
Showcasing the best of UK medical technology Focus on the UK at Medica Future Watch Clinical Need New technology and innovations Issue 21 2022 Meeting unmet clinical needs Going Global Cracking international markets People & Places Influential people and places in the industry Updates and expert advice Finance and funding news Money Regulatory DISCOVERING HEALTH TECHNOLOGY INSIDE: Medilink UK largest attendance ever ABHI Presentation Theatre showcasing innovation
Medilink UK has over 25 years’ practical experience of delivering specialist support to organisations across the life science sector (private and public). Our services include: To find out more about how Medilink UK can become your trusted service provider contact MedUK@medilink.co.uk Innovation, Commercialisation & Regulatory • Assessments - market, competitor & technology assessments • Health Economic Analysis –assessment of the cost effectiveness of an intervention • Clinical Evaluation Reports – CER strategy, creating CER plans and authoring full report Marketing, Communications & Design • Publishing – magazines, reports & brochures • Event Management – large scale national events, exhibitions, conferences • Marketing Campaigns – digital advertising, email, social media and offline marketing • Branding and Design - design and production of digital, print and exhibition materials International • Market Access – understanding market dynamics, demand assessment, market comparisons • Regulatory Pathways – navigating complex landscapes in international markets • Exhibitions – organising presence / support at major international exhibitions Medilink UK Serving the life science sector
Welcome to Lifescience Industry magazine
Lifescience Industry brings together news, expertise and comment from leading organisations in the UK life sciences sector. A dynamic, innovation led, global sector that includes, medical technology, biotechnology, pharmaceuticals, and digital health.


This edition focusses on the UK at Medica 2022. Held annually in Düsseldorf, Germany, Medica is the worlds largest

medical technology trade show, bringing together over 5.600 exhibitors from more than 50 countries.

In this issue our introduction to the UK at Medica is provided by Medilink UK. Our Future Watch section includes new developments in diagnostics and drug development. In the Clinical Need section we focus on AI and early detection.
In Going Global we spotlight the ABHI, UK Pavilion, Presentation Theatre at
Medica. People and Places introduces the work of SEHTA, and new manufacturing facilities in Wales.
The Money section includes a look at how Medilink Midlands have supported the sector and our Regulatory section examines clinical evaluation and developments in new data legislation


DISCOVERING HEALTH TECHNOLOGY UK at Medica 2022 Medilink UK are delivering their largest UK pavilion at Medica 2022 4-7 23 11 14 18 Hand-held haemoglobin analyser with secure POC connectivity UK Pavilion Presentation Theatre at MEDICA Opportunities for international partnership in data-driven healthcare Future Watch Clinical Need People & Places Regulatory 30 Major global events and Lifescience Industry magazine activities A message from the editor”
Gwyn Tudor
Editor Lifescience Industry online – visit www.lifescienceindustrynews.com for the latest news 8 APIS Assay Technologies and Moffitt Cancer Center collaborate on TROLL Biomarker assessment 9 From drug substance to drug product: benefits of working with an integrated services provider 10 UK regional anaesthesia system added to largest GPO in the US 11 EKF introduces hand-held haemoglobin analyser with secure POC connectivity 21 SEHTA - understanding the needs of small business Scaling up medical device contract manufacturing capabilities in Wales Opportunities for international partnership in data-driven healthcare 14 Greater adoption of AI-guided imaging has potential to transform cancer diagnosis 16 Supporting patients and the NHS through early detection 27 Understanding the approach to successful clinical evaluation Reports 28 Data: Which Direction? Published by Teamworks. www.teamworksdesign.com Editor: Gwyn Tudor, editor@lifescienceindustrynews.com Advertising Sales: info@lifescienceindustrynews.com Art direction: Lee Gillum. www.lifescienceindustrynews.com The views expressed in this publication do not necessarily represent the opinions of individual partners unless explicitly stated. © Teamworks. 2022 Supported by 2012 ISSUE 4 7 Schooner Way, Atlantic Wharf, Cardiff CF10 4DZ Tel: 029 2047 3456 Web: www.mediwales.com The views expressed in this publication do not necessarily represent the opinions of individual Medilink UK members unless explicitly stated. © MediWales Ltd. 2012 Produced by MediWales for Medilink UK Designed by Teamworks Design & Marketing Contact: Editor: Jess Fisher jess@uklifescienceindustry.com Advertising: Charlotte Tyson ruth@uklifescienceindustry.com Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad ullamcoin eu orem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum. Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur orem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in
Lwww.medilinkuk.com Issue 5 Jess Fisher Editor
Greater adoption of AI-guided imaging has potential to transform cancer diagnosis
Going Global Money 18 Showcasing UK HealthTech expertise at MEDICA 20 Innovative medical training models showcased on ABHI UK Pavilion at MEDICA 2022 24 D&O insurance - 10 risks facing life science businesses 25 Medilink Midlands helps life sciences companies secure nearly £80 million in investment
Medilink UK deliver largest ever UK pavilion to support life sciences
In its 20th year of attendance, Medilink UK are delivering their largest UK pavilion with their record-breaking numbers of exhibitors at MEDICA 2022.
As a leading industry organisation for health technology, Medilink UK has been supporting UK life sciences innovators for over 25 years.
Over the last 20 years, their presence at MEDICA has continued to grow and as the Lead UK Partner of Messe Düsseldorf, 2022 will see the highest number of exhibitors on their pavilion, with over 60 companies attending with Medilink UK.

In addition to this, Medilink will also be supporting a further 40 companies attending trade missions with the Department for International Trade (DIT) from the North of England and Midlands regions.
For UK organisations who are eager to build their international business, the exhibition presents a prime opportunity for both pioneering start-ups and market leaders to showcase their latest innovations, technologies, products and services to key buyers and distributors on a global platform.

”“As this year will mark our 20th year at the exhibition, along with over 25 years of supporting the healthcare and life sciences sector, I am particularly proud of this also being our largest presence to date.
“International trade fairs can serve as great arenas for companies keen on exploring overseas trade opportunities and industry trends. Our aim is to increase the visibility of UK enterprise at the MEDICA trade fair and assist them in forging new business relationships and strengthen existing ones and I look forward to this year being no exception.”
Tom Elliott Managing Director Medilink North of England
Wider support available for UK exhibitorsTo
Companies exhibiting with Medilink UK will benefit from access to a wide-ranging team of specialists who have in-depth understanding of large-scale events, sector-specific international markets and the regulatory landscape. In addition to that, exhibitors are also supported by the UK based advisors from the Department for International Trade (DIT).
This year, Dr Patrick Trotter, Head of Innovation and Commercialisation at Medilink, will be on hand to discuss how to de-risk and fast track innovation and product development to increase the probability of commercial success and profitability. He will be delivering a presentation on his regulatory expertise as part of the Medilink programme of informative presentations delivered on their pavilion.
Medilink International Advisor, Dr Rashmi Raju will be on hand to discuss market access projects with anyone looking for additional information and guidance on opportunities in the market,
4 Issue 21
understanding international healthcare settings, market dynamics, assessment criteria, reimbursement policies and regulation.
Also, bigger and better for MEDICA 2022, is the Life Sciences Directory app which Dr Rashmi Raju has collaborated with DIT to bring to the exhibition. The app provides a platform for UK companies to highlight their expertise and products at MEDICA, can be used during a physical or virtual event or as a web directory used to promote active UK organisations to potential partners and opportunities in overseas markets. The app can be downloaded from the Apple app store and Google play store.
Marketing and Communications Manager
Carly Ludlam will also be in attendance to assist companies needing additional marketing and PR support to raise their profile during the event and advise on longer term marketing strategies.
Medilink are also happy to welcome two of their corporate partners to their stand this year; market leading law firm Hill Dickinson who help companies to navigate the legal and regulatory landscape as well as Saffery Champness, a top 20 firm of Chartered Accountants and Registered Fiduciaries.
The Medilink UK pavilion is located in Hall 16, which is a sought-after location at this event, guaranteeing great visibility. Exhibiting companies are offered multiple space options ranging in sizes, including smaller spaces for new exporters, to suit their budget and goals.

