
CF RATED
Challenges arise with offthe-shelf parts p.10
INTELLIGENT SENSORS
Pontosense pioneers wireless sensor tech p.12
MEMORY BOOST
MRAM is enhancing reliability in medical devices p.16

















Challenges arise with offthe-shelf parts p.10
Pontosense pioneers wireless sensor tech p.12
MRAM is enhancing reliability in medical devices p.16
















EDITORIAL
MedTech is making Canada proud
6 WEST TECH REPORT
Microlynx builds trust where needed
7 THINK GREEN
U.K. Royal Mint mines gold in E-waste
In every issue
18 NEW PRODUCTS
20 SUPPLY SIDE
21 AD INDEX
22 DEV BOARDS
Arduino Pro Edge AI/ ML vision & speech kit
8 ABLE TO ASSIST
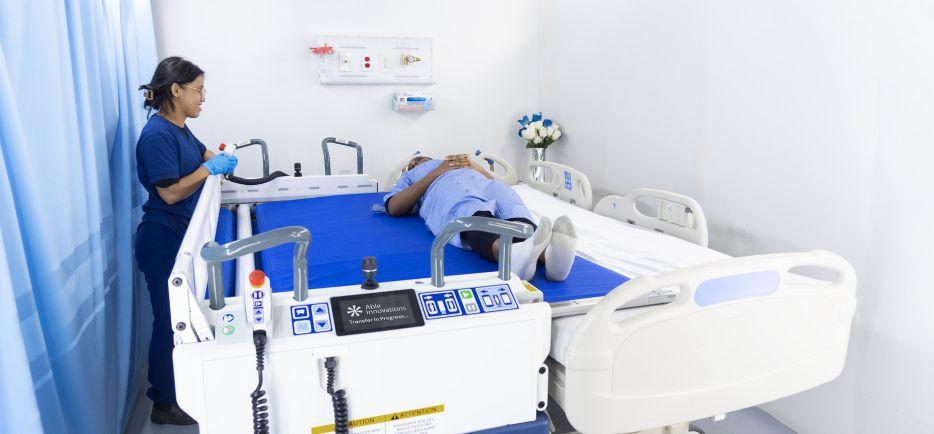
Toronto-based medtech design group puts patients to bed.
10 OFF-THE-SHELF
High-speed MRAM is emerging as the key to ensuring data integrity.
12 SMARTY PARTS
Applications transformed by the power of AI-driven human sensing.


16
MEDTECH RELIABILITY
MRAM memory chips are enhancing trustworthiness of designs.


• Low interwinding capacitance to minimize EMI and achieve high CMTI (Common Mode Transient Immunity)
• Optimized for isolated bias supplies for SiC and GaN gate drivers, such as the UCC25800-Q1 from Texas Instruments
• Ideal for automotive OBC and traction inverters in EV/HEVs

The medical electronics design market has been experiencing a remarkable period of transformation in Canda. This has been propelled by a convergence of technological advancements, increased healthcare demands and strategic governmental support. In response to pressing needs for innovative, patient-centric solutions, Canadian companies have risen to the challenge, creating devices that are smarter, more connected, and capable of delivering advanced healthcare outcomes.
These developments signal both a robust present and an exciting future - one defined by intelligent sensors, miniaturized systems and the integration of artificial intelligence (AI).
As the population ages and healthcare systems worldwide aim a move toward more personalized, preventive care models, demand for advanced medical electronics has intensified. Canadian companies, supported by a thriving ecosystem of research institutions, manufacturers and specialized investors, are uniquely positioned to lead this charge.
The country’s commitment to fortifying key ecosystem elements has strengthened domestic design and fabrication capabilities. As a result, Canadian firms are increasingly able to prototype, manufacture and distribute medical devices domestically, reducing dependence on overseas suppliers and minimizing risks associated with global supply chain disruptions. In recent years, AI has emerged as a vital tool for enhancing the capabilities of medical
electronics. AI is transforming diagnostics, imaging and decision support systems, enabling devices that not only collect data but also analyze and interpret it to guide healthcare providers.
Able innovates with ALTA
Toronto-based Able Innovations’ ALTA platform for robotic patient transfers is an example of a solution that leverages AI to improve safety and efficiency in hospital environments. By minimizing manual lifting and transferring, the ALTA platform reduces the risk of injuries among healthcare staff and patients, providing a glimpse into how AI can improve operational efficiency in healthcare.
You can read all about this trailblazing tech marvel, featured as the cover story in this issue (page 10). My interview with Able Innovations co-founder and CEO Jayiesh Singh is another example of the brilliant braintrust this country is afforded in healthcare development.
One of the most notable areas of progress has been in medical sensing technology, where companies like Pontosense Inc., also based in Toronto, have made impressive strides. Known for their work on intelligent wireless sensors, Pontosense develops sensors that capture critical patient data such as heart rate, respiration, and movement in non-invasive ways.
These sensors are particularly useful for continuous monitoring and in-home care, where comfort and ease of use are paramount. Such advancements reflect Canada’s capabilities in wireless communication, miniaturization and battery efficiency — all essential components for the effective deployment of Internet
of Things (IoT) technologies in healthcare.
The incorporation of IoT has allowed for seamless data collection, leading to more informed decisions and real-time interventions. By facilitating the constant exchange of information between devices, doctors and patients, IoT devices represent a major step toward proactive and responsive care. This edition of EP&T also provides an in-depth look at Pontosense, as presented by the Q&A format interview on page 12.
Government & industry have both recognized the potential of the medical electronics sector, and several initiatives are in place to bolster growth. Programs aimed at stimulating R&D, supporting start-ups, and enhancing manufacturing capabilities have provided substantial support.
Looking forward, collaborations between industry and Canadian research universities promise to drive further innovation. These partnerships aim to address pressing issues in healthcare, such as the demand for cost-effective solutions in remote and underserved communities.
By leveraging Canada’s expertise in AI, IoT and microelectronics, the industry is poised to continue developing cutting-edge devices that can improve health outcomes in these areas. In addition, government-backed initiatives for skill development and talent retention will ensure that the workforce is prepared to tackle the complexities of the future.
Canada’s information leader for electronic engineers and designers
NOV/DEC 2024
Volume 46, Number 8
READER SERVICE
Print and digital subscription inquiries or changes, please contact Angelita Potal Tel: (416) 510-5113 Fax: (416) 510-6875
Email: apotal@annexbusinessmedia.com
Mail: 111 Gordon Baker Rd., Suite 400 Toronto, ON M2H 3R1
EDITOR Stephen Law slaw@ept.ca · (416) 510-5208
WEST COAST CORRESPONDENT
Mike Straus · mike@brandgesture.ca
ACCOUNT MANAGER Joanna Malivoire jmalivoire@ept.ca · direct (866) 868-7089
ACCOUNT COORDINATOR Shannon Drumm sdrumm@annexbusinessmedia.com
MEDIA DESIGNER Jaime Ratcliffe jratcliffe@annexbusinessmedia.com
AUDIENCE DEVELOPMENT MANAGER Anita Madden amadden@annexbusinessmedia.com (416) 510-5183
GROUP PUBLISHER Paul Grossinger pgrossinger@annexbusinessmedia.com
CEO Scott Jamieson sjamieson@annesbusinessmedia.com
EP&T is published eight times per year by ANNEX BUSINESS MEDIA 111 Gordon Baker Road, Suite 400 Toronto, ON M2H 3R1 Tel (416) 442-5600 Fax (416) 510-5134 annexbusinessmedia.com
SUBSCRIPTION RATES
Canada – $59.67 one year; $95.88 two years USA – $136.68 (CAD) per year International – $187.17 (CAD) per year Single copy – Canada $15.00
ISSN 0708-4366 (print) ISSN 1923-3701 (digital)
PUB. MAIL AGREEMENT NO. 40065710 Return undeliverable Canadian addresses to: EP&T Circulation Department, 111 Gordon Baker Rd. Suite 400, Toronto, ON M2H 3R1

