Early-Stage Pancreatic Cancer Screening Test
Pancreatic cancer, a lethal tumor of the pancreas, has one of the highest mortality rates among all major cancers. Every year, this disease claims approximately 466,000 lives worldwide, ranking it as the seventh most common cause of cancer-related
Cont’d on page 24
Metagenomic sequencing can offer antimicrobial resistance predictions for treating bloodstream infections much faster than conventional laboratory tests, thus saving lives and improving antibiotic management.
See article on page 17
Simple Paper-Based Test Simultaneously Quantifies Multiple Cardiac Biomarkers
Centralized lab testing has long been the go-to method for diagnosing common illnesses. However, this approach often necessitates costly medical equipment and complex procedures that can only be carried out by highly trained profes -
sionals within a medical facility. These factors prolong testing time and hinder the widespread application of diagnostics in remote and resource-poor areas due to limited access to central labs. In response to these challenges, point-of-care (POC)
Cont’d on page 13
Detecting Parkinson’s in Brain Cell Proteins
It’s estimated that 1% of individuals aged over 60 are affected by Parkinson’s disease. Unfortunately, diagnosing this kind of neurodegenerative disease is challenging, with cognitive and movement tests sometimes taking over a year to confirm the diagnosis. Early diagnostic molecular tests could speed up interventions and help Parkinson’s patients receive treatment faster.
Cont’d on page 14
New Procalcitonin Testing Guidelines
Although procalcitonin tests have been employed in Europe for years, they were only introduced in the U.S. in 2017 when the FDA approved their use for guiding antibiotic treatment and predicting the likelihood of death within 28 days for sepsis patients. Given their recent arrival in the U.S., there remains uncertainty about the appropriate use of these
Cont’d on page 28
AI-Powered Automatic Hematology Analyzer Features RET Indicator

As the amount of information generated by hematology analyzers increases, clinicians can be affected by excessive workload, resulting in overlooking of critical information. Now, a cutting-edge hematology analyzer applies artificial intelligence (AI) analysis to generate truly interpretable information which helps decrease
Cont’d on page 12
Joint World-European Laboratory Medicine Congress Breaks Attendance Records


The 25th IFCC WorldLab and 25th EFLM EuroMedLab were jointly held at the new Rome Convention Center “The Cloud”, on May 21-25, 2023. Hosted by the Italian national society SIBioC, the event was attended by 11,500 professionals plus some 2,000 online participants from over the
world. The congress featured a rich program of plenary lectures, accredited symposia, educational workshops, as well as 2,140 poster presentations. LabMedica’s review of exhibition highlights starts on page 4, while page 29 features a conference wrap-up by Congress President Prof. Sergio Bernardini.

®
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
Metagenomics Much Faster in Diagnosing Antibiotic Resistant Bloodstream Infections Metagenomics Much Faster in Diagnosing Antibiotic Resistant Bloodstream Infections GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE
® INTERNATIONAL Vol.40 No.4 • 7/2023 VISIT DAILY CLINICAL LAB NEWS ISSN 1068-1760 WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
WorldLab ’23 Report . 4 LabMedica EXPO . 6-28 Clinical News .. .10-28 IFCC News ...... . 29 Industry News . .. . 33 Events Calendar . . 34
Qnostics | Molecular Infectious Disease Controls
THE MOST COMPLETE QC SOLUTION FOR MOLECULAR TESTING

Whole Pathogen
Capable of monitoring the entire testing process including extraction, amplification, and detection. As whole pathogen controls, the Q Control range can be used to effectively monitor the performance of the entire testing process including extraction, amplification and detection.
Flexible
Our controls are whole pathogen and contain the full organism genome, therefore mimicking the patient sample to the best ability.
Third Party Controls
Manufactured independently of instrument and assay manufacturers providing a true third-party control to provide unbiased assessment of performance.
Traceability
Organisms traceable to WHO standards where possible.
Multi-Analyte Material
Where appropriate, Qnostics provide multi-analyte controls designed for use with multiplex assays.
PORTFOLIO
Exotic Diseases Central Nervous System Gastrointestinal Diseases Transplant Associated Diseases Respiratory Infections Blood Borne Viruses Sexually Transmitted Infections
marketing@randox.com randox.com Product availability may vary from country to country. Some products may be for Research use Only. For more information on product application and availability, please contact your local Randox Representative. Drug Resistance 102 LMI-07-23 LINKXPRESS COM BOOTH 3223

103 LMI-07-23 LINKXPRESS COM
WorldLab-EuroMedLab 2023: Highlights of Technical Exhibition

INTERNATIONAL
labmedica.com
EDITORIAL BOARD
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Jocelyn M. Hicks United States
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom
A GLOBETECH PUBLICATION
Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com
Dan Gueron
The 25th International Congress of Clinical Chemistry and Laboratory Medicine (WorldLab) and 25th European Congress of Clinical Chemistry and Laboratory Medicine (EuroMedLab) were hosted by the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC, Rome, Italy; www.sibioc.it), on the occasion of their 55th Annual Congress, at the new Rome Convention Center “The Cloud” from May 21st to 25th, 2023. Organized by MZ Events Srl (Milano, Italy; www.mzevents. it), EuroMedLab-WorldLab 2023 featured innovative and diverse educational workshops, including lectures, symposia, recent advancements in clinical practice and science, poster presentations, and industry exhibits.
The biennual WorldLab and EuroMedLab congresses both continue to act as leading forums in the field, bringing together scientists, laboratory specialists, clinicians, and industry to enable scientific exchange and advancement. This year, the congress adopted a combined approach of both physical and virtual interactions. There was particular focus
on scientific and technological advancements, as the main objective of the meeting was not only to contribute to the advancement of laboratory medicine and to the dissemination of advanced knowledge, but also to foster the creation of an opportunity to establish professional and scientific links/bridges among the participants.
The technical exhibition featured latest advances by 110 industry leaders from around the world. Here’s LabMedica’s review of the exhibition highlights:


Roche (Basel, Switzerland; www.roche. com) showcased its innovative laboratory solutions and digital diagnostics through"Innovation Past, Present and Future". Roche featured new innovations, such as several molecular systems from its growing PCR portfolio – the cobas 5800 and the new LightCycler PRO System, which will be launched later this year. A prototype for the new cobas Mass Spec system was unveiled, bringing mass spectrometry into the routine testing environment. Roche also introduced navify Sample Tracking, its cloud-based digital solution for labs to track patient test samples before even reaching lab premises.
Siemens Healthineers (Erlangen, Germany; www.siemens-healthineers.com) presented its newest innovations and trends in clinical diagnostics and POC testing at the industry event. On display at the Siemens booth were the company’s two new solutions for high-volume hematology testing, the Atellica HEMA 570 Analyzer and the Atellica HEMA 580 Analyzer. Both hematology analyzers offer integrated automation and intelligence to eliminate barriers that hamper workflow efficiency and deliver faster patient results.
Snibe (Shenzhen, China; www.snibe.com) showcased its latest products with high-performance features, including the newest member of its MAGLUMI X-series of new-generation
Cont’d on page 5
David Gueron
Sanjit Dutt
Carolyn Moody, RN
Simone Ciolek
Parker Xu
Karina Tornatore
HOW TO CONTACT US
Subscriptions:
Send Press Releases to:

Advertising & Ad Material:
Other Contacts:
www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
ADVERTISING SALES OFFICES
SUBSCRIPTION INFORMATION
LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field.
To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222.
For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com
ISSN 1068-1760
Vol.40 No.4. Published, under license, by Globetech Media LLC; Copyright © 2023. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services.
Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com
Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi
3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
4 LabMedica International June-July/2023
Publisher
USA Miami, FL 33280, USA Carolyn.Moody@globetech.net Tel: (1) 954-932-6215 GERMANY,
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007 OTHER
&
Miami,
Carolyn.Moody@globetech.net Tel: (1)
JAPAN Tokyo, Japan Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335 CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877 OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838 Founder & Editorial Director Marc Gueron
Managing Editor News Editor Regional Director Regional Director Regional Director Reader Service Manager
SWITZ., AUSTRIA Bad Neustadt, Germany
EUROPE
UK
FL 33280, USA
954-686-0838
WorldLab / EuroMedLab 2023 Report
chemiluminescence immunoassay (CLIA) analyzers. MAGLUMI X Tech is the core technology powering the X series of fully automatic CLIA analyzers which possess powerful capabilities such as patented pipetting technology, accurate incubation technology, stable and precise measuring technology, multiple expansion solutions, and user-friendly and high-efficiency loading design. These latest high-end features help the MAGLUMI X series CLIA analyzers to provide better immunology diagnostic capabilities for laboratories and hospitals.
Mindray (Shenzhen, China; www.mindray.com) demonstrated its latest technologies and solutions in in-vitro diagnostics, including the company’s ALL IN ONE hematology solution that brings greater efficiency to hematology testing with simplified workflows and ease of operation. Mindray's ALL IN ONE Hematology Solution enables end-toend automation throughout the process, from tube sorting through to analysis of CBC, ESR, CRP, SAA, and HbA1c with a single EDTA tube, smear preparation, and slide reading. The solution is ideal for core or centralized laboratories with mid-to-high sample volumes and a high demand for turnaround times.
QuidelOrtho (San Diego, CA, USA; www.quidelortho.com) demonstrated Savanna – a true sample-to-result point-of-care multiplex molecular solution – alongside its Vitros XT 7600 Integrated System which offers a wide-ranging menu of over 160 assays. Also featured at the industry event was the TriageTrue High Sensitivity Troponin I Test that empowers medical professionals to make informed decisions that lead to better patient outcomes. QuidelOrtho also participated in the EuroMedLab 2023 Satellite Meeting – Point-ofCare Testing: Home, Hospital and Beyond event. The company’s Vendor Talk by Dr. Holger Gundelach, Head of EMEA POC-IA Business Unit and Clinical Value at QuidelOrtho, on "The time is now – Accelerate patient management at the point-of-care" provided valuable insights and explored the future of POC testing.

Randox (Crumlin, UK; www. randox.com) introduced the new Acusera third-party controls designed for comprehensive test menu consolidation in laboratory internal quality control. These third-party controls are "true," meaning they are not biased towards or optimized for any specific reagent, method, or instrument, and hence offer an objective assessment of performance. Also on display at the industry event was Randox's Vivalytic, an all in one molecular diagnostic solution. This compact benchtop platform consolidates the complete molecular workflow, including extraction, PCR amplification, and detection.
Abbott (Lake Forest, IL, USA: www. abbott.com) introduced GLP Systems, an innovative total laboratory automation (TLA) solution, offering proven

High precision at clinically relevant decision points

High onboard stability combined with low calibration frequency
5 LabMedica International June-July/2023 105 LMI-07-23 LINKXPRESS COM
Homogeneous methods using block polymer detergents
Wide measuring ranges for convenient detection of diagnostically relevant results
DiaSys. Total confidence in patient results. www.diasys-diagnostics.com High quality solutions for reliable cardiovascular risk assessment HDL-c direct FS LDL-c direct FS Cont’d from page 4
on
6 WorldLab / EuroMedLab 2023 Report BOOTH 2567
Cont’d
page
AUTOMATED SAMPLE PREPARATION AUTOBIO DIAGNOSTICS


WorldLab / EuroMedLab 2023 Report

Cont’d from page 5
technology with more flexibility and options to meet high volume needs. The GLP TLA system can markedly boost the efficacy of laboratory testing. It allows labs to concentrate on crucial tasks by maximizing uptime with infinite redundancy, streamlining workflow, and reducing turnaround time.
DiaSorin (Saluggia, Italy; www.diasorin.com) presented its industryleading offering of fully-automated chemiluminescent immunoassay (CLIA) panels, designed specifically for diagnosing infectious diseases. Among its products showcased at the event, the highlight was the LIAISON MeMed BV test – DiaSorin's groundbreaking solution for rapidly and accurately distinguishing between bacterial and viral infections, thereby facilitating quicker, more informed decisions regarding treatment and patient management. The LIAISON MeMed BV test is the first of its kind, fully automated solution that uses data based on the host's response.
Sebia (Lisses, France; www.sebia.com) highlighted its new Alegria 2 instrument, a fully automated, sample-to-result solution. The instrument utilizes ORGENTEC’s unique and comprehensive Alegria Monotest portfolio, boasting over 100 parameters. A key distinguishing feature of the Alegria system is its flexibility to run any test from the wide ORGENTEC portfolio at any given time. Physicians can request tailor-made biomarker profiles to be analyzed for individual patients, ensuring the best possible patient care.
Binding Site (Birmingham, UK; www.bindingsite.com) showcased its Optilite system, the leading solution for multiple myeloma and immune status testing. Optilite represents a significant breakthrough in special protein testing. The innovative system has been fully optimized to simplify complex analytical processes, delivering unparalleled efficiency, workflow optimization, and confidence in test results. With an array of intelligent features, Optilite sets a new standard in the protein laboratory field by seamlessly integrating cutting-edge technology and advanced software.
Werfen (Barcelona, Spain; www.werfen.com), a silver sponsor of the Congress, highlighted its comprehensive and integrated line of acute care diagnostics. This included the company’s blood gas family, featuring the GEM Premier and Avoximeter product lines, as well as the whole blood hemostasis family, featuring the ROTEM, Hemochron, and VerifyNow product lines. Werfen also promoted two educational sessions that took place during the event and sponsored the Satellite
Meeting “Point-of-Care Testing: Home, Hospital and Beyond”
a forum for companies and industry experts to discuss topics around innovations and the future of POCT.


Menarini Diagnostics (Florence, Italy; www.menarinidiagnostics. com) introduced its brand new PRIME MDx all-in-one solution. This fully automated sample-to-result system is specifically designed to streamline laboratory workflows for real-time PCR diagnostic assays, offering exceptional efficiency, flexibility, and ease of use. Dedicated software enables automatic result generation and interpretation, leading to high-quality results, workflow standardization, and improved laboratory efficiency. The continuous workflow feature allows users to initiate a new loading process (extraction/setup) while a previous PCR is still running.