MEDICA 2023 is already open for booking If you would like to be a part of the Medilink UK pavilion, find out more about this event or see how Medilink UK’s International team can support you, please email : international@medilink.co.uk or you can find them in person in Hall 16.
“Continuing to provide a comprehensive support offering was key to our growth at MEDICA and we are happy to provide our exhibitors with access to a widerange of specialists, giving exceptional value to exhibiting on our UK pavilion that would not be available elsewhere.
“We also offer full support at all stages, from logistics, accommodation, travel, pre-event preparation and marketing, all designed to help exhibitors capitalise on the event and see the highest return on their investment.”
Melissa Erwin Senior International Officer
“We have participated in multiple exhibitions with Medilink over the years, as they have involvement with the largest healthcare events in the World. Medilink offers us a platform to showcase our products to a wider audience, allowing us to build and maintain important relationships.
“We are always pleased with the benefits of exhibiting on the pavilion, such as the location and networking opportunities.”
“We were incredibly impressed with the additional opportunities offered by the Medilink UK Pavilion. We secured global leads ranging from South America and Middle East into Asia reaching India. The support from Medilink was priceless making the experience so easy for us.”
Atlantamed Ltd
For daily lifescience news visit www.lifescienceindustrynews.com 5
Rachel Tobin, Marketing Executive, Precision UK
”” ”
Exhibiting with Medilink UK MEDICA 2022
Attracting 6,000 exhibitors from across 70 countries, and over 120,000 visitors, there is no doubt why MEDICA is often referred to as ‘the place where medicine meets.’ This is why the event is not-to-be-missed for those who want to connect with a global audience of dealers, buyers, suppliers, and distributors.
Over the last 20 years Medilink UK has been taking UK businesses to the trade fair, growing year on year to become the Lead UK Partner of Messe Düsseldorf. This year Medilink UK are attending with their largest ever presence, with over 60 companies exhibiting on their UK pavilion.

Tom Elliott, Managing Director at Medilink North of England, gives his insight into why organisations should consider attending MEDICA with Medilink UK:
“Over our 20 years at MEDICA, we have supported hundreds of UK companies of different sizes and exporting experience. It provides the perfect platform for embarking on new partnerships and showcasing medical devices and solutions on a global scale. This year we are taking more companies than ever before, a true testament to the value of exhibiting on our pavilion.
“It’s fantastic to see the organisations we support come away with tangible successes as a result of their presence at MEDICA. Having met with hundreds of new and existing contacts, gaining a greater knowledge of international trade in the region, most of our exhibitors come away with a number of strong commercial leads.
“We have examples of companies who have grown their international presence drastically thanks to being regular stand holders at MEDICA, with some businesses resulting in a 50/50 split between UK and international sales. This shows that it should be seriously considered by those who want to take their medical solutions overseas.”
A4P Consulting Ltd (J10) Consulting services
Abingdon Health (K06) Diagnostics
Accoson (H15-5) Blood pressure monitoring equipment
Advanced Healthcare Technology Ltd (H21-8) Medical equipment manufacturing
Advena Limited (H15-6) Consulting services
AG Instruments Ltd (J16-5) Medical instruments
ASep Healthcare Ltd (H15-3) Disposable articles for hospitals
Askorn (J10) Medical devices
Atlantamed Ltd (H15-1) Treatment room and IC equipment
Bedfont Scientific Ltd (J10) Diagnostic equipment
Bluetree Medical (H21-1) Medical consumables
Carelight Limited (H25) Wearable technologies and smart textiles
Celler8 (J16-3) PEMF technology
Chemence Ltd (H21-4) Adhesives and sealants manufacture
Conductive Transfers Limited (H21-6) Printing of stretchable electronics
MEDICA has been running for 40 years and helps exhibitors generate a huge number of leads, resulting in a great return on their investment. Booking for next year is already open, email international@medilink.co.uk to book with Medilink UK or you can find them in Hall 16.
CPI: Centre for Process Innovation (J16-6) Immuno assay testing
6 Issue 21
Tom Elliott
Managing Director
Medilink North of England
”
” ”
Atlantamed Ltd
Globus Group
Department for International TradeMidlands Region (H21-7) Trade and investment

Department for International TradeNorthern Powerhouse Region (H21-6) Trade and investment
EMT Healthcare Ltd (H25)
Blood pressure monitoring equipment
European Device Solutions Ltd (H25) Consulting services
Excellentcare Medical Ltd (K06) Medical and surgical equipment
eXroid International (H12) Haemorrhoid treatment technology
Globus Group (H03) Respirators
Haddletons (H25) Health insurance and professional services
Henleys Medical Supplies (J16-7) Blood pressure monitoring equipment Hill Dickinson (H25) International commercial law firm
Intradys (J10) Software for interventional neuroradiology
IS Instruments (J10) Spectrometers
IVDeology (J10) Consulting services
Kent County Council (J10) Local government
Lattice Medical (J10) Soft tissue reconstruction
LEEC Limited (H10) Medical instruments and equipment
LocaMed Ltd (H11) Surgical instruments and apparatus
Marsden Weighing Machine Group (H15-7)
Scales and body fat testing
Medezine Ltd (J16-4) Healthcare product manufacture
Medical Engineering Technologies Ltd (J10) Medical device validation and testing
Medilink North of England (H25) Specialist Life Science Association
Medilink UK (H25) Specialist Life Science Association
Morgan Innovation & Technology Ltd (J10) Electrosurgical instruments
Patient Guard Ltd (J16-8) Consulting services
PD-M International Ltd (H21-3) Consulting services
Plasticom (J10) Glucose testing
Rapid Fluidics (H25) Molecular diagnostics testing
Relab (J10) Administrative information systems
Renfrew Group International (H25) Analyser systems and equipment
Saffery Champness (H25) Accountancy
Santander (H25) Banking
Seers Medical Ltd (J16-9) Equipment for wards and examinations
SEHTA (H25)
Health technology network
Speciality Fibres and Materials Ltd (H21-9)
Instant wound dressings
Sterimedix Ltd (J16-2)
Disposable articles for hospitals
Surgical Innovations Group Plc (H15-2) Instruments for minimal invasive surgery
Team Consulting (H21-5) Consulting services
The Rodnight Partnership (H15-8) Global channel management
TSL Healthcare (J16-1) Universal medical commodities
Viamed Ltd (H15-4) Treatment and monitoring systems
Virtysens (J10)
and outpatient facilities
Vytruve (J10)
Orthopaedic implants and prostheses
West Midlands Growth Company (H25) Investment promotion
West of England Combined Authority (H21-2)
authority
Woundcare Solutions (K06)
Care
For daily lifescience news visit www.lifescienceindustrynews.com 7
“MEDICA is one of the sector’s leading events and we experienced great success there; we secured a large number of new global leads from the last show.”
“The communication, support and overall management of the UK pavilion by Medilink was really professional and we quickly re-booked for the following year”
Inpatient
Local
Wound
APIS Assay Technologies and Moffitt Cancer Center collaborate on TROLL Biomarker assessment
Cancer is a multi-pathway disease, whereby it is now clear that personalised genomic medicine is required in the diagnosis and stratification of treatment, and in the prediction and prevention of the disease. There is a real clinical need for validated biomarkers that can demonstrate clinical impact in the decisions made for cancer treatment pathways.
The most frequent genetic alterations across multiple human cancers are mutations in the TP53 gene and the activation of the PI3K/AKT pathway, two events crucial for cancer progression.
APIS Assay Technologies has entered into a Research & Development agreement with the Laboratory of Elsa Flores, Ph.D., and Marco Napoli, Ph.D. characterising the suitability of long non-coding RNAs (lncRNAs) TROLL-2 and TROLL-3 as predictive biomarkers of cancer progression with the goal to assess the role of TROLLs as markers of response to chemotherapy.
The laboratory of Dr. Elsa R. Flores has recently demonstrated that the crosstalk between p53 gene mutations
and the AKT pathway (pathway that promotes survival and growth in response to extracellular signals) is mediated by two long non-coding RNAs (lncRNAs), called TROLL-2 and TROLL-3, which promote tumour formation and progression in several orthotopic models of human cancers.
The published and patented data provide preclinical rationale for the implementation of these lncRNAs and WDR26 as novel therapeutic targets for the treatment of human tumours dependent upon mutant TP53 and/or the PI3K/AKT pathway.
The first target of the R&D collaboration will be triple-negative breast cancers (TNBCs). TNBCs are among the most therapeutically challenging human cancers and are frequently characterised by the hyperactivation of the PI3K/ AKT pathway and the gain-offunction mutation of the tumour suppressor TP53.
TNBCs have higher levels of both lncRNAs compared to non-TNBC cases. A pan-cancer analysis of TCGA datasets and human cancer tissue microarrays showed that these two lncRNAs
are prognostic in breast cancer and that they are novel biomarkers of cancer progression in at least 6 different tumour types, underlying the relevance of TROLL-2 and TROLL-3 across multiple human tumours.
The aim of the research collaboration between APIS and Moffitt will be to determine the feasibility of these lncRNAs as a diagnostic tool for the prediction of efficacy of treatment pathways and prognosis of treatment outcome in chemoresistant TNBCs and further analysing NSCLC and melanoma as potential diagnostic targets for the TROLL Biomarkers.
Image: Performing a PCR assay within Manchester based APIS Lab (as used for the TROLL Biomarker development)