STEPHEN LAW Editor slaw@ept.ca
© 2024 EP&T. All rights reserved. Opinions expressed in this magazine are not necessarily those of the editor or the publisher. No liability is assumed for errors or omissions or validity of the claims in items reported. All advertising is subject to the publisher’s approval. Such approval does not imply any endorsement of the products or services advertised. Publisher reserves the right to refuse advertising that does not meet the standards of the publication. Occasionally, EP&T will mail information on behalf of industry-related groups whose products and services we believe may be of interest to you. If you prefer not to receive this information, please contact our circulation department.
PRINTED IN CANADA
DMZ, a global startup ecosystem located in Toronto, has announced its new Advisory Council. The group of visionary leaders will guide DMZ through an ambitious phase of growth and innovation, enhancing support for startups and reinforcing the hub’s role in national and global entrepreneurial ecosystems.
The council will unlock new opportunities, forge strategic partnerships and solidify DMZ as an ecosystem developer. Renowned for its community, an investment arm and a global network of strategic joint ventures, DMZ equips founders to build, validate and scale impactful startups while also producing skilled professionals for the innovation economy through expert-led courses.
In a bold move set to reshape the global semiconductor industry, Taiwanese electronics giant Foxconn has announced plans to build the world’s largest Nvidia semiconductor manufacturing facility in Mexico. The ambitious project will focus on producing cutting-edge chips, aimed at supporting Nvidia’s rapidly growing demand in AI, automotive, and gaming sectors.
The facility, set to be located in Mexico’s northern region, is expected to play a critical role in addressing the global semiconductor shortage. Foxconn’s decision to establish the factory underscores its strategy to diversify production away from Asia while capitalizing on the North American market.
The partnership with Nvidia comes at a time when AI-driven technologies, including machine learning, autonomous vehicles and data centres, are pushing chip makers to ramp up production.
Mercedes-Benz is partnering with the Ontario Vehicle Innovation Network (OVIN), the Government of Ontario’s flagship initiative for the automotive

and mobility sector. The purpose is to expand startup creation and scouting activities in North America and to promote the commercialization of automotive innovation. The OVIN Incubators Program will focus on identifying and fostering innovation in future software & AI, future vehicle components and future electric drive. Working with startups and in partnership with OVIN, Mercedes-Benz will help progress projects through the provision of its specialist background and use cases. Selected projects will also benefit from the international Mercedes-Benz Startup Autobahn network. Separately, the firm intends to start a research collaboration with the University of Waterloo, with a focus on neuromorphic computing for automated driving applications.
“Innovation is part of Mercedes-Benz DNA. In our global R&D strategy, open innovation gives us rapid and direct access to the latest ideas and developments around the world. We are therefore delighted to further expand our activities in Canada as a founding partner of the OVIN,” says Markus Schäfer of Mercedes-Benz Group AG.
Newark has added RIGOL Technologies to its test and instrumentation portfolio in Canada, the U.S. and Mexico. This expansion brings a new level of affordability and quality to test equipment users across a wide range of industries and applications.
With a broad range of products, RIGOL offers oscilloscopes, arbitrary
waveform generators, spectrum analyzers, RF signal generators, power supplies, dc loads, and multimeters, providing versatile solutions.
According to research from BlackBerry Ltd., 75% of embedded software developers and engineers globally admit that they are frequently pressured to compromise on safety requirements to meet tight project deadlines. The survey, which included 1,000 developers, highlights the increasing strain on software professionals as they balance the demands of rapid innovation with the need for safety and security.
While developers cite security (54%), cost control (52%) and safety certifications (48%) as their ‘top’ considerations when selecting an operating system, the survey also highlights significant downstream challenges in each of these areas that have made the vast majority (74%) of respondents open to changing their existing OS. Indeed, of those required to meet international safety standards, 61% say it is extremely or very challenging to meet these specific standards with their current OS. Security concerns (36%) and lackluster performance issues (28%) are the two main reasons respondents are considering changing their current OS.
Open-Source operating systems are the preferred foundational platform for almost half (44%) of developers, with an additional 25% indicating no preference for either Open-Source or proprietary offerings.
BY MIKE STRAUS, WEST TECH CORRESPONDENT
Since 1984, Microlynx Systems has been an in-demand provider of electronics design services for a variety of industries. General manager Clayton Myhill says the company’s culture and personality is what has kept it in business for 40 years; Microlynx also stands behind its work and provides exceptional service, leading to significant repeat business.
“Microlynx Systems was started by Ken Mouratidis, the original architect of the company,” Myhill explains. “He founded the company in 1984 and he’s still here today. He and the other majority partner, Bill Durtler, are interested in retirement, but still like to work.They like being busy, and they enjoy working on interesting projects.”
In 1984, Mouratidis was a university graduate with an engineering degree; he had a desire to perform one-on-one client work and oversee his own career, which led him to start Microlynx. 40 years later, Microlynx’s culture and personality has kept it humming along. The company doesn’t advertise; its clients come via word-of-mouth, and the firm sees significant repeat business due to their high quality of work.
“Stand by our work”
“When I first came here in 2015, I liked the feel of the culture and the people that were here,” Myhill notes. “We had like-minded people – people who are not only great at their jobs but are good, kind, caring, and considerate – and we take that into consideration when dealing with customers as well. We are vested in our clients’ success and we make sure to stand by our work. That’s why a lot of our customers are repeat customers.”
Myhill says the company operates more like a family business; all the employees are on a first-name basis, and anyone can speak their mind when issues arise. Microlynx Systems’ flexible working hours and interesting variation of projects have led to significant employee retention, which is important given the challenges in sourcing engineering talent.
“Finding the right talent is a