Bioperfectus (Shanghai, China; www.bioperfectus.com), a bronze sponsor of the Congress, showcased the SAW-48 automated nucleic acid extraction workstation, an in-vitro diagnostic medical device that combines sample loading, nucleic acid purification, and PCR setup functions (PCR master mix preparation and nucleic acid pipetting) into one instrument. The SAW-48 offers the same basic functions as the SAW-96, but in a smaller and lighter form factor. Also on display at the Bioperfectus booth was the SSNP-2000B nucleic acid extraction system, a laboratory medical device that integrates state-of-the-art technologies to enable automatic nucleic acid extraction of up to 32 samples at a time.

Greiner Bio-One (Monroe, NC, USA; www.gbo.com) collaborated with Tracie Healthcare Solutions (Munich, Germany; www.tracie. health) to jointly present a complete concept of a digitalized sample Cont’d on page 8
6 LabMedica International June-July/2023
The AutoMimo 1200 is an automated sample preparation system for use with the Autof ms1000 MALDI-TOF that can effectively solve the problem of poor repeatability and uniformity caused by manual micro-dosing errors.
MONONUCLEOSIS HETEROPHILIC ANTIBODY TEST SEKISUI DIAGNOSTICS
The OSOM Mono Test is intended for the qualitative detection of infectious mononucleosis heterophilic antibodies in serum, plasma or whole blood. It delivers results in five minutes and has >99% sensitivity and 96% specificity.
202 LMI-07-23 COM
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
203 LMI-07-23 LINKXPRESS COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
–
ICU Patient Studies Show Critical

Importance of Ionized Magnesium

Patients Undergoing Continuous Renal Replacement Therapy (CRRT)
Hutten et. al.1 found that patients receiving CRRT with citrate anticoagulation had normal tMg levels, but low iMg levels. This is due to magnesium ions being bound by citrate, and the citrate-magnesium complex being measured as tMg. These patients are actually hypomagnesemic but would not be recognized as such if only tMg were measured. Adult

Surgical ICU Patients
Yeh et. al.2 found that 21% of tMg tests which were reported as normal were hypermagnesemic based on iMg. This exposes patients to potential risks associated with undetected hypermagnesemia, including prolonged days on the ventilator, muscle weakness, QT prolongation, and cardiac arrhythmia. In addition, there were many patients with low tMg and normal iMg, which led to unnecessary Mg supplementation and repeat blood draws.
107 LMI-07-23 LINKXPRESS COM Test Menu pH, PCO2,
Contact us for a bibliography of more than 25 recent publications about the importance of Mg++ in disease processes. novabiomedical.com/iMglink
PO2, SO2%, Hct, MCHC, Na+ , K+ , Ca++ , Mg++ , Cl, TCO2, Glu, Lac, BUN, Creat,
HHb, O2Hb, MetHb, COHb, tHb, ePV, HbF, tBil
2.Yeh, et al. Total and ionized magnesium testing in the surgical intensive care unit - Opportunities for improved laboratory and pharmacy utilization. J Crit Care, 2017, 42, 147-151.
tMg (mg/dL)
iMg (mg/dL)
Adult
1.Hutten et al., Ionized and not total magnesium as a discriminating biomarker for hypomagnesaemia in continuous venovenous haemofiltration patients. Nephrol Dial Transplant, 2021.
novabiomedical.com Low Normal High Low Normal High tMg Measured iMg Measured Adult tMg (mg/dL) Adult iMg (mg/dL) Low Normal High Low Normal High iMg Measured tMg Measured BOOTH 2135
AUTOMATIC WESTERN BLOT ANALYZER SHENZHEN YHLO BIOTECH

CREATININE TESTING SYSTEM NOVA BIOMEDICAL


collection process. Tracie, an innovative digital healthcare company, provides full support for the sample collection process to eliminate errors and ensure the best possible diagnosis for patients. Through the implementation of Tracie's software, the entire sample collection process is digitized, eliminating the need for manual steps and enabling clear patient identification. This not only enhances patient safety and simplifies day-to-day operations but also improves the overall quality of medical care.
Sansure Biotech (Hunan, China; www.sansureglobal.com), a bronze sponsor of the Congress, demonstrated its comprehensive range of IVD solutions, including the iPonatic III portable molecular workstation which stands out for its ability to deliver extremely accurate test results within 8 to 45 minutes. Equipped with innovative one-tube fast release and rapid PCR amplification technology, it enhances point of care testing (POCT) by optimizing steps such as sample handling, nucleic acid extraction, purification, PCR amplification, and result processing. Sansure also presented its newly-launched Natch 16S nucleic acid extraction system which employs magnetic bead technology and boasts ten different mixing modes.
Diagnostica Stago (Asnières-sur-Seine, France; www.stago.com) unveiled its new coagulation line - the sthemO and sthemE product ranges. The sthemO 301, the first analyzer in the sthemO hemostasis series, is a high-throughput, fully automated coagulation analyzer designed for medium to large laboratories and can be integrated into automated tracks. Also included in the new range of sthemO analyzers by Stago is the sthemO 201. This bench-top analyzer, suitable for smaller laboratories, offers the same level of efficiency and analytical performance as the sthemO 301. In order to optimize these two novel systems, Stago has also launched new eSolutions under the sthemE umbrella. The sthemE Manager is designed to facilitate data and information exchange between one or several in vitro diagnostic analyzers and laboratory information systems.
Beckman Coulter (Brea, CA, USA; www.beckmancoulter.com) unveiled DxI 9000 Access, its brand-new high throughput immunoassay analyzer that is designed for superior laboratory performance and demands no daily maintenance. The DxI 9000 is the most efficient immunoassay analyzer in terms of footprint, boasting the ability to process up to 215 tests per hour per square meter (tests/hr/m2). Its unique ZeroDaily Maintenance feature further enhances the device's uptime performance, as confirmed by beta users who report that it redefines their workday by eliminating daily maintenance tasks and reducing yearly maintenance routines by up to a staggering 96%.
Thermo Fisher Scientific (Waltham, MA, USA; www.thermofisher. com) demonstrated the Thermo Scientific B·R·A·H·M·S KRYPTOR compact PLUS fully automated, random-access benchtop immunanalyzer that delivers remarkable precision, thereby improving patient outcomes. Thermo Fisher also displayed the Thermo Scientific Indiko, a fully automated benchtop analyzer for clinical chemistry and specialty testing. This device offers genuine walk-away time for the operator once it's loaded. Also on display was the Thermo Scientific TSQ Altis triple quadrupole mass spectrometer, which delivers unparalleled accuracy and precision
Cont’d on page 9



8 LabMedica International June-July/2023
The Tenfly Phoenix Blot Analyzer is a new generation blotting system that offers automatic sample loading, drying, scanning and reading, along with 50 incubation positions and 60 reagents positions.
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The StatSensor Creatinine Hospital Meter System is a handheld analyzer and miniaturized single-use biosensor for whole blood creatinine testing. The system uses StatSensor Creatinine’s advanced, patented Multi-Well technology. 205 LMI-07-23 COM 206 LMI-07-23 LINKXPRESS COM
108 LMI-07-23 LINKXPRESS COM
Cont’d from page 6
for low-level compound detection and quantitation in complex matrices.