8 Issue 21 Future watch
“The collaboration with Dr. Flores and the Moffitt team expands the APIS Biomarker portfolio by targeting the p53 family pathway, with potential companion diagnostics applications.”
Dr. Ian Kavanagh, COO, APIS Technologies Ltd
Dr Flores added: “The R&D collaboration with APIS will provide a clear and realistic path to provide new diagnostic applications to the patients.”
From drug substance to drug product: benefits of working with an integrated services provider
Quotient Sciences is a drug development and manufacturing accelerator, providing customers with integrated drug substance and drug product solutions from the early stages of molecule selection and development through to the clinic and beyond.
Supporting Candidate Selection to First-in-Human
Quotient engages with customers from the point of candidate drug selection, helping to ensure that a lead compound can be manufactured in sufficient quantities to support initial toxicology and preclinical studies, while also being equipped to scale up the manufacturing processes to produce clinical drug substance to pharmaceutically acceptable standards (Good Manufacturing Practice: GMP). When developing a drug substance manufacturing process, the chemistry of the impurities generated is as important as the purity of the compound itself. Quotient has a significant breadth of expertise in analytical sciences, enabling them to identify, quantitate and ultimately control the impurities generated in the manufacturing process. Small differences in impurities can have a profound impact on the toxicological profile of the compound.
Bridging from Drug Substance to Drug Product
The drug substance/drug product interface is a key part of any successful drug development program. The activities relating to each are often the responsibility of different companies, or siloed departments of the same organisation. This creates inefficiencies when we consider the complexity of today’s molecules and target product profiles, with timeline pressures ever present.
A fully integrated drug substance and drug product manufacturing service results in a more efficient and accelerated development plan. Integrating all activities under a single organisation encourages better workflows, processes, and builds close relationships between multidisciplinary experts. Process chemists can work alongside analytical
and solid-state chemists to ensure rapid development and optimisation of a drug substance. Formulation scientists and solid-state teams can together provide clear and unambiguous data for optimising drug substance form, leading to rapid drug substance, dosage form design and drug product manufacturing.
Seamless coordination between drug substance and drug product manufacturing results in a more efficient and accelerated development plan. Integrating all activities under a single organisation in a nonsiloed way encourages close relationships between multidisciplinary experts, creating a more agile approach to pharmaceutical development. The ultimate benefit is a significant shortening of the timeline from candidate selection to clinical development. On average drug development timelines will be reduced by 2-4 months, translating into significant R&D cost savings and shortens the faster provision of new medicines to patients.
9For daily lifescience news visit www.lifescienceindustrynews.com Future watch
Candidate Selection First-inHuman Proof-ofconcept NDA Commercial Product DRUG SUBSTANCE DRUG PRODUCT CLINICAL TESTING Preclinical Phase 1 Phase II/III Market www.quotientsciences.com
UK regional anaesthesia system added to largest GPO in the US
Medovate, a medical device development company based in Cambridge, is taking their revolutionary technology for regional anaesthesia to new heights.
The company has announced that their SAFIRA® (SAFer Injection for Regional Anaesthesia) has now been added to the Vizient Group Purchasing Organisation (GPO) alongside Konica Minolta’s SONIMAGE HS2 ultrasound system in the United States.

The SAFIRA® system automatically limits injection pressure to a specified threshold to help reduce the risk of nerve injury and improve patient safety during regional anaesthesia. In addition, the technology also transforms the practice into a one-person procedure by enabling a single clinician, an anaesthetist, to conduct the entire nerve block process. The novel technology, developed alongside specialist clinicians in the UK’s NHS, is designed to increase patient safety during ultrasound-guided regional anaesthesia.
Medovate and Konica Minolta Healthcare – a US-based provider and market leader in medical diagnostic imaging and healthcare information technology – established a partnership in January 2021 to promote best practices in regional anaesthesia across the US. The SAFIRA® technology was added to Konica Minolta’s UGPro® Solution, which brings together education, procedures and imaging equipment, such as the SONIMAGE HS2, to further expand the use of regional anaesthesia and enhance patient safety nationwide.
Konica Minolta has established ultrasound contracts for its next-generation point-of-care portable ultrasound system SONIMAGE HS2 with USA healthcare company Vizient – the largest GPO in the US, serving over 5,000 notfor-profit health system members.
This latest development means that healthcare providers in the USA can now
access the SONIMAGE HS2 alongside the additional patient safety benefits provided by Medovate’s SAFIRA® system.
Both companies are committed to promoting safer regional anaesthesia with solutions that deliver clinical efficiency, simplify use and advance better outcomes for patients.
“We are delighted that through our co-promotional relationship with Konica Minolta, our ground-breaking SAFIRA® system is now available alongside the SONIMAGE HS2 through Vizient, the largest GPO in the US. This is a great opportunity for clinicians in the United States to be able to realise the benefits of using these technologies in combination as best practice to improve patient safety during peripheral nerve blocks.”
Stuart Thomson Managing Director Medovate
10 Issue 21 Future watch
www.medovate.co.uk
EKF introduces hand-held haemoglobin analyser with secure POC connectivity
The hand-held haemoglobin analyser now connects securely to the company’s point-of-care (POC) middleware, enabling additional features including a calculated haematocrit value and data management. Available globally, the DiaSpect Tm has received an IVD CE-mark, FDA clearance and is registered in many more countries across all continents.
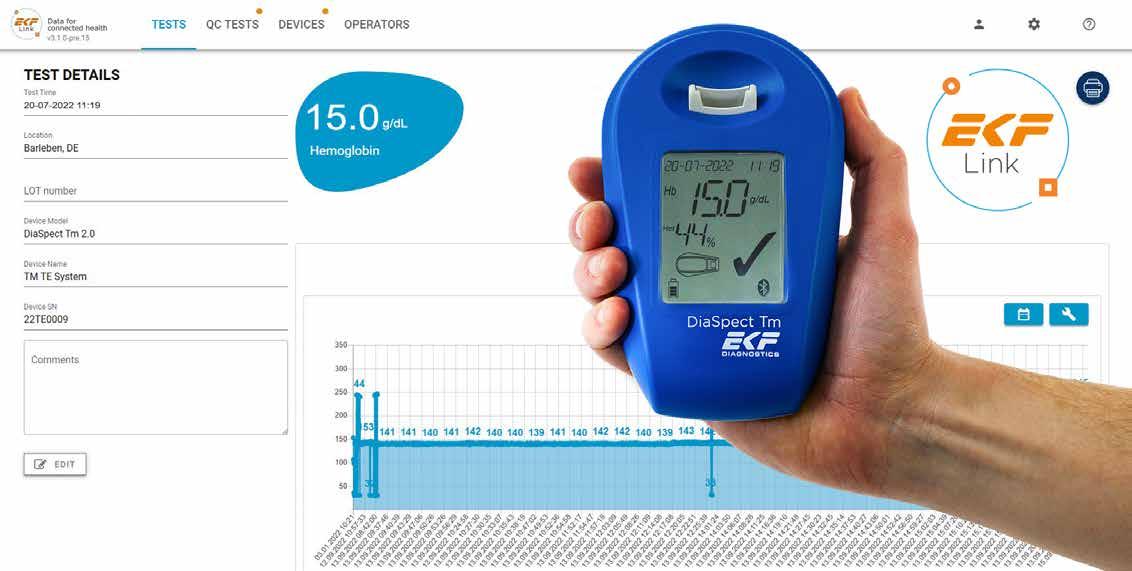
The enhanced DiaSpect Tm can now be connected to the recently released middleware, ensuring test results are stored and accessed securely on a centralised platform. This expands on their growing list of analysers that are compatible with their new POC data management platform. EKF’s DiaSpect Tm is also equipped with Bluetooth, internal storage for 4,000 tests and QC results, as well as the capability to provide a calculated haematocrit value alongside the usual haemoglobin result.
The DiaSpect Tm results are easily accessible from a web browser, through EKF Link, while being securely protected on the servers already available in most laboratories and hospitals. Patient and operator identification allow for better tracking of both staff competence and test results, and a Quality Control reminder function ensures compliance with regulations.
Enabling rapid decision making, the DiaSpect Tm simultaneously delivers haemoglobin with calculated haematocrit results in under two seconds from just 10 µL of capillary or venous blood collected in microcuvettes and inserted directly into the analyser and requires minimal training. In addition, no calibration is required as it is
factory calibrated against the HiCN reference method, and turbidity is also measured and compensated for.
The DiaSpect Tm is POC haemoglobin analyser is palm-sized, lightweight, robust and easily transportable. It can be used in any screening setting, even in challenging climatic environments. This is because its reagent-less microcuvettes have up to a 2.5 year shelf life, even after opening, and are unaffected by temperature and humidity. Its ‘always-on’ technology enables professionals to start using the device immediately. The rechargeable internal battery, which provides up to 40 days/10,000 tests continuous use, offers the flexibility of not needing a power source for weeks at a time.
“Our new DiaSpect Tm will enable POC coordinators, operating rooms and screening programmes to connect this fast and reliable photometric haemoglobin analyser to Laboratory Information Systems (LIS) or Patient Information Systems (PIS) through the EKF Link. Now with the EKF Link connectivity, DiaSpect Tm is an ideal POC solution enabling users to rapidly upload results from anywhere and power up their performance, delivering results directly alongside the patient or blood donor.”
David Wojahn Haematology Product Manager Medovate
11For daily lifescience news visit www.lifescienceindustrynews.com Future watch
EKF Diagnostics, a global in vitro diagnostics company, announces the launch of an enhanced DiaSpectTm
www.ekfdiagnostics.com