Clayton
challenge,” Myhill says. “There’s sometimes a disconnect between what’s on paper and what a person is like in their ability to do the job. Engineers are typically about the black and white, the ones and zeros. However, it is critically important to ensure a person fits well into the amazing culture we have here. We want them to be set up for success as well as to be an effective contributor.”
Since 2015, they have revamped the company’s hiring process to ensure they are prioritizing cultural fit among new hires. Myhill says he would sooner hire someone with strong intuition, natural talent, and a good work ethic than someone who has all the right qualifications but isn’t a team player.
In addition to its electronics design services, Microlynx Systems also manufactures test fixtures. Myhill says the test fixture arm of the business started because customers would require designs to be tested when taking them to contract manufacturers. Microlynx Systems noticed there was a gap between services that were available for customers on the market and what customers actually needed; for customers needing a test fixture but could not justify the cost for a full robotic style, due to lower production numbers, but needed something more robust than a small-scale low volume fixture, there was no available service.
“We found ourselves making a fixture for our clients that was an effective middle solution,” Myhill explains. “We created something that could handle 10,000 repetitions without falling out of tolerance. Our test fixtures are put together with aircraft-grade aluminum frames, so they’re pretty robust. We found at that point that our solution was one that filled the gap for many companies and we found ourselves with new clients that only needed this kind of test fixture.We since developed years of experience getting the most out of that test fixture without having to break the bank.”
Microlynx Systems’ test fixtures have been used by Sony and Motorola; the company has shipped its fixtures all
around the world. These test fixtures have a variety of applications, from aerospace and defense to medical equipment development and more. Myhill says that each client’s needs are different and Microlynx can easily scale up or down as needed to give clients the right fixture for their needs. Some companies’ circuit boards must go through testing as well as programming, so it is no surprise that each project is a bespoke bed-of-nails test fixture that is designed and built specific to their needs.
The company also offers obsolescence management services; Myhill says obsolescence management is an interesting area of work.
“One of the things we do quite well is a form-fit-function replacement for a subsystem or complete system if required,” Myhill explains. “We have worked on several aerospace and defence projects over the years, including projects to replace and improve electronics for the CF-18 and other aircraft in order to keep their systems and maintenance effective years longer than they were originally intended to be used for.”
Myhill says Microlynx Systems’ engineers are skilled at modernizing old equipment, as it is a unique skill to find was to make older things work and integrate into more modern electronics systems.
Microlynx is a primary subcontractor for Raytheon Canada, working on an over-the-horizon radar system for the Federal Government. They delivered on that project and are now upgrading the tech for the next system.
“Why do people come to us? Well, it really is about trust. We are trusted when the cost of failure is very high,” Myhill notes. “That means aviation, defense, aerospace and medical. That is something we are all proud of here at Microlynx. This has been a fantastic company to work for; it has great people and a good culture.”
www.microlynxsystems.com
Mike Straus is EP&T’s West Coast correspondent. mike@brandgesture.ca
The Royal Mint in the U.K. is pioneering a new factory that provides a more sustainable source of gold and reduces reliance on mining. The 3,700-square-metre facility uses world-first patented chemistry from Canadian clean tech company Excir, extracting gold from printed circuit boards (pcbs) found in everyday items, such as TVs, laptops and mobile phones, in minutes. Excir’s chemistry works at room temperature, creating a more energy efficient and cost-effective method of gold recovery.
The factory has scaled the innovative technology from laboratory to an industrial level for the first time and has the capacity to process up to 4,000
tonnes of printed circuit boards (pcb) from e-waste every year. It provides the UK’s oldest company with a new, more sustainable way to ‘mine’ high-quality 999.9 purity gold. Recovered gold is already being used in the luxury jewellery collection, 886 by The Royal Mint.
The factory delivers a more sustainable solution to growing environmental challenges. It has been designed to ensure that valuable finite resources are recovered and other materials are appropriately treated for onward processing.
“We are not only preserving finite precious metals for future generations, but we are also preserving the expert craftmanship The Royal Mint is famous for by creating new jobs and reskilling

opportunities for our employees,” said Anne Jessopp, of the Royal Mint.
As part of its commitment to being a leader in sustainable precious metals, The Royal Mint has also been actively engaging with major industry bodies to help produce the first standard by the ISO for the definition of
recycled gold, helping to provide clarity to the industry, end consumer and other stakeholders.
“The factory allows us to reduce our reliance on mined materials and is another example of how we’re working to decarbonise our operations,” stated Sean Millard, at The Royal Mint.

BY STEPHEN LAW, EDITOR EP&T
Able Innovations Inc., a Toronto-based startup, has emerged as a trailblazer in the field of healthcare automation with the introduction of its ALTA Platform. This robotic system is designed to facilitate patient transfers in hospital environments, significantly reducing the physical burden on healthcare staff while enhancing patient safety and comfort.
Founded in 2018 by Jayiesh Singh and Philip Chang, the firm’s leading-edge technology addresses challenges in healthcare and is transforming how hospitals approach one of the most physically demanding aspects of patient care. Infused with a bevy of sensors, haptics, actuators and motorized drives, this turbo-charged bed is not only improving operational efficiency in hospitals, but also adding to a broader trend toward automation and smart solutions in the medical field.
“Within hospital environments, it is very common to transfer a non ambulatory individual who can’t move. It could be orderlies, PSWs and/or nurses to physically move them over to the next surface. That’s always a strenuous task, but also requires an assembly of staff that are very scarce. So, our device does all the heavy lifting,” Singh said.
As the healthcare sector faces increasing pressure to deliver high-quality care amidst workforce shortages and growing patient populations, Able Innovations is at the forefront of a technological revolution that promises to alleviate strain while improving care standards. With automation built in, Singh says the unit is very easy to operate, so the user can focus on the patient. The bed detects the surface, before rolling under the patient and then transfers them onto a new surface.
“I think we’ve completely
reimagined how patients can be moved. In the past, you may have required four to six individuals to move a patient,” he noted. Now, with the addition of a lot of technological endeavors - we can do that with one person.”
The ALTA Platform is a complex system, that has an array of components and intelligence all built into maneuvering humans. As a result, a very high level of duty and care is required with its design, according to Singh. The system has multiple checkpoints for every actuation, as complications can arise during the act of rolling underneath a patient to pick them up. Through the design process, Singh and his engineering team had to observe many considerations, such as exercising caution with the patient’s skin and skin integrity during the transfer process.
“We also had to factor in how the bed manages obstacles, such as patient dressings, tubes, splints - things that you come across. So, we’ve implemented a lot of sensory technology,” he stated. “While it
Jayiesh Singh, CEO of Able Innovation, has combined the latest automation and smart sensor technology when designing the Alta Platform robotic patient transfer bed.