LumiraDx (London, UK; www.lumiradx.com) presented its LumiraDx Platform which is designed to perform multiple tests on one single instrument wherever needed. The LumiraDx Platform is similar to a portable laboratory, compact yet capable of delivering lab-grade results in a variety of care settings, thereby bringing clinical decision-making closer to the patient. This rapid, high-performing, POC diagnostic solution employs active microfluidic technology, providing results for a range of analytes, including NT-proBNP, HbA1c, and CRP, within minutes.
LGC Clinical Diagnostics (Middlesex, UK; www. lgcgroup.com) demonstrated how automated processes with its Multichem independent QC and IAMQC QC data management software can enhance QC efficiencies in the laboratory. LGC’s brands include three IVD manufacturers of QMTs (Maine Standards Company, SeraCare and Technopath Clinical Diagnostics) and one manufacturer of viral and bacterial antigens and antibodies (The Native Antigen Company). LGC showcased various product offerings, including Multichem Independent Quality Control, VALIDATE calibration and verification materials, as well as its leading serology portfolio – ACCURUN, alongside its Clinical Genomics portfolio including Seraseq for NGS.
Diesse Diagnostica Senese (Rigoni, Italy; www.diesse.it) unveiled its new innovative immunochemistry analyzer, the CHORUS EVO which has been designed in line with the company’s aim of developing instruments featuring cutting-edge technology that meet Italian design and its vision of "Diagnostics Evolution". CHORUS EVO is a new-generation analytical instrument that integrates innovative digital technology, paving the way for the integration of artificial intelligence functions. In order to achieve this, DIESSE is collaborating with the SAIHUB consortium, a network of companies that specialize in applying artificial intelligence in life sciences.
The record number of scientific abstracts (over 2100) submitted by scientists and laboratory professionals from across the world for presentation, and record number of corporate members and non-members participating in the industry exhibition made Roma 2023 the best attended and most successful EuroMedLab event ever held. The 26th WorldLab will be convening in Dubai, UAE, on May 26-30, 2024. The 26th EuroMedLab is scheduled for May 18-22, 2025, in Brussels, Belgium.

VACUETTE ® CAT Serum Fast Separator Tube

9 LabMedica International June-July/2023
WorldLab / EuroMedLab 2023 Report
The VACUETTE® CAT Serum Fast Seperator Tube combines the speed of a plasma tube with the advantages of serum.
Greiner Bio-One GmbH / Kremsmünster, Austria E-MAIL office@gbo.com / We are a global player / Find the contact details of your local partner on our website. 109 LMI-07-23 LINKXPRESS COM
ONLY 5 MINUTES TO PROCESSING DON'T
LOSE ANY TIME
BOOTH 3511
Microarray-Based Test to Detect Complex Urinary Tract Infections


While Urinary Tract Infections (UTIs) are typically straightforward to diagnose and treat, there is a subset of patients for whom this is not the case. These individuals suffer from what are referred to as 'complicated UTIs,' where conventional treatments fall short and a specific combination of antibiotics and treatments tailored to each unique case is required. Understanding the 'microbiome' and its interaction with the infectious pathogen is also a key concern for infectious disease specialists. Now, a single test can identify over 20 organisms and an extensive range of drug-resistant genes, facilitating the development of targeted treatment plans that maximize therapy effectiveness.


PathogenDx’s (Scottsdale, AZ, USA; www.pathogendx.com) first UTI assay is built on its unique D3 Array technology, a groundbreaking approach to multiplexed arrays and a crucial asset in the evolution of infectious disease testing. The D3 Array, standing for Dynamic, Dimensional, Detection Technology, bridges the technological gap between traditional PCR-based assays, arrays, and Next-Generation Sequencing (NGS). It

offers multiplexed targeted sequence detection that is affordable, quick, and highly accurate and sensitive. The D3 Array is designed to mimic a three-dimensional structure or scaffold, providing a larger surface area than traditional two-dimensional microarrays. This design allows for a higher density of molecular probes attached to the surface, and the sample can move freely within the 3D structure. This movement, similar to a solution-like environment, increases the likelihood of probe-sample binding events, reducing the time needed for hybridization and clinical result reporting.


The D3 Array is quick, shortening the time to results. It can simultaneously provide identification and drug-resistance results in a single test, and detect a wide variety of organisms (bacteria, viruses, fungi), as well as identify drug-resistant genes. This allows for the creation of advanced, multiplexed panels in a single 'well' test. If necessary, the D3 Array can detect over 100 targets in a single reaction, with a single shift time to result. Its unique 3D architecture optimizes the test's speed and sensitivity and enables simplified multiplexed testing. The D3 Array provides multiplexed targeted sequence detection that is cost-effective, quick, and highly accurate and sensitive.
Rapid and precise identification of infectious pathogens is essential to maximize patient care and ensure positive outcomes. Rapid identification of the causative organism and the presence or absence of drug-resistant genes can enhance and guide therapy, promoting effective patient management and preventing serious complications. The D3 Array technology fits perfectly between PCR-based assays, known for their reliable pathogen identification, and NGS sequencing, recognized as a crucial discovery tool. This technology becomes especially valuable when multiple PCR-based assays are needed, which can be costly and time-consuming, or when NGS sequencing goes beyond what is required.
10 LabMedica International June-July/2023
CAPILLARY ELECTROPHORESIS ANALYZER
The CAPILLARYS 3 TERA TLA capillary electrophoresis analyzer offers a large menu of tests for diagnosis and follow up of myeloma, diabetes and hemoglobin disorders. It is able to process serum and whole blood at the same time.
INTEGRATED CLINICAL CHEMISTRY & IMMUNOASSAY ANALYZER SIEMENS HEALTHINEERS
The Atellica CI 1900 integrated clinical chemistry and immunoassay analyzer can run up to 600 photometric, 400 IMTs, and 120 immunoassay tests per hour and has an onboard capacity of 113 assays.
208 LMI-07-23 COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
209 LMI-07-23 LINKXPRESS COM
110 LMI-07-23 LINKXPRESS COM
HIT Testing in Minutes.

The on-demand solution that saves more than time.
Fast, accurate HIT antibody detection. Prompt detection of HIT antibodies is critical to selection of the most appropriate therapy. Only Werfen provides a fully automated HIT assay on Hemostasis testing systems, ready-to-use, 24 hours/day, 7 days/week. Complete HIT testing solutions—now on-demand for ACL TOP® testing systems.