13For daily lifescience news visit www.lifescienceindustrynews.com The life science network for Wales bringing together industry, academia and the clinical community to support the advancement of human life science in Wales and create collaborations and business opportunities for our members / Over 30 years supporting the life science community / An independent and not-for-profit network / Connecting with national and international partners / Focus on collaboration to further business opportunities / Developing activity to meet the sector’s diverse needs MEDIWALES NHS COLLABORATION CONFERENCE For more information please contact MediWales or visit: mediwalesconnects.com The all Wales NHS collaboration conference, bringing together the health and care communities in Wales is back for 2023 www.mediwales.com JUNE 2023
Greater adoption of AI-guided imaging has potential to transform cancer diagnosis
Tim Simpson General Manager, Hologic UK & Ireland
Over recent years we have seen exciting developments in the use of artificial intelligence (AI) guided imaging for diagnostics and the positive impacts this could have for the NHS and patients. In particular, there is a huge opportunity to utilise the power of AI for cancer screening, to ensure earlier detection and speedy and accurate diagnosis.
The Industrial Strategy AI Mission (UK Gov) has set out a goal to use data, AI and innovation to transform the prevention, early diagnosis and treatment of chronic diseases by 2030. It predicts that within 15 years better use of AI and data could result in over 50,000 more people each year having their cancers diagnosed at an early rather than late stage. While progress has been made already, there are still challenges to overcome to make sure health services have the data capabilities to make the best use of AI.
Efficient and accurate diagnosis
We have seen first-hand the positive impact of AI-guided imaging with the UK’s first pilot of digital cytology in cervical cancer screening at University Hospital Monklands. The technology rapidly reviews test slides providing the screener with the most diagnostically relevant cells. The initial results are promising with the hospital reporting increased capacity of around 25% in slide assessment and improved analysis turnaround times.
Incorporating AI into breast cancer screening could also boost efficiency and accurate diagnosis. Digital breast tomosynthesis (DBT) detects more invasive breast cancers as compared with traditional 2D mammography.
Imaging solutions, harnessing the power of AI, can reduce the number of images to review, ultimately reducing interpretation time and allowing radiologists to read more cases per hour.
It is also crucial to utilise AI to identify those most at risk of developing breast cancer, such as women with dense breasts, which is a key risk factor. Innovative hospitals have adopted breast density software systems, using a machine-learning algorithm to analyse a patient’s breast pattern and texture to provide an objective assessment of breast density. Finding those most at risk of developing breast cancer could help identify women who may benefit from more regular screening and ensure early diagnosis.
Power of AI to reduce burden on HCPs


AI can complement the work of health care professionals, freeing up their time to focus on difficult to diagnose cases and in turn reducing the burden on an already overstretched NHS. For example, AI guided imaging has the potential to remove the current requirement for breast images to be reviewed by two radiologists.
Based on a comparison with the average time taken to read a breast screening image,
14 Issue 21 Clinical Need
Clinical
the use of AI could mean up to 13% less time is needed to read a mammogram. Radiologists would be able to report more cases per day and the efficiency with which images are reviewed would be improved resulting in significant overall time savings for clinicians. This is especially relevant given the global shortage of radiologists. In 2020, the Royal College of Radiologists warned that the shortage of breast radiologists is highly likely to rise over the next five years.
Challenges to overcome
While the potential of AI applied to imaging is promising, there are still several key challenges to overcome. The 2021 ECIBC guidelines on breast cancer screening recommended the use of DBT or digital mammography in screening.
However, using DBT means that file sizes are larger and there are more images to read which can lengthen reporting times. The Hologic Research and Development team has already gone some way to addressing these well reported issues by introducing software solutions that reduce the Picture Archiving and Communication (PACS) storage requirements for tomosynthesis images. Healthcare organisations will also need to carefully evaluate image storage requirements as these technologies become more routinely used.

Also, in order for the NHS to more widely adopt AI-based imaging technologies, there will need to be greater provision of storage, so images can be accessed in real-time. National portals will also need to be created which enable data sharing ensuring there are large enough data sets which are diverse enough and accessible to HCPs across the UK, rather than being siloed in smaller regions.
Investing in AI innovation
Investment in AI innovation has been crucial, such as the £36 million investment in AI technologies to revolutionise NHS care, via the AI in Health and Care Award. This included funding at Imperial College London to evaluate the potential of AI for analysing images from routine mammograms. We now need to see the NHS capitalise on these technologies to ensure they are adopted more widely.
The 2019 NHSX report on AI in health and care identified key considerations for the NHS to capitalise on AI opportunities within diagnostics. This included ensuring good public engagement with the concept of AI, validating the results of algorithms in recognising malignancies in the mammograms of people with different ethnicities, and considering how
quicker diagnosis would impact on individual pathways and the system as a whole. For example earlier diagnosis may lead to longer anxious waits for treatment, if the capacity of the system to treat is not increased as well.
To fully unlock the potential of AI in imaging, these considerations need to be addressed and will require collaboration between regulatory bodies, the NHS, innovators and developers so that we have clear, proportionate and innovation-friendly regulation of AI technologies. Creating the appropriate data storage, accessibility and data sharing foundations will also be critical for AI to reach its full potential and aid in the earlier detection of cancer, ultimately making a real difference to patients, the NHS and our society.
At ABHI, it is our vision to make high quality diagnostic technologies accessible to all who need them, so that diseases and health conditions can be detected and treated earlier.
15For daily lifescience news visit www.lifescienceindustrynews.com
Need
SEHTA Membership+ Offer 1) A free SEHTA Surgery+. A three-hour 1:1 session, on the topic of your choice, with assigned specialist resource from SEHTA 2) A Free Grant Application Review Service (GARS), proven to optimise the chances of a successful application 3) Free Matchmaking utilising our database of over 14000 contacts, to provide a basis for collaboration to innovate 4) Full access to the SEHTA curated library of Publications and Webinars In addition - 10% discount on services provided by: • ORCHA – Digital Health Technology assessment • Psephos – Regulatory help and support • Hill Dickinson – all legal matters concerning health technology • SEHTA – NICE Meta tool • SEHTA – Daily consultancy rate for commissioned work • SEHTA – Paid webinar and annual Expo (discounted on the SEHTA member rate) SEHTA Membership+ Interested? Contact info@sehta.co.uk or scan the QR code to register The NEW SEHTA Membership+ offers SEHTA members the opportunity to upgrade their FREE membership, in order to take advantage of the additional services that SEHTA offers
Supporting patients and the NHS through early detection
Nishan Sunthares Managing Director, Diagnostics, ABHI