looks like a stretcher or bed, there is a lot of algorithm development that goes into making it work every time, but also easy to operate.
Through the entire design process, Singh mused that it has felt like they were engineering a car, rather than some other electronic gadget.
“The Alta Platform has a lot of the same subsystems and it has so many unique parts built from the ground up. We dealt with everything from front-end UI (user interface) development to finding new materials, while negotiating algorithm and machine learning development. This is because there are no existing models for what we’re doing,” Singh said.
As a result, the Able Innovation team endured a lot of trial and error, experimentation and integration of various disciplines and systems. In the end, Singh says it was all very rewarding, but also a very complex engineering endeavor.
“If you break everything apart, each system is elegant - perhaps

even simple. But, when you align this array of systems - they need to work together in perfect sync. For example, we have seven computers on the bed platform that work at high frequency, to make sure that the motors are in conjunction with what sensors expect,” he added.
From start to finish, Singh estimates that approximately 17 engineers, technicians and technologists have had a hand in the design of the ALTA Platform. That level and depth of expertise was necessary, as there are so many different departments and clinical-use areas in hospitals that the product needs to be compatible with.
“We need to mesh with most surfaces that patients are on, which includes such medical equipment as MRI machines or X-Ray tables,” Singh added. “All we’re doing is reducing the need for people at that point of transfer.”
While operated by medical staff using a joystick, AI enables the
ALTA Platform to make real-time decisions during the transfer process. Using machine learning algorithms, the system can autonomously adjust its movements based on the specific conditions of the patient and the environment. For example, the platform can detect variations in patient weight, size, or positioning and adapt the transfer accordingly to ensure smooth and safe movement from one surface to another (e.g., bed to stretcher).
“This is a complex piece of machinery that needs to be very easy to operate. Consider a nurse who is into the eleventh hour of their 12-hour shift – it must be able to operate intuitively,” Singh said.
A bit heavier than a standard stretcher, ALTA even features haptic sensors in the handlebars, so that it can detect when staff are holding, pushing or directing it. Machine learning algorithms power the Smart Move Technology within the unit, enabling the system to learn
from data gathered by its sensors. The system learns to anticipate and accommodate slight shifts in a patient’s posture, minimizing discomfort or risk of injury during the transfer. This predictive ability allows the platform to continuously improve its transfer methods over time, making each operation safer and more efficient.
“We’ve now introduced a set of stress sensors, so we can detect when somebody walks in front of the Alta. In the future, that will advance into semi-autonomous driving, perhaps,” Singh stated.
Wherever possible, Singh and his design team tried to leverage existing electronics and software, aiming to eliminate another source of variability.
“We purposely selected components that have been tried and tested in the market, so that we could focus on reliability - especially because we’re moving humans. We’re leveraging motors actuators that have already undergone a lot of lifecycle testing,” he added.
When it comes to meeting regulatory safety standards relating to the user experience, Singh says his team went above and beyond what is required.
The ALTA Platform allows for robotic transfer of hospital patients.
The tech filled beds are the creation of Toronto-based Able Innovations.
“It’s been baked into the core design of our device, which features multiple redundant systems that track safety and provides multiple fail-safes,” he stated. “The battery we used is ‘over spec’ – with the capacity to always hold a decent amount of reserve power. We track that, so that the user is never in a situation where the patient is mid-transfer, and the battery runs out,” he noted.
Singh anticipates that the Alta Platform will evolve in the next few years, including new features, applications, or even markets.
“We see ourselves getting more specialized in certain clinical areas to provide the most amount of value to them,” concludes Singh “We’re seeing a lot of specialized clinical areas wanting to use our technology with some bespoke modifications, whether it’s handling a very specialized surface or even a patient in a very unique position.” www.ableinnovations.com
A look
BY CONOR DUFFY, VICE-PRESIDENT, MARKETING, SYSTEM POWER AT ADVANCED ENERGY
As our population ages, the reliance on healthcare has grown rapidly to treat a vast range of chronic diseases, from cancer to heart disease. This has led to significant advances in technology and equipment, with enhanced treatments and better outcomes for these conditions.
Safety is a (if not the) primary concern for any medical device developer, and this increases with the level of body / operator contact. There are multiple standards/requirements that have been implemented to address this. IEC 60601-1 is arguably the most important.
This article examines the standards surrounding technology in medicine and the use of pre-qualified off-the-shelf components and subsystems in such devices, with a focus on power supplies.
As a population, we are more aware of our own health than at any period in history. A 2023 UN report predicts the number of people aged over 65 will more than double by 2050 – to 1.6 billion people. This is leading to greater pressures on medical services that treat chronic symptoms of old age to evolve rapidly. Without these changes, the demands on these facilities can push them to full capacity even before the seasonal increase in admissions. Therefore, monitoring and treatment is increasingly being performed remotely, which relies on advances in medical device technology.
Fortune Business Insights predicts this will lead to a market for medical devices worth almost a trillion dollars (US$ 886B) by 2032, a CAGR of 6.3%. With such significant returns for system developers, it’s vital that standards to ensure safety are both implemented and upheld.

Safety is an important consideration with all types of electronic equipment, especially in the medical sphere. Medical equipment comes into contact with patients and operators when used. To ensure an adequate (universally agreed) level of safety, standards are devised and published by the industry’s regulatory bodies, for example the IEC.
The standards apply to finished products as well as components and sub-systems. Third-party assessment and certification confirm that requirements are met and the units will be safe in all normal usage. The most stringent of standards also demand safety during one or more fault conditions.
For medical electrical equipment, IEC 60601-1 is the primary international safety standard. It categorizes products according to risk and type of application.
For risk, Class I devices (for example hand-held surgical instruments, manual wheelchairs) are generally considered safe and present minimal risk to patients and users. Class II (infusion pumps, imaging devices, etc.) pose a moderate risk with greater regulatory controls needed. Finally, Class III (pacemakers, implantable defibrillators, etc.) present
a high risk and are often life-sustaining or life-supporting, and therefore require rigorous regulatory oversight as well as extensive clinical data and pre-market approval.
To manage these risks and define design requirements correctly, IEC 60601-1 specifies three types of applications:
• Type B (Body) classifications refer to non-conductive devices that can be disconnected from the patient rapidly, for example ultrasonic equipment or a blood pressure monitor.
• Type BF (Body Float) classifications refer to devices that make conductive electrical contact, albeit not in close proximity to the patient’s heart.This includes ECG devices.
• Type CF (Cardiac Float) classifications are determined where the system makes electrical contact directly to, or close to, the patient’s heart during normal use. Cardiac catheter electrodes fall into this category.
Only Type B devices can have an earth connection and Type BF and Type CF must ‘float’ with respect to earth.
While a power supply would never be connected directly to a patient, it can form an essential part of the barrier between any mains voltage and the patient. To ensure this, electrical isolation is one key parameter stipulated by IEC 60601-1.
While the primary focus is on patient safety, equally important is the safety of the operator who runs a slightly lesser risk from a malfunction of the equipment. To address this, IEC 60101-1 discusses ‘Means of Protection (MOP)’, which are sub-divided into ‘Means of Patient Protection (MOPP)’ and ‘Means of Operator Protection (MOOP)’. The number of MOPs, the levels of electrical isolation and the permissible leakage in normal (NC) and single fault conditions (SFC) are all defined in the standard.
Pre-certified off-the-shelf power supplies can be used for Types B and BF applications. However, due to the strict requirements and complexity of Type CF applications, custom-designed power supplies were often the only option. This often involves modifying an existing power supply, which is usually time-consuming and expensive.
Although it is possible, this approach
involves considerably higher design risks and additional costs. Beyond the design, certification requires an extensive understanding of the regulations and risk assessments (to ISO 14971), testing and validation, documentation, compliance testing (in conjunction with a third-party lab) and the development of quality management systems (compliant to ISO 13485) -- all before regulatory submission and post-market surveillance.
In Types B and BF devices, use of off-theshelf equipment has made the process easier, reducing scope of testing, streamlining regulatory submission and leveraging existing documentation provided by the power supply manufacturer. This has led to a strong demand for pre-qualified Type CF power supplies.
However, now, power supplies that are CF rated, meeting the IEC 60601-1 standard, are arriving in the market. For example, in September 2024, Advanced Energy announced the first fanless convection cooled CF-rated solution, the NCF150 series of open frame power supplies.The series delivers maximum output power up to 150W and offers voltages of 12V, 15V, 19V, 24V and 48V.


