Isolation of Extracellular Vesicles using a Weak Anion Exchange Functionalized Nanofiber Matrix
Sujeong Yang, Emma Burman, Ian Scanlon
Astrea Bioseparations, Horizon Park, Barton Road, Comberton, Cambridge, CB23 7AJ, UK
Abstract
Extracellular vesicles (EV), including exosomes, have a promising role in regenerative medicine, diagnostics, and delivery of gene therapies. Mild processing conditions for EV isolation are crucial to maintain the integrity and functionality of these nanosized membrane-bound structures. There is a need for scalable isolation techniques that achieve
Methods and materials
Extracellular vesicles EVs were produced from two different cell lines, human mesenchymal stem cells (EVerZom, France) and HEK 293 cells (EchoBiotech, China).
Feedstock preparation
In both cases EVs were harvested, clarified and diafiltered into Phosphate Buffered Saline (PBS), concentrated, and loaded directly onto the fiber. The diafiltered concentrate was then loaded directly onto the chromatography capsule
Chromatography downstream purification EV isolation was performed at each CDMO facilities using Exo
AstreAdept® Novel composite electrospun nanofiber adsorbent with expansive flowpath
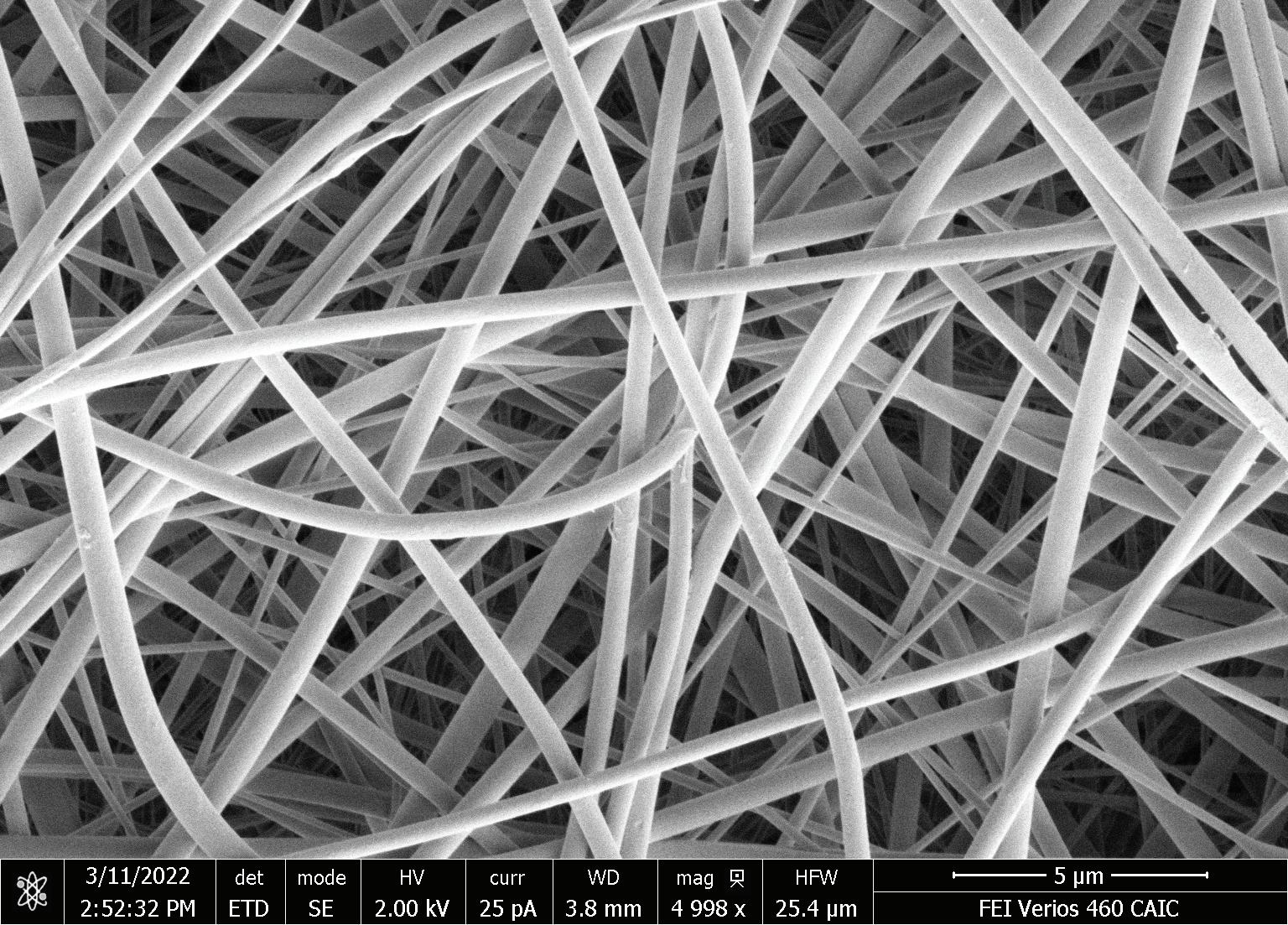
In this study, EVs were isolated from mesenchymal stem cell (MSCs) and from HEK 293 cell culture, using ExoHERO®, a novel chromatography capsule containing a unique electrospun composite nanofiber, AstreAdept® Primary

Primary Capture of EVs from Mesenchymal Stem Cells: ExoHERO® Weak Anion Exchange adsorbent chemistry
Search: Astrea Bioseparations
Breakthrough study on EVs from HEK 293 cells: ExoHERO® Weak Anion Exchange adsorbent chemistry
Breakthrough of particles in flowthrough by nanoFCM
The



Purification of EVs by total protein analysis: ExoHERO® - Weak Anion Exchange adsorbent chemistry ExoView data from load and elution
“The tetraspanin colocalizations remain identical before and after chromatography. This indicates that the technology doesn’t select an
0 10 20 30 40 50 60 70 80 90 100 -50 0 50 100 150 200 250 300 0 5 10 15 20 25 Conductivity (mS/cm) Volume (mL) UV Primary capture of EVs from Mesenchymal cells UV Conductivity
high
high
damage.