The benefits of diagnostics are well understood. It is now time for the appropriate investment to realise their capabilities for the NHS and the patients they serve.
Getting a fast, accurate diagnosis is a fundamental element of modern healthcare. It guides clinical decisions on appropriate treatments or interventions and determines ongoing healthcare needs.
Through the COVID-19 pandemic, awareness of the role diagnostic solutions play in disease detection and management grew profoundly. What might the future look like if this model is expanded to help address more health conditions?
At ABHI, it is our vision to make high quality diagnostic technologies accessible to all who need them, so that diseases and health conditions can be detected and treated earlier.
Earlier diagnosis is critical to improving the survival rates of those who have a serious condition. For example, 92% of patients with bowel cancer diagnosed at stage 1 survive their disease for at least five years, compared to 10% of patients diagnosed at stage 4. Early disease detection opens the door to planning the care and support for those in need.
Not only can diagnostics bring significant benefits to patient care, but they can also cut waiting times and alleviate NHS workforce burdens. In clinical oncology, for example, AI-powered medical imaging has the potential to revolutionise clinicians’ workflow and automate time-consuming tasks. Rapid test platforms also have the capability to reduce testing turnaround times.
Yet despite the well-recognised importance of diagnostics, and the fact that 95% of all clinical pathways rely on patient access to pathology services, including cancer diagnosis, funding for pathology only accounts for 2% of the NHS budget.
Appropriate investment and a shift towards early diagnosis, rather than simply just treatment, would therefore lead to significant improvements, not only in patient outcomes, but in the workload of those working within the NHS.
Diagnostic tools and tests are fundamental to modern healthcare. They need to be funded properly and fully integrated into clinical strategies to enable our healthcare workers to utilise them effectively. At ABHI, we are currently working with the health technology industry, government, the NHS and national partners to make this vision a reality.
16 Issue 21 Clinical Need
www.abhi.org.uk
Powered by ABHI, the UK Healthcare Pavilion is a unique partnering platform helping overseas customers discover, connect and innovate with the UK’s thriving healthcare and life sciences sector. Its aim is to inspire cross-border collaborations that can realise large scale impact in response to local and global health challenges.
The platform was created out of a need to provide a single front door showcasing the strengths of UK healthcare and life sciences. Its mission is to provide overseas buyers looking for UK solutions with a simple, insightful way to identify and engage UK industry and healthcare organisations, and to support UK-based medical device, diagnostics, and digital health exporters looking to engage customers around the world.
The UK Healthcare Pavilion provides a global platform showcasing the very best of the sector, and highlights the strengths the UK has to offer.
For more information, visit ukhealthcarepavilion.com

17For daily lifescience news visit www.lifescienceindustrynews.com Clinical Need www.brandon-medical.com
Showcasing UK HealthTech expertise at MEDICA

The world’s largest event for the medical sector returns to Düsseldorf, Germany on 14-17 November, giving UK companies a chance to showcase UK expertise on the world stage.
Here are some key exhibitor highlights:
UK medical equipment manufacturer Adam,Rouilly will be showcasing their new innovative range of medical training models to support healthcare professionals perfect all manner of essential clinical and nursing skills.
ligator for the treatment of internal haemorrhoids.
For the first time ever there will be a ‘UK Pavilion Presentation Theatre’ at MEDICA to give UK innovators a platform to demonstrate their technologies to international audiences.
Organised by the Association of British HealthTech Industries (ABHI) and the Department for International Trade (DIT), and co-sponsored by Welsh Government and law firm Sidley Austin, the dedicated space will run for the full four days at the show. Its aim is to give exhibiting UK companies greater opportunities to showcase their strengths and build new partnerships.
Presenting UK companies will have a unique platform to showcase their latest innovations to all markets. Representing the Department for International Trade, Dr Neil Ebenezer, MedTech and Genomics Specialist, will be presenting on a number of topics including the Potential Future UK MedTech Regulatory Framework, the UK MedTech Landscape and changes to IP since the end of the Brexit transition period. The full listings of presentations, as well as all the UK activity at MEDICA 2022 is being captured on the dedicated MEDICA page on the UK Healthcare Pavilion virtual platform. This is a chance to discover and connect with innovative UK life sciences companies, hospitals, clinics and key organisations.
www.ukhealthcarepavilion.com
Showcasing UK HealthTech
The fair – which has grown considerably over the past 40 years – is open to trade visitors from all over the world. Today more than 5,600 exhibitors from more than 50 countries present in its 17 halls.
This year, ABHI – A leading UK industry association for health technology – has also joined forces with DIT to co-deliver a joint UK Pavilion stand, bringing together a diverse mix of UK companies, and national partners, who all supply a range of high-quality and innovative HealthTech and healthcare services.
GlucoRx will be demonstrating the world’s first multi-sensor non-invasive continuous glucose monitor (CGM), heralding a new era of needle-free monitoring for people with diabetes.
UK manufacturer Haemoband Surgical will be showcasing their innovative range of band ligation and proctology products. The company’s primary device, the Haemoband-Plus, is the first multiaction, pre-loaded disposable
Healthcare Innovation Consortium (HIC) will be launching their new healthfocused International Accelerator Programme (IAP), which will be open for application in October 2022 to support the growth of international technology innovators in the UK.
UK manufacturer JEB Technologies Ltd will be showcasing a novel device called CamPROBE (Cambridge Prostate Biopsy Device), a new development that is used to facilitate the acquisition of prostate biopsy tissue through the transperineal route under local anaesthetic.
18 Issue 21 Going Global
Seating Matters
Speed Plastics will showcase the range of bespoke engineering services they offer healthcare companies. Speed Plastics offers a complete design and development service from concept idea through to the finished commercial product.
Rober will be showcasing their full range of pioneering ‘zero pressure’ mattress solutions. Over the last few years, Rober has invested heavily in R&D to develop a complete range of pressure ulcer mattresses that cater for a variety of needs.

Leading manufacturer Surgitrac Instruments will be showcasing their range of top-quality surgical instruments. Surgitrac® Instruments’ CE / UKCA marked ophthalmic range is ethically manufactured and complies with international standards.
Also joining ABHI is Nuline Medical. The company was
launched in 2017 with a vision to create a medical device company that makes a difference, delivering high quality, competitively priced products with outstanding customer service.
PolyNovo UK Ltd will be showcasing their revolutionary NovoSorb BTM (Biodegradable Temporising Matrix) technology, developed to fulfil the need for a synthetic biodegradable product that could be safely used in medical devices.

UK manufacturer Seating Matters will be promoting their newest innovation in seating, Sydney GoFlat™ - an innovative lie-flat chair for critical care patients that is helping with early
mobilisation and rehabilitation in hospitals around the world.
Surgical Holdings will be demonstrating how hospitals can manage and care for their surgical instruments in a sustainable and cost-effective way. To help hospitals cut their carbon footprint, they have introduced an expert Smart Repair service covering a range of surgical instruments.

Timesco Healthcare – a manufacturer of innovative medical devices for anaesthesia, surgery, podiatry
and primary care – will be launching a brand-new video laryngoscope, adding to their extensive range of anaesthesia products and airway management portfolio.
Armstrong Medical (part of Eakin Healthcare) will be showcasing their range of high-quality, innovative, respiratory care products for use in critical care, neonatal and perioperative sectors. The company exports to more than 60 countries.
19For daily lifescience news visit www.lifescienceindustrynews.com Going Global
MEDICA is taking place from 14 – 17 November 2022 in Düsseldorf, Germany. Throughout the event, visitors will be able to visit the joint ABHI & DIT UK Pavilion in Hall 16, Block J48.
Surgical Holdings
PolyNovo
Armstrong Medical
Rober
www.abhi.org.uk
Innovative medical training models showcased on ABHI UK Pavilion at MEDICA 2022
UK medical equipment manufacturer Adam,Rouilly will be joining the ABHI UK Pavilion at MEDICA 2022 to showcase their new range of medical training models to support healthcare professionals perfect all manner of essential clinical and nursing skills.
The company will be showcasing some of their latest products, including the pioneering GlucoHand® Glucometer Simulator. About 422 million people worldwide have diabetes, and effective diabetes management is now a growing part of the general skills which all clinicians must possess.
The device has been developed in collaboration with Nina Godson at Coventry University, to aid understanding and teaching of blood sampling and the interpretation of glucose level data and its implications in patient treatment planning.