In 25 years the world will be home to an additional 850 million people over 65, accounting for 1 in 6 people. To give context to this age group’s growth rate, the over 65s make up just 1 in 10 people now. Plus, the number of people over 80 is growing at an even faster rate. The aging population comes with a rise in chronic conditions associated with age, and a requirement for equipment such as pacemakers and other cardiac-related devices.
There is a growing need to develop systems and technologies to enable more effective and available healthcare, often from the patients’ homes, to help prevent overwhelming existing facilities.
While essential to ensure safety, vital standards and regulations – such as IEC 606011 – can slow development, requiring custom developed, or custom modified componentry such as power supplies. The use of pre-qualified and fully tested off-the-shelf equipment classified for Type B and Type BF devices has helped to reduce the risk, cost and time-tomarket. The rise of CF-rated power supplies will reduce this risk for even the highest level of device type.
www.advancedenergy.com


BY STEPHEN LAW, EDITOR EP&T
When it comes to saving lives or improving patient care in medical environments, innovation is often driven by the need for smarter, more efficient solutions. Pontosense Inc., a Toronto-based pioneer in wireless sensor technology, is at the forefront of this tech movement. Specializing in intelligent sensors designed for medical applications, Pontosense is reshaping how data is collected and utilized in healthcare settings. These cutting-edge devices are enabling non-invasive,
real-time monitoring, significantly improving the accuracy and convenience of medical devices.
In this exclusive Q&A, EP&T Magazine sits down with the team at Pontosense to explore their latest breakthroughs, the challenges of designing for medical environments and the critical role of wireless technology in the future of healthcare.
Q. Can you explain the core technology behind Pontosense’s intelligent wireless sensors?
Pontosense was founded on a legacy of innovation, with our founders holding over 500 patents collectively. At the heart of our product, Silver Shield, is a groundbreaking millimeter-wave (mmWave) sensor, small enough to fit on a coin, yet powerful enough to measure critical biometrics in real time. This is not just another sensor; it’s a disruptive force in AgeTech.
Unlike conventional wearable devices or existing RADAR-based fall detection systems, which often suffer from high false positives and
limited accuracy in identifying true falls, our technology offers a superior solution. By harnessing proprietary signal processing and achieving a 40 dB signal-to-noise reduction, we ensure precise, interference-free measurements. This advanced engineering enables consistent, reliable tracking of motion, presence, and even the early detection of critical health changes—all while safeguarding privacy, as it operates without the need for cameras or microphones.
Through continuous innovation, Pontosense aims to go beyond just creating products—we are redefining healthcare technology by making it smarter, more efficient, and truly non-intrusive, empowering individuals and caregivers alike.
Q. What specific medical applications are your sensors used in?
Falls are a major global health concern, ranked as one of the leading causes of injuries and injury-related deaths. In fact, 1 in 4 older adults falls each year, creating serious risks to their health, independence, and quality of life. Addressing this challenge, we developed a solution designed to ensure safety across various environments—whether in hospitals, long-term care facilities, or private homes.
One of the most significant challenges we encountered was the lack of clear, standardized regulations in the market. Many competing solutions fail to meet the necessary benchmarks for accuracy and reliability, leading to widespread false alarms and inconsistent performance. We overcame these challenges by focusing on precision and safety—developing a solution that outperforms existing products, even in an environment where regulatory frameworks are lagging.
Our approach raises the bar by delivering industry-leading performance, giving both individuals and caregivers the confidence that our technology will function reliably across diverse medical and non-medical settings.
Q. How do your wireless sensors contribute to improving patient outcomes?
It’s not just about diagnosis—it’s about being proactive in ensuring safety, security, and empowering individuals. The line between medical and non-medical environments is blurring, with homes increasingly becoming the new frontier of care. Healthcare is shifting from a
reactive model, where intervention happens after incidents occur, to a proactive approach that focuses on prevention and early detection.
Our sensors bridge the gap between the hospital and the home, extending the reach of telemedicine beyond traditional care settings. By monitoring and identifying risks early, we help prevent adverse events like falls or worsening health conditions before they escalate into emergencies.
This proactive model improves outcomes, reduces the burden on care facilities, and promotes independence, giving individuals and families peace of mind.
Being proactive isn’t just better—it’s transformative. It shifts healthcare from responding to crises to preventing them altogether, making care smarter, faster, and more accessible for everyone. We’re not waiting for the future; we’re building it now, creating a new standard where safety


