
12 minute read
Exploring Skin of Colour and Laser Hair Removal
Exploring SOC and Laser Hair Removal
Dr Baldeep Farmah provides an introduction to how laser hair removal can be made more accessible for patients with skin of colour
Advertisement
The global laser hair removal industry is growing and is expected to be worth $3.4 billion by 2026, according to market experts Acumen Research and Consulting.1 Despite this buoyant growth prediction, laser hair removal has historically been a subject of avoidance for patients who have darker skin tones, specifically those identified as skin types IV, V and VI on the Fitzpatrick skin type scale.2,3 Predominantly, this can be attributed to many professional laser hair removal devices being unsuitable for use on these skin types due to the possibility of certain adverse effects. These include (but are not limited to) hyperpigmentation,4 hypopigmentation,5 blistering and scarring.6 The previous inability within the aesthetics industry to treat patients with skin types IV, V and VI has been problematic to patient and practitioners alike. This treatment exclusion meant that patients were not able to experience the same level of technology as their fair-skinned counterparts and were left having to resort to more traditional, and less sophisticated and effective methods of depilation including shaving, waxing, depilatory creams and electrolysis. The situation is thankfully, if somewhat slowly, changing. Ongoing technological advancements coupled with improved awareness and education within the specialty in recent years means that laser hair removal devices can now reliably treat patients with darker skin tones, providing excellent outcomes.
The initial consultation One UK survey by the Black Skin Directory revealed that 92% of black women said it was a challenge to find a skincare professional who could meet their needs.7 In addition, 90% of respondents said the experience and knowledge of treating skin of colour (SOC) was the primary reason they chose a particular clinician.7 These statistics highlight how imperative it is that aesthetic practitioners are fully educated and informed about all skin types, and to avoid a ‘one size fits all’ approach. There are various physiological differences between dark and fair skin types that should be acknowledged, including the stratum corneum cell layers,8 ceramide levels,9,10 skin pH,11 transepidermal water loss (TEWL),12 and pigmentation.13 Specific knowledge and understanding should be applied, with particular focus on understanding melanin distribution in skin types, and how melanin reacts to laser wavelengths. It is also important to acknowledge the potential psychological impact of former treatment exclusion and how treatment disadvantage can be stressful and upsetting to explain. The consultation itself should be structured as such so that concerns about safety and efficacy on darker skin types are answered before questions are even asked. This is in addition to standard consultation discussion points such as treatment goals and expectation, current method of hair removal, how laser treatments work, different types of laser technology and why certain treatments are more effective than others, the treatment process and any factors that may affect outcome, medical history, pre- and post-treatment treatment care, cost and frequency and anticipated outcome. In addition, in my clinic, I will always perform a patch test to observe epidermal responses, establish the best treatment parameters for the specific skin type, and ascertain any adverse reactions 48-72 hours pre-treatment.