Adam,Rouilly’s GlucoHand® model features a simulated glucometer device which can be pre-set by the trainer to give a low, normal, high or completely randomised blood glucose reading – allowing control for training or simulation scenarios. It also includes a realistic adult sized hand, with two refillable finger pads in the middle and ring fingers containing mock blood. Once a sample of mock blood has been successfully obtained from the fingertip and placed on the test strip, the glucometer will display the predetermined reading which must then be interpreted by the student.
Also being showcased is the Cricoid Pressure Trainer®, developed in collaboration with Consultant Anaesthetist Dr Osman Abdelatti, to help facilitate training in the application of the correct amount of cricoid pressure, which is routinely used during emergency and obstetric anaesthesia and ventilation.
Studies have shown that 95% of clinicians were unable to identify the correct amount of force required to occlude the oesophagus and that 87% were incapable of applying the correct amount of force. The Cricoid Pressure Trainer aims to significantly enhance training utilising the device’s realistic anatomical structures and instantaneous electronic feedback.
In addition, Adam,Rouilly will be presenting their Rectal Examination Model which has been improved in collaboration with the Spinal Injuries Association to offer additional functionality making the model more versatile and lifelike. The model now provides training in stool assessment, general rectal examination and examination of an abnormality, manual stool extraction as well as enema training and insertion of liquid rectal medication.
“We are delighted to be showcasing our innovative medical training models and simulators at MEDICA 2022. Our large range of products have been purposely designed and developed in direct response to customers’ requirements and through a combination of robust research and development and collaboration with leading clinicians and world-class medical institutions. Our experienced and knowledgeable team are always pleased to give advice about our products, and we look forward to joining the ABHI at this year’s show to showcase how our exciting new solutions can support the training needs of healthcare professionals worldwide.”
Tariq Shahab Director of Sales & Marketing Adam,Rouilly
20 Issue 21 Going Global www.abhi.org.uk
SEHTA - understanding the needs of small business
SEHTA (South East Health Technologies Alliance) was founded in 2005 as an organisation to understand and meet the needs of small healthcare businesses.
With over 1,400 members, SEHTA is one of the largest networks of individuals from Academia, Business and Care/Clinicians (ABC), with the purpose of improving the health and care of the citizens of the UK as well as increasing wealth. They do this by offering ‘one-to-one’ business support services and ‘one-to-many training, workshops’ and seminars and larger events.
BresMed’s new global HQ at Steel City House in Sheffield is the heart of the company’s worldwide operation. Commenting on the move, CEO Nic Brereton said: “I am so proud to be expanding BresMed to these premium premises in the city. The building very much fits with our brand – it’s iconic with a quirky feel and, like us, it dares to be different.”
The company has come a long way since Nic moved into a one-person office in the city many years ago, adding: “Actually, it was more a cupboard! However, it helped me launch the business.”
Neil Roberts CEO SEHTA
The SEHTA Grant Appraisal Service provides feedback on proposals, including the best ways to strengthen an application, including consideration of SEHTA inclusion in the project and directs clients to the most suitable funding sources. The appraisal is based upon detailed discussion with the client articulating the technology/service proposal they need funding for, what they want to do with the funding and how much funding they need.
The Grant Review Service then provides a rapid analysis of a completed draft grant application articulating ways to improve it to increase the chance of success.
The NICE META Tool supports and informs a face-to-face discussion between medical technology developers and trained advisers in order to identify gaps in product development and evidence generation plans.
SEHTA works closely with a number of organisations including the Greater London Authority (GLA) & London South Bank University, as well as international organisations like Business Finland, and has partnered with a leading life sciences international law firm Hill Dickinson, medical devices regulatory consultancy Psephos
Biomedica, and ORCHA (the Organisation for the Review of Care and Health Applications). As part of their work with the SimDH (Simulation for Digital Health) at London South Bank University, SEHTA mentors up to 100 companies.

SEHTA has developed close relationships with EU partners and has worked on 9 EU projects, including 2 current projects. EU Interreg AiBle - is a 3-year UK/France cross-border EU Interreg project to improve the recovery experience of stroke patients by developing an upper-limb rehabilitation exoskeleton robot based on AI and cloud computing. EU Interreg IMPULSInnovation in Manufacturers of Products Used in Life Sciences aims to help small life science and nutrition businesses from South East England and Northern France to collaborate, innovate and access business opportunities on the other side of the Channel. Both of these EU Interreg projects are funded by the ERDF (European Regional Development Fund), which works transnationally.
SEHTA also delivers a series of paid for and free of charge webinars to members on subjects ranging from intellectual property (IP), regulation (MDR and IVDR), and how to access the NHS.


21For daily lifescience news visit www.lifescienceindustrynews.com People and Places
“I am delighted to work with my team to help support MedTech SMEs across our sector with access to grant funding, legal/regulatory advice, medical device regulatory advice, and design technology support, as well as supporting the digital health community with our partners.”
www.sehta.co.uk
Scaling up medical device contract manufacturing capabilities in Wales
Following a surge in demand for elective care, Eakin Surgical – a long-established UK single-use surgical instruments manufacturer based in Cardiff – are scaling up their contract manufacturing services.
After a number of years successfully delivering ethylene oxide (EtO) sterilisation services and sterile packing services from its UK-based clean rooms, Eakin Surgical is expanding its business in the medical device contract manufacturing segment to offer more value-added services to its customers.
The covid-19 pandemic has led to a surge in demand for sterile packaging solutions and single-use medical equipment as healthcare facilities seek to curb the spread of infections.
Backed by over 20 years’ experience, Eakin Surgical’s latest expansion will help medical device manufacturers (OEMs) reduce costs, meet rising demand for sterile packaging and weather supply shortages caused by
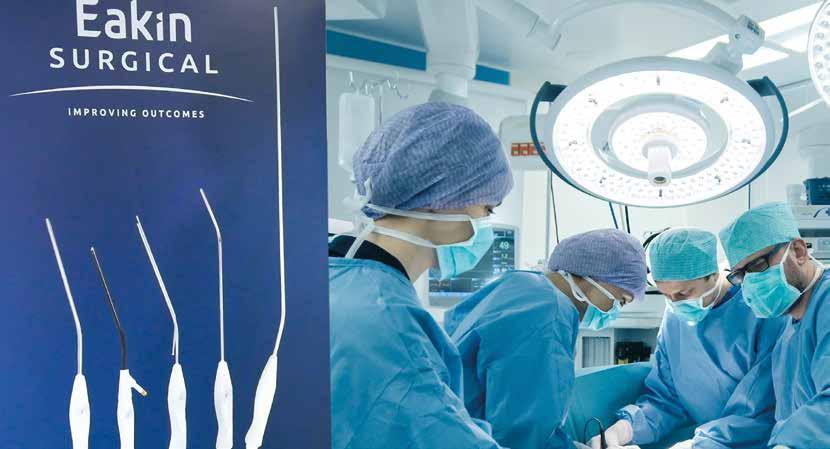
the current climate. The company offers expertise across all facets of medical device manufacturing from plastics injection moulding to assembly and packaging in an ISO Class 8 cleanroom, and EtO sterilisation. Since the end of 2021, the company has almost doubled its third-party EtO business.
With the continued growth in its manufacturing capabilities, the company can now also offer experience and expertise in sourcing hard-to-find parts and components for complex medical devices. Helping businesses to realise cost efficiencies and optimise their supply chain in light of rising demand and logistics disruptions.
As local systems work hard to recover elective services to pre-pandemic
levels – and many manufacturers are still struggling to catch up with sudden increased demand for parts and products, Eakin Surgical aims to help OEMs meet challenges, whether it’s reducing operating costs, navigating regulatory hurdles, or shortening time to market.
BresMed’s new global HQ at Steel City House in Sheffield is the heart of the company’s worldwide operation. Commenting on the move, CEO Nic Brereton said: “I am so proud to be expanding BresMed to these premium premises in the city. The building very much fits with our brand – it’s iconic with a quirky feel and, like us, it dares to be different.”
The company has come a long way since Nic moved into a one-person office in the city many years ago, adding: “Actually, it was more a cupboard! However, it helped me launch the business.”
“Eakin Surgical is a well-respected manufacturer of single-use-surgical instruments that are sold worldwide, and we have long been a trusted partner to the NHS. Having serviced the industry for over 20 years, the creation of a new contract manufacturing service is an exciting development that allows us to offer our knowledge and expertise to support the recovery of elective care and meet rising demand for sterile packing and single-use instruments worldwide, offering businesses access to high-quality, cost-effective and reliable solutions.”
Matthew Krolak Business Development Manager Eakin Surgical
22 Issue 21 People and Places
www.eakinsurgical.com
Opportunities for international partnership in data-driven healthcare
Whilst athletes competed in Birmingham 2022, at UK House: The Commonwealth Business Hub, the collaborative spirit of the games was being harnessed to create international partnerships in data driven healthcare.
There can be no doubt that COVID-19 has advanced the use and acceptance of digital health worldwide - both within the healthcare professions and across the wider public.
Thanks to the daily use of lateral flow tests, for example, the public perception of Point of Care tests has shifted from the occasional use of home pregnancy kits to being something that could be deployed for a range of conditions, with patients using digital devices to self-report their data via apps or QR codes. This reliance on digital technologies will remain post-pandemic, accelerating long-term growth in the digital economy and digital market sectors.
Birmingham is well placed to foster the connections that will bring these changes to the world thanks to its ABC (Academic, Business and Clinical) ecosystem, combined with the most broad and stable diverse population in the UK.
The region is large enough to have a great range of businesses, institutions, NHS trusts and universities, but compact enough for people to work together and forge strong relationships. In the clinical setting, the West Midlands has specialist, well recognised
centres working on genomics, microbiology, diagnostics and pathology, amongst others.
What academic, business and clinical organisations all have in common is the growth of digital products and services. This underpins, enhances and accelerates their activities. The West Midlands is the UK’s biggest multi-city 5G testbed. Support from a variety of communication providers allows digital health developers opportunities to prototype, trial, validate and commercialise 5G-enabled health solutions with regional health and care providers.
Another key element is staffing. The region has the highest number of young adults in the UK. They come from varied and diverse ethnic and cultural backgrounds, giving not only a youthful perspective and enthusiasm, but also a diverse set of ideas. The high number of universities and colleges in the West Midlands provides a pool of individuals studying digital and healthcare data qualifications. This represents an available future workforce that will be looking for career opportunities with cutting-edge companies.
This fertile ground has led to a number of exciting developments from the region, including projects looking at the use of AI in medical applications; how regulations adapt and can facilitate the greater usage and development of technology; new approaches to diagnostics; and the exploration of remote
care and digitising clinical trials, to alleviate care and health staff shortages and ensure a greater diversity of patients are catered for.