Experience unparalleled reliability and ease of use with the high-performance E300.
Designed to handle a variety of wires, cables, and insulation materials with precision, the E300 ensures top-quality results. Seamlessly integrate the E300 with a wide range of peripherals to create an automatic processing line, boosting efficiency and performance.
Processes conductor cross sections from 0.05 to 10 mm² (36 to 8 AWG)
Intuitive interface simplifies job creation and setup
Quick software-assisted troubleshooting
Programmable clamping axis for greater accuracy
Two-in-one quick-change feeding unit, including a short piece kit for processing short cables
Wire Solutions for a Connected World schleuniger.com 905-827-1166
starts at home, and care is always within reach.
Q. What kind of technical challenges have you encountered and how have you overcome them?
We don’t integrate with other devices—we create our own. We design and build the complete solution ourselves, from the ground up. This vertical integration ensures that every element meets our high standards without relying on external components or systems.
However, we understand that interoperability is essential in healthcare, which is why our solution is designed to integrate easily with existing care management systems. This ensures smooth, secure data exchange and seamless alignment with current workflows to facilitate incorporation of our technology within their established infrastructure.
Our approach enables efficient collaboration across platforms, helping streamline operations without disrupting familiar processes. With this combination of end-to-end development and interoperability, we ensure both cutting-edge technology and compatibility with broader ecosystems, supporting care providers to maximize efficiency.
Q. Can you provide some insight into the regulatory challenges of wireless sensing.
One of the key challenges in introducing wireless sensing technology to healthcare is the lack of clear and consistent
regulations. Ensuring reliable performance is critical, as undetected events can pose serious risks to patient safety. Our focus is on delivering accurate, dependable solutions that make a meaningful impact, prioritizing safety and effectiveness over promises.
At Pontosense, we don’t compromise when it comes to safety and health. That’s why we’re pushing to work with governments, industry leaders and lobbyists to establish higher standards and stricter regulations in wireless technologies. It’s time the industry stopped cutting corners, because people’s lives are on the line.
Q. How do you ensure the accuracy and reliability of the data collected by your sensors?
Accuracy and reliability are at the core of everything we do. Our commitment is backed by deep expertise—before founding Pontosense, we built a global business in compliance testing, trusted by Fortune 500 companies for technologies that drive everyday life. This experience has given us invaluable insight into the standards of precision and performance, and we bring that same dedication to advancing healthcare solutions with Pontosense.
We’ve always been committed to pushing the boundaries of precision and performance, and that commitment continues with Pontosense. We’ve invested in a state-of-the-art lab in London, Ontario, where we run continuous AI-based testing to validate our sensors and refine their performance in real-time. This helps us go beyond just meeting industry
standards—we’re working to set new benchmarks in wireless sensing. What role does AI or machine learning play in processing the data gathered by your sensors, and how is it enhancing medical decision-making?
Data precision is the foundation of our technology. Our sensors capture readings with 10,000 times more accuracy than competing solutions, setting new industry standards. This level of precision changes everything. When you have data that good, it lightens the load on AI and machine learning (ML) models, allowing them to focus on generating deeper insights more quickly without being weighed down by noise or inaccuracies.
With advanced AI and ML algorithms, we significantly reduce false positive reports, ensuring reliable alerts crucial in areas like fall detection where excessive alarms can lead to caregiver fatigue and hinder patient response times.
Beyond fall detection, our AI-driven analytics unlock more sophisticated insights, such as proof of life, vital sign tracking, gait analysis, and early indicators of chronic health conditions. Our data-first approach allows AI and ML not only to perform better but also to learn faster— pushing the boundaries of what predictive care and monitoring can achieve.
Q. How do you foresee this technology evolving?
We’re at the forefront of remote patient monitoring which extends the time individuals can live safely and independently at home. Our partners are already

deploying Silver Shield in homes around the world. As we continue to innovate and adapt, our technology will evolve to enhance the patient experience even further, ensuring that remote monitoring is not just a necessity but a seamless part of daily living. We’re committed to redefining the standard of care, making it smarter, more accessible, and deeply integrated into the fabric of home life.
Q. What innovations in sensors are you most excited about?
While our innovations are proprietary, we’re on the cutting edge of sensor technology. We’re collaborating with four of the five largest chipset companies in the world to develop their next generation of chipsets, specifically designed for wireless sensing capabilities.
These advancements will deliver unprecedented power and efficiency, revolutionizing the way healthcare electronics function on a mass scale.
Q. How does your R&D process work?
While I can’t divulge all the details of our latest breakthroughs, our R&D process is relentless and innovative. We’ve made significant strides this year by reconfiguring and enhancing the output of our product. What might appear to be incremental changes have led to a remarkable tenfold improvement in our system’s performance within months.
Can you discuss the importance of energy efficiency in your sensors and the strategies you employ to extend battery life in medical applications?
Our product is not battery-operated, removing concerns around battery life and replacement cycles that can complicate medical applications. Instead, it is designed as a low-power solution, ensuring energy efficiency while maintaining continuous, reliable operation, minimizing
maintenance needs for seamless performance in healthcare environments.
Q. With cybersecurity being a major concern in medtech, how do you ensure data is secure?
We understand that the data we collect is not only sensitive but also essential for ensuring patient safety and maintaining trust in healthcare technology. Our commitment to security is a key differentiator, comple menting our industry-leading accuracy.
Pontosense’s team has a strong background in encryp tion and security measures, drawing on expertise from in dustry leaders known for their robust security protocols. Technologies developed by these leaders have been trusted by high-profile users, including government offi cials, due to their exceptional encryption capabilities. This expertise informs our ap proach to securing the data transmitted by our sensors, ensuring it’s protected by our advanced multi-layered secu rity strategy and compliance with global data protection standards.
Q. What are the biggest trends ahead?
The future of healthcare is shifting. The biggest trend we see is the move away from traditional care settings like hospitals and long-term care homes toward ‘aging in place,’ where people can live com fortably and independently in their own homes. COVID reinforced this shift when we saw that over 70% of deaths occurred in long-term care facilities. People today don’t want to be confined or tethered to machines and wires; they want the freedom to live out their lives naturally at home for as long as possible.

Pontosense Inc. develops AI-powered contactless human-centric sensing. www.pontosense.com
BY JOE O’HARE, EVERSPIN TECHNOLOGY

