A Nanofiber Based Adsorbent to Address Purification Requirements in the Delivery of mRNA Therapies via Extracellular Vesicles
2 Preparation Of Linearised Plasmid For mRNA Production
Background
mRNA therapeutics, for example certain covid vaccines, are manufactured using an enzymatic reaction.
• The desired sequence is expressed as a plasmid from e coli. Super coiled and open circular DNA are digested enzymatically to give a linear template.
• In Vitro Transcription (IVT) reaction uses the linearised plasmid to create multiple copies of the mRNA sequence required.
Challenges
Purity of the linearised template is critical
• Residual host cell DNA impurities or other nucleic acid contaminants will give rise to off-target mRNA products. These consume costly reagents and are difficult to remove.
Low Productivity Processes
Bead-based processes restrict the size of the templates which can be purified



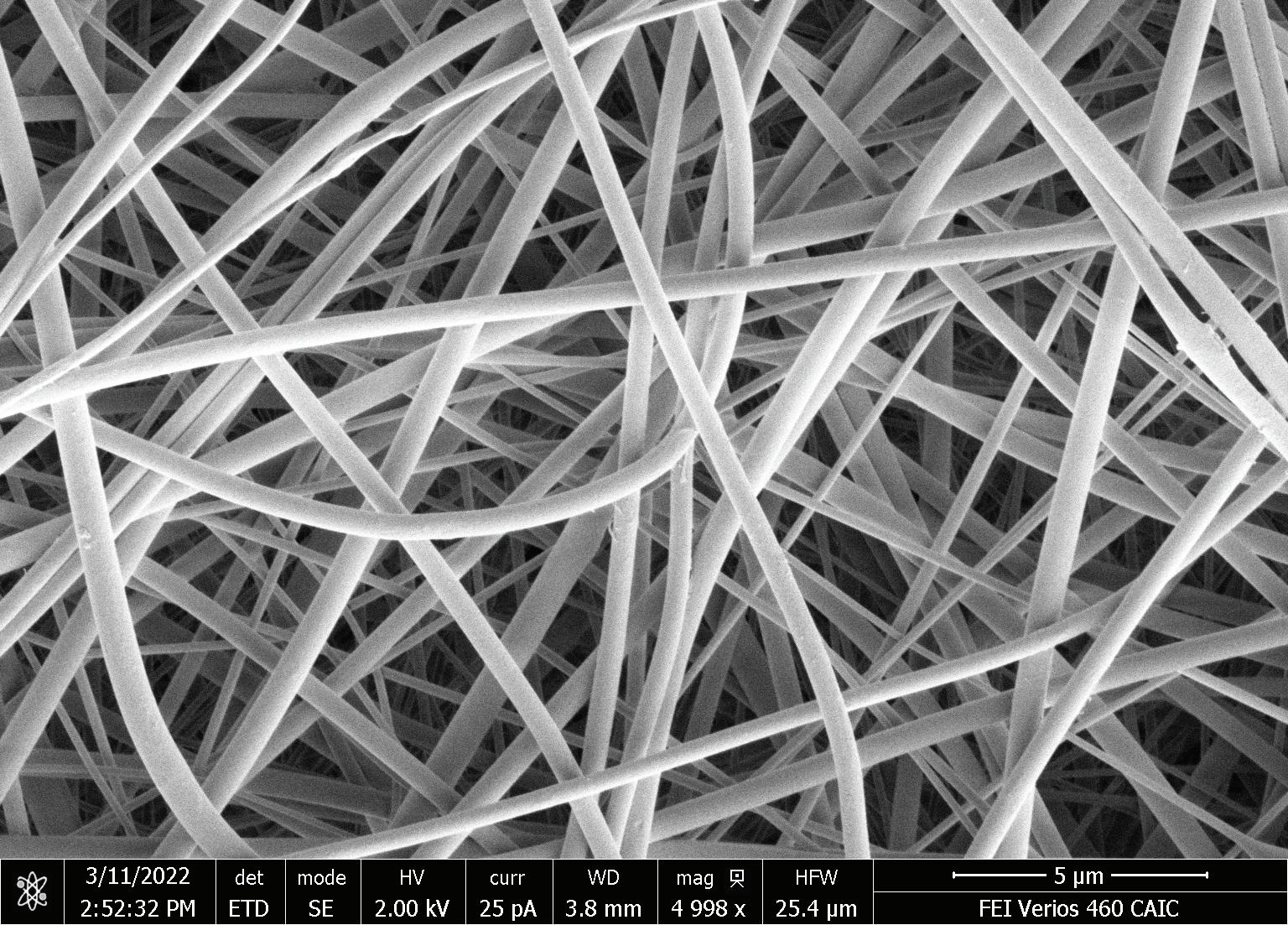
Bead based adsorbents, where a large portion of the surface area is contained within the pores, are limited in application for EV purification due to the size of the target versus the size of the pores.