West Midlands companies which have successfully navigated this path already include Binding Site, a manufacturer of specialist diagnostic products. With nine international sites and an overseas market accounting for 90% of its sales, Binding Site has capitalised on the region’s world class facilities, highly skilled talent pool, and connectivity to domestic and international markets. Its refined diagnostics products are combined with an excellent business model that has gained the company recognition as a trusted supplier worldwide. Another company that has chosen to locate in the West Midlands is Dignio - a medical software company established in Norway which chose Birmingham as the location for its first UK office in 2017.
For medtech and digital healthcare companies based in the region, the opportunity now is to engage with international partners. Bringing a technology to market in the UK will create products that help patients, not only in the UK but throughout the world.
23For daily lifescience news visit www.lifescienceindustrynews.com People and Places
Chris Dyke, Medilink Midlands, explores how UK medtech companies are ready to deliver datadriven healthcare from Birmingham to the Commonwealth and beyond.
www.medilinkmidlands.com
D&O insurance - 10 risks facing life science businesses
Jon McArdle, Life Sciences Insurance Specialist; Arthur J. Gallagher, Insurance Brokers
Directors of life sciences companies carry a huge weight of responsibility, and yet many companies, especially in the start-up phase, may not recognise the true value of a directors’ and officers’ (D&O) insurance policy

These are 10 reasons why a D&O insurance policy can protect your business, its reputation and its balance sheet.
1. Regulatory obligations
The life sciences industry operates in an increasingly complex regulatory landscape. Unforeseen adverse events or manufacturing defects may expose a business, or its directors, to litigation—from illness or injury claims through to product recall.
2. Medical research studies and clinical trials
Clinical trials create significant risk exposure by their very nature, whether they are focused on vaccines, new drugs or therapies - sponsors of clinical trials often require a D&O policy, which may respond in the event that legal action is taken against a company director for a wrongful act.
3. Cyber risk and the protection of intellectual property
The protection of sensitive data is paramount and the consequences of a breach can be extremely damaging. While businesses may have cyber insurance in place, a D&O policy can offer protection to senior executives in the event that they’re deemed liable for certain data breaches.
4. Investor expectation
Most investors in life science businesses will typically tolerate higher levels of risk and it
is common for investors to require evidence of a comprehensive risk management strategy, including a D&O policy.
5. Shareholder expectation
Directors are expected to create value for shareholders. If the business performs badly, shareholders may decide to bring a claim against the directors for negligence or breach of duty/trust.
6. Attracting board members and non-executive directors to the board
Any life science business that fails to protect its senior executives may struggle to recruit the calibre of senior leader it needs to drive the organisation forward.
7. Customer and supplier relationships
Many parties that you work with will have their own risk management policies, and this may include a requirement for your business to have D&O insurance.
8. Mergers and acquisitions
Mergers and acquisitions can lead to significant growth opportunities, but directors could end up liable for lack of due diligence, overpayment or poor integration. A D&O policy can offer long-term financial and reputational protection of involved parties.
9. Overseas expansion
D&O cover can protect directors who make frequent trips abroad, including the provision of cover for legal representation and extradition protection should they be detained.
10. Environmental impact
Businesses are under increased focus in terms of their environmental sustainability. A D&O policy could help to protect a business’s senior executives if it’s alleged that certain environmental responsibilities are not met.
Gallagher offer Insurance and risk management services to the life sciences industry. They provide over 3,000 organisations globally with tailored insurance programmes & risk management solutions extending to intellectual property infringement, supply chain exposures, industry mergers and IPO’s.
24 Issue 21 Money
www..ajg.com
Medilink Midlands helps life sciences companies secure nearly £80 million in investment
Analysis by the life sciences industry association Medilink Midlands reveals it has helped the region’s life sciences companies to secure investment of £77 million.
Medilink Midlands’ analysis also reveals that it has been instrumental in the creation of over 190 new high level jobs in the Midlands, 47 university collaborations, 34 traineeships for life sciences businesses, and has engaged with 1,700 SMEs in the Midlands’ life sciences sector.
One of these companies is Dignio, which is taking connected care to a new level, with its proven, integrated care platform that is easy to use with Bluetooth connected devices, and alerts healthcare professionals if they need to act.
Already well established in Norway, the company wanted to expand into the UK. By working with Medilink Midlands and the West Midlands Academic Health Science Network (WMAHSN), Dignio set up a UK base in Birmingham earlier this year. The Dignio integrated care platform is now used in multiple care settings across the UK.
Another example is Spirit Healthcare, one of the UK’s fastest-growing companies. It was recognised as one of Technology’s 50 ‘Best Companies to Work For’ in 2021, named in the UK’s top 100 best mid-sized companies, and won the Medilink UK Advances in Digital Healthcare Award 2022.
Spirit develops innovative products and services that empower people to take control of their own health. It has combined its health and social care expertise with technological brilliance to develop the innovative digital health platform CliniTouch Vie, which is powering virtual wards and remote patient monitoring.
Warwickshire MedTech business POWERbreathe International manufactures a range of respiratory muscle training
devices that strengthen breathing muscles that are often described as ‘a set of dumbbells for your lungs’. Support from Medilink Midlands has included an introduction to Advena when POWERbreathe needed help with a regulatory issue for its operations manual and international distributors.
Simon Himsworth comments: “Medilink Midlands remains focused on championing the region’s life sciences industry, and supporting them to overcome barriers to growth and together boost the Midlands’ economic output from life sciences and medical technologies.