Reliability in medical devices is crucial, especially in life-critical systems that require continuous operation without failure. Magnetoresistive random access memory (MRAM) is emerging as the key to persistent, highspeed memory that ensures data integrity even during power loss. By addressing the gaps left by traditional memory technologies like DRAM and Flash, MRAM provides an optimal solution for medical systems that demand both endurance and reliability.
MRAM leverages magnetic states rather than electrical charges to store data. This eliminates the need for backup power sources such as batteries or capacitors, making MRAM particularly well-suited for medical devices that must function reliably in environments such as hospitals. MRAM’s standard interfaces, both parallel and serial, allow for seamless integration, offering low-latency storage and retrieval.
Modern
In electronic medical devices, this combination of non-volatility and fast, high-bandwidth writing is essential. MRAM handles intensive memory workloads and ensures data integrity in mission-critical environments where power failure cannot result in data corruption. This makes MRAM especially integral for the next generation of medical devices, particularly in applications that require continuous data logging and reliable operation under unpredictable conditions.
One example of a medical device which is reliant on MRAM technology is a hemodialysis machine. In its simplest form, hemodialysis machines consist of a blood pump, a dialyzer (often called an artificial kidney), a dialysis fluid supply system, and a set of tubes connecting the patient to the machine. The blood pump circulates the patient’s blood through the dialyzer, where waste products and excess fluids are filtered
out. Dialysis fluid (dialysate) flows on the opposite side of the membrane in the dialyzer, allowing the exchange of waste and electrolytes while preventing the loss of essential proteins and blood cells.
The machine precisely controls the flow of blood and dialysate to match patient-specific parameters, adjusting pressure and flow rates to optimize the efficiency of the treatment.
Modern systems are often equipped with monitoring systems that track critical variables such as blood pressure, temperature, and flow rates, as well as alarms for air bubbles, blood leaks, or other mechanical failures. Automated safety features ensure the machine continues to operate in case of power failure, and systems are designed with redundancy to avoid single points of failure. MRAM’s role is to log critical system performance and patient data reliably, without the risk of data loss or corruption. Its non-volatile memory ensures that all data remains intact during power fluctuations.
Currently, Everspin’s clients use MRAM in hemodialysis machines because of its ability to write data continuously over the life of the equipment without concern for data reliability.With MRAM’s virtually infinite endurance, designers do not need to worry about wearout, which is a common concern of other non-volatile memories such as Flash.Within a hemodialysis machine, and many other types of treatment and monitoring equipment, there is a need to:
• store configuration data that specifies how the system boots up or initializes;
• measure and record sensor data such as pressure or flow;
• log any system alarms; and
• retain operational data when the system is turned off until the system is again turned on.
MRAM offers significant advantages over conventional memory technologies. Its near-unlimited write endurance ensures that it can handle the constant data logging required in medical devices such as patient
monitoring systems and insulin pumps. Unlike NAND flash memory, which can degrade after repeated write cycles, MRAM provides a durable solution, maintaining consistent performance throughout a device’s lifecycle.
Additionally, MRAM is immune to soft errors caused by radiation or alpha particles, a common concern in high-radiation environments like hospitals and clinical settings. This protection against data corruption further enhances the reliability of medical equipment.
Traditional memory solutions such as DRAM and Flash come with inherent tradeoffs. DRAM, while fast, is volatile, meaning it loses all stored data when power is cut. In life-saving medical equipment, this can result in the loss of critical data. Flash memory, while non-volatile, suffers from limited write endurance, making it less suitable for devices that require constant data logging. MRAM bridges this gap by providing both non-volatility and unlimited write endurance, positioning it as a superior alternative for many medical applications. Another approach to protecting data is to use SRAM with a battery

backup. However, batteries present their own long-term risk and have to be replaced on regular schedule.
For medical device manufacturers, the adoption of MRAM can result in long-term cost savings. MRAM’s high reliability reduces the risk of device failures and product recalls, minimizing warranty claims and extending the lifespan of medical devices. Long product life cycles are also needed given the need to support deployed equipment for many years.
As the demand for more advanced and reliable medical devices grows, MRAM stands out as a robust solution that enhances performance, reliability, and safety in healthcare technology. Its non-volatility, unlimited endurance, and tolerance to radiation-induced errors will continue to make it the preferred choice for medical applications where data integrity is critical to patient care.
Everspin Technologies Inc., Chandler AZ, develops and manufactures discrete magnetoresistive RAM or magnetoresistive random-access memory products. www.everspin.com
The world’s largest continuous source of semiconductors.
• Over 15 billion finished devices in stock
• Over 200,000 part numbers in stock
• Over 12 billion die in stock
• Over 20,000 device types manufactured
• Capability to manufacture over 70,000 device types


CIRCUIT BREAKERS’ MAGNETIC MODULE PROTECTS WITHIN MILLISECONDS SCHURTER
Models TA35 and TA36 2-pole classic circuit breakers are optionally equipped with an additional magnetic module, which delivers reliable protection within milliseconds in the event of very high overcurrent (short circuit). No additional fuse is required when using a thermal-magnetic type. Depending on the application, the magnetic modules are available either with a slow or a fast acting characteristic. Both models are designed for snap-in mounting and with finely graduated rated currents. www.schurter.com/en/ datasheet/TA35_Rocker_2Pole
SMD SINGLE-FUSE BOOSTS AMPERAGE
LITTELFUSE
871 Series ultra-high amperage SMD fuse provides a single-fuse, surface-mounted solution, eliminating the need for parallel fusing configurations. The small-sized SMD fuses deliver ratings of 150A and 200A, previously available in much larger through-hole fuses. https://www.littelfuse.com/

INFINEON
StrongIRFET 2 power MOSFET 30V portfolio is optimized for high robustness and ease-of-use. Devices are specifically designed to meet the requirements of a wide range of mass market applications, enabling high design flexibility. Device offers up to a 40% R DS(on) improvement and up to a 60% reduction in Q G compared to the previous generation of devices. www.infineon.com/strongirfet2_30v
ADVANCED ENERGY
Artesyn ADH1300-48S28 ultra-efficient and highly reliable dc-dc converter module converter is designed for telecom wireless base stations and other equipment that have radio frequency power amplifier’s (RFPA’s). The 1,300W device delivers a 28V output in an industry-standard half-brick form factor, reducing energy consumption while offering integrated remote digital control capabilities. Converter has an industry-leading efficiency of nearly 96% at full load.
www..advancedenergy.com/ en-us/products/dc-dc-conversion-products/telecom-48v-inputpcb-mount-bricks/rfpa/adh1300/
COMPACT INDUSTRIAL
3KW PROGRAMMABLE POWER SUPPLY DELIVERS CONSTANT VOLTAGE
TDK-LAMBDA
HWS3000 programmable 3000W ac-dc power supplies in a 270 x 150 x 61mm case size deliver nominal output voltages of (24V, 48V, 60V or 130V) and output currents are fully programmable (CV/CC) from zero up to their maximum rating. Products are available with an 85 - 265Vac single-phase or three-phase 170265Vac input. Output programming can be achieved using a serial RS485 interface (MODBus protocol) or analog 1 - 5V or 4 - 20mA signals. www.product.tdk.com/en/power/
INTEL
Altera Agilex 3 next-generation, power and cost-optimized FPGAs are designed to meet the power, performance and size requirements of embedded and intelligent edge applications. Devices deliver higher levels of integration, enhanced security and higher performance in a compact package, with densities ranging from 25K-135K logic elements. Products feature an on-chip dual Cortex A55 ARM hard processor subsystem with a programmable fabric infused with AI capabilities.
https://www.intel.com/ content/www/us/en/products/ details/fpga/agilex/3.html
SAMTEC
JTAG-compliant connector series is available on both 2.54 mm and 1.27mm centerlines. Common sizes are 10 pin (2 x 5), 14 pin (2 x 7), and 20 pin (2 x 10) and are available in both through-hole and SMT orientations and with shrouded or unshrouded insulators. The most popular JTAG-compliant connectors are the FTSH terminal strip, TST shrouded terminal strip, and FFSD micro pitch cable assembly.
www.samtec.com
MEK

