
17 minute read
Case Study: Fat Dissolving Complications
Having unique services and being an expert in what you provide is important both for a successful business and for patient safety and satisfaction. This includes being well-prepared for managing the complications. This article discusses the possible adverse effects following deoxycholic acid for the purpose of fat reduction. It also presents a case study with long-lasting cutaneous adverse events associated with deoxycholic acid administration for local excessive adipose tissue and our approach to management. Intralipotherapy and its safety profile The history of using chemical compounds such as phosphatidyl choline (PDC) and/or deoxycholic acid (DCA) to treat local adipose tissue deposit can be traced back to the early 2000.1 Widely known as injection lipolysis, DCA is extensively used for non-surgical body or face contouring, targeting localised excess fat pocket. The mechanism of DCA is leading to the lysis of local adipocytes, resulting in permanent improvement in contouring.2,3 According to the literature, DCA has a high safety profile, with a low percentage of patients experiencing adverse effects such as nodules lasting more than one month (0.9%) and skin necrosis (0.01%).4 There are two categories of side effects, which are all localised within the treatment areas.5 These include the common and normally relatively short-term complications, and those that are extremely rare and long-term.6,7
Common and short-term There is a study by Amore et al. which showcases the common and short-term adverse effects associated with fat dissolving treatment well. In the study of 221 participants:4 75% experienced swelling (oedema) within three days post treatment, 60% had tenderness lasting more than 12 hours, 41% presented with ecchymosis, 39% experienced warmth to the treatment area, 30% reported temporary altered sensation (numbness), 28% presented with redness/ erythema and 14% of lumps/nodules which resolved with one month. Rare and long-term Reasons for extremely rare and possibly long-term major adverse events to occur are normally associated with the intralipotherapy technique, including uneven administration, bolus deposition, and superficial placement of the agent. Patient age (over 60) is also a factor whereby reduced metabolism could lead to inadequate treatment intervals, and overdosing will also cause issues, as will treating a patient too soon (before four weeks) after their initial session.8 Case study: cutaneous adverse effect Dr Olha Vorodyukhina and Dr Tracy Xu explore the A 26-year-old lady presented to Dr Xu’s clinic for a fat dissolving treatment. Her possible adverse effects following deoxycholic acid main area of concern was the upper part injections for fat reduction and present a patient of the lateral thighs, superficial to the skeletal prominence of greater trochanter. case study It was agreed that DCA would be a good treatment for this patient because she had a healthy lifestyle with regular gym trips three times per week. All other body parts were within normal proportion, except a localised secondary fat pocket, which had been present at both lateral thighs since puberty. Dr Xu has been using the DCA brand DesoBody for body contouring since it was first available in the UK in 2018 and has achieved a good portfolio of satisfactory treatments, so this was the product of choice. For her treatment, after the skin was thoroughly disinfected three times with chlorhexidine to minimise complications, each 10ml vial of DCA was mixed with 1ml Lignospan Special (20mg/ ml lidocaine and 12.5ug/ml adrenaline) before being administered to the area of interest. Two vials were administered to the left lateral thigh (20cm2 surface area of soft tissue thickness 4cm), and one