the disease can be terminal due to a high risk of intracranial haemorrhage. Interestingly, the Japanese meaning of Moya Moya is “puff of smoke” relating to the anatomical image of tangled blood vessels formed in the brain to compensate for an occlusion 2 .
CASE REPORT
A 24-year-old female was admitted to the Emergency Department with a sudden lack of coordination and right upper limb weakness, most marked in the right hand when writing and combing her hair. The patient also experienced difficulty gripping objects in right hand. She denied any weakness in left side, headaches, vertigo or altered gait. She was prescribed 100mg of aspirin and 75 mg of clopidogrel for a minimum of three months for stroke prevention and symptoms began to improve over two weeks after initial admission. MRI and DSA were performed, which suggested Moya Moya disease, with bilateral internal carotid artery (ICA) stenosis and left middle cerebral artery (MCA) territory infarction.
Fifteen days later, the patient presented to the Department of Molecular imaging and Therapy for a SPECT brain scan with Acetazolamide challenge. Acetazolamide is used a cerebral vasodilator to induce an increase in cerebral blood flow, allowing for comparison with baseline brain perfusion. Brain perfusion imaging was performed as a baseline study with 708MBq 99m Tc-ECD administered intravenously, with the patient resting and eyes closed. SPECT/CT imaging was performed 30 minutes post-injection on the GE 670 Discovery DR. A low dose CT (120kV, 40mA axial CT) was performed for purposes of attenuation correction and anatomical localisation.
Two days later, the patient presented for the acetazolamide challenge imaging. 1000mg of acetazolamide was infused intravenously over 10 minutes. At 15 minutes post-acetazolamide infusion, 722MBq 99m Tc-ECD was administered intravenously with the patient resting and eyes closed. SPECT imaging was acquired 30 minutes post injection of 99m Tc-ECD. Images were also compared to a normal subject SPECT database using GE Neurostat 3D-SSP software, to assist in assessing perfusion changes.
FINDINGS OF BRAIN IMAGING
On baseline SPECT/CT imaging, a focal area of severe hypoperfusion was identified in the left dorsal frontal cortex with extensive mild hypoperfusion throughout anterior and lateral temporal cortex, sparing the medial frontal cortex. For the Acetazolamide challenge, SPECT images compared to the baseline study showed that there was significant worsening of the perfusion in the left frontal lobe and anterior temporal cortex with partial extension to the parietal lobe (Figure 1 - right page).

Charter Main is a newly created medical device company established to provide consumables for the safe and e cient delivery of Technegas from a Technegas generator into your patient for a V/P S.P.E.C.T. lung scintigraphy scan.
Charter Main also provides your servicing needs on the Technegas generator, supply masks, and spare crucibles.

While the company is new, its support team is not, having over 150 years of global experience in servicing, maintaining, commissioning, decommissioning, training and repairing Technegas and TechnegasPlus generators.
Carbon Crucible