recovery and
purity at scale, while minimizing EV aggregation or
of EVs from Mesenchymal cells
capture
HERO®, a novel chromatography capsule containing a unique electrospun composite nanofiber, AstreAdept® Analysis Particle load/mL was assessed by a nanoparticle tracking analysis (NTA) or nanoFCM, and purity of the load and elution pools were assessed for total protein content. Capacity of the capsule, as well as recovery and purity of the EV purification, are reported for each EV feedstock. Experiments kindly performed by Everzom Main Populations: Triple positive EV CD63/CD81+ EV CD63+ EV
5 6 1 2 4
TARGET DIAMETER (nm) EXTRACELLULAR VESICLES 30-150 Recovery and purity in elution pool EV Recovery Fold increase in purity MsC EV single step PBS +0.4M NaCl elution 50% 1.8* HEK 293 EV elution 1 PBS 0.4M NaCl 37% 0.7875 HEK 293 EV Elution II PBS 0.85M NaCl 22% 5.23#
3
Experiments kindly performed by EchoBiotech Experimental data kindly provided by EchoBiotech and by EVerZom Experiments kindly performed
EVerZom
by
EV
*Purity ratio of 108 particles/ µg of protein. These values are comparable to the ratio achieved by current SEC method. #significantly improved ratio compared to current strong AEX method used for EV isolation (2 fold increase reported) • Average mean flowpath diameter 860 nm • Giving excellent flow characteristics for ATMP targets METHOD Buffers A: Equilibration buffer PBS B: Elution buffer PBS +1M NaCl (elution at 40% B ~400 mM NaCl in PBS) Load EV solution from MSCs cells Diafiltrated into PBS Concentrated 10x Load concentration measured by NTA ~4E+10 particles/mL 5 mL load Adsorbent volume 0.12mL OUTCOME Recovery ~50% Capacity for MSCs EVs : 1.7E+12/ mL of adsorbent Conclusions
sub-population.” EVerZom
data from two different EV expression systems shows the applicability of the weak anion exchange nanofiber material for the purification of EVs, with recoveries comparable to those reported by our partners for platform processes. Purity results from these initial test are already promising, and with additional optimisation of the elution conditions it may be possible to achieve greater clearance or impurities and recovery of the particles. The weak anion exchange functionality allows an additional optimisation parameter of the buffer pH to achieve separation of impurities. This could be utilised in the load conditioning to prevent binding of proteins, or during elution to selectively remove targeted impurities. METHOD Buffers A: Equilibration buffer PBS B: Elution buffer PBS 0.55 M NaCl C: Elution buffer PBS, 0.85 M NaCl Load EV solution from HEK 293 cells Diafiltrated into PBS Concentrated 10x Load concentration: 2.17E+10 particles/ mL, measured by nanoFCM ~2.17E+10 particles/mL Adsorbent volume 0.12mL Results shown normalised to Particles loaded/ mL adsorbent METHOD Biophysical characterization using ExoView based on fluorescent antibody detection of tetraspanins using Single Particle interferometric reflectance imagining sensor (SP-IRIS) Results shown normalised to Particles loaded/ mL adsorbent OUTCOME Capacity for HEK 293 EVs ~1.5 E+12/ mL of adsorbent OUTCOME Weak AEX fiber can be used to separate EVs from protein impurities, with greater efficiency than either SEC or strong AEX MINIMAL POPULATIONS CD63+ CD81+ CD9+ LOAD 91% 63% 50% ELUTION 88% 72% 60% CD63 CD81 CD9 CD63/CD81 CD63/CD9 CD81/CD9 Triple+ LOAD 25% 0% 2% 25% 10% 6% 31% ELUTION 14% 1% 3% 25% 10% 8% 36% -2.00E+09 0.00E+00 2.00E+09 4.00E+09 6.00E+09 8.00E+09 1.00E+10 1.20E+10 1.40E+10 0 20 40 60 80 100 Particles/ mL mL of feed/ mL of adsorbent Breakthrough of particles in flowthrough by NTA 10% breakthrough Particle breakthrough by NTA QB10 Any data or results provided are only examples and do not provide any guarantee of similar results in future. The products of Astrea Bioseparations may be covered by or for use under one or more patents: astreabioseparations.com/patents All trademarks, trade names, trade dress, product names and logos are the property of Astrea UK Services Ltd. © 2024 Astrea Bioseparations Ltd. All rights reserved