vial to the right lateral thigh (10cm2 surface area of soft tissue thickness 4cm). This dosage was according to the treatment protocol based on the physical assessment. A 23 gauge, 100mm intralipotherapy needle was used, injecting from the midpoint of the lateral thigh towards the cephalic side, using a fanning technique with multiple retrograde linear threads. The linear thread stopped at 1cm from the entry points to avoid agent accumulation. The fanning angles between each thread was 2-3 degrees and the angles were only changed when the needle had been retracted back to the dermis layer to avoid the needle bending. There was only one needle entry point on each of the lateral thighs. In total, 40 passes of 0.5ml each thread (20ml in total) was
Advertisement

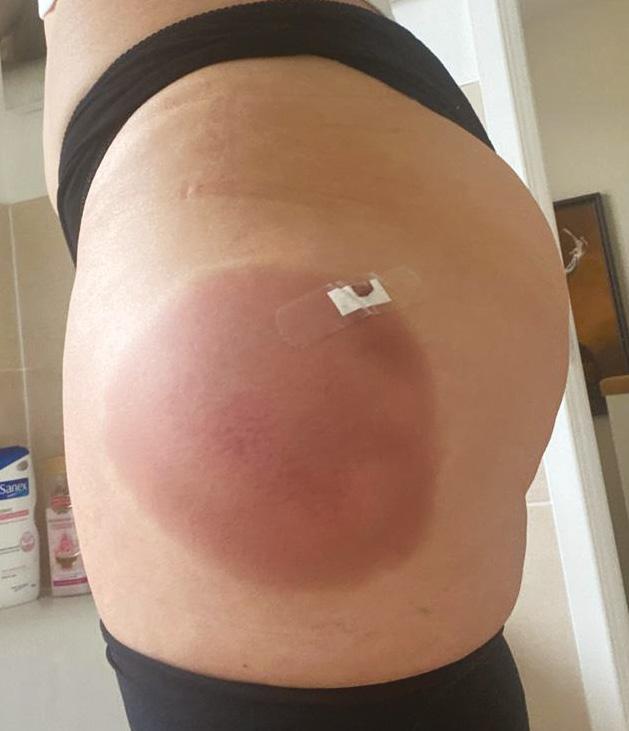
Figure 1: Patient one day following DCA treatment to the left lateral thigh.
administered to the left lateral thigh, and 20 passes of 0.5ml (10ml in total) linear threads to the right lateral thigh. The whole treatment procedure was smoothly done without major discomfort from the patient. Immediately post procedure there was some redness at the treated area, especially on the left side, where a higher dosage was administered. The red area was padded with sterile gauze before helping her to put her tightening garment on, and she was advised to keep wearing it for 72 hours from the treatment, then keep it on at night when sleeping for seven days. The tightening garment is an essential part of the protocol in fat-dissolving injections for minimising swelling and assisting with lymphatic drainage post treatment. The patient was booked for a review in five weeks.
The adverse event This patient emailed the clinic the next morning as she was experiencing extreme swelling and pain, induration, and difficulty getting dressed due to swelling, particularly the left thigh. Judging from the pictures she sent of her left thigh (Figure 1), the treated area had more than average swelling and looked inflamed. The patient was booked for a review appointment at the next available time, and in the meantime advised to take arnica tablets, massage with arnica gel, avoid contaminating the injection site, and keep a detailed track of image record of the changes. On day three post-treatment, the patient reported that the swelling had settled down, so she decided not to present to the clinic for review. She was advised to return for a clinic review at five weeks, where she would be assessed for a second treatment. At the five-week assessment, there was no redness, and soft tissue thickness reduced to 3cm, which was a good response in terms of result. However, there were three lumps on the left, and one lump on the right thigh and the sensation on the left lateral thigh was slightly reduced. There was an area of discolouration with a diameter of approximately 10cm on the left lateral thigh, and a periodical shooting pain at the right lateral thigh, scoring 1/10, which is not exacerbated by physical pressure (Figure 2). From clinical examination, Dr Xu felt that the soft lumps were most likely caused by uneven distribution of the agent so another treatment was carried out using a 20 gauge 100mm cannula to help break down the lumps. A total of 17.5ml of DCA was administered. The next day, the patient reported low degree of pain scoring 0.5/10, and said the swelling was very subtle compared to the first treatment. The picture sent by the patient seven days after showed improvement of the induration and bruise (Figure 2). At the four-week review, the lump sizes were reduced by 50%, but the discoloration had very little improvement. The initial shooting pain the patient was describing was not reported since this point, and all clinical examination showed normal sensation.