Skin type scales Part of the consultation process should include an analysis of skin type. The current industry standard is to use the Fitzpatrick scale that classifies skin into six broad categories (I being the fairest skin types and VI being the darkest) and is used to establish how much melanin is in the skin.14 As the population continues to diversify, there is conversation within the industry as to whether the Fitzpatrick scale is still relevant. As a result, some practitioners use a combination of different scales. One such example is the Roberts Skin Type Classification System, which observes both the Fitzpatrick and Glogau scales, and considers propensity to pigmentation, scarring and inflammation which can be useful when planning a patient’s treatment regimen.15,16 What current skin type scales do not explain and is useful to acknowledge are the physiological differences in skin types. This is important to ensure that different skin types are treated appropriately and safely, and to form an understanding of why difficulties in treating darker skin types can arise. A key difference pertaining to laser hair removal lies in skin pigmentation. Up to 8% of the cells in the stratum basal level of the epidermis is a melanocyte.17 Melanocytes make the pigment called melanin. When laser and light energy passes through the epidermis to target its chromophore, the energy has to go through the melanocytes in the epidermis. Melanocytes in the hair bulb, epidermal melanocytes and the melanin within them can act as competing chromophores and absorb this energy, resulting in an increased risk of complications and a higher frequency of adverse events such as erythema. Longer
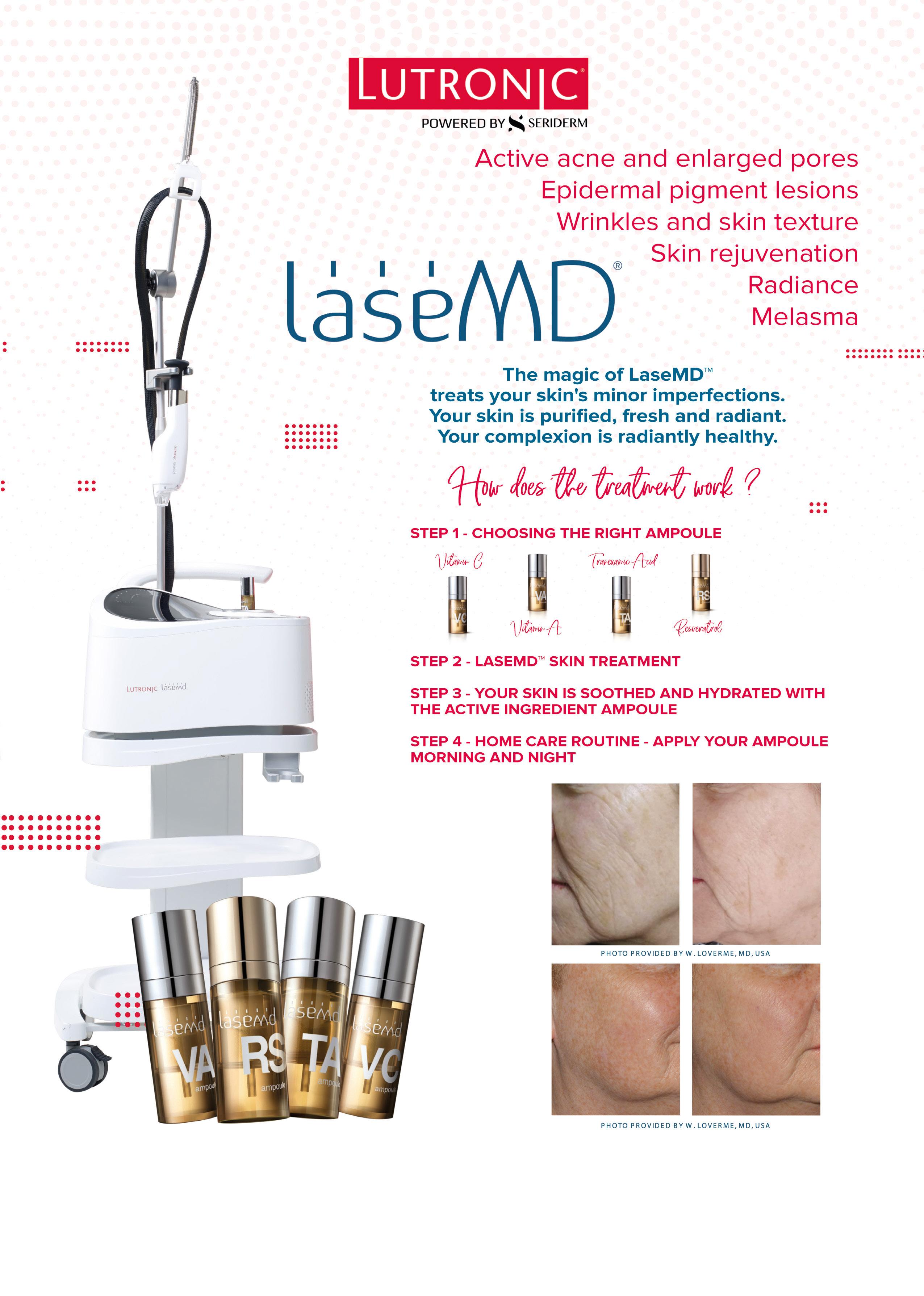
wavelengths are considered to be more effective on darker skin types as they are absorbed less by melanin.17 In addition, longer pulse durations are also recommended to prevent overheating. Excessive overheating of melanocytes in darker skin types can result in post-inflammatory hypopigmentation which is a widely recognised adverse event of laser hair removal.17
Available technology Given the wealth of technological capabilities within the sector, it’s almost unbelievable to conceive that only a decade ago, laser hair removal on darker skin tones was unheard of. Part of the historic problems lie in the basic fundamentals of how lasers work. Many devices were simply not sophisticated enough to distinguish sufficiently between the increased amount of epidermal melanin in skin types IV-VI and melanin present in the hair follicles and shaft. It is this technological ‘confusion’ that led to a higher frequency of adverse effects. Technology is fundamental to aesthetic clinics becoming more inclusive and it’s essential for practitioners to carefully consider what a laser hair removal device can offer their clinic and patients before making an investment commitment. Different types of laser hair technology include Ruby, Alexandrite, Diode and Nd:YAG.