For more information, contact your local Werfen representative. werfen.com

Thrombocytopenia
Heparin-Induced
ACL, ACL AcuStar, ACL Elite, ACL TOP, HemosIL, ReadiPlasTin, RecombiPlasTin, SynthAFax and SynthASil are trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 111 LMI-07-23 LINKXPRESS COM BOOTH 1447
RAPID HBSAG IMMUNOASSAY WAMA DIAGNÓSTICA



MICROWELL ELISA & CHEMILUMINESCENCE

AI-Powered Automatic Hematology Analyzer Features RET Indicator
Cont’d from cover
turn-around time, minimize technical misclassifications and reduce the workload of clinicians.
AUTOMATED PROCESSOR
The new DH-615 hematology analyzer from Dymind Biotechnology Co., Ltd. (Shenzhen, China; www.dymind.com) based on fluorescent nucleic acid staining technology and the company’s proprietary AI Cube technology combines 6-DIFF with RET, greatly improving identification of abnormal cells and enhancing the ability to analyze hematological diseases such as leukemia, anemia, etc. In comparison to the traditional flag alarm functions, AI Cube technology provides more comprehensive information which is truly interpretable for the analysis of diseases, rather than simply providing conclusive data. Using Al technology, the DH-615 provides the graphics of the normal group and the corresponding diseases of different degrees for the reference of clinicians, which is more intuitive and clear. The DH-615 hematology analyzer perfectly combines AI analysis technology and DMS (Data Management System) for technical integration and higher compatibility.
ISO 13485:2016


an automated ELISA/CLIA analyzer: An open, programmable, walk away system.

As one of the most common laboratory tests for anemia diagnosis, blood routine examination has an important initial screening significance for further diagnosis of anemia. Some of the key indicators associated with anemia in blood routine are RBC counting, HGB concentration, MCV, and Reticulocytes (RET). The DH-615 is the first cutting-edge hematology analyzer with RET, which improves efficiency and accuracy for clinical detection in anemia and other diseases. Using nucleic acid fluorescence staining technology, DH-615 can summarize the four types of RET into three parameters according to the different nucleic acid content in different types of RET (different fluorescence intensity), which are HFR (High Fluorescent Ratio), MFR (Middle Fluorescent Ratio) and LFR (Low Fluorescent Ratio). The detection of reticulocytes en hances testing for bone marrow erythropoiesis and related diseases.
In addition, the DH-615 supports automated sampling of venous and capillary whole blood, which reduces the pressure of blood collection while increasing the detection velocity and reducing the risk of biological contamination. With the function of automated capillary blood sampling, it completely solves the pain points of the complicated operation process in the laboratory. The DH615 series adopts self-developed software to achieve full-process management, which provides accurate and fast test results for the outpatient and emergency laboratory without manual operation.
12 LabMedica International June-July/2023
The Imuno-RÁPIDO HBsAg is a rapid chromatographic immunoassay kit for the qualitative detection of Hepatitis B surface antigen (HBsAg), using monoclonal and polyclonal antibodies for selective identification in serum specimens
MOLECULAR DIAGNOSTIC SYSTEM XI’AN TIANLONG SCIENCE & TECHNOLOGY
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Tianlong Panall 8000 All-in-one Molecular Diagnosis System is easy-to-use and secure, integrating the functions of sample tube decapping/ capping, sample loading, nucleic acid extraction, PCR setup, detection and result analysis. 211 LMI-07-23 COM 212 LMI-07-23 LINKXPRESS COM
sales@awaretech.com
PPID via bar code
LIS enabled
Option for disposable tips
Robust and reliable
unique and easy to use software allows customization of every operation the analyzer performs, making ChemWell®2 Flexible for many lab applications.
•
•
•
•
ChemWell®2’s
is
ChemWell®2
AACC Booth 2955
112 LMI-07-23 LINKXPRESS COM
Simple Paper-Based
Test Simultaneously Quantifies Multiple Cardiac Biomarkers



Cont’d
sensors were developed as alternative diagnostic tools, characterized by their simplicity, rapid operation, compact size, and affordability. The most prevalent type of POC tests are paper-based sensors, also known as lateral flow assays (LFAs), where the injected sample fluid flows horizontally and reacts with specific test regions (test lines) to generate, for instance, a color change. Despite their ease of use and cost-effectiveness, existing LFAs have certain drawbacks, such as lower sensitivity and challenges with multiplexed testing for disease biomarkers.

To address these shortcomings, researchers at University of California (UCLA, Los Angeles, CA, USA; www.ucla.edu) have devised a novel paper-based biosensor that utilizes a fluorescent multiplexed vertical flow assay to rapidly and simultaneously measure three cardiac biomarkers from human serum samples. This new paper-based POC sensor’s vertical flow design allows for multiple test regions with up to 100 individual test spots on a single disposable cartridge. The powerful sensor operates with just a small serum droplet and can be easily used by a minimally trained individual in under 15 minutes per patient. Along with its multiplexing capabilities, the paper-based sensor also boasts high sensitivity, achieving a detection limit better than ~0.5 ng/mL for each biomarker — less than one billionth of half a gram per milliliter of serum.
Additionally, the UCLA researchers have created a mobile phone-based, low-cost handheld fluorescence reader and a deep learning-assisted signal analysis pipeline to automatically and accurately quantify the three target biomarkers in a user-friendly manner. The team tested their paper-based multiplexed sensor for the quantification of three biomarkers of acute coronary syndrome (ACS), including myoglobin, creatine kinase-MB (CK-MB), and heart-type fatty acid binding protein (FABP). ACS is a cardiovascular condition that demands prompt diagnosis in emergency situations, and these target markers are released into the bloodstream shortly after symptom onset. The newly-developed paper-based sensor was evaluated on human serum samples, and the measured concentrations for all three cardiac biomarkers aligned well with the benchmark measurements obtained by a standard laboratory test. Given its accuracy, speed, user-friendliness, and affordability, this deep learning-enabled paper-based multiplexed sensor offers an attractive POC testing option for various applications in remote and resource-limited settings.

“Compared to a commonly used linear calibration method, our deep






Image: Deep learning-enabled multiplexed POC sensor using paper-based fluorescence vertical flow assay (Photo courtesy of UCLA)
learning-based analysis benefits from the function approximation power of neural networks to learn non-trivial relationships between the multiplexed fluorescence signals from the paper-based sensor and the underlying analyte concentrations in serum,” said Artem Goncharov, a graduate student at UCLA Electrical & Computer Engineering Department. “As a result, we have accurate quantitative measurements for all three biomarkers of interest despite the background noise present in clinical serum samples.”
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International June-July/2023
113 LMI-07-23 LINKXPRESS COM
from cover
Detecting Parkinson’s in Brain Cell Proteins
Cont’d from cover





Now, researchers have devised a new technique that can reveal signs of Parkinson’s disease in urine samples.
Researchers at Purdue University (West Lafayette, IN, USA; www.purdue.edu) developed a method that potentially enables the detection of alterations in LRRK2 (leucine-rich repeat kinase 2) proteins and their downstream pathways in urine samples of Parkinson’s patients. LRRK2 proteins are known to be associated with Parkinson’s disease. This innovative approach might also pave the way for noninvasive testing for other neurodegenerative disorders and cancers. Among several methods to study the effect of LRRK2, tracking its biological pathway is feasible through analysis of urine, blood, and cerebrospinal fluid.