Managing the complication A few treatment options were proposed to restore normal circulation and break down the lumps of fatty tissues (cellulite). The first was deep tissue massage that the patient can perform with a manual massager at home. The second was carboxytherapy treatment in clinic. Although this treatment approach is not discussed in the literature for treating DCA complications, its therapeutical properties,9,10 and historical use to treat diabetic ulcers11 provided confidence that this would be a good treatment of choice for this patient. Carboxytherapy has also been recommended by UK DesoBody trainer and Botulinum Toxin Club founder Dr Harry Singh, as well as the inventor of DesoBody, Professor Roberto Amore, for the improvement of cutaneous and subcutaneous adverse effects from lipolysis injection.12 The first effect following CO2 injection is a strong vasodilation of the vessels; therefore increasing oxygenation in the treated area. It also improves regenerative activities and stimulates the formation of new capillaries – also known as angiogenesis. As a result, it increases circulation and stimulates localised metabolic activity of the tissues, which is known as the Bohr effect.13,14 About 1% of absorbed CO2 converts into carbonic acid CO2+H20=H2CO3; further reacting to leave bicarbonate dissolved in the blood plasma H+HCO3. These reactions cause the pH of the blood to decrease, and release oxygen to the tissues.13 The patient was prescribed 10 sessions of weekly carboxytherapy treatments with Dr Olha Vorodyukhina. Injection depth were subdermal and intradermal for both circulation stimulation and cellulite reduction. The total volume of gas administered at each
Before After

Before After
Figure 3: Patient before carboxytherapy and after 10 sessions.

treatment was 80cc (left) and 40cc (right). After five sessions of CO2, the right side had full positive resolution. After 10 sessions on the left side, there was significant improvement in colour and further lump reduction. The patient was advised to have another five to 10 sessions of CO2 for the right thigh, and after five more sessions her skin had almost returned to normal.
Discussion The safe dosage regime and techniques, pharmacokinetics, pharmacodynamics and adverse events of DCA injection have been well researched and documented in the past 30 years.15 DCA is highly selective for adipose tissue, and very small interaction with protein-rich tissues such as muscle tissue, blood vessels, and skin.2,8,13,15 On the other hand, as DCA causes lysing effect to the targeted adipose tissue with disruption to the cell membrane, leading to cell death and tissue inflammation, its vasoconstrictive nature could result in undesired cutaneous effects, if dosage or treatment technique have not been optimal.16 Duncan et al. also described in a clinical safety survey about the consensual poor outcome at inner or outer thigh, compared with other areas such as abdomen, flanks and submental chin.17 The main reasons for the thighs to be a poor area to treat include: 1. It is usually accompanied by the skin laxity, which could mask the true effect of fat reduction 2. This area has a high incidence of cellulite, which makes the adipose tissue to form more lumps and therefore more difficult for a targeted medicine distribution 3. Higher incidence of fibrosis especially post lipolysis treatment 4. The distribution is normally over a broad area instead of the ideal bulky, rounded shape areas such as abdomen
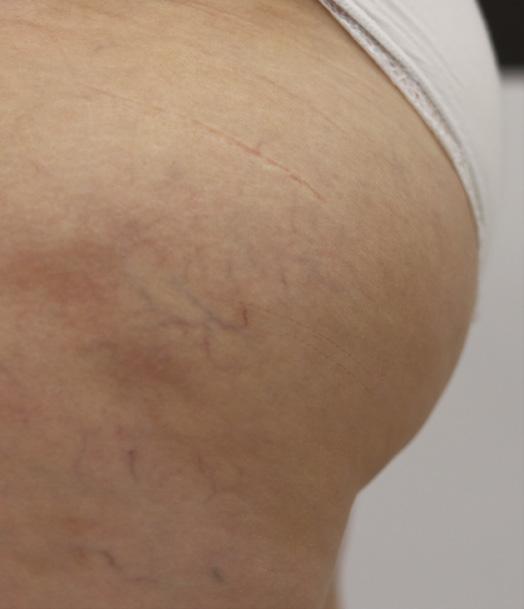