Ruby (694.3 nm) A Ruby laser produces pulses of red visible light at a wavelength of 694.3 nm. It is very well absorbed by the melanin pigment in the hair, making this system very effective for people of skin tones I to III on the Fitzpatrick Scale. However, due to the shorter wavelength of the Ruby laser system being absorbed more by melanocytes, is not recommended for individuals with darker skin tones due to the possibility of pigmentation issues and scarring in skin tones IV to VI.18
Alexandrite (755 nm) The Alexandrite is a red-light laser that uses an Alexandrite crystal as a medium and operates at a shorter wavelength of 755 nm. This makes it ideal for targeting the melanin in the hair follicles of individuals with light to olive skin tones. Extreme caution is recommended in patients with skin types IV to VI as the laser can destroy melanin, resulting in hypopigmentation.19,20
Diode (808 nm) A precursor to the arrival of Nd:YAG technology, Diode lasers use semiconductor technology combined with a single narrow spectrum light beam to target specific chromophores in the skin. The 808 nm diode laser wavelength has a high abruption rate in melanin and uses the principle of selective photothermolysis (SPTL) to target melanin. The laser selectively heats the melanin and destroys the root and blood flow to the hair follicle, which results in the disruption of hair growth and regeneration. Suitable for use on all Fitzpatrick skin types, a diode laser can be complemented by cooling technology, or other pain reducing methods which improve treatment efficacy and patient comfort.21
Nd:YAG (1064 nm) In my view, practitioners looking to improve their inclusivity and widen their patient demographic should ensure that the device they choose includes Nd:YAG technology. It’s generally accepted that the use of Nd:YAG wavelength at 1064 nm is the most suitable for treating skin types IV-VI as the absorption by epidermal melanin is much lower than with other wavelengths such as Alexandrite, that are better suited to treating skin types I-III. Nd:YAG is also highly absorbed in oxyhemoglobin, which affects the blood supply to the hair follicle.22,23
Choosing a device I personally choose to use a device that combines both Nd:YAG and Alexandrite in my clinic. My device of choice is the Lumenis SPLENDOR X system, although there are many other devices available which are suitable for treating SOC. When choosing a device, I would always encourage practitioners to assess the clinical studies relating specifically to SOC for the specific device and look for a device that is customisable so you can tailor the treatment to fit the patient’s specific skin type, hair colour and thickness for the most effective treatment. You also want to consider speed for hair removal, so be sure to enquire about the spot size. This is because larger spot sizes allow the laser to penetrate deeper into the epidermis and allows the practitioner to cover larger areas more quickly.24
Consider advancing technology Technological advancements in the aesthetics industry and specifically laser hair removal have opened a wealth of opportunities to patients and practitioners alike. Practitioners should ensure that they research available system technology thoroughly and choose an option that not only allows them to treat a diverse patient base, but that also offers them the ability to expand their expertise, knowledge and training – all fundamental elements for procuring trust between patient and practitioner. All decisions should involve an element of inclusion so that all patients feel respected and treated as an individual and their physiological and psychological needs are considered and fulfilled simultaneously.
Dr Baldeep Farmah is the medical director of Dr Aesthetica clinic. His aesthetics skills are uniquely combined with years of experience as a psychiatric consultant to encourage a more holistic approach to aesthetic treatments. He specialises in offering patients natural-looking results that leaves a positive impact on their mental and emotional wellbeing. Qual: MBBS, RCPSYCH
TO VIEW THE REFERENCES GO ONLINE AT WWW.AESTHETICSJOURNAL.COM
BOCOUTURE® was the first neurotoxin treatment approved for upper facial lines1
FEEL GOOD LOOK GOOD
Bocouture® (botulinum toxin type A (150 kD), free from complexing proteins) 50/100 unit vials*. Prescribing information: M-BOC-UK-0432. Please refer to the Summary of Product Characteristics (SmPC) before prescribing. Presentation: 50/100 units of Clostridium Botulinum Neurotoxin type A, free from complexing proteins as a powder for solution for injection. Indications: Temporary improvement in the appearance of moderate to severe upper facial lines (glabellar frown lines, crow’s feet lines, horizontal forehead lines) in adults ≥18 and <65 years when the severity of these lines has an important psychological impact for the patient. Dosage and administration: For intramuscular use only. Unit doses recommended for Bocouture are not interchangeable with those for other preparations of botulinum toxin. BOCOUTURE should only be administered by an appropriately qualified healthcare practitioner with expertise in the treatment of the relevant indication and the use of the required equipment, in accordance with national guidelines . The intervals between treatments should not be shorter than 3 months. Reconstitute with 0.9% sodium chloride. Glabellar Frown Lines: Total recommended standard dose is 20 units. 