• Size exclusion techniques can be used, but are not applicable at process scales, and the resolution of product related impurities (i.e. other large particles) is limited.
• Commercially available convective adsorbers are strong anion exchangers, meaning they require high salt concentrations to elute large macromolecules, which are often less stable at high salt concentrations. EVs were eluted with a low salt buffer, by using nanofiber adsorbent functionalising a weak AEX ligand. This also allows the use of pH of the elution and binding buffers for additional selectivity.
Conclusions
Extracellular vesicles (EVs), including exosomes, are potential tools for delivery of nucleic acid therapeutics. Their native tropism, stability, and lack of inherent immunogenicity can deliver therapeutic payloads with increased efficacy and are of particular interest in oncology. mRNA sequences can be delivered using EVs as an alternative to systemic delivery via lipid nanoparticles (LNPs), so avoiding potential issues of LNP instability, toxicity, and low drug load efficiency. Purification of the raw materials used to produce both the payload and the vector in these new systems can be challenging since both modalities are larger than can be dealt with easily by most chromatographic adsorbents. This study demonstrates that AstreAdept® nanofiber adsorbent can produce pure linearised plasmid for mRNA production and isolate EVs from their biological sources, and so support early process development to quickly deliver the critical quality attributes required for clinical use. AstreAdept® nanofiber adsorbent was functionalised with ion exchange (WAX) or hydrophobic (HIC) ligands, and then encapsulated in a disposable housing. After sanitization, the pre-packed capsules were used to purify raw material components for the non-viral vector gene therapy delivery Chromatogram of linear DNA purification AGE analysis of linearisation reaction Effective EV Isolation With Weak Anion Exchange Nanofiber Adsorbent EVs Can Be Separated By Relative Charge AstreAdept® technology: Novel Nanofiber Adsorbent With Expansive Flowpath Purity And Recovery Of Linearised DNA Purifying Linearised Plasmid On Hydrophobic Nanofiber Adsorbent
Abstract
Ian Scanlon1, Christie Childers1, Emma Burman2, Marc Hummersone1 1. Astrea Bioseparations, Horizon Park, Barton Road, Comberton, Cambridge, CB23 7AJ, UK 2. Department of Biochemical Engineering, University College London, UK 5 6 1 4 3 TARGET DIAMETER (nm) EVS 30-150 PDNA 4-5 KB ~100 PDNA 20 KB 275-286 PDNA 56 KB 313-342 • Average mean flowpath diameter 860 nm Giving excellent flow characteristics for both EVs and nucleic acids METHOD 1 mL of hydrophobic nanofiber produced 6 mg of a 6 kb plasmid sequence from E Coli lysate. The purified plasmid was buffer exchanged into RO water; 4 mg was linearised with HIND III, adjusted to 2.25 M ammonium sulphate and bound to the HIC adsorbent. Bound DNA was eluted using a step to TE. The enzymes and protein used in the linearisation are removed in the flowthrough.
Raw materials for the next generation of advanced therapeutics can be purified quickly and efficiently using the novel nanofiber adsorbent described. This technology can be used to speed up the discovery, process development and manufacturing processes. By extrapolating our results, we predict that clinically relevant volumes of linearized DNA and EVs, would only require a 100 mL adsorbent capsule. METHOD Buffers A: Equilibration buffer PBS B: 100% gradient to Elution buffer PBS +1M NaCl Flow rate: 10mv/min • EV solution Diafiltrated into PBS • Concentrated 10x Load concentration measured by Nanoparticle Tracking analysis (NTA) Load concentration (particles) 1.17 e10/mL Multiple ligands High Binding Capacity Fast Flow Rate Reduced Footprint
and operate at very low flow rates due to the high viscosity of
nucleic acid solutions. • All steps run at 5 mL/min, including 0.5 M NaOH CIP. Total process took 45 minutes. Delta column pressure remains below 0.2 bar through the process.