BresMed’s new global HQ at Steel City House in Sheffield is the heart of the company’s worldwide operation. Commenting on the move, CEO Nic Brereton said: “I am so proud to be expanding BresMed to these premium premises in the city. The building very much fits with our brand – it’s iconic with a quirky feel and, like us, it dares to be different.”
“Our mission is to be at the forefront of delivering direct support to the region’s life sciences industry. Working with companies in the sector to help them secure almost £80 million in funding since 2019, is clear evidence we are fulfilling this.”
The company has come a long way since Nic moved into a one-person office in the city many years ago, adding: “Actually, it was more a cupboard! However, it helped me launch the business.”
Simon Himsworth CEO Medilink Midlands
“With our skills, knowledge, expertise, network and connections, Medilink Midlands has the resources to deliver relevant support that creates opportunities and networks, and offers members a gateway for essential connectivity to the academic, business and clinical communities across the Midlands, and helps companies form new collaborative partnerships and navigate the increasingly complex health innovation landscape.”
25 Money
For daily lifescience news visit www.lifescienceindustrynews.com www.medilinkmidlands.com



27www.lifescienceindustrynews.com
Medilink North of England Clinical Evaluation Specialists, Patrick Trotter & Stefanie Lowry, offer insight into
Data: Which Direction?
Richard Parker, Legal Director, Hill Dickinson LLP, examines how political disruption has impacted the progress of data protection and digital information legislation.

Recently, the volume and velocity of big political announcements, has made following the direction of government policy from one day to the next a challenging task. This has prompted many to admit to longing for a more predictable, and boring form of politics. The uninitiated may hope that the government’s plans for data protection reforms could offer some respite. However, despite the proclamations of “a new direction” for data protection over a year ago, and the recent introduction of a new Bill, the legislative process has stalled. The future direction and extent of the reforms are now uncertain, and susceptible to political turbulence.
Following a consultation process, the government introduced the Data Protection and Digital Information Bill. The new culture secretary, Nadine Dorries MP, said: “Out of the EU, our new Data Reform Bill will ensure everyone can take back control of their personal data,” and promised an end to “pointless paperwork”.
The Bill had its first reading in parliament in July 2022 and was due its second reading in September 2022. However, following a cabinet reshuffle by Liz Truss, this was postponed, “to allow ministers to consider the legislation further’.
The reality
As always, the devil is in the detail. Ministers can be forgiven for needing extra time to consider the Bill. In keeping with tradition for data protection legislation, the Bill’s 192 pages, 113 clauses and 13 schedules are not an easy read, even for veterans of previous reforms. However, once digested, the reality is not easily reconciled with the rhetoric.
Richard Parker, legal director at Hill Dickinson LLP, who advises clients in the life sciences, health and care sectors on all aspects of information governance, has been following the reforms and explores how we got here, the uncertainty about which direction we will be heading in next, and the detail of the Bill.
What was said
In September 2021, the government launched a consultation on data protection reforms in the UK, Data: a new direction. Then culture secretary, Oliver Dowden MP, said: “Now that we have left the EU, we have the freedom to create a bold new data regime: one that unleashes data’s power across the economy and society for the benefit of British citizens and British businesses whilst maintaining high standards of data protection.”
At the Conservative Party Conference in October 2022, another new culture secretary, Michele Donelan MP, stated: “We inherited GDPR from the EU, and its bureaucratic nature is still limiting the potential of our businesses … we will be replacing GDPR with our own business and consumer-friendly, British data protection system … it will be simpler and clearer for businesses to navigate … No longer will our businesses be shackled by lots of unnecessary red tape … a truly bespoke, British system of data protection”.
Whether the Bill accomplishes the aim to do away with “pointless paperwork” and “unnecessary red tape” will be hotly debated. The “bureaucracy” is not eliminated entirely, but mostly replaced with similar, but modified, requirements. The net margin of deregulation for
28 Issue 21 Regulatory
organisations will therefore require careful comparison of the old and new schemes and will vary from one organisation or context to another. The costs of migrating to a new regime, as well as the burden of complying with diverging regimes in the UK and EU (for organisations established or targeting individuals in both territories), will also need to be factored in.
Furthermore, the Bill modifies - rather than “replacing” - the existing UK General Data Protection Regulation (GDPR) retained from EU law after Brexit and the accompanying Data Protection Act 2018 (DPA). It is not “a truly bespoke, British system of data protection”; at best it is an off-the-rack European system of data protection inherited from our neighbours, albeit with substantial alterations. Nor is it “simpler and clearer …
to navigate”; particularly given there is no consolidation of the EU-derived GDPR and DPA into a single Act.
Therefore, whatever it was that the culture secretary was announcing at conference, taken at face value, it was not this Bill.
What was announced would require such a substantial rewrite of the Bill that it would be easier to start afresh, suggesting something more ambitious, dramatic, and divergent is in the works. But even that statement is in doubt only weeks after it was made, as the latest changes at the top of the government appear to usher in a more frugal, circumspect, and technocratic government to reassure the markets. This may point things in yet another new direction, prioritising stability of the regulatory framework rather than diverging from EU law
at this challenging time, with the risks to the free flow of personal data across the English Channel that entails.
Time will tell, but it is already looking ambitious for the Bill, in any form, to complete its passage into law before the next general election. Nonetheless, many of the reforms as they stand have found support during the consultation so are likely to proceed in some form at some point, so remain worthy of analysis.
Richard Parker’s full detailed assessment of the Bill can be read online at www.lifescienceindustrynews.com
29For daily lifescience news visit www.lifescienceindustrynews.com Regulatory
www.hilldickinson.com
14th - 17th November 2022 Medica, Germany 15th - 16th November 2022 Future Surgery 2022, London 23rd -26th November 2022 CME, China 24th November 2022 UKCA Regulatory Webinar 28th November 2022 ABHI Sustainability Conference 30th November 2022 ABHI Digital Health Conference 8th December 2022 MediWales Innovation Awards 8th December 2022 Women in Biotech, BIA 9th - 11th January 2023 Biotech Showcase, San Francisco 18th - 20th January 2023 Medical Japan 30th January2nd February 2023 Arab Health 9th February 2023 IVDR Webinar March 2023 BioWales in London 16th March 2023 Medilink Midlands Awards 20-23 March 2023 BIO-Europe Spring ,Switzerland 23rd March 2023 Medilink NOE Healthcare Business Awards 28th - 29th March 2023 Making Pharmaceuticals, Coventry 20th April 2023 Anglonordic Life Science Conference, London 20th April 2023 ISO13485 Implementation Webinar May - June 2023 Africa Health 7th June 2023 Medilink UK National Awards 7th - 8th June 2023 Med-Tech Innovation Expo June 2023 MediWales Connects NHS Collaboration Conference 21st - 23rd June 2023 FIME, USA November December January February March April May June 2022-23 Event Calendar 2022 2022 2023 2023 2023 2023 2023 2023 MIDLANDS


31VALUE ENGINEERINGPRODUCT DESIGN www.gxgroup.com +44(0)1291 673437 Drawing on our 35 years of knowledge, GX is proud to be part of the team who created an affordable rapid Covid-19 tester. Find out how we could help you develop your next medical product. AT GX WE FIND PRACTICAL WAYS TO BRING A DESIGN IDEA TO LIFE. TURNING BRILLIANT IDEAS INTO COMMERCIAL REALITY
32 Medilink Midlands East Midlands Office Charnwood Campus Bakewell Road Loughborough LE11 5RB Tel: +44 (0)121 452 5630 www.medilinkmidlands.com West Midlands Office 4 Greenfield Crescent Edgbaston Birmingham B15 3BE Tel: +44 (0)121 452 5630 www.medilinkmidlands.com Medilink South West c/o Institute of Bio-Sensing Technology University of the West of England Coldharbour Lane Bristol BS16 1QY www.medilinksw.co.uk DISCOVERING HEALTH TECHNOLOGY Medilink North of England Hydra House Hydra Business Park Nether Lane Sheffield S35 9ZX Tel: +44 (0)114 232 9292 www.medilink.co.uk Elliot House, 151 Deansgate, Manchester M3 3WD Tel: +44 (0)7734 383 407 Written by the sector, for the sector. In print and online, Lifescience Industry magazine is supported by some of the UK’s most respected medical organisations. Partner organisations MediWales The Maltings East Tyndall Street Cardiff CF24 5EA Tel: +44 (0)29 2047 3456 www.mediwales.com South East Health Technologies Alliance Atlas Chambers 33 West Street Brighton East Sussex BN1 2RE Tel: +44 (0)7905 201857 www.sehta.co.uk National & Regional PartnersMedilink UK Partners BioPartner UK 16 Old Queen Street London SW1H 9HP Tel: +44 (0)20 7193 7815 www.biopartner.co.uk ABHI Suite 2, 4th Floor 1 Duchess St London W1W 6AN Tel: +44 (0)20 7960 4360 www.abhi.org.uk Better together










