4 units into 5 injection sites (2 injections in each corrugator muscle and 1 injection in the procerus muscle). May be increased to up to 30 units. Injections near the levator palpebrae superioris and into the cranial portion of the orbicularis oculi should be avoided. Crow’s Feet lines: Total recommended standard dosing is 12 units per side (overall total dose: 24 units); 4 units injected bilaterally into each of the 3 injection sites. Injections too close to the Zygomaticus major muscle should be avoided to prevent lip ptosis. Horizontal Forehead Lines: The recommended total dose range is 10 to 20 units; a total injection volume of 10 units to 20 units is injected into the frontalis muscle in five horizontally aligned injection sites at least 2 cm above the orbital rim. An injection volume of 2 units, 3 units or 4 units is applied per injection point, respectively. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Generalised disorders of muscle activity (e.g. myasthenia gravis, Lambert-Eaton syndrome). Infection or inflammation at the proposed injection site. Special warnings and precautions: It should be taken into consideration that horizontal forehead lines may not only be dynamic, but may also result from the loss of dermal elasticity (e.g. associated with ageing or photo damage). In this case, patients may not respond to botulinum toxin products. Should not be injected into a blood vessel. Not recommended for patients with a history of dysphagia and aspiration. Caution in patients with botulinum toxin hypersensitivity, amyotrophic lateral sclerosis, peripheral neuromuscular dysfunction, or in targeted muscles displaying pronounced weakness or atrophy. Bocouture should be used with caution in patients receiving therapy that could have an anticoagulant effect, or if bleeding disorders of any type occur. Too frequent or too high dosing of botulinum toxin type A may increase the risk of antibodies forming. Should not be used during pregnancy unless clearly necessary. Should not be used during breastfeeding. Interactions: Concomitant use with aminoglycosides or spectinomycin requires special care. Peripheral muscle relaxants should be used with caution. 4-aminoquinolines may reduce the effect. Undesirable effects: Usually, undesirable effects are observed within the first week after treatment and are temporary in nature. Undesirable effects independent of indication include; application related undesirable effects (localised pain, inflammation, swelling), class related undesirable effects (localised muscle weakness, blepharoptosis), and toxin spread (very rare - exaggerated muscle weakness, dysphagia, aspiration pneumonia). Hypersensitivity reactions have been reported with botulinum toxin products. Glabellar Frown Lines: Common: headache, muscle disorders (elevation of eyebrow). Crow’s Feet Lines: Common: eyelid oedema, dry eye, injection site haematoma. Upper Facial Lines: Very common: headache. Common: hypoaesthesia, injection site haematoma, application site pain, application site erythema, discomfort (heavy feeling of frontal area), eyelid ptosis, dry eye, facial asymmetry, nausea. For a full list of adverse reactions, please consult the SmPC. Overdose: May result in pronounced neuromuscular paralysis distant from the injection site. Symptoms are not immediately apparent post-injection. Legal Category: POM. List Price: 50 U/vial £72.00, 50 U twin pack £144.00, 100 U/vial £229.90, 100 U twin pack £459.80. Product Licence Number: PL 29978/0002, PL 29978/0005 Marketing Authorisation Holder: Merz Pharmaceuticals GmbH, Eckenheimer Landstraße 100,60318 Frankfurt/Main, Germany. Date of Preparation:August 2021. Further information available from:. Ground Floor Suite B, Breakspear Park, Breakspear Way, Hemel Hempstead, Hertfordshire, HP2 4TZ Tel: +44 (0) 333 200 4143
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK. Adverse events should also be reported to Merz Pharma UK Ltd at the address above or by email to UKdrugsafety@merz.com or on +44 (0) 333 200 4143.

*Botulinumtoxin type A, purified from cultures of Clostridium Botulinum (Hall strain)2 References: 1. Merz Pharma GmBH & Co. Merz Announces European Approval of Bocouture for the Treatment of Upper Facial Lines. Available at: https://www.merz.com/blog/news/merz-announces-european-approvalof-bocouture-for-the-treatment-of-upper-facial-lines/. Accessed November 2021. 2. BOCOUTURE® (incobotulinumtoxinA) Summary of Product Characteristics. Merz Pharmaceuticals GmbH.
M-BOC-UK-0445 Date of Preparation: December 2021