Extracellular vesicles (EVs) are minute packages utilized by cells for molecular delivery. EVs can contain phosphoproteins that are potential markers for early cancer detection or disease progression monitoring, as suggested in previous studies. EVs offer a way to target disease markers, as they are released by specific types of cells. However, sampling such biomarkers from the brain via spinal tap is a highly intrusive process.
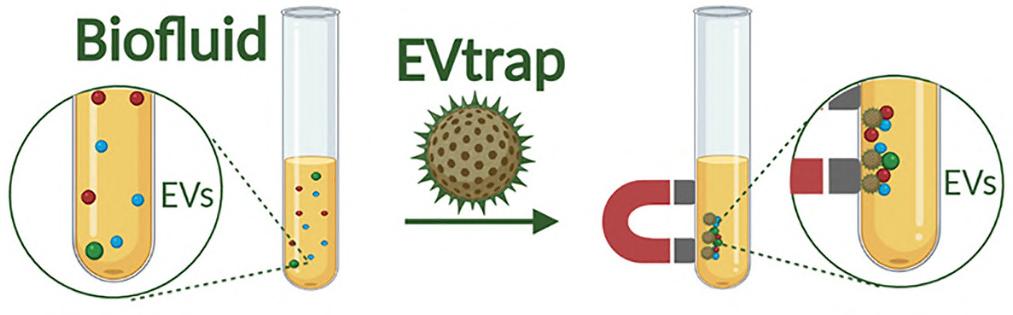

The potential of urine as a source of brain-related chemical markers was previously unknown. Although urine samples contain proteins that might serve as disease markers, many are involved in general cell maintenance and unrelated to diseases. Interestingly, these EVs can readily cross the blood-brain barrier. Upon being discharged from the brain into the bloodstream, they become concentrated or filtered into the urine. In this research, the team successfully isolated EVs quickly from urine samples, using the EVtrap (Extracellular Vesicles total recovery and purification) method. The EVtrap method offers a simple way to monitor changes in urine, which is routinely collected in various clinical studies.




“It’s going to be a big new area in diagnostic development, especially for neurodegenerative diseases and cancer,” predicted co-author Anton Iliuk. “This kind of analysis opens a new frontier in noninvasive diagnostics development. It’s showing that biomarkers previously thought to be undetectable have become uncovered and do a really good job of differentiating disease from non-disease state.” The researchers published their findings in the journal Communications Medicine on May 10, 2023.




14 LabMedica International June-July/2023
PCR CONSUMABLES
Accumax range of PCR tubes, strips, plates and seals are the outcome of the company’s impeccable standards of quality, resulting in tubes, plates and seals that guarantee precise and error free results
IMMUNOASSAY CONTROL LGC CLINICAL DIAGNOSTICS
Multichem IA Plus Control is intended for use as a third party, multi-constituent QC material to monitor the precision of laboratory testing procedures for immunoassays, containing 86 analytes for a range of disease markers.
214 LMI-07-23 COM
to
code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
215 LMI-07-23 LINKXPRESS COM
To receive prompt and free information on products, log on
www.linkXpress.com or scan the QR
114 LMI-07-23 LINKXPRESS COM
Image: The EVtrap technology uses magnetic beads to rapidly isolate and identify large quantities of proteins from extracellular vesicles (Photo courtesy of Purdue University)


We make diagnostics that matter At SEKISUI Diagnostics, what matters to you matters to us. Our whole purpose is to partner with you to provide intelligent solutions that enable you to make a timely difference. Because we both understand that there is a patient behind every answer—and that’s what matters most. For more information about our assays and systems, please visit sekisuidiagnostics.com or email us at questions@sekisui-dx.com Clinical Chemistry • Point-of-Care • Enzymes • Pre-Analytic Systems © 2023 SEKISUI Diagnostics, LLC. All rights reserved. Because every result matters is a trademark of SEKISUI Diagnostics, LLC. 115 LMI-07-23 LINKXPRESS COM BOOTH 1919
Seegene and Werfen to Jointly Develop Syndromic qPCR Assays


Seegene, Inc. (Seoul, Korea; www.seegene.com) has entered into discussions with Werfen (Barcelona, Spain; www.werfen. com) to expand their ongoing collaboration within the Spanish and Portuguese markets through Seegene's OneSystem Business. Seegene and Werfen have a long-standing relationship, and the extended partnership will enable the co-development of syndromic qPCR assays suited for the healthcare systems of Spain and Portugal at Werfen's OEM Technology Center in Spain. These assays will include those for sexually transmitted infections and drug resistance.
The objective of Seegene's OneSystem Business is to share its syndromic quantitative PCR technologies and its SGDDS (digitalized development system). This system allows researchers, regardless of their experience level, to develop assays. Furthermore, Seegene aims to provide automated manufacturing technologies to create syndromic PCR assays compatible with its standardized OneSystem instruments. Through this partnership, Spain and Portugal will gain the knowledge and technology to develop and manufacture syndromic qPCR assays,


Are

thus creating an infrastructure capable of responding swiftly to future pandemics without depending on foreign products and resources.
Seegene's OneSystem Business is pursuing its ultimate goal of cre-



16 LabMedica International June-July/2023
ROTAVIRUS ONE STEP CARD TEST CERTEST BIOTEC
CerTest Rotavirus one step card test is a colored chromatographic immunoassay for qualitative detection of Rotavirus in stool samples, offering a simple and highly sensitive screening assay for diagnosis of Rotavirus infection.
ELISA & IFA AUTOMATED PROCESSOR DAS SRL
217 LMI-07-23 COM 218 LMI-07-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
APE IF ELITE is a combined ELISA and IFA automated processor that can manage 10 methods on line with four ELISA microplates at different temperatures and features a three needle system that allows fast processing on 16 IFA slides.
you still spending more than 3 seconds on sed rates? Come see how the iSED® ESR analyzers can bring efficiency to ESR Booth 4588
22 116 LMI-07-23 LINKXPRESS COM
Image: The Seegene All-in-One Platform is a unique streamlined automation system (Photo courtesy of Seegene) Cont’d
on page
Metagenomics Much Faster in Diagnosing Antibiotic Resistant Bloodstream Infections

Bloodstream infections can quickly progress to sepsis, multiple organ failure, and even death. Timely and appropriate antibiotic therapy is crucial for managing the infection. Antimicrobial resistance (AMR) poses a significant challenge in treating bloodstream infections. Current clinical methods for identifying the causative pathogen are lengthy and labor-intensive, involving two culture and sensitivity tests that take at least 1 to 3 days to complete—first isolating and identifying the pathogen and then performing antimicrobial susceptibility testing. Now, new research presented at ECCMID 2023 demonstrates that metagenomic sequencing can offer rapid and actionable AMR predictions for treating bloodstream infections much faster than traditional laboratory tests, potentially saving lives and improving antibiotic management.
The study conducted by researchers at the University of Oxford (Oxford, UK; www.ox.ac.uk) reveals that rapid metagenomics can provide accurate results within just six hours of detecting bacterial growth in a blood sample. Clinical metagenomics sequences all genetic material, including infectious pathogens, in a sample simultaneously, reducing the time spent on running tests, waiting for results, and conducting additional tests. For their study, the Oxford researchers randomly selected 210 positive and 61 negative blood culture specimens for metagenomic sequencing, using the Oxford Nanopore GridION platform to sequence DNA. The sequences were utilized to identify the pathogen species causing infections and to detect common species that can contaminate blood cultures.