On reflection of this case, it is likely that the dosage for the areas intended to treat was higher than this patient’s soft tissue tolerance level. This indicates that, when assessing for fat dissolving at the area of skeletal prominence, the standard protocol of dosages should be lowered, halved, or even avoided completely, to minimise significant short-term and long-term complications such as redness, swelling, pain, and lumps. From the above we have learnt that when treating the lateral thigh, especially over the greater trochanteric area, the treatment should be performed with great caution at a minimal dosage, or avoid treating at all if less desired. To minimise the risk of possible cutaneous effects, when treating areas superficial to major skeletal prominence such as great trochanter, we propose: • Assess the skin laxity, the soft tissue fibrosis and severity of cellulite carefully • Start from the smallest dose required • Use less traumatic techniques, such as cannulas for administration • Evenly distribute the agent and avoid accumulative dose at certain areas leading to localised fat liquefaction • The layer of distribution is also important to avoid too superficial deposition of DCA, which could lead to compromised systemic absorption of the emulsified adipose tissue18
Consider complications It’s important to be aware of the possible complications when administering DCA treatments and know how to handle both short and long-term adverse events. Carboxytherapy can be considered to correct the lumps, induration and discolouration from fat dissolving treatment and should be a widely equipped service for clinics providing fat reduction services.
Dr Tracy Xu is an advanced aesthetic doctor with more than 10 years of experience in cosmetic surgery and injectable treatments. Dr Xu was trained as a resident trainee in facial plastic surgery, as well as aesthetic injectables since her Bachelor of Medicine and Bachelor of Surgery (Hons) degree in 2009. She completed her Level 7 (master) degree in aesthetic injectables and Level 4 Cosmetic Dermatology with Harley Academy. Dr Tracy is an advanced aesthetic trainer for Cosmetic Courses. Qual: MBBS(hons), PhD in Life Sciences
Dr Olha Vorodyukhina is a dental surgeon, aesthetic practitioner, the clinical lead for training provider Cosmetic Courses and a trainer for Sinclair Pharmacy. She has been practising aesthetics since 2011 and specialises in injectables. Dr Vorodyukhina is the owner and founder of Shine Medical and Angels Twelve clinics in the Midlands, which have both been selected as centres of excellence in the Midlands by Sinclair for their hyaluronic acid filler MaiLi. Qual: Dip Stom Ukrainian Medical Stomatological Academy 2005
REFERENCES
1. Rotuna AM, Suzuki H, Moy RL, Kolodney MS. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution.
Dermatologic Surg. 2004;30(7):1001-1008. 2. Shridharani SM, Behr KL. ATX-101 (deoxycholic acid injection) treatment in men: insights from our clinical experience. Dermatologic Surg. 2017;43:S225-S230. 3. Liu M, Chesnut C, Lask G. Overview of Kybella (deoxycholic acid injection) as a fat resorption product for submental fat. Facial Plast Surg. 2019;35(03):274-277. 4. Amore R, Amuso D, Leonardi V, et al. Evaluation of Safe and Effectiveness of an Injectable Solution
Acid Deoxycholic Based for Reduction of Localized Adiposities. Plast Reconstr Surg Glob Open. 2018;6(6):e1794. 5. Pinto H, Hernandez C, Turra C, et al. Evaluation of a new adipocytolytic solution: adverse effects and their relationship with the number of vials injected. J Drugs Dermatol 2014;13:1451-5. 6. Pinto H, Hernandez C, Turra C, et al. Evaluation of a new adipocytolytic solution: adverse effects and their relationship with the number of vials injected. J Drugs Dermatol. 2014;13:1451–1455. 7. Yagima Odo ME, Cucé LC, Odo LM, et al. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg. 2007;33:178–188; discussion 188. 8. Duncan DI, Chubaty R. Clinical safety data and standards of practice for injection lipolysis: a retrospective study. Aesthet Surg J. 2006 Sep-Oct;26(5):575-85. Erratum in: Aesthet Surg J. 2009