The consumables work in the same way as those of the competitor. C.E. marked and fully approved by the responsible Australian regulatory authority, (ARTG No. 319260) for the e cient, safe and optimal generation and delivery of Technegas into a patients’ lungs.
Charter Main, however, o ers a fully registered crucible with 0.3μl bowl capacity
which is very convenient on low-activity days reducing the number of simmers required to achieve the activity desired for delivery to your patient.
Additionally, the generator can be e ciently serviced and maintained by our expert service engineers with over 35 years of experience, and its operating life cycle extended through routine servicing and regular attention to maintenance issues.
Plastic Delivery Sets
Education & CPD Case Study
The baseline images that were compared with the GE 3D-SSP Neurostat database, indicated that there was a significant reduction in perfusion in the left frontal lobe and lateral temporal cortex, which worsened on the post-acetazolamide images. The reduction in perfusion is linked to the Z-score values ranging from -4.5 to -6.5 that is shown on the 3D-rendered images with associated colours relating to Z-score value on the colour bar (Figure 2 - below).
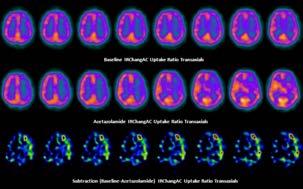
Figure 1: The SPECT/CT images illustrates extensive hypoperfusion in the left middle cerebral artery distribution with evidence of a small area of dorsal frontal cortical infarction.
Brain SPECT IRChangAC Images. Top Row: Baseline SPECT; Middle Row: Post-acetazolamide SPECT; Bottom Row: Subtraction of post-acetazolamide from baseline SPECT
Figure 2: The surface rendered images of the GE 3D-SSP Neurostat database illustrates significant reduction in Z-scores in the left frontal and lateral temporal lobes on post-acetazolamide scan Surface Rendered GE 3D-SSP Neurostat Images
Education & CPD Case Study
DISCUSSION
This case is particularly interesting as the SPECT/CT images allow us to appreciate the definitive lack of perfusion in the left MCA vascular territory when comparing baseline images to acetazolamide challenge images, consistent with left MCA territory ischemia with positive steel phenomena suggestive of Moya Moya disease. These findings correspond to the those on the MRI and DSA imaging. By confirming the presence of steel phenomena on the acetazolamide challenge cerebral perfusion scans, the patient will continue on the dual anti-platelet therapy until further follow up and continue to be closely monitored by the stroke prevention clinic. A possible treatment pathway in the future may consist of surgical revascularisation which aims to improve cerebral blood flow by anastomosis of a well perfused ‘donor’ artery and the recipient artery 3 .
References 1) Kim, J. (2016). Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. Journal Of Stroke, 18(1), 2-11. doi: 10.5853/jos.2015.01627 2) Abdulgafoor M. Tharayil, Adel E. Ahmed Ganaw, Nissar Shaikh, Sujith M. Prabhakaran, Arshad H. Chanda, Simi Praveen, Ajith Kumar Choran and Qazi Zeeshan ul Haq (August 20th 2019). Moyamoya Disease: A Rare Vascular Disease of the CNS, Vascular Malformations of the Central Nervous System, Bora Gürer and Pinar Kuru Bektaşoğlu, IntechOpen, DOI: 10.5772/intechopen.88770. 3) Kim, J. (2016). Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. Journal Of Stroke, 18(1), 2-11. doi: 10.5853/jos.2015.01627
Education & CPD What’s that
What's That? The Case of The Missing Lung
Author: Bryce Drowley, Monash Health
INTRODUCTION
A 23-year-old patient presented with shortness of breath and haemoptysis for investigation. She initially was investigated with CTPA which yielded non-diagnostic result due to poor contrast opacification of pulmonary vasculatures secondary to altered cardiopulmonary vascular anatomy. No further relevant medical history was provided from the patient prior to the commencement of the V/Q scan.
Technegas was administered to the patient until 0.8 –1 kct/sec was achieved. After acquiring 8 static views for the ventilation images, 167MBq of 99m Tc MAA was administered intravenously in the right cubital fossa and the same views were acquired in reverse order. Is there any immediate concern?

Figure 1: Nuclear medicine V/Q planar statics.
METHOD
Technegas is produced by instilling 0.1-0.2mL of 99m Tc Sodium pertechnetate into a carbon crucible placed in between two electrodes which are fixed in the Technegas machine. This carbon crucible is then heated to approximately 2500 degrees Celsius in the presence of Argon gas 1 . Once burnt, the chamber consists of small spherical particles which are inhaled by the patient via the Patient Administration Set and then trapped in the alveoli of the lungs 2 . Particle size of 99m Tc MAA is also important. When particles of 20 - 40 µm are administered intravenously, they are trapped in the capillary beds of the lungs and can therefore be imaged 3 .
Gamma Gazette is the official publication of the Australian and New Zealand Society of Nuclear Medicine.