Sequencing successfully identified 99% of infecting pathogens, including polymicrobial infections and contaminants, and yielded negative results in 100% of culture-negative samples. In some cases, sequencing detected probable infection causes that routine cultures missed, while in others, it identified uncultivable species when a result could not be determined. Sequencing could also detect antibiotic resistance in the 10 most common infection causes. A total of 741 resistant and 4047 sensitive antibiotic-pathogen combinations were examined, with traditional culture-based testing and sequencing results agreeing 92% of the time. Comparable performance could be achieved using raw reads after just two hours of sequencing, with an overall agreement of 90%. The average time from sample extraction to sequencing was four hours, with complete AMR prediction achieved two
hours later, providing actionable AMR results 18-42 hours sooner than conventional laboratory methods.
“Antibiotic resistant bloodstream infections are a leading killer in hospitals, and rapidly starting the right antibiotic saves lives,” said Dr. Kumeren Govender from the John Radcliffe Hospital, University of Oxford, who led the study. “Our results suggests that metagenomics is a powerful tool for the rapid and accurate diagnosis of pathogenic organisms and antimicrobial resistance, allowing for effective treatment 18 to 42 hours earlier than would be possible using standard culture techniques.”
“This is a really exciting breakthrough that means we will be able to diagnose the cause of


patients’ infections faster and more completely than has been possible before,” added David Eyre, Professor of Infectious Diseases at the University of Oxford, who co-led the study. “We are working hard to continue to overcome some of the remaining barriers to metagenomic sequencing being used more widely, which include its current high cost, further improving accuracy, and creating improved laboratory expertise in these new technologies and simpler workflows for interpreting results.”

LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 17 LabMedica International June-July/2023
117 LMI-07-23 LINKXPRESS COM
3125
BOOTH
GOLD STANDARD
AI-Powered Deep Learning Model Accurately Counts Cell Types in Whole Slide Images
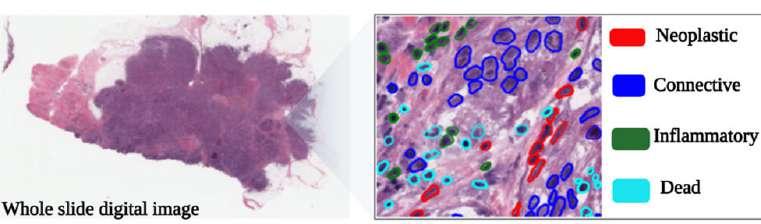
Improved methods for counting cell types in pathology images using deep learning approaches are much needed. Current techniques based on segmentation and regression face challenges such as the necessity for precise pixel-level annotations, difficulties in handling overlapping nuclei or obscured regions, and insufficient information on individual cell type locations. Moreover, probabilistic models tend to yield uncertain predictions and can lead to overconfident predictions. Researchers have now developed an advanced deep learning model to predict and count various cell types in the tumor microenvironment, which is expected to enhance the accuracy and efficiency of cancer diagnostics and treatment planning.

Identifying the different cell types in the tumor microenvironment can offer valuable insights into the tumor's histology and underlying biology. Precise and reliable cell type counting is also crucial for research and clinical applications. In addition, cell counts can be used to study the distribution of different cell types in the tumor microenvironment and its correlation with patient outcomes. In clinical settings, cell counts can help monitor therapy response and track disease progression. Researchers from the University of Eastern Finland (Kuopio, Finland; www.uef.fi) have proposed a new evidential multi-task deep learning approach, called CT-EMT, to overcome the limitations of current methods for cell type counting in whole slide tumor images. This approach formulates cell type density estimation and cell type counting as regression tasks, and nuclei segmentation as a pixel-level classification task.




The proposed cell type segmentation and counting approach has outperformed state-ofthe-art HoVer-Net and StarDist models, with relative improvements of 21% and 12% in terms of mean panoptic quality. The developed model can deliver persuasive interpretations of diverse cell types and can be applied to various computational pathology tasks, such as tumor grading, prognosis, and treatment planning. This work will pave the way for the
Cont’d on page 22



18 LabMedica International June-July/2023
AUTOMATED LABORATORY WORKSTATION
The Auto-LiPA 48 laboratory workstation offers fully automated processing for the complete range of INNO-LiPA and INNO-LIA tests from hybridization to color development, with pre-programmed test methods controlling temperature.
MINI BLOCK HEATER GREINER BIO-ONE
220 LMI-07-23 COM 221 LMI-07-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The EU PLUG Mini Block Heater is intended only for indoor use and as a general laboratory tube heating device (dry bath), providing excellent temperature uniformity and accuracy for heating of tubes from 0.2 mL up to 50 mL.
HPLC HbA1c Analyzer
AFFINITY POCT HbA1c Analyzer JULY 23-27 ANAHEIM, CA Goldsite Diagnostics Inc. en.goldsite.com.cn GSH-60 A1c Go Visit us at Booth number: 4478 118 LMI-07-23 LINKXPRESS COM
BORONATE
Image: A deep learning framework estimating cell types in a whole slide digital pathology image (Photo courtesy of University of Eastern Finland)
Take control, molecular control
Widest range of independent third-party controls, valid for any molecular testing platform



> More than 140 pathogens available
• Contain purified nucleic acid, complete microbial genome
• Any target can be amplified
• Precise concentration in copies/µl verified by qPCR.

• Non-infectious material provided with inactivation certificate.
• Lyophilized presentation ensures stability and reduces transport costs.