May-Jun;29(3):262. PMID: 19338944. 9. Riyad abdulhamza G, Al-Omary H, Physiological Effects of Carbon Dioxide Treatment on
Diabetic Foot Ulcer Patients. IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN:2278-3008, p-ISSN:2319-7676. Volume 13, Issue 5. 10. Brochado TMM, de Carvalho Schweich L, et al. Carboxytherapy: Controls the inflammation and enhances the production of fibronectin on wound healing under venous insufficiency. Int Wound J. 2019 Apr;16(2):316-324. Epub 2018 Nov 22. 11. Brandi C, Carboxytherapy. Practical Manual with Clinical Indications and Protocols, 2019, pp.27-99. 12. Eldsouky F, Ebrahim HM. Evaluation and efficacy of carbon dioxide therapy (carboxytherapy) versus mesolipolysis in the treatment of cellulite. J Cosmet Laser Ther. 2018 Oct;20(5):307-312. Epub 2018
Jan 17. 13. Sakai Y, Miwa M, Oe K, et al. A novel system for transcutaneous application of carbon dioxide causing an “artificial Bohr effect” in the human body. PLoS One. 2011;6(9):e24137. 14. Bunyatyan ND, Drogovoz SM, Shtroblya AL, Kononenko AV, Zelenkova H, Prokofyev AB, Sapovsky
MM, Nikolaeva LL. The mechanism of the pulmoprotective action of carboxytherapy. Vopr Kurortol
Fizioter Lech Fiz Kult. 2019;96(4):58-62. Russian. 15. McGraw Hill, Emergency Medicine Procedures Chapter 106, Subcutaneous Abscess
Incision and Drainage. 2013.<https://accessemergencymedicine.mhmedical.com/content. aspx?bookid=683§ionid=45343748> 16. Dayan SH, Humphrey S, Jones DH, et al. Overview of ATX-101 (deoxycholic acid Injection): a nonsurgical approach for reduction of submental fat. Dermatologic Surg. 2016;42:S263-S270. 17. Walker P, Lee D. A phase 1 pharmacokinetic study of ATX-101: serum lipids and adipokines following synthetic deoxycholic acid injections. J Cosmet Dermatol. 2015;14(1):33-39. 18. Ramirez MR, Marinaro RE, Warthan ML, Burton CS. Permanent Cutaneous Adverse Events After
Injection With Deoxycholic Acid. Dermatol Surg. 2019 Nov;45(11):1432-1434.
96% patient satisfaction with BOCOUTURE®, you can be confident of results that your patients come back for.1
FEEL GOOD LOOK GOOD

Bocouture® (botulinum toxin type A (150 kD), free from complexing proteins) 50/100 unit vials*. Prescribing information: M-BOC-UK-0432. Please refer to the Summary of Product Characteristics (SmPC) before prescribing. Presentation: 50/100 units of Clostridium Botulinum Neurotoxin type A, free from complexing proteins as a powder for solution for injection. Indications: Temporary improvement in the appearance of moderate to severe upper facial lines (glabellar frown lines, crow’s feet lines, horizontal forehead lines) in adults ≥18 and <65 years when the severity of these lines has an important psychological impact for the patient. Dosage and administration: For intramuscular use only. Unit doses recommended for Bocouture are not interchangeable with those for other preparations of botulinum toxin. BOCOUTURE should only be administered by an appropriately qualified healthcare practitioner with expertise in the treatment of the relevant indication and the use of the required equipment, in accordance with national guidelines . The intervals between treatments should not be shorter than 3 months. Reconstitute with 0.9% sodium chloride. Glabellar Frown Lines: Total recommended standard dose is 20 units. 4 units into 5 injection sites (2 injections in each corrugator muscle and 1 injection in the procerus muscle). May be increased to up to 30 units. Injections near the levator palpebrae superioris and into the cranial portion of the orbicularis oculi should be avoided. Crow’s Feet lines: Total recommended standard dosing is 12 units per side (overall total dose: 24 units); 4 units injected bilaterally into each of the 3 injection sites. Injections too close to the Zygomaticus major muscle should be avoided to prevent lip ptosis. Horizontal Forehead Lines: The recommended total dose range is 10 to 20 units; a total injection volume of 10 units to 20 units is injected into the frontalis muscle in five horizontally aligned injection sites at least 2 cm above the orbital rim. An injection volume of 2 units, 3 units or 4 units is applied per injection point, respectively. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Generalised disorders of muscle activity (e.g. myasthenia gravis, Lambert-Eaton syndrome). Infection or inflammation at the proposed injection site. Special warnings and precautions: It should be taken into consideration that horizontal forehead lines may not only be dynamic, but may also result from the loss of dermal elasticity (e.g. associated with ageing or photo damage). In this case, patients may not respond to botulinum toxin products. Should not be injected into a blood vessel. Not recommended for patients with a history of dysphagia and aspiration. Caution in patients with botulinum toxin hypersensitivity, amyotrophic lateral sclerosis, peripheral neuromuscular dysfunction, or in targeted muscles displaying pronounced weakness or atrophy. Bocouture should be used with caution in patients receiving therapy that could have an anticoagulant effect, or if bleeding disorders of any type occur. Too frequent or too high dosing of botulinum toxin type A may increase the risk of antibodies forming. Should not be used during pregnancy unless clearly necessary. Should not be used during breastfeeding. Interactions: Concomitant use with aminoglycosides or spectinomycin requires special care. Peripheral muscle relaxants should be used with caution. 4-aminoquinolines may reduce the effect. Undesirable effects: Usually, undesirable effects are observed within the first week after treatment and are temporary in nature. Undesirable effects independent of indication include; application related undesirable effects (localised pain, inflammation, swelling), class related undesirable effects (localised muscle weakness, blepharoptosis), and toxin spread (very rare - exaggerated muscle weakness, dysphagia, aspiration pneumonia). Hypersensitivity reactions have been reported with botulinum toxin products. Glabellar Frown Lines: Common: headache, muscle disorders (elevation of eyebrow). Crow’s Feet Lines: Common: eyelid oedema, dry eye, injection site haematoma. Upper Facial Lines: Very common: headache. Common: hypoaesthesia, injection site haematoma, application site pain, application site erythema, discomfort (heavy feeling of frontal area), eyelid ptosis, dry eye, facial asymmetry, nausea. For a full list of adverse reactions, please consult the SmPC. Overdose: May result in pronounced neuromuscular paralysis distant from the injection site. Symptoms are not immediately apparent post-injection. Legal Category: POM. List Price: 50 U/vial £72.00, 50 U twin pack £144.00, 100 U/vial £229.90, 100 U twin pack £459.80. Product Licence Number: PL 29978/0002, PL 29978/0005 Marketing Authorisation Holder: Merz Pharmaceuticals GmbH, Eckenheimer Landstraße 100,60318 Frankfurt/Main, Germany. Date of Preparation:August 2021. Further information available from:. Ground Floor Suite B, Breakspear Park, Breakspear Way, Hemel Hempstead, Hertfordshire, HP2 4TZ Tel: +44 (0) 333 200 4143
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK. Adverse events should also be reported to Merz Pharma UK Ltd at the address above or by email to UKdrugsafety@merz.com or on +44 (0) 333 200 4143.

*Botulinumtoxin type A, purified from cultures of Clostridium Botulinum (Hall strain)2 References: 1. Prager W, et al. Clin Cosmet Investig Dermatol. 2012;5:53–58. 2. BOCOUTURE® (incobotulinumtoxinA) Summary of Product Characteristics Merz Pharmaceuticals GmbH.
M-BOC-UK-0450 Date of Preparation: March 2022