PowerSpector JSAz 550 larger 3D desktop AOI system is a larger L-size model, capable of accommodating printed circuit boards (pcb) up to 550x550mm (21.7”x21.7”). Unit features a completely redesigned chassis that includes upgraded motors, drives and spindles. While the optics and software remain consistent with firm’s other desktop models, the chassis design ensures that it meets the demands of inspecting larger pcbs with efficiency and precision.
AOI system is designed for maximum defect coverage, while maintaining short programming times.
www.marantz-electronics.com
FEATURE USB 3.2 GEN 2
FISCHER CONNECTORS
Core Series and UltiMate Series are available with USB 3.2 Gen 2 up to 10 Gbit/s with optimal signal integrity to meet the growing need for high-speed data transmission in demanding environments. Products join the portfolio of USB 3.2 connectors and cable assemblies for harsh-environment applications where SWaP (size, weight and power) is critical. Devices are suitable for a wide variety of applications, from surgical equipment in operating rooms and small computers and peripherals for soldiers, to outdoor test & measurement applications and civil and military drones.
https://fischerconnectors.com/ en/circular-connectors/ultimate-series/

MC33777 battery junction box integrated circuit (IC) integrates critical pack-level functions into a single device. Unlike conventional pack-level monitoring solutions that require multiple discrete components, external actuators and processing support, product consolidates essential BMS functions. IC reduces design complexity, qualification and software development effort and cost for OEMs, while enhancing the overall performance of the system.
https://www.nxp.com/products
Visit ept.ca for the latest new products, news and industry events.

Infineon Technologies has succeeded in developing 300mm power gallium nitride (GaN) wafer technology. The German-based semiconductor player has developed the new technology in an existing and scalable high-volume manufacturing environment, which will move to help drive the power semi market.
Chip production on 300mm wafers is technologically more advanced and significantly more efficient compared to 200mm wafers, since the bigger wafer diameter fits 2.3 times as many chips per wafer. GaN-based power semiconductors find fast adoption in industrial, automotive and consumer, computing & communication applications, including power supplies for AI systems, solar inverters, chargers and adapters, and motor-control systems.
State-of-the art GaN manufacturing processes lead to improved device performance resulting in benefits in end customers’ applications as it enables efficiency performance, smaller size, lighter weight, and lower overall cost.
San Jose-based provider of optical networking equipment, software and services, Infinera Corp. has agreed to receive up to USD$93 million in federal funding to expand operations, including in Lehigh Valley PA.
“Infinera has been a significant part of the extensive semiconductor sector in the Lehigh Valley that goes back to the development of the transistor by Bell Labs and Western Electric,” said Don Cunningham, president & CEO of Lehigh Valley Economic Development Corp. “Lehigh Valley was the original Silicon Valley, and many of the talented engineers and develops remain here.”
The proposed funding, from the CHIPS and Science Act, would support construction of a new advanced test and packaging facility in Bethlehem and a new fab in California.
“The federal government’s effort and investment through the CHIPS Act to increase domestic chip production has led to several new projects in the Lehigh Valley including the expansion of Infinera. The region is in a prime position to help America meet its objective to reclaim leadership in chip production.”


Raytheon has been awarded a three-year, two-phase contract from DARPA to develop foundational ultra-wide bandgap semiconductors, or UWBGS, based on diamond and aluminum nitride technology that modernizes semiconductor electronics with increased power delivery and thermal management in sensors and other electronic applications.
During phase one of the contract, the Raytheon team will develop diamond and aluminum nitride semiconductor films and their integration onto electronic devices. Phase two will focus on optimizing and maturing the diamond and aluminum nitride technology onto larger diameter wafers for sensor applications.
“This is a significant step forward that will once again revolutionize semiconductor technology,” said Colin Whelan, Raytheon president of advanced technology. “Raytheon has extensive proven experience developing similar materials such as Gallium Arsenide and Gallium Nitride for Department of Defense systems. Combining that pioneering history and our expertise will work to mature these materials towards future applications.”


















VENDOR: ARDUINO
PRO
EDGE AI/ML: VISION & SPEECH KIT
AKX00058 is a Pro Edge AI/ML vision and speech kit that provides an opportunity to learn and create solutions focused on artificial intelligence using computer vision, speech recognition and machine learning on the Edge. Practice with Edge AI tools to implement automation systems capable of image recognition and classification for object shape and position detection.
Get familiar with Speech Recognition tools to realize voice-controlled applications, capable of being part of hands-free systems or out-of-reach equipment. Integrate all with remote control thanks to the Arduino Cloud, experiencing the Arduino C++ based programming easiness.
Typical applications include industrial automation (goods quality control and counting, predictive maintenance, voice-controlled machines), smart cities (building automation, condition monitoring, appliances control systems), healthcare (patient monitoring).
The Arduino PRO Kits are development enablers designed to offer end to end solutions to deep dive the technological experiences offered by the Arduino Ecosystem, targeting specific verticals. Made of a set of selected products, accessories, tools and guides, are easy and ready-to-use bundles to build professional applications and proof of concepts.


Applications
Industrial Automation:
• Goods quality control and counting
Predictive Maintenance
• Voice controlled machines
Smart Cities
• Building Automation
• Condition monitoring
• Appliances control systems
• Healthcare
Patient monitoring
Kit Contains
• Arduino Portenta H7
• Arduino Portenta Vision Shield Ethernet Arduino Nicla Vision

• Arduino Enclosure for Nicla Vision Software Included:
• 2x Arduino Speech Recognition Engine
• Arduino Cloud for Business 3 months voucher
• Machine Learning Tools for IoT Cloud Service & Support Included:
• 1hour remote consulting for Product Selection with an Arduino or Arduino Partner expert 2 hours remote Technical Support from an Arduino Expert
Scan here for more information:
Thank you to our incredible sponsors, exhibitors and attendees for making this year’s EPTECH electronics trade shows a success! Your support has fueled innovation, sparked new connections and showcased the latest in electronics technology across Canada.
From cutting-edge components to breakthrough solutions, your participation helped EPTECH remain the premier platform for professionals in the electronics industry. We are grateful for your commitment to advancing the industry and sharing your expertise with peers.

Lanyard Sponsor Gold Sponsors




To see list of all of our 2024 EPTECH exhibitors, please visit eptech.ca
STAY TUNED FOR DETAILED DATES AND REGISTRATION INFORMATION FOR FUTURE EPTECH EVENTS IN 2025.
Hammond has over 20 million dollars of in-stock inventory and over 16,000 unique product skus to choose from.

Low voltage power transformers, high-end audio transformers and chokes, medical grade isolation.

Diecast aluminum, extruded aluminum, and plastic enclosures in thousands of sizes and configurations.


15A and 20A heavy duty outlet strips for commercial/industrial, rack mount, and benchtop applications.






Junction, wall mount, and freestanding enclosures in painted steel, stainless steel, aluminum and non-metallic.


19” racks, cabinets, and accessories for test & measurement, data communications and more.