Founded in 1969, the ANZSNM is the major professional Society for those practising Nuclear Medicine in Australia and New Zealand.
AVA I L A B L E I N PRI NT & D I G ITAL Don't miss all the latest Free for members
Case Studies
Papers & Abstracts
Industry Specific Reports
Industry News
Events and Awards
Member Profiles R E A D I T T O DAY anzsnm.org.au
Education & CPD What’s that
FINDINGS
There was relatively homogenous decreased uptake of tracer in the right lung on the perfusion images with preserved ventilation to both lungs. The differential diagnoses include isolated right proximal pulmonary trunk embolus or mediastinal mass compressing the right proximal pulmonary trunk.
However, once a thorough patient history was conducted, it was discovered that the patient had a Fontan’s procedure as a child for his hypoplastic left heart syndrome.
BACKGROUND OF FONTAN’S PROCEDURE
A Fontan’s procedure, or Fontan’s circulation is a tedious surgery to re-direct blood from the lower body straight to the lungs. This is completed by disconnecting the inferior vena cava (IVC) from the right atrium of the heart and reattaching to the inferior side of the pulmonary artery. The superior vena cava (SVC) is also disconnected from the right atrium and connected to the superior side of the pulmonary artery 4 .
The reason a Fontan’s procedure was completed for this patient was due to an unbalanced AV septal defect which resulted in a small left ventricle and a dominant right ventricle. This caused a compromise at the origin of the right pulmonary artery (RPA), therefore not allowing enough blood to be pumped into the lungs.
DISCUSSION
Figure 2 – Fontan’s Circulation 4
The reason that only one lung was perfused was un-apparent at first. However, after visualising the patient’s anatomy on their previous CT scan, the pathway of blood flow became much easier to understand. As you can see in Figure 3 (right page), the SVC tracks down and enters the RPA superiorly. Similarly in figure 4 (right page), the IVC is seen to track upwards into the inferior side of the RPA.
Due to the significant difference in blood volume supplied via the IVC versus the SVC, there is a pressure gradient difference. The increased volume of blood would cause a greater pressure in the IVC and therefore push any blood from the SVC into the left lung. Hence any blood supplied from the IVC would then track into the right pulmonary artery and eventually into the right lung.
Education & CPD What’s that
Figure 3. Axial slice of CTPA at level of SVC.
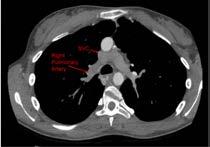
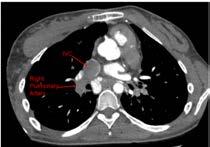
Figure 4. Axial slice of CTPA at level of IVC.
In order to perfuse the right lung upon injection, ideally, the IVC needs to supply the lungs with the 99m Tc MAA. This can be achieved by cannulating and injecting in a lower limb. This technique has been supported by CT imaging modalities for CTPA and angiography which both use inferior limb and superior limb injections of contrast5. The same technique can be utilised for VQ scan as half of the dose can be administered in an upper limb and the other half in a lower limb. This will then eliminate the need to correctly time the injections of contrast in CT, as 99m Tc MAA is trapped in the capillary beds of the lungs.
CONCLUSION
The Fontan’s procedure is a rare surgery which can affect the pathway of 99m Tc MAA. In order to combat these issues next time, a more thorough patient history would greatly benefit the patient’s diagnosis. Once a more thorough patient history is gathered, an altered VQ procedure would see a dual injection of tracer in an upper limb and lower limb in order to perfuse both lungs. Thus avoiding a false-positive finding.
References
1. Howarth, D., Lan, L., Thomas, P., & Allen, L. (1998). 99mTc Technegas Ventilation and Perfusion Lung Scintigraphy for the Diagnosis of Pulmonary Embolus. Society Of Nuclear Medicine, 40(4). 2. Lloyd, J., Shields, R., Taylor, C., Lawson, R., James, J., & Testra, H. (1995). Technegas and Pertechnegas particle size distribution. European Journal Of Nuclear Medicine, 22(5), doi: 10.1007/bf00839062 3. Draximage. (2019). DRAXIMAGE® M A A. Retrieved 10 December 2019, from https://www.accessdata.fda.gov/drugsatfda_docs/ label/2009/017881s010lbl.pdf. 4. Heart Information Center: Heart Anatomy | Texas Heart Institute. (2019). Retrieved 5 November 2019, from https://www.texasheart.org/hearthealth/heart-information-center/topics/heart-anatomy/ 5. Sandler, K., Markham, L., Mah, M., Byrum, E., & Williams, J. (2014). Optimizing CT angiography in patients with Fontan physiology: singlecenter experience of dual-site power injection. Clinical Radiology, 69(12), e562-e567. doi: 10.1016/j.crad.2014.09.011