the
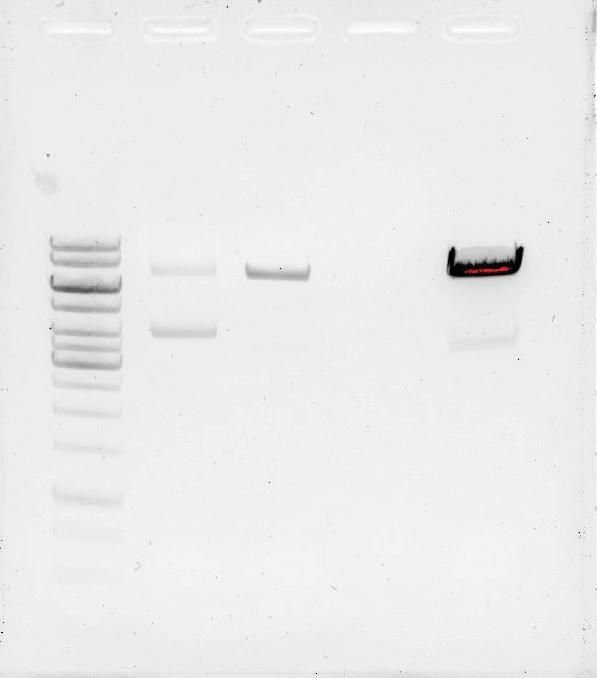
1: Ladder 2: Purified pDNA (mixture of sc/oc) 3: DNA post linearisation with HIND III 4: Flowthrough from HIC adsorbent 5: Elution from HIC adsorbent 1 2 3 4 5 0 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0.09 0.1 50 100 150 200 250 300 350 400 450 500 0 0 50 100 150 200 pressure (MPa) mL UV (mAU) Conductiviy (mS/cm) Chromatogram of linear DNA purification UV 1_280 UV 2_260 mS/cm MPa DNA mg/mL ml Total DNA (mg) Pre-Linearization 0.19 21 4.0 HIC adsorbent elution pool 0.68 5 3.4 DNA mg/mL* ml Total DNA (mg) Pre-Linearization 0.19 21 4.0 HIC adsorbent elution pool 0.68 5 3.4 Yield of linearised DNA: 85% Removal of Protein: 99% *Based on densitometry Protein measured by Bradford total protein
Isolation of EV particles using the nanofiber adsorbent for primary capture EVs from two different CDMOs were used to assess adsorbent capacity 0.00E+00 2.00E+11 4.00E+11 6.00E+11 8.00E+11 1.00E+12 1.20E+12 1.40E+12 1.60E+12 1.80E+12 2.00E+12 Particles per mL absorbent mSC HEK 293 OUTCOME • Load EVs : 9.26E+11/ mL of adsorbent • Total Recovery of EVs ~85%
Purification of Linearised DNA Isolation of EVs HIC Adsorbent Size (mL) Linear DNA Yield (mg) 1 ~3-4 10 ~30-40 100 ~300-400 1000 3000-4000 Weak AEX Adsorbent Size (mL) EV Yield Total particles 1 8E+11 10 8E+12 100 8E+13 1000 8E+14 0 20 40 60 80 100 120 140 160 180 0 5 10 15 20 25 30 0 10 20 30 40 50 60 70 80 Conductivity mS/cm UV (mAU) Elution Pool mL mAU Conductivity Any data or results provided are only examples and do not provide any guarantee of similar results in future. The products of Astrea Bioseparations may be covered by or for use under one or more patents: astreabioseparations.com/patents All trademarks, trade names, trade dress, product names and logos are the property of Astrea UK Services Ltd. © 2024 Astrea Bioseparations Ltd. All rights reserved Search: Astrea Bioseparations The average standard dosing for an mRNA therapeutic is reported at between 1-2 mg/Kg, and the efficiency of the IVT reaction has been reported between 60-100x production of mRNA per mg of linearised pDNA Doses for EV therapeutics are quantified by protein or nucleic acid, or by particle size. In this case it has been reported that a systemic delivery of EVs would require 2E+10 particles/ Kg REFERENCES 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8641981/ 2.https://www.sciencedirect.com/science/article/pii/S277304172300008 *Dependent on sequence length
Primary Capture
Of EVs From HEK293 Cells