AMPLIRUN®
info@vircell.com For Research Use Only in the United States. Not for use in diagnostic procedures. 119 LMI-07-23 LINKXPRESS COM
BOOTH 1153
Puritan® patented HydraFlock® — ahead of the flock in collection and elution. Our flocked swabs are available in various tip shapes and sizes and provide some of the highest collection and elution capacities available on the market today — that means you get the maximum amount of available specimen with each sample. That efficiency helps ensure the most accurate results possible — the first time. Flocked technology also available in patented PurFlock Ultra.®




LEARN MORE ONLINE info.puritanmedproducts.com/flock



AI Algorithm Predicts Diabetic Kidney Disease Through Blood Tests
Diabetes is globally recognized as the main contributor to kidney failure. Notable advancements have been made in devising treatments for kidney disease in diabetic patients. Yet, evaluating an individual's risk for kidney disease based solely on clinical factors can be challenging. Consequently, identifying who is most susceptible to developing diabetic kidney disease is a vital clinical need. Now, scientists have created a computational method that predicts the likelihood of a person with type 2 diabetes developing kidney disease, a common yet severe diabetes complication. This could aid physicians in preventing or improving the management of kidney disease in type 2 diabetes patients.
The new algorithm developed by researchers from Sanford Burnham Prebys (La Jolla, CA, USA; www.sbpdiscovery.org) and the Chinese University of Hong Kong (CUHK, Hong Kong; www.cuhk.edu.hk) relies on measuring a process known as DNA methylation, which is the accumulation of subtle changes in the DNA. DNA methylation can provide essential insights into gene activation and deactivation and can be easily measured via blood tests.
Utilizing comprehensive data from over 1,200 type 2 diabetes patients registered in the Hong Kong Diabetes Register, the researchers constructed their model which they also tested on an independent group of 326 Native Americans with type 2 diabetes. This confirmed the model's predictive power for kidney disease across diverse populations. The researchers are presently fine-tuning their model and extending its application to address other health and disease-related inquiries, such as why some cancer patients do not respond favorably to certain treatments.
“This study provides a glimpse into the powerful future of predictive diagnostics,” said co-senior author Kevin Yip, Ph.D., a professor and director of Bioinformatics at Sanford Burnham Prebys. “Our team has demonstrated that by combining clinical data with cutting-edge technology, it’s possible to develop computational models to help clinicians optimize the treatment of type 2 diabetes to prevent kidney disease.”
“Our computational model can use methylation markers from a blood sample to predict both current kidney function and how the kidneys will function years in the future, which means it could be easily implemented alongside current methods for evaluating a patient’s risk for kidney disease,” added Yip. The results of the study were published in the journal Nature Communications on May 15, 2023.

20 LabMedica International June-July/2023
MALARIA ANTIGEN TEST LUMIQUICK DIAGNOSTICS
The QuickProfile Malaria pf/pv Antigen Test Card is a qualitative immunochromatographic assay for rapid detection and differentiation of P. falciparum-specific HRP-2 and P. vivax-specific LDH in human whole blood specimens.
POC BLOOD LEAD POISONING TEST MERIDIAN BIOSCIENCE
223 LMI-07-23 COM 224 LMI-07-23 LINKXPRESS COM WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
LeadCare II is the only CLIA waived POC test for blood lead poisoning. It can screen with a simple finger-stick in just three minutes. The system includes the LeadCare II Blood Lead Analyzer and the LeadCare II Test Kit.
When it comes to flock, Puritan® is out in front.
VISIT US IN PERSON
AACC Booth #4115
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device 120 LMI-07-23 LINKXPRESS COM
Blood Tests Can Reveal Brain Damage Caused by Neurosurgery




urrently, magnetic resonance imaging (MRI) is the go-to method following brain surgery to determine potential damage to the patient’s brain. MRI is useful for detecting signs of hemorrhage and areas of the brain affected by ischemia due to poor blood flow. However, its limitations lie in providing an accurate estimate of the degree of cell injury. Now, it may be possible to assess damage to the brains of patients operated on for brain tumors by measuring biomarkers in the pre-and post-surgery. The increase in these markers is correlated with the damage caused by inadequate blood flow, according to the findings of a study published in the journal Neurosurgery on May 5, 2023.
Researchers at the University of Gothenburg (Gothenburg, Sweden; www.gu.se) investigated the biomarkers, namely neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and tau protein, in patients operated on for brain tumors. The role of these biomarkers in neurological diseases, especially Alzheimer’s and other dementias, and in patients with traumatic brain injuries, has been well researched. NfL is indicative of nerve cell fiber damage, GFAP signifies injury to the brain’s supporting cells, while tau points to nerve cell impairment.
In the present study, 34 adult patients diagnosed with glioma, a common brain tumor type, were included. The biomarkers’ concentration was first measured a day before surgery, then successively on days one, three, five, and ten post-operation. The study found that the blood markers’ levels were in sync with both the degree of injury from oxygen deprivation during surgery and the severity of the neurological deficit experienced by the patients. This means that the practice of measuring blood biomarkers could potentially pave the way for a novel approach to assess neurosurgery-induced injuries, consequently facilitating the comparison of different surgical methodologies.

“It’s also conceivable that high levels of these markers might be signs of damage that could cause brain fatigue or other cognitive problems for the patients in the somewhat longer term,” said Isak Michaëlsson, a doctoral student at the University of Gothenburg and resident in neurosurgery at Sahlgrenska University Hospital. “If so, the markers could be used to identify patients at an early stage so that they get the right kind of rehabilitation.”
WE ARE DREAMERS+ DISRUPTORS LEADERS + LUMINARIES SEEKERS + SEARCHERS

LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 21 LabMedica International June-July/2023
C JULY 23-27 I ANAHEIM, CA
GLOBAL LAB MEDICINE COMMUNITY Register for Digital Pass Select Anaheim at meeting.aacc.org Anytime Access with Digital Pass Select Can’t join us in person for 2023 AACC? Connect with your community and stay ahead of changes in the field of laboratory medicine with Digital Pass Select. This is your pass to discover the topics and discussions you don’t want to miss: •The Sunday plenary session •16 select scientific sessions •Poster sessions (e-posters only) Watch it live or on demand, at your own pace, between July 23 - August 28, 2023, starting 24 hours after each session first airs. Be one with us.
C. DIFFICILE TEST PRO-LAB DIAGNOSTICS



AI-Powered Deep Learning Model Accurately Counts Cell Types in Whole Slide Images
Cont’d from page 18
creation of more accurate and robust digital pathology tools that can support pathologists and clinicians in diagnosing and treating cancer patients.
“The UEF Cancer AI research team aims to explore the potential of using deep learning technology in cancer and health data analysis,” said senior researcher Hamid Behravan of the University of Eastern Finland. “Our study will involve the development and evaluation of cutting-edge deep learning algorithms for analyzing cancer and various types of health-related data, including medical images, genomic data, and electronic health records. We believe that this approach has the potential to significantly improve the accuracy and efficiency of breast cancer diagnosis and treatment planning, as well as to facilitate the discovery of new insights and patterns in cancer data. We hope that our research will contribute to the advancement of precision medicine and the development of more effective and personalized approaches to breast cancer prevention and prognosis.”
Seegene and Werfen to Jointly Develop Syndromic qPCR Assays


Cont’d from page 16 ating a world free from all diseases, including cancer and infectious diseases that affect not only humans but all organisms. To fulfill this objective, Seegene is building an open innovation global network where scientists worldwide can collaborate to develop syndromic quantitative PCR assays tailored to local needs. As part of this initiative, Seegene is actively looking for additional partners from other European countries to join its global network and anticipates further agreements to be concluded throughout the year to expedite the expansion of its OneSystem Business.
"We will share our PCR technologies and know-how with any company in any country wishing to develop syndromic quantitative PCR assays," said Dr. Jong-Yoon