

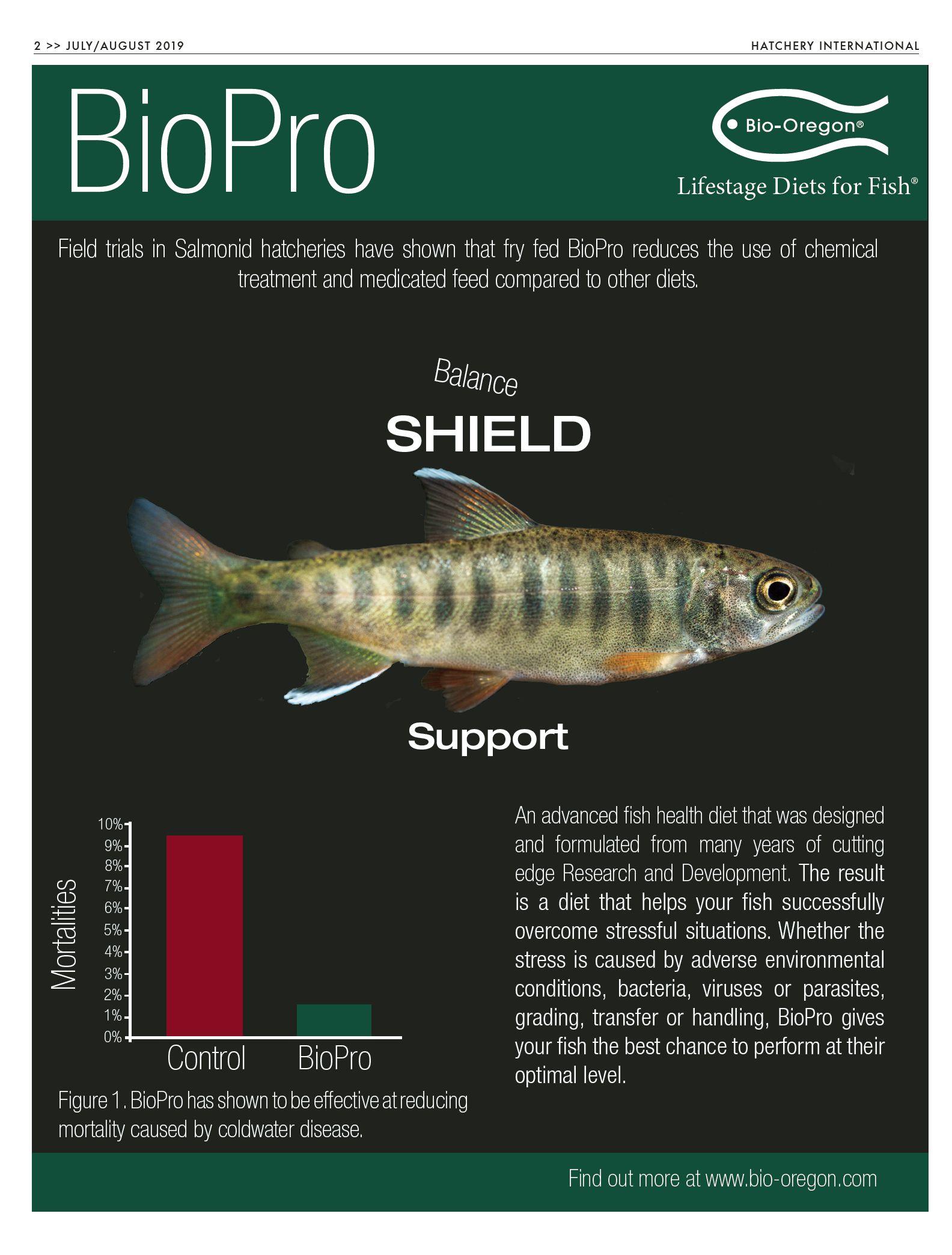
Researchers show promise for Blue crab farming
BY BONNIE WAYCOTT
Blue crabs (Callinectes sapidus) have played an important part in Mississippi’s history and economy for years. Consumers enjoy the species as appetizers and seafood products, but blue crab populations in the wild are declining, putting a strain on local fishermen.
Established in the early 2000s, the University of Southern Mississippi’s Blue Crab Hatchery is working to make farmed blue crabs a reality. It’s hoped that these efforts will allow fishermen to supplement their income by developing fisheries based on cultured blue crabs.

In the U.S., a blue crab hatchery at the University of Southern Mississippi is showing significant potential for diversifying the blue crab industry
“The idea took off when wild crab harvests in Chesapeake Bay were really low,” said Dr. Kelly Lucas, aquaculture director at the Thad Cochran Marine Aquaculture Center in Ocean Springs, Mississippi.
“The University of Maryland worked out techniques to mass-produce blue crab larvae to experiment with supplanting the wild harvest in the Bay. Our hatchery was established using protocols developed by the Maryland facility with modifications to those protocols, based on data gained from years of experimentation.”
continued on page 22
New RAS for salmon eggs opens in Norway
After just over 12 months of construction, Benchmark’s new land-based salmon egg production facility, SalmoBreed Salten in Sørfold, Norway, officially opened on May 22nd. The facility is touted by the company as the “most advanced land-based facility for the production of salmon eggs in the world.”
Located in northern Norway, SalmoBreed Salten has the capacity to produce 150 million eggs annually, the equivalent of approximately half a million tonnes of harvested salmon. The land-based site is designed to hold stock from egg to final mature broodfish resulting in the highest standards of biosecurity in the industry.
continued on page 30

RAStech 2019 debuts as industry touts bright prospects for RAS
BY MARI-LEN DE GUZMAN
WASHINGTON, D.C. – Recirculating aquaculture systems (RAS) and developments in biotechnology will help address the world’s increasing food supply challenges, said Sylvia Wulf, president and CEO of Aquabounty in her keynote address at RAStech 2019.
“We have to be able to steward the environment in a different way moving forward,” Wulf told 275 attendees at the conference held in Washington, DC, on May 13 and 14. Sustainable food production through RAS will enable fish farming wherever the consumers are, Wulf said.
Aquabounty’s AquAdvantage salmon is the first genetically engineered seafood that passed regulatory approvals from both Canada and the U.S. for commercial production. They have been developed to be raised in RAS environments, alleviating concerns about fish escapes that can impact wild fish populations, Wulf said.
“In 30 years, we will have two billion more people to feed,” Wulf said in her keynote. “I believe a hundred per cent of seafood growth is going to come from aquaculture, and RAS is going to become increasingly important.”
continued on page 28
Mail Agreement #PM40065710
UNDELIVERABLE CANADIAN ADDRESSES TO 111 Gordon Baker Road, Suite 400, Toronto, ON M2H 3R1
Female crabs carry their eggs externally, and each egg mass or “sponge” contains ~2 million eggs. Credit: Harriet Perry, senior research scientist, professor emerita, The University of Southern Mississippi (USM)
FROM THE EDITOR
VOLUME 20, ISSUE 4 | JULY/AUGUST 2019
Editor Mari-Len De Guzman, mdeguzman@annexbusinessmedia.com
Regular Contributors Lynn Fantom, Ruby Gonzalez, Ron Hill, Matt Jones, Colin Ley, Liza Mayer, John Mosig, Vladislav Vorotnikov, Tom Walker, Bonnie Waycott
Advertising Manager Jeremy Thain, jthain@annexbusinessmedia.com
Tel. +1-250-474-3982, Toll-Free (North America) 1-877-936-2266
Circulation Manager Barbara Adelt, badelt@annexbusinessmedia.com
Tel: 416-442-5600 ext. 3546
Production Svetlana Avrutin, savrutin@annexbusinessmedia.com
COO Scott Jamieson, sjamieson@annexbusinessmedia.com
South, Simcoe, ON N3Y 4N5
Hatchery International is published six times a year by Annex Business Media
The authority for statements and claims made in Hatchery International is the responsibility of the contributors. Reference to named products or technologies does not imply endorsement by the publisher.
A subscription to Hatchery International (six issues) is $37.00 within Canada, $37.00 US within North America and $47.50 US outside North America. To subscribe visit our website at www.hatcheryinternational.com
Subscriptions Angie Potal, apotal@annexbusinessmedia.com
Tel: 416-410-5113 • Fax: 416-510-6875 or 416-442-2191
The future is now
n every industry one of the challenges for the future has always been developing and retaining the skills and talent that would propel the industry forward and ensure consistent growth. Aquaculture is no different.
IThis relatively young industry boasts some of the smartest minds and most experienced professionals from across the globe, who have spent decades perfecting their craft and accumulating valuable knowledge that can only be beneficial to such a growing industry. As with other industries, we are faced with a huge generation of baby boomers that are getting ready for some well-deserved retirement –or semi-retirement.
A recent article from Investopedia estimates around 10,000 baby boomers are reaching retirement age per day. About 21 percent of people employed in Canada are 55 years old or older. The population of adults in retirement age (those 65 years or older) will comprise nearly 30 percent of the population in the European Union by the year 2080. In Asia, the outlook is very similar. Almost 28 percent of Japan’s total population are made up of people over the age of 65. In China, the declining number of young adults and the aging population are causing labour shortages in certain industries.
The reality is that a huge chunk of great minds and skills will be leaving the industry in droves over the next 10 years. The hope is that they will have passed on their knowledge to their younger counterparts to continue the watch and help take the profession and the industry to the next level. Succession planning in every organization should be an essential part of the mentoring process for any young professional joining this industry.
As much as we celebrate the contributions of our veteran aquaculture professionals, we also want to applaud the young men and women in the industry who are truly making a difference. Those who help shape the future of the aquaculture industry. And we begin where every aquaculture operation begins: in the hatcheries.
Next
The advertising deadline for the September/October issue is July 10th. Don’t miss the opportunity to be part of this exciting aquaculture publication. For more information, or to reserve space in the next issue, call our Advertising Department at +1.250.474.3982 jthain@annexbusinessmedia.com
editorial deadline for the September/October issue is July 10th. Contact editor Mari-Len De Guzman at mdeguzman@annexbusinessmedia. com for details. Material should be submitted electronically with prior arrangement with the editor.
Hatchery International is proud to launch our first-ever Top 10 Under 40 program, recognizing outstanding men
BY MARI-LEN DE GUZMAN

Hatchery International is proud to launch the first-ever Top 10 Under 40 program, recognizing outstanding men and women under the age of 40 working in hatcheries across the globe.
and women under the age of 40 working in hatcheries across the globe. Whether you work at a commercial hatchery, a research institution with hatchery or fish culture operations, or at a not-for-profit helping to restore and conserve fish populations in our water systems, we would like you to nominate a colleague that you think deserves to be among the most promising young hatchery professionals in the industry.
We are looking for young professionals who: shows a deep understanding and knowledge of fish culture; demonstrates a strong work ethic; has the ability to lead and innovate; possesses a strong passion and commitment to sustainable and responsible production; and commits to the highest standards of hatchery practices.
It’s time to shine the spotlight on some of these deserving individuals who are making a difference in this profession. Online nominations are currently open and will end July 31st.
Just by getting nominated all these young professionals are already winners in their own right, but we have to choose the Top 10 among them. We will announce their identities and feature their stories in the November/December 2019 issue of Hatchery International.
To nominate a deserving young professional, visit www. hatcheryinternational.com/top-10-under-40.
Have you got a story tip or a hatchery operation with a great story to tell? Send me an email at mdeguzman@annexbusinessmedia.com.











Branzino producer Ideal Fish plans new RAS hatchery build

Washington, D.C. – A new hatchery may be in the works for Branzino producer Ideal Fish in Waterbury, Connecticut. Ideal Fish founder and CEO Eric Pedersen told Hatchery International at the RAStech 2019 conference that his company is planning to add a hatchery to its 63,000-sq.ft. RAS facility. Although the timelines are contingent upon the company securing funding, the chief executive is optimistic that the new hatchery will be operational by 2021.
Ideal Fish is the only commercial scale producer of European seabass (Dicentrarchus labrax), also known as Branzino, in the U.S., and one of only a few producers in the world successfully rearing marine species in recirculating aquaculture systems (RAS). It produces 125 metric tons of Branzino per year.
Pedersen said his company is currently importing Branzino fry from Les Poissons du Soleil outside of Montpellier in France. “It just makes sense to have a hatchery in our facility,” Pedersen said, adding the long trans-Atlantic trip from France can be hard on the fish.
In a later development Ideal Fish has announced it has received fresh funding (amount undisclosed as of press time), from a consortium of US and international investors. The new funding will, among other things, allow the producer to increase production capacity to 175 metric tons and expand its operations across the U.S. in the near future.
– Mari-Len De Guzman
Focus shifts from tilapia to shrimp
Chinese tilapia producer Hainan Xiangtai Fishery Co. is shifting its focus from tilapia production to the domestic shrimp market, the SeafoodSource reported.
The company is building shrimp farms in Thailand, and wants to add significantly to its processing operations, according to Xiangtai director Liu Rong Jie, the report said.
“The Thai project will farm and process 4,000 tons of vannemei shrimp a year, according to Jie, who said
his firm’s investments in the next five years will add CNY 1.5 billion (US$221.4 million) in revenue to the Xiangtai annual earnings sheet," the SeafoodSource reported.
"A 3,000-ton-per-year collagen plant will be built in Hainan alongside expanded processing lines for shrimp and tilapia, which will incorporate higher mechanization through artificial intelligence,” the report read.
Imported shrimp causing concern over
bacteria, NaturalShrimp says
After a March 2019 investigation in Canada revealed that grocery stores were selling imported shrimp containing antibiotic-resistant bacteria, Dallas, Texasbased NaturalShrimp is reaffirming its antibiotic-free shrimp production system.
NaturalShrimp said it produces shrimp economically in an indoor, all-natural environment, without the use of drugs of any kind. “Our patented technology ensures that harmful organisms cannot harm or destroy the shrimp. Shrimp is the single most consumed seafood in the world, with nine billion pounds eaten annually,” the company stated in a release.

The investigation found shrimp containing bacteria like E.coli and staphylococcus aureus, which are resistant to traditional antibiotics. NaturalShrimp said it uses an enclosed, recirculating salt water system without the use of antibiotics or other toxic chemicals.
“We have developed a safe way to grow shrimp that solves the concern with antibiotics and other drugresistant bacteria,” said Bill Williams, chairman and CEO of NaturalShrimp. “We believe our system will create a disruptive revolution in the growing and harvesting of the world’s most popular seafood.”





Eric Pedersen
NEWS BRIEFS
California’s Kern River Hatchery back in action following renovations
The Kern River Hatchery in California recently reopened following three years of extensive renovations. The updates will allow the nearly 100-year old facility to improve operations and capabilities into the future, including raising and planting Kern River Rainbow Trout (Oncorhynchus mykiss).
“Things were a little bit run down and they wanted to come up with a facility that had newer raceways, a spawning facility, some more modern round tanks, and they dropped in five wells, so that we could get cooler water,” said hatchery manager Tony Holland. “We have the ability to run them off the grid or standby power. They have a backup generator.”
For years the hatchery has struggled with water too warm to effectively raise fish in the summers, Holland said. Being able to tap into a well will allow the facility to rear fish year-round. One side of the hatchery raises triploids which come from a different hatchery and are planted throughout the Kern River drainage.
“What this program will allow us to do is have diploid Kern River rainbows that are native to this water and they’ll be reared in this new area. This summer, in August, we’re going to pull fish out of the Sequoia National Forest and we’re going to helicopter them out. There’s going to be 50-100 fish that we’re going to use as the broodstock for this program. The new mission is to bring those in and raise them into a broodstock and in the future be able to plant them out.”
Holland said the goal of the renovations and the new rainbow trout program is not only to provide fishing opportunities and to mitigate the impacts of the fishery, but to help with restocking of Kern River watersheds.
– Matt Jones





A look at the new facilities at the Kern River Hatchery, including new raceways and round tanks which can be routed river water or well water.
Inside the new RAS building, where an automatic transfer switch will protect in case of power outages.

FISH TRANSPORT TANKS
Sales and Rental Options
• Unapparelled strength
• Aluminum interior and fiberglass exterior
• Large hatch openings for easy access
• Designed for use with fresh and salt water
• All SS hardware for extremely long life
View our gallery of completed fish transport tanks at www.reiffman.com

Toll Free 1-800-835-1081 or 509-525-1081
Jim Brennan at jim@reiffman.com




OFAH and Honda Canada team up to provide equipment to hatcheries

The Ontario Federation of Anglers and Hunters (OFAH) have long supported volunteer-based fish stocking efforts in the province, through means such as the Community Hatchery Program. Their efforts have inspired other bodies to get involved and, in 2017, the OFAH and Honda Conservation Partnership was formed, dedicated to providing equipment to worthy volunteer hatcheries.
“It was actually on Honda’s initiative,” said Robert Pye, manager, Business Development and Corporate Messaging for OFAH. “They have a great connection to grassroots outdoor life in Ontario. They recognize that people in the outdoors community have a passion for conservation work. They found an opportunity to support the OFAH, which in turn supports the volunteers leading the charge for conservation initiatives across the province.”
Through the partnership, the OFAH has provided volunteer hatcheries with pumps, outboard motors, generators and even a snow blower in one case.
“We have hatcheries that are sometimes in very remote locations,” said Pye. “When you think about the work that our hatchery volunteers take on throughout the year, every little bit helps. If the hatchery volunteers find they need to purchase a snow blower so the volunteers can safely get into the hatchery, then that donation keeps money in the local club so they can put it towards the resource.”
The partnership also allows OFAH members to purchase certain Honda equipment at a reduced rate.
After being approached by Honda, the OFAH put the call out to their members to find out where the greatest need was and how to spread Honda’s support evenly cross the province.
“These are all volunteer hatcheries – really the volunteer effort for supporting our fisheries in Ontario is just incredible,” said Pye. “Our fisheries wouldn’t be the same in Ontario if it wasn’t for the volunteers. There’s clearly a lot of people who are very passionate about it and very giving of their time.”
Every year over 900 volunteers donate nearly 60,000 hours of their time to over 40 community hatcheries, raising millions of fish for public waters, Pye said. In 2018, the Community Hatchery Program alone stocked over 5.5 million fish.
“Nobody ever said that you have to take this on,” he said. “Volunteer-run hatcheries came about because people had a passion to make a difference and they put together the infrastructure and worked with government biologists and they worked with groups like the OFAH to put together a framework to provide fish stocking opportunities.”
– Matt Jones
(From left) OFAH’s Robert Pye and Honda Canada’s Jeremy Ham present a Honda generator to two volunteers from Ontario’s Metro East Anglers.

funding for freshwater mussel hatchery in Pennsylvania



In Pennsylvania, depleted mussel beds in the Delaware and Susquehanna river basins will soon get a helping hand with a new US$7.9 million funding agreement to build a freshwater mussel hatchery in southwest Philadelphia’s Bartram’s Garden. The Partnership for the Delaware Estuary signed the funding agreement with the Pennsylvania Infrastructure Investment Authority.
Freshwater mussels filter large quantities of microscopic polluting particles and healthy mussel beds help keep the water clean and clear. But they’re also some of the nation’s most imperilled animals due to dams, toxic spills and runoff and changing water flows.

“We’ve been restoring native mussels to the Delaware Estuary for over 10 years but there are so few mussels left and we don’t want to continue relying on vestigial mussel beds,” said Dr. Danielle Kreeger, science director at the Partnership for the Delaware Estuary.
“Most existing hatcheries focus on saving rare species. We need greater numbers of the more common mussel species that do the most good for water quality. Then we’ll put them in appropriate places that need water quality improvement,” she added.
The hatchery will stock enough mussels to jump start the recovery process and allow populations to expand naturally where that’s possible. In areas where natural reproduc-
tion can’t occur but where stocked mussels can persist, they might also be stocked repeatedly if the water quality benefits are substantial enough.
As mussels grow, their water filtering capacities increase dramatically, resulting in clearer water, more light for bottom plants and better ecosystem health. Baby mussels will be reared in nursery ponds before moving to sites within the watersheds.
“We’ll also investigate ways to include mussel assemblages in engineered habitats in impaired areas such as urban waters and supply mussel seed for diverse outreach and research,” said Kreeger.
-Bonnie



models) lamps are rated 80% efficient at the end of 12,000 hours
Dr. Danielle Kreeger at the Fairmount Water Works in Philadelphia (Photos: Partnership for the Delaware Estuary)
Using a syringe to gently flush glochidia larvae from a female yellow lampmussel (Lampsilis cariosa) for use in propagation at the Mussel Hatchery at Fairmount Water Works.
Juvenile alewife floaters, Utterbackiana implicata, about five months old.
Waycott
Land deal puts Salmon Evolution closer to building ‘largest’ land-based salmon farm in Europe

Norwegian firm Salmon Evolution is a giant step closer to realizing its plans to build Europe’s largest land-based salmon farm with the recent acquisition of a property at Indre Harøy in Fræna, Norway.
The industrial site is currently owned by Fræna local authority, and is partly a quarry and undeveloped land zoned for commercial development, according to Salmon Evolution. The land will be the future development site of the company’s planned flowthrough salmon farm.
“This is an important milestone, and fully in line with our timetable for realising Europe’s largest land-based fish farm,” said Kristofer Reiten, chair of Salmon Evolution. He describes the strategic and structural conditions as optimal for developing a land-based aquaculture facility.
“We have unlimited access to clean and fresh seawater, adequate electricity supplies, our own deepwater quay and opportunities for expansion and growth. In addition, we are optimally positioned in terms of distance to market,” Reiten said in a statement.
Fræna council has followed Salmon Evolution’s work on developing land-based fish farming at Indre Harøy with great interest.
“It’s very gratifying that the company is now ready to take the next step,” said Anders Skipenes, chief administrative officer at the local authority. “Salmon Evolution’s project at Indre Harøy will make an important contribution to building a national powerhouse for seafood production. The new Hustadvika local authority could scarcely have got off to a better start in terms of forward-looking industrial development.”
Early this year, Salmon Evolution announced it has raised NOK50 million (US$5.7 million) through a private placement. This capital infusion will help finance the first phase of construction of the land-based farm, as well as to fund “organizational development and the appointment of key personnel.”
Estimated cost for the entire project is NOK3 billion (US$367.2 million).
Salmon Evolution plans to build its land-based salmon farm using flow-through system with CO2 aeration. The company’s chair Reiten calls the concept, “sea-based aquaculture on land.” The planned salmon farm is expected to have an annual production capacity of 28,800 metric tons, and a maximum standing biomass of 13,300 tons.


AquAdvantage eggs make their way to Indiana
WASHINGTON, D.C. –
AquaBounty Technologies is ramping up commercial production of its genetically-engineered AquAdvantage salmon with the shipment of some 120,000 salmon eggs to its Albany, Indiana facility by end of May.
AquaBounty president and CEO Sylvia Wulf said the company will be harvesting the last batch of conventional salmon from the 1,200-metricton Indiana facility, and will then focus production for its fast-growing AquAdvantage salmon.

“We have conventional or non-transgenic salmon being grown in (the Indiana) facility since last June. We expect to put our AquAdvantage salmon by the end of this month, so we will be growing those to market weight in Indiana,” Wulf told Hatchery International in an interview, following her keynote presentation at RAStech 2019 in May.
Last March, the U.S. Food and Drug Administration lifted its ban on imports of genetically-engineered salmon, and in April, Environment and Climate Change Canada approved the commercial production of the AquAdvantage salmon at AquaBounty’s Rollo Bay facility in Prince Edward Island. These two recent regulatory developments have cleared the way for the full commercial production of the first genetically-engineered seafood on the market.
The company will also begin stocking its 250-metric-ton Rollo Bay RAS facility in Prince Edward Island, Canada, with AquAdvantage salmon fry, as it completes the construction of the grow-out portion of the facility, which is expected in August, Wulf said. AquaBounty will also be building a hatchery and broodstock facility in Rollo Bay, part of a $12-million construction project to build a RAS production facility to grow the genetically-engineered salmon from egg to harvest size exclusively on land.
AquAdvantage salmon eggs are currently being produced at AquaBounty’s R&D facility in Bay Fortune, P.E.I. The company expects to harvest its first commercial batch of AquAdvantage salmon in the fourth quarter of 2020.


– Mari-Len De Guzman
Indre Harøy in Fræna local authority (Photo: Vasco Pinhol, Salmon Evolution)
Photo: AquaBounty Technologies
Philippines university tech detects WSSV in shrimp
Auniversity in the Philippines has developed a detection tool that aims to help the Philippines shrimp industry in its battle against the white spot syndrome virus (WSSV).
The JAmp (Juan Amplification) WSSV Diagnostic Kit, developed by Dr. Mary Beth Maningas, a professor at the Manila-based University of Santo Tomas, can amplify target viral DNA using one amplification temperature and give results in one hour.

The JAmp WSSV Diagnostic Kit. Early detection and monitoring efforts are the most effective strategy in minimizing impact of WSSV, according to developer, Dr. Mary Beth Maningas.
WSSV is characterized by presence of white spots in the carapace of the shrimp in the later stage of its infectious cycle. Massive mortalities occur three to 10 days after infection.
(Photo: DOST-PCAARRD S&T Media Service)
The test can be done for shrimps at any life stage and any shrimp tissue can be used.
The kit is accessible to the ordinary Filipino shrimp farmer in several ways. The total cost of reaction is almost 60 percent less compared to conventional diagnostic procedures. The execution and interpretation of results are designed to be used by farmers. The test has been designed and tested to be used for rapid and timely monitoring.
“It was developed to bridge the gap between the latest research in diagnostics and its practical application in the field,” Maningas said.
The tool, which took years to develop, was among those

included at the government’s recent National Technology Transfer Day held in Manila.
Maningas said molecular detection is currently the most popular method in diseasemonitoring and mitigation, and many kits using this technology are widely available in the market.
Several factors, however, keep small-to-medium scale farmers at bay. Besides being expensive, operating these requires a tedious process and highly trained technical personnel.
JAmp’s two-step process involves the extraction of the target DNA and the LAMP (loop mediated isothermal amplification) reaction. The results of the diagnostic kit per tube is either positive or negative, and done by visual examination. The kit uses a fluorescence dye that binds to DNA when amplified.
A positive result is indicated by the fluorescence of the sample. This shows that at the time of testing, the shrimp sample had a significant WSSV infection.
“The WSSV affects major cultivated shrimp species in the Philippines, such as P. monodon, P vannamei and M. rosenbergii. With no available cure, early detection and monitoring efforts are the most effective strategy in minimizing the risk of a prolonged infection and huge economic loss,” Maningas said.
-Ruby Gonzalez



























































































































































































GENOMICS
Family factor
Atlantic salmon production in Chilean regions benefit from tailored genetics
BY BAS WOLKENFELT AND ROBBERT BLONK
erformance of genetically improved stock of Atlantic salmon differs between regions in Chile, according to a data analysis of Hendrix Genetics’ breeding program.
PLarge differences were found in the ranking of families from the breeding program for the expected performance in Chile’s region 11 and region 12. This implies that for optimal performance in a specific region, specific families should be selected. However, selection of specific families requires that field performance for the genetic stock is collected in each region. By selecting stock based on performance data collected in region 12, an 804-gram improvement in harvest weight can be realized compared to the situation where selection is based on performance data collected in region 11.
ENVIRONMENTAL TRAITS
With future expansion most likely to take place in region 12, assessing genetic performance of Atlantic salmon in this region is of high importance for the efficiency of its salmon production (Alvial et al., 2012). The main question that we need to address, is whether a breeding program which is entirely based on performance recorded in region 11, is also effective for improving the performance in region 12. In the absence of genotype by environment interaction, the answer to this question would be “yes.”
In this study, we investigated the degree of genotype by environment interaction between region 11 and region 12 in Chile. This study was conducted based on sentinels from the Hendrix Genetics breeding program placed in these two regions.
FIELD INTELLIGENCE
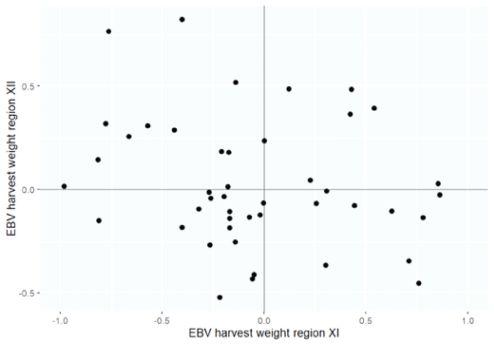
1: Mean estimated breeding value (EBV) for harvest weight per family for region 11 and region 12)
under normal production circumstances including commercial densities, commercial diets, and sanitary treatments. At harvest, sentinels are followed through regular slaughter processing lines to obtain slaughter characteristics.
DATA COLLECTION AND ANALYSES
2019 Hatchery Ad Campaign | Theme: LPB Intro | Design: B | Version: 3
The breeding program run by Hendrix Genetics in Chile started in the late nineties, based on imported material from the Scottish Landcatch strain. The genetic strain underwent continuous genetic selection for the Chilean environmental and production conditions. For more than 15 years, the breeding nucleus is raised in high-biosecurity units, protected from environmental and sanitary risks. The selection on the broodstock animals is based on family information from the field. An important source of field information comes from siblings raised in production environments (called “sentinels”). With this information, more accurate selection can be obtained for performance in commercial environments as these are notably different from the biosecure nucleus environment.
International | Size: Qarter-Page Island Horz | Dimensions: 170mm x 125mm (6.7” X 5”)
Following the large-scale outbreak of the infectious salmon anemia (ISA) virus between 2008 and 2010, stricter regulations for the Chilean salmon industry were created (Mardones et al., 2009; Niklitschek et al., 2013). These new regulations include expansion of the production surface area and the reduction of stocking densities (Alvial et al., 2012; Niklitschek et al., 2013). Together with the already limiting space in the Los Lagos region (region 10), these regulations have triggered salmon farmers to move southward to Aysén (region 11) and Magallanes (region 12) over the past years (Mardones et al., 2009; Iizuka and Zanlungo, 2016). The environmental characteristics differ substantially between the salmon culture regions in Chile, with extreme changing photoperiod, and colder and less saline waters in the more southern areas (Ibieta et al., 2011; Niklitschek et al., 2013). In different genotype by environment (GxE) studies on salmonids, strong reranking of families was found between environments (Correa et al., 2018; Sae-Lim et al., 2013).
More economical,
With the strategic move of the industry towards region 12, Hendrix Genetics also started to place sentinels in that environment to be able to respond to market needs going forward. To that end, relatives of families in the nucleus are marked, transported to production facilities at customer sites in various regions in Chile, and followed throughout the commercial production process. Typically, sentinels are raised
convenient, longer shelf life
Grow more and larger bivalves, shrimp, and sea cucumbers. An excellent nutritional supplement and live algae replacement for all life stages, day 7+. Shelf life of up to 2 years when kept frozen.
For this study, full- and half-siblings of 48 families were tagged and sent to two sentinels, in region 11 and in region 12. By the end of the production cycle, the tagged fish were separated from the commercial fish and harvest information was collected. The harvest characteristics measured included: harvest weight, gutted weight, fillet weight, deformity, maturity, and fillet color.
Fixed effects for the genetic model were selected based on the general linear modelling procedure in R 3.5.1 (R Core Team, 2018). All potential interactions between fixed effects were tested and selected for the genetic model based on the condition of the p-value being < 0.05. Significant effects on harvest weight were found to be sex, deformity and maturity. Additionally, there was also a cage effect present for the sentinel group in region 11, where individuals were split up over two cages.
COOK DESIGN
As phenotypes for each fish were available for only one of either locations, performances in the different regions were treated as different traits. Through phenotypic information of, and relatedness between (half-)siblings at the different locations, estimated breeding values and genetic parameters were obtained through the use of BLUP software of the BLUPF90 family (Misztal et al., 2002). For differences in production periods, feed and other management practices were corrected in the genetic analyses.
For harvest weight, the following genetic models were used:
yXI = m+ Sex+ Deformity + Maturity+ Cage +Animal + e
yXII = m+ Sex+ Deformity + Maturity+Animal + e
where yXI and yXII are the observations of harvest weight for individual animals in region 11 and region 12, respectively, and µ represents the population mean. The model includes fixed effects for sex, deformity, maturity, and in the case of region 11, an effect of cage, and is complemented by a random animal effect, and the random residual error e Genotype by environment was also investigated for fillet colour, deformity and maturity. GxE was estimated as the genetic correlation for the trait measured in the two different regions. In the animal model for estimating the genetic correlation for fillet colour the following fixed effects were included: sex, maturity and sea cage (for region 11 only). The fixed effects for both deformity and maturity were sex and sea cage (for region 11 only).
RESULTS
General characteristics of the harvest data for the three cages are summarized in Table 1.
Mean harvest weight in region 12 (7.4 kg) was significantly (p < 0.001) higher than in region 11 (5.5 kg and 6.2 kg), which is most likely caused by the difference in production period. Heritability estimates for harvest weight were found to be 0.40 ± 0.001 and 0.12 ± 0.002 for region 11 and 12, respectively.
DANIEL
FIGURE
TABLE 1: Overview cage characteristics
Sea cage
Stocking
Individuals
TABLE 2: Top five families in region 11 with their average EBV (estimated breeding value, in kg) for both regions. In between brackets the rank in region 12.
Rank FamilyEBV 11EBV 12
1 (25)
2 (19)
3 (36)
4 (47)
5 (43)
CHIOI15_0280.864-0.026
CHIOI15_0040.8550.028
CHIOI15_0150.781-0.137
CHIOI15_0270.761-0.455
CHIOI15_0290.713-0.346
Average gain 0.795-0.187
TABLE 3: Top five families in region 12 with their average EBV (estimated breeding value, in kg) for both regions. In between brackets the rank in region 11. TABLE 4: Variance components and heritabilities for the
Rank FamilyEBV 12EBV 11
1 (36)
2 (41)
3 (22)
4 (15)
5 (9)
CHIOI15_0120.822-0.402
CHIOI15_0330.764-0.761
CHIOI15_0190.518-0.138
CHIOI15_0070.4860.123
CHIOI15_0390.4840.430
Average gain 0.615-0.150
The genetic correlation for harvest weight between the two different cages in region 11 was 0.93 ± 0.001. This suggests that, even with the difference in production period, there is hardly any genotype by environment interaction between cages in region 11, and therefore these cages were treated as the same environment.
However, between regions 11 and 12 the genetic correlation for harvest weight was -0.16 ± 0.006. This indicates a strong impact of the environment on the expression of genetic potential performance, resulting in a substantial reranking of families between both regions (Figure 1). Also, when using a more simplified model, excluding effects for deformity and maturity, the degree of genotype by environment interaction remained the same.
To illustrate the implications of these results, the top ten percent best-performing families in region 11 and in region 12 are displayed in Table 2 and Table 3, respectively. Based on these results, when selecting the ten percent best families in region 11, a genetic gain of 0.795 kg could be realized in that particular region. However, when stocking the exact same families in region 12, there will be a genetic loss of 0.187 kg. In contrast, when selecting for region 12 based on performance data from that specific region, there will be a 0.615 kg genetic gain. Adding this to the loss of 0.187 kg in case of selections based on performance data from region 11, a total gain of 804 grams could be realized if specific selections take place.
In addition to the harvest weight, strong genotype by environment interactions were found to be present for fillet colour, deformity and maturity with correlations of 0.57 ± 0.01, 0.57 ± 0.02, and 0.02 ± 0.002, respectively. The variance components and heritabilities for the investigated traits can be found in Table 4.
σ²A = genetic variance; σ²E = residual variance; h2 = heritability; XI = region 11 (Aysén); XII = region 12 (Magallanes).
CONCLUSIONS
The genetic correlations between traits measured in the two regions, indicate strong genotype by environment interactions for harvest traits of Atlantic salmon between the regions 11 and 12 in Chile. Particularly, the correlation of -0.16 ± 0.006 for harvest weight expresses a strong reranking of families between regions. Therefore, the best families in region 11 are different from the best families in region 12. This means, that for optimal salmon production in region 12, fish should be selected based on performance data specifically from region 12 and not from region 11. Similarly, fish destined for region 11 should be selected using data from region 11.
Based on the results of this study, selecting the ten percent best families for production in region 12, based on performance data collected in that same region, 804 g of genetic gain can be realized, compared to when data is collected in region 11. With a harvest size of 6 kg, this means an increase of approximately 14 percent.
Also, for the other harvest traits investigated, strong genotype by environment interactions were found. This result indicates the need for separate selections for the two regions. To optimize the selections of its breeding program, Hendrix Genetics currently has additional sentinel groups in different locations in Chile.
Bas Wolkenfelt is a geneticist at Hendrix Genetics in the Netherlands. Robbert Blonk is director of research and development at Hendrix Genetics Aquaculture.

LITERATURE
• Alvial, A., J. Forster, J.M. Burgos, R. Ibarra, and S. St-Hilaire. 2012. The recovery of the Chilean salmon industry. ISA Cris. Its Consequences Lessons. Study Glob. Aquac. Alliance co-sponsored by World Bank, Undersecretariat Fish. Chil. Salmon Ind. Assoc. (SalmonChile). Puerto Montt, Chile.
• Correa, K., R. Figueroa, J. Yáñez, and J. P Lhorente. 2018. Genotype-environment interaction in Atlantic salmon body weight in fresh and seawater conditions. Page.
• Ibieta, P., V. Tapia, C. Venegas, M. Hausdorf, and H. Takle. 2011. Chilean salmon farming on the horizon of sustainability: review of the development of a highly intensive production, the ISA crisis and implemented actions to reconstruct a more sustainable aquaculture industry. InTech.
• Iizuka, M., and J.P. Zanlungo. 2016. Environmental collapse and institutional restructuring: The sanitary crisis in the Chilean salmon industry.
• Mardones, F.O., A.M. Perez, and T.E. Carpenter. 2009. Epidemiologic investigation of the re-emergence of infectious salmon anemia virus in Chile. Dis. Aquat. Organ.. doi:10.3354/dao02040.
• Misztal, I., S. Tsuruta, T. Strabel, T. Druet, and D. Lee. 2002. BLUPF90 and related programs (BGF90). Page in Proc. 7th World Congr. Genet. Appl. to Livest. Prod.
• Niklitschek, E.J., D. Soto, A. Lafon, C. Molinet, and P. Toledo. 2013. Southward expansion of the Chilean salmon industry in the Patagonian Fjords: Main environmental challenges. Rev. Aquac.. doi:10.1111/raq.12012.
• R Core Team. 2018. R: A language and environment for statistical computing. Vienna R Foudation Stat. Comput.. doi:10.1007/978-3-540-74686-7.
• Sae-Lim, P., A. Kause, H.A. Mulder, K.E. Martin, A.J. Barfoot, J.E. Parsons, J. Davidson, I.I.I. Rexroad C. E., J.A.M. van Arendonk, and H. Komen. 2013. Genotype-byenvironment interaction of growth traits in rainbow trout (Oncorhynchus mykiss): A continental scale study1. J. Anim. Sci. 91:5572–5581.






IN DEPTH CLIMATE CHANGE

Gearing up for change
Hatchery strategies to counter effects of climate change
BY TOM WALKER
Concerns around climate change are increasingly being felt in the aquaculture industry, ranging from how these changes affect natural fish reproduction to hatcheries’ ability to rear species effectively.
Todd Pearsons, senior fisheries scientist with the Grant Public Utilities District in central Washington state in the U.S., spoke about strategies to mitigate the impacts of climate change on trout and salmon hatcheries in his presentation at the 69th Northwest Fish Culture Concepts last December.
“Most of us have read papers and heard a lot of talk about the predicted climate change effects on salmonids,” says Pearson. “We know that stream flows will change dramatically in terms of when they come off and the amount of water running. When you combine this with warmer drier weather, water temperatures will be higher and water levels will be lower.”
These factors will challenge the ability of fish to rear naturally in some areas they have historically, Pearson adds, and they will also challenge hatchery operators in the same locations.
WATER WOES
The first concern is simply water availability. “It is becoming more challenging to keep our intakes in the water during higher spring flows,” says Pearson. “And then we can have water levels drop later in the year, so an intake can go dry.”
Pearson adds, they may need stronger intakes to be able to deal with higher flows and more debris coming down. Having a back-up source using a portable intake will also be important.
“Having flexible water rights and multiple sources so you can access sufficient ground and surface water in the future is going to be really critical,” he says.


Making the best use of the water that you have will become a higher priority, Pearson notes.
“When I poll this room I find that maybe 20 percent of hatchery operators are currently using some form of recirculation,” he says. “One-pass water is going to be a luxury of the past. We need to be focusing more on water recirculation for water conservation.”
“The ability to imprint fish on water that is not adjacent to your hatchery is an area of research we need to focus on, because we may not have the acclimation sites we do right now,” Pearson adds.
Temperature control will become increasingly important. “We need to provide the capability for plenty of cold water,” says Pearson. “This might mean that we are going to need to recirculate more of our water so that we can keep it cold by running it through chillers.”
Insulation of vessels and piping, and providing more shading and cover, will also be necessary. “We may need to do more over-winter acclimation at remote sites, if our core sites are warming up,” Pearson adds
FISH COLLECTION
The effects of climate change may also lead to increasing challenges in gaining access to broodstock.
“If we have a lot of stream drying in the future, we are not going to be able to collect target broodstock in areas that we have collected them in the past,” Pearson notes.
“We might need to collect fish at downstream locations where there is more water, and tissue identification approaches will be needed to identify and segregate the genetic lines that we need.”
Another factor to consider is that phosphorus discharge may become a bigger issue in the future if there are lower




volumes in receiving water. “That means we are going to have less dilution of the amount of phosphorus that we are discharging into the system,” Pearson explains.
“Being able to produce things like low discharge feeds and having more efficient ways to get the feed to the fish so that we have less phosphorus being discharged into the environment will be needed.”
In some cases, hatcheries may even need to produce smaller size fish, so there is less discharge into the environment, he adds.
CHANGE IS INEVITABLE
All of these factors will now be part of the process when selecting a site for a new hatchery Pearson notes.
“It is likely in the future that successful hatchery locations are going to be limited to mainstem areas or spring fed locations where we know we have good access to water,” he says.
Historical numbers on previous drought and flood intervals may no longer be useful in selecting new locations, Pearson says. Taking a more predictive approach will be key.
“We are probably not going to be able to tinker our way out of this,” Pearson says. “We are going to have to have changes in policy and different ways to operate our hatcheries in order to make some of these kinds of things work, and we are going to have to change the way we implement policy.”
The good news, Pearson says, is that hatchery operators have more than a century of experience in adjusting hatchery conditions for the benefit of fish.
“We should just expect this and plan to do the hard work and the science up front,” says Pearson. “We need to be proactive in developing these strategies, so that when we actually need this stuff, need it to be on the ground, we are ready.”



Sea cucumber
Hatchery trials probe potential for sea cucumber farming in Oman
BY RUBY GONZALEZ
Despite successful outcomes with fertilization, a sea cucumber (Holothuria scabra) hatchery trial conducted in Oman resulted in mass mortality during the first few days post-fertilization (PF).
This could have been caused by using equipment of “concomitant hatcheries,” according to the research team, composed of Al-Rashdi KM, Claereboudt MR, Eeckhaut. They said this could be prevented in the future by “emphasizing the need for high levels of training in sterilization and decontamination and a sepsis.”
The hatchery trials comprised first phase of the firstever initiative in Oman to develop hatchery techniques for sea cucumber for aquaculture development and stock enhancement programs. The authors documented this in their research article, “First trials on hatchery and larval development of the sea cucumber, Holothuria scabra (Jaeger 1883),” published this year in the HSOA Journal of Aquaculture and Fisheries, and funded by the Agricultural and Fisheries Development Fund.
HIGH-VALUE PRODUCT
Sea cucumber is a recognized high-value product in Oman, where the dry weight costs over $1,000 per kilogram. Spurred by Asian markets, high demand for the slow-

Sea cucumber is a recognized high-value product in Oman, where the dry weight costs over $1,000 per kilogram.
growing echinoderm has taken an “alarming” toll on Omani population of the species. Observed stock densities had decreased to less than one individual per hectare.
“Aquaculture of this species has developed as a response to the overfishing problem but has not been yet studied in Oman,” they said.
For the study, in vitro maturation and fertilization were conducted using an artificial maturation substance extracted from sea urchin oocytes. Four hatchery runs were conducted between September 2009 and November 2010, at the Aquaculture Research Unit of Sultan Qaboos University in Al-Hail. Land-based outdoors tanks were used to keep the broodstock.
Ninety percent fertilization and larval development rates were achieved, producing more than 400,000 larvae over four independent in vitro fertilization trials, and developed successfully into normal embryos and larvae.
However, mass mortality was observed across all trials during the first three days PF, reaching as much as 70 percent. From day four of development, survival rate remained almost stable.
During the daily microscopic observations of the larvae, abundant ciliate protozoans and copepods were observed in the larval tanks.
ANTISEPTIC ENVIRONMENT
Contamination by the feeding solutions had been ruled out because the mortality occurred before feeding. They said the presence of abundant copepods and ciliates could be responsible for the day three PF mortality.
Citing previous studies, the authors explained copepods attack larvae either directly or by repeated collisions, causing bodily damage to the larvae. Mortality due to ciliates had been observed.
In tracing the possible source of the copepods and ciliates, they said, “Since the water used for larval rearing was filtered and UV-treated, it is unlikely the source of the copepods and ciliates, but some of the equipment used to siphon the water, transfer the larvae from one container to another, the container themselves may have been contaminated by a concomitant fish hatchery and emphasize the need for high levels of training in sterilization and decontamination and a sepsis.”
Overall, the study said Oman’s potential to develop sea cucumber aquaculture is high. It has natural sites suited for sea ranching, such as the Bay of Mahout. Human resources and basic technology potential are in place.
“For example, less than one percent of the surface of the Bay of Mahout Bay could support the sea ranching of 4 M of sea cucumbers H. scabra if it is supplied by an efficient farm having 50,000 m2 of ponds and an efficient hatchery with 600,000 liters of adequate tanks.”
Since 1958, Faivre has been developing and manufacturing high quality equipments for the aquaculture industry




















Researchers show promise for Blue crab farming
HATCHERY
The current research program is based on a three-phased approach: hatchery production, intermediate raceway grow-out and a final grow-out in quarter-acre ponds to a soft crab or a live bait crab for recreational fishing.
“We have six independent larval rearing systems, seawater preparation and storage, rotifer (Branchionus rotundiformis stype) and Artemia production, and wet and dry laboratories,” said Harriet Perry, senior research scientist and professor emerita at the University of Southern Mississippi’s Gulf Coast Research Laboratory. “Each culture system consists of a 1,400-liter fiberglass larval rearing tank with 1,000 liters of water and a round 400-liter filter reservoir tank. The reservoir tank has a trickling biofilter, protein skimmer and activated carbon/crushed coral up-flow filter.”
Seawater (28‰) is prepared with filtered, dechlorinated city water. There are also semi-continuous, high-density rotifer culture systems in place with a 200-liter coneshaped culture tank, oxygen concentrator, feeding pump, air supply and heater. The heaters maintain water temperature at 25°C and the Artemia culture system includes a water table with five 20-liter cone-shaped hatchers.
SPAWNING
Females with recently laid egg masses are obtained from commercial fishermen, disinfected, held in individual quarantine tanks and examined for parasites and disease. Crabs free of disease are transferred to 100-liter hatching tanks for spawning. The spawn of a single female is used to stock tanks. Female crabs are then returned to the wild. Newly hatched zoeae are fed rotifers.
LARVAE
Zoeae are harvested with netting, counted and placed in culture systems at 100,000/system (total culture capacity is 600,000 individuals). The systems are pre-stocked with feed – 50 million enriched rotifers and algae.
“During the later stages, zoeae are also fed adult copepods and newly hatched and enriched Artemia once a day,” said Perry. “Daily plunge samples are taken from the tanks to determine the zoeae’s stage of development and number, and the number of rotifers and Artemia. Temperature, salinity, pH, ammonia, nitrite and nitrate are monitored over the culture period. Tanks are harvested following the appearance of the final larval stage, called a megalopa, usually within 24-30 days of stocking.”
MEGALOPAE
Harvested megalopae are placed in 6’x 22’ raceways with ~8,000 liters of seawater. Structure (pom-poms) is added to the tanks to provide refuge and reduce cannibalism. Temperature, salinity, pH, ammonia, nitrite and nitrate levels are monitored regularly. Juveniles are fed pelleted


food, frozen Artemia and copepods, with feeding regimes adjusted to coincide with predicted growth.
After the first week of grow-out, freshwater is added to the systems to gradually lower salinities and allow juveniles to acclimate from 25‰ to 3‰. They are then transferred to low salinity ponds (average depth is three feet) at the Mississippi Department of Marine Resource’s Aquaculture Facility near Gulfport. Artificial sea salts are added to bring the salinity to 1‰. A diet of pelleted finfish is given during the first week at 15 percent of body weight, followed by small, aquacultured koi from week two until harvest. The crabs are stocked at 2,500 to 3,000 crabs per quarter-acre pond.

ponds that produce bait crabs contain submerged aquatic vegetation. Smaller crabs are fished out with minnow traps, the larger ones with crawfish traps and sub-adult and adult crabs with standard crab traps. The ponds that produce soft crabs have no vegetation and crabs are harvested using bushlines that are run twice a day to check for crabs that are molting. Those showing signs of shedding are removed and the days until molting estimated. They’re then transferred to closed recirculating seawater systems. The length of stay in the ponds varies and depends on temperature and size requirements for production.
The
After being checked for disease, females are put into individual aquaria. The eggs hatch as a zoeal larvae, which are then transferred to culture tanks.
When crabs reach ~1 to 1 and 1/2 inches they are removed from raceways and placed in ponds. Peelers or molting crabs are harvested using bushlines.
Adding structure to the raceways
(Photos: Harriet Perry, senior research scientist, professor emerita, University of Southern Mississippi)
A SUSTAINABLE ALTERNATIVE
The Blue Crab Hatchery’s main goal is to support the livelihoods of blue crab fishermen in Mississippi.
“We’re working to offer another economic opportunity for fishermen or farmers,” said Lucas. “Farming can supply bait crab production for anglers, and cocktail or smaller soft shell crabs for the restaurant market. Some fishermen may decide to farm crabs and some farmers may diversify their ponds by adding crab production. Therefore, it could be another income string for fishermen at a time when it’s not conducive to be on the water, while people who farm can add a new product. Our technology is very much in the developmental stage but there have been a lot of people who are willing to try it and want to diversify.”
THE ROAD AHEAD
Lucas says there are two challenges to overcome: cannibalism and maintaining broodstock.
“First, we need to develop a captive broodstock program to control production year round,” she said. “We are looking to start such a program. We have a geneticist at the Aquaculture Center and the reproductive physiology of blue crabs is known. We’re working to find funding for this aspect of the research.”
“Megalopae, juvenile and adult crabs are highly cannibalistic, and this greatly increases mortality,” she continued. “But there are ways to reduce cannibalism, like decreasing stocking density or providing shelter in tanks.”
It may be some time before the researchers have the answers they need, but Lucas has high hopes for their work. Following the recent funding of a joint National Sea Grant proposal with North Carolina, Perry, along with the crab team, will work with the NC researchers and private industry to transfer their hatchery and production technology to the Atlantic Coast. If their research pans out, more blue crabs in the U.S. could be the result of a new, thriving industry.
RESEARCH
(Photos: Eric Gigli)
Spotted seatrout
Spawning, larviculture of spotted seatrout at low salinity
Protocols of spawning and larviculture of the spotted seatrout (Cynoscion nebulosus), which are currently performed at high salinity, could be done at low salinity, according to a study by graduate student, Eric Gigli, at the University of Southern Mississippi’s Thad Cochran Marine Aquaculture Center (TCMAC).
“ The ability to do marine aquaculture in places where a consistent supply of high salinity salt water is not available would greatly expand opportunities for developing a domestic aquaculture industry,” Gigli told Hatchery International.
This portion of his thesis aimed to evaluate the effects of salinity on spotted seatrout embryos and early larvae, and the feasibility of low-salinity culture at these stages.
TCMAC is located in the Mississippi sound area, where salinity of coastal waters is low and seasonally variable.
The findings likewise offer positive cost implications. “Currently, the center uses artificially mixed salt and well water for the entire culture cycle of spotted seatrout. The ability to culture animals in low salinity reduces the cost of artificial saltwater, which represents as much as 10 percent of the production cost, and potentially allows the use of local water,” Gigli said.
Fertilized embryos from a volitional spawn produced by local broodstock conditioned at 29 psu (practical salinity units) were stocked in 100-liter incubators at 25, 18.75, and 12.5 psu.



Although survival in the 25 and 18.75 psu groups was twice that of the 12.5 psu group throughout, the study recommended a volume of 12.5 psu.
Gigli explained, “Higher mortality in this group was only observed pre-hatch and we believe this mortality was primarily due to embryos falling out of suspension and clustering at the bottom of the incubator, where they succumbed to insufficient oxygen levels or overall reduced water quality.
“Following this initial mortality, the larvae in the 12.5 psu treatment survived and grew as well as those at the other salinities.
“Accordingly, if the incubation procedure can be modified to improve suspension at low salinity, this excess initial mortality may be avoided. Further work is needed to determine if incubation conditions at low salinity can be modified to maintain suspension without affecting survival.”
Salinity can affect the fitness of embryos and early larvae in many ways because buoyancy is a first trait of importance to embryonic and early larval fitness.
Spotted seatrout frequents habitats featuring a broad range of salinity from nearly fresh water upper estuaries to high salinity barrier islands.
-Ruby Gonzalez




Spotted seatrout larva
Spotted seatrout fertilized embryo



WANT HEALTHIER FISH?




Ginger root powder
African catfish fingerlings
Adietary supplementation of ginger root powder improves the performance of African mud catfish (Clarias gariepinus) fingerlings, according to a study conducted in Nigeria.
The inclusion level of one percent ginger root powder in the fish diet was recommended by authors, Adegbesan SI, Obasa SO, Akintokun AK, and Abdulraheem I, in their article published in the Journal of Aquaculture and Fisheries.
Among four diets, this posted the highest and best growth response in terms of percentage weight gained specific growth rate and apparent net protein utilization.



mortality rate.
The higher levels of phytobiotics, they said, have lower palatability and could have caused the reduction in the total feed intake because of the presence of tannin in ginger root powder.
Reduction in survival rate in fish fed with ginger root powder diets in the experiment could also be a result of some phytochemicals present in the phytobiotics, the study said.
Phytobiotics, plant-derived products added to feed in order to improve performance, are valuable materials in promoting growth and reducing pathogenic diseases in cultured fish diets.


























Inclusion of two percent could be antibacterial and antifungal and may improve gastrointestinal microbes.
The fingerlings were fed with 40 percent crude protein diets containing zero and three concentrations of one, two and three percent ginger root powder.
Fish fed with diet containing varying levels of ginger root powder posted better growth performance and had “greatly reduced” microbial population compared to the control group.
In preparing the fish diet, fresh rhizomes of ginger were dried and grinded. The powder was mixed directly with the basal diet and added into the diets. The compounded feeds were then pelletized before being sun-dried.
The study assessed the growth, nutrient utilization and anti-microbial potentials of cultured C gariepinus fed varying inclusion levels of ginger root powder.















Data collected showed inverse correlations. Survival rate decreased as concentration of ginger root powder increased. The total bacterial counts and total fungal counts decreased as inclusion levels of the supplements increased which were different from the control.
Fish fed two percent and three percent ginger root powder recorded the highest
The antimicrobial properties of ginger root powder were considered because catfish, like all fish, “live in microbe-rich environment and are vulnerable to invasion by pathogenic and opportunistic micro-organisms.”
Ginger rhizome has been reported to possess a broad spectrum of prophylactic and therapeutic activities, and is known to be beneficial to growth and immune systems in aquatic animals.
-Ruby Gonzalez

Fresh ginger rhizomes were dried and grinded for the fish diets.
(Photo: Ruby Gonzalez)


Collective culture
New algae curator at Milford Lab talks shop with shellfish hatcheries
BY LYNN FANTOM
The woman, seated in front of the biosafety cabinet, reaches under the protective window and meticulously pipettes a green liquid containing Tetraselmis chui into a 10-milliliter vial. She moves with the precision of a scientist, yet – dressed in jeans and a plaid flannel shirt –looks more like a shellfish farmer.
Striking that balance in many ways is what her job calls for as the curator of the Microalgal Culture Collection of the Milford Laboratory, based in Milford, Connecticut.
Lisa Guy is approaching her one-year anniversary (in September) at the Connecticut lab, which is part of Northeast Fisheries Science, the research arm of NOAA Fisheries in that region of the U.S. As algologist of this unique and historic archive, she maintains over 200 strains of microalgal cultures that are available to academic researchers and commercial shellfish hatcheries – at no cost.
Says her boss and longtime director of the lab, Dr. Gary Wikfors, “it’s a national and international resource.”
In addition to offering “starter cultures,” shipped free in small vials, the lab provides technical assistance. As Wikfors sees it, it’s a combination of the Burpee’s Seed Catalog of microalgae and an extension service for hatchery operators.
Guy, who holds degrees in biology and environmental science, has a foot in each of those worlds. She spent nine years at Horn Point Oyster Hatchery, which produces one billion oyster spat annually. Starting as an intern, she not only learned all aspects of oyster culture from broodstock management to outplanting but also gained experience on a working oyster farm. She advanced to become a faculty research assistant and served for four years as the head algologist.
Guy moved into the role of Milford’s algal curator after it was vacant for more than two years following the retirement of its longtime head. “We think it is a big deal for the shellfish and aquaculture communities to know that the lab has recommitted to maintaining this important algae culture and continuing to provide this extension service,” says Kristen Jabanoski, science communications specialist at Milford Laboratory.
Guy nurtures the algal archive in 24/7 climate-controlled incubators and a “light-room” of flasks and test tubes. She maintains copies of each of the 200-plus strains in three different media (filtered seawater, artificial seawater fortified with vitamins and minerals, and agar).
This redundancy is important as a hedge against equipment failures and unexpected water-quality variations.
Much of Guy’s time is spent transferring the cultures to the different media and keeping them growing. “It must be


a bear to maintain,” says Taras Pleskun, who is the hatchery manager responsible for algae at Mystic Oysters in Connecticut. “It’s a back-up bank of pure strains.”
Pleskun says three or four strains are the workhorses in his hatchery, but he may cultivate up to eight for special purposes.
A tendency exists to keep on using the same algae, according to Guy. People say, “I learned from somebody. They used it, so I always use it.” Some popular strains are T-Iso Tisochrysis lutea (T-Iso), Isochrysis sp. (C-Iso), Tetraselmis chui (Ply-429), Chaetoceros calcitrans (Chaet cal), Pavlova lutheri (Mono), and Nannochloris sp. or Nannochloropsis sp. (Nanno).
“Just don’t be afraid to try something new,” she adds. “Water quality really is a factor and can vary from year to year, even in a lab.” Trial-and-error is an important principle at a hatchery.
PREDICTABILITY
So much affects what happens in the hatchery, from fundamental biology to feeding to operational elements. Says Maine oyster farmer, Bill Mook, “I want to make it a predictable process.”
One way he strives for that is by consulting with Milford, “where you can combine their knowledge of biology with your own observations.”
Lori Mayer, a marine biologist who manages the hatchery for New Jersey-based Clam Daddy’s, which she owns with her husband, says she turns to Milford with questions like: Are growth rates being affected by the nutrients I’m using? What if we go from fluorescent to LED lighting?
“The Milford Lab has been invaluable to our hatchery,” she says. “We have been using their starter cultures successfully for 14 years to grow algae to feed our larval and post- set clams.”
Lisa Guy enjoys trouble-shooting by phone. “Some of my best conversations have been in sharing experiences, bonding, even when things go wrong. The call ends with them thinking, ‘It’s not just me.’”
When people call Guy, they are also tapping into the knowledge at the lab overall. Guy attends monthly staff meetings with some 25 researchers and post-doc students investigating queries like shellfish responses to ocean acidification and fish interaction with aquaculture gear.
But it isn’t always that formal, since the director fosters collaboration with coffee breaks every morning at 10:30. Someone
scoops espresso into the coffee press; another passes scones from a favorite bakery. One day a local grower joined them and was treated to a lively session of expert troubleshooting.
In the past, the team has conducted the Milford Microalgal Culture Workshop for researchers and hatchery managers who want to learn how to grow microalgae to feed their shellfish stock.
Every year Milford Laboratory also presents research results at either the Milford Aquaculture Seminar or the Northeast Aquaculture Conference and Exhibition.
Despite these efforts, Jabanoski believes that in many ways the lab is “still a hidden gem.” Guy adds, “There are so many new players in aquaculture who are asking ‘Where do I get my algae?’”
RUSSIAN INFLUENCE
The lab began in 1919 with one federal researcher and the support of local oyster businesses, according to NOAA. But it was later, with the hiring of Victor Loosanoff, who served as director from 1935 to 1962, that the lab produced foundational research on shellfish biology and culture.
A native Russian, who learned English while working as a lumberman and commercial fisherman, Loosanoff earned his Ph.D. in zoology at Yale. Under his direction, the lab developed a methodology to spawn bivalves nearly year-round and rear them through the embryonic, larval, and adult stages. Researchers cultured algae to nourish the shellfish during each life stage – which became the Milford Lab Culture Collection. It dates back to the 1950s.
“At the time, no one in the world could consistently maintain and develop substantial numbers of bivalve mollusk eggs to a large enough size to be planted as seed,” according to Maille Lyons in an article for National Shellfisheries Association.
Today, the scientific resources of the Milford Lab include not only the microalgal culture collection but also a shellfish hatchery and tank farm, a flow cytometry facility, and a 49foot research vessel, the R/V Victor Loosanoff.
And Milford’s work is likely to be increasingly important in the future as the climate changes, and hatcheries are needed to protect shellfish during vulnerable stages. Says Mook, “The availability of all of the microalgal strains and the intellectual power [at Milford] will be more and more essential to keeping people with shellfish on their plates.”



Lisa Guy nurtures the algal archive in 24/7 climate-controlled incubators.
Lisa Guy prepares a starter culture vial for a farm that grows oysters, quahog, and sea scallops.
Live Algae On Site



RAStech 2019 debuts as industry touts bright prospects for RAS
Building technologies for the future
Innovation for land-based, sustainable aquaculture projects
More than 30 years leading innovative water recirculation technologies worldwide.

“Our (AquAdvantage) fish was specifically designed to operate in a (RAS) environment. We continue to optimize those growing conditions so that we are recirculating close to 99 per cent of the water,” the AquaBounty chief executive added.
Aquaculture professionals from 21 countries gathered for the RAStech 2019 tradeshow and conference. The conference featured two days of RAS-focused education sessions with over 70 speakers from around the world with presentations on shrimp farming in RAS, energy optimization, engineering innovations, feeds management as well as health and disease management in RAS.
The team from The Conservation Fund’s Freshwater Institute presented some of its latest research on RAS, including: results from a preliminary investigation on integrating membrane biological reactors within RAS; effects of photoperiods in growth, health and maturation of Atlantic salmon in RAS; the economies of scale; fillet and product quality, among other things.
In her keynote address, Wulf enumerated some of the benefits of RAS production in sustainable aquaculture, including the ability to put RAS facilities “close to consumption” limiting the environmental impact of food production, and the biosecurity of RAS which reduces the risk for diseases and eliminates the need for antibiotics.
“There is such a great environmental story that goes along with RAS,” Wulf said. “As we think about the sustainability story, consumers are more and more aware and asking questions about the sustainability of how their food is made.”
Alejandro Roxas, AquaBounty’s chief operating officer, also spoke at the opening session highlighting the importance of people in the success of RAS operations.
“When we think about RAS, we have the image that we are in a box,” Rojas said. However, he added, RAS operations involve a diverse array of expertise and skills to ensure optimal performance –from water quality monitoring and energy efficiency to vendor and supplier management, construction and transportation.
SKILLS DEVELOPMENT, KNOWLEDGE-SHARING
Rojas stressed the need for aquaculture organizations to engage the universities and colleges in the communities they operate to ensure the educational institutions are producing the quality of people with the necessary skills to operate efficiently from multiple fronts in RAS environments.
Attendees at RAStech generally agree there is thirst for knowledge and information sharing when it comes to RAS. Still in early stages but quickly gaining momentum, land-based aquaculture is touted as a significant shift in sustainable seafood production.



For Australian Scott Parkinson, CEO of Ornatas in Tasmania, RAStech has been a “fantastic” opportunity to connect and engage with like-minded professionals in the RAS industry. Ornatas is developing the world’s first-ever commercial hatchery for tropical rock lobsters.
Parkinson and Greg Smith, University of Tasmania associate professor and the director of the ARC Research Hub for Commercial Development of Rock Lobster Systems, were among the international attendees at this year’s RAStech conference.
FUTURE GROWTH
Attendees also expressed optimism about the future of RAS.
Eric Pedersen, founder and CEO of Ideal Fish, believes RAS is going to be an “explosive” area of growth. Ideal Fish grows European seabass (Dicentrarchus Labrax) to harvest size in a 63,000-sq.ft. state-of-the-art RAS facility in Waterbury, Connecticut in the U.S.
“I think (RAS is) going to be an explosive area of growth in aquaculture, particularly in the United States, where aquaculture is just beginning to get a toehold in the seafood production chain,” Pedersen told Hatchery International.
“Having conferences like this that brings professionals from academia, from industry, from finance together to support this industry is going to be an increasingly crucial part of this development.”
For Robin Muzzerall, vice-president for aquaculture at Icy Waters Arctic Charr in Whitehorse, Yukon Territory in Canada, the conference is an opportunity to learn about RAS technologies.
“We currently don’t have RAS where I am so part of my reason for coming here is to educate myself to see what is out there now compared to about five years ago, and start making good choices when we speak to designers,” Muzzerall told Hatchery International.
RASTECH 2020
Making plans to attend the next RAStech? Save the date now for November 16 and 17, 2020 for RAStech 2020 to be held in South Carolina, U.S.A. Check www.rastec.com regularly for more details.
PIT TAGS & SCANNERS






Virginia Tech associate professor and RAStech 2019 conference director David Kuhn welcomes attendees at the opening session.
In her keynote address, AquaBounty CEO Sylvia Wulf stressed the role RAS will play in finding sustainable ways to address a growing global demand for seafood.
New land-based salmon egg production opens in Norway

“The opening of our new facility in Salten is a very important milestone for Benchmark which will allow us to capitalize on our leading market position in salmon genetics and the favourable long-term market trends in the industry,” said Jan-Emil Johannessen, head of Benchmark Genetics.
Land production of salmon eggs gives the company “complete control of the spawning season,” allowing it the ability to supply its customers every week throughout the year and produce “in an environment with the highest standards of biosecurity,” Johannessen added.
The opening event will kicked off with a closed seminar on how breeding and genetics can contribute to the sustainable growth of the aquaculture industry, with guest speakers including: Erland Bullvåg, dean of Nord University; Kristian Eikre, investment director at Ferd; Klemet Steen, chief advisor smolt at Lerøy; Knut Rønningen, senior advisor at the Food Safety Authority; and Morten Rye, Benchmark’s genetic expert.
The facility was officially opened by Kjell-Børge Freiberg, Norway’s Minister of Petroleum and Energy, and followed by a tour of the site. The opening event will also include cultural entertainment and talks from Johan H. Andresen, owner of Ferd; Geir Wenberg of Salten Aqua; Ingegjerd Eidsvik, CEO of Artec Aqua; and Benchmark’s CEO Malcolm Pye and Stig Joar Krogli, general manager at the site.
The event concluded on May 23rd with a full-day seminar with customers focusing on genetics, production and product innovation.
“We look forward to welcoming our customers, investors, and key actors in the salmon industry to celebrate the opening of our state-of-the-art facility,” Johannessen said. “Together we can help producers to increase the quality, yield, health and welfare of their stock to support a sustainable and profitable salmon industry.”
New project in Wester Ross
The Scottish Salmon Company (SSC) has announced “major” new freshwater projects that will see the company invest approximately £10 million (US$12.6 million) in its operational infrastructure and Scotland’s rural economy.


A new facility in the Applecross estate in Wester Ross, ‘Applecross Kishorn’ is due for completion in 2020 and will create a centre of excellence in freshwater production, reinforcing the company’s commitment to innovation and best practice.
The SSC has also acquired two freshwater facilities in the area. These include on-shore hatchery ‘Appleburn Couldoran’ and a nearby facility at Loch Damph, which will support increased smolt production as the business continues to deliver its ambitious strategy for responsible, sustainable growth.
To mark the new developments ‘Applecross Kishorn’ and ‘Appleburn Couldoran’, the SSC’s chief executive Craig Anderson planted Scottish heritage apple trees at both sites.
The new investments are part of the SSC’s ongoing commitment to Scotland’s rural communities and economies, and will provide long-term job security for 21 skilled, full-time members of staff. They follow record results for the company in 2018, that saw revenues reach £180.1 million (US$227.8 million) and export volumes hit more than 60 percent of sales with particular success in key export markets North America and the Far East.
“We are making a significant investment in the Highlands and Islands which will strengthen our freshwater operational infrastructure and deliver greater capacity to meet the increasing global demand for our quality Scottish salmon.”

“We are making a significant investment in the Highlands and Islands which will strengthen our freshwater operational infrastructure and deliver greater capacity to meet the increasing global demand for our quality Scottish salmon,” Anderson said.
“We are committed to responsible business growth in the communities in which our staff live and work, and to sustainably building on our positive economic impact in these rural areas,” he continued. “These infrastructure projects will mean long-term job security and more spending in the local area through our local sourcing policy.”


Craig Anderson, Scottish Salmon Company chief executive, and Richard Polanski, recirculation project manager. (Photos: Scottish Salmon Company)
Nick McNeil, SSC freshwater technician, Craig Anderson, SSC chief executive, and Niall McCallum, SSC freshwater area manager (mainland).
22 million triploid eggs from Iceland coming to Atlantic Canada

Iceland-based StofinFiskur has reached a five-year agreement with Norway’s Grieg Group to supply over 22 million triploid eggs for a planned salmon farming and processing project in Newfoundland, Canada.
The land-based hatchery will produce up to seven million triploid smolt annually, which will be sold to salmonid aquaculture farms in the province and will be developed on approximately 10 hectares of serviced land.
To comply with the strict environmental requirements from the Canadian authorities, the selected eggs will produce sterile salmon (triploid) to avoid reproduction and settling if escapes were to occur. These eggs are genetically selected for growth, high quality characteristics, and improved resistance to a variety of diseases. Assuming the project is completed, StofnFiskur says it will be the only foreign company to supply Atlantic salmon genetics to Canada.
Through research, triploid salmon have shown to have a range of characteristics that differentiate them from standard diploid salmon, aside from sterility. Scientists at the Norwegian Institute for Nature Research conducted analysis of fillets showing that total lipid and fatty acid quantities were significantly lower in triploid than in diploid Atlantic salmon fillets. However, when fatty acids were normalized to total lipid content, triploid fillets had significantly higher relative levels of important omega-3 long-chain polyunsaturated fatty acids.
Triploid salmon have shown to have a range of characteristics that differentiate them from standard diploid salmon, aside from sterility.

Photo: StofinFiskur


Breakthrough tuna diet launches in Japan
Aquaculture nutrition solutions provider Skretting has developed a new diet aimed at helping tuna farmers avoid the biosecurity risks and sustainability issues associated with baitfish diets.
Now available in Japan, MaGro is a soft extruded feed for bluefin tuna that has been created using patented production technology. The arrival of the breakthrough is ideally timed for Japan’s tuna farmers, who are seeing rising consumer demand for Japanese bluefin in domestic markets and further afield, including Asia and North America.


MaGro is the result of more than 20 years of dedicated R&D at Skretting to establish a viable alternative to baitfish feeding protocols. During that time, Skretting evaluated several feed types – everything from wet mashes to a wide variety of sausage formats – and these appraisals led to the finding that a soft extruded diet was by far the best option for tuna farming moving forward. The resulting diet takes the specific nutritional needs of bluefin into consideration and delivers it in a form that the fish want to consume. With a texture that is much softer than the pellets tailored for other fish farming sectors, MaGro still has a much lower water content than baitfish. As such, it offers a much better feed conversion ratio. The formulation is also very consistent, comprising fully-traceable ingredients, whereas the nutritional profile of baitfish can fluctuate dramatically depending on the species of baitfish used, when and where it was caught, and the storage system used. Baitfish also needs to be frozen – from the point of being caught through to the point of being fed to the tuna, which in itself requires freezing and thawing systems to be in place.
Furthermore, the practical, easy-to-use format of MaGro means that farmers can utilise semi-automatic “canon” feeding systems, which also support much more efficient, cost-effective production. Such user-friendly systems contribute to feed and manpower savings, while also diminishing water pollution. www.skretting.com
BIO - INCUBAT
Incubation system of salmon eggs

Ergonomic design

ADVANTAGES
Control and regulation of flows
Easy to assemble and handle Safer
Lower requirements of water
5 TRAYS 7 TRAYS
Unit of 5 trays with individual trays of 500mm x 500mm
Total capacity per unit: 112.000 eggs
Maximum water requirement per unit: 40 LPM
Sustainable Shrimp Partnership teams up with IBM for traceability
The Sustainable Shrimp Partnership (SSP) has joined the IBM Food Trust ecosystem to help provide traceability of SSP shrimp from farm to fork. The platform will use blockchain technology to deliver greater accountability and transparency to customers and consumers for every element of SSP premium Ecuadorian farmed shrimp's production and journey to the consumer plate.

As part of the Food Trust ecosystem, SSP’s members, comprised of responsible shrimp producers based in Ecuador, will enter data about how the shrimp is produced onto the blockchain. Ultimately, retailers around the world will be able to see this data and trace it in every stage so they can ensure the quality of the shrimp they are selling to consumers. SSP plans to enable consumer access via an app, enabling individuals to view provenance data about the shrimp they buy.
Food Trust enables real-time, end-to-end and immutable traceability of a food product to verify supply chain history; and can also provide verification of the shrimp’s SSP qualification– including confirmation it used zero antibiotic, approved and certified to the Aquaculture Stewardship Council (ASC) Standard. Food Trust provides a secure platform to which data can be uploaded and shared, and can help verify the authenticity of product claims. The technology will be accessible to buyers, retailers and consumers, and allow permissioned parties to have visibility into key product information.
SSP shrimp is produced to the highest social and environmental standards – ASC certified, use of zero antibiotics, and with neutral impact on local water quality. With the introduction of blockchain technology, SSP shrimp will be the first shrimp products on the IBM Food Trust solution. www.sustainableshrimppartnership.org
Unit of 7 trays with individual trays of 500 mm x 500 mm
Total capacity per unit: 157.500 eggs
Maximum water requirement per unit: 56 LPM

Triploid salmon egg technology could prevent cross breeding
Scientists at the Norwegian Institute for Nature Research (NINA) recently revealed that triploid salmon may play a significant role in the sustainable development of the aquaculture industry.
In a new study titled, “Comparisons of reproductive function and fatty acid fillet quality between triploid and diploid farm Atlantic salmon (Salmo salar),” researchers demonstrated that triploid salmon do not pose a significant threat to the wild salmon population, have good growth performance and high fillet quality. Triploidy could prevent escaped farm salmon breeding in the wild, while also improving nutrient quality within farmed fillets.

New chilling and heating incubators ideal for zebrafish eggs and larvae
Torrey Pines Scientific has announced the new, Larger EchoTherm Chilling/Heating Incubators ideal for incubating zebrafish eggs and larvae below, at, or above room temperature.
The 100-liter incubators are Peltier-based for heating and chilling. They have no compressors or CFCs and operate exceedingly well between 25°C to 33°C, the ideal temperatures for incubating zebrafish eggs and embryos. Other applications include incubating marine samples below room temperature, enzyme reaction and deactivation, protein crystallization, hybridization, ligations, storing oocytes, and general lab incubations.
There are two models. The IN55 is fully programmable and the IN50 is simple digital. They are settable from 4ºC to 70ºC and feature PID temperature control to ± 0.1°C, digital display to 0.1°C, and accuracy to ± 0.2ºC. They have RS232 I/O port for remote control and data collection, digital timer in hours, minutes and seconds with user settable auto-off, temperature ramping both up and down, and audible alarms.
Chamber size is 24” high by 20” deep by 14” wide and come with eight stainless steel racks with room for twelve.
The units are shipped with universal power supplies that take AC line inputs from 100VAC to 265VAC, 50/60 Hz and converts that to 12 volts DC for the unit, AC line cord for the country of use, user’s manual and full 12-month warranty. They are UL, CSA, and CE compliant. www.torreypinesscientific.com
Through many years of R&D, Atlantic salmon ova producer StofnFiskur has developed a secure method for triploidization of salmon ova. In the same way as in humans, the genes in salmon are normally placed on two sets of chromosomes. StofnFiskur can produce triploid ova by exposing fertilized eggs to high pressure in combination with specific temperature levels, at the correct time early in the production process. Then, the fertilized ova get three pairs of chromosomes instead of the natural two, producing salmon that are unable to reproduce. Through strict testing procedures at its land-based production facilities, StofnFiskur ensures that salmon ova sold as triploid are sterile. www.stofnfiskur.is



Photo: StofinFiskur













August
SEPTEMBER
Sept. 9-12
19th International Congress on Disease
Sept. 10-11
Aquaculture Innovation Europe London, UK aquaculture-innovation.com
70th NWFCC
DECEMBER 3 – 5,
Northwest

OCTOBER
Oct. 7-10
Aquaculture Europe 2019 Berlin, Germany www.aquaeas.eu
DECEMBER
Dec. 3-5
70th NWFCC Victoria, Canada gofishbc.com/nwfcc


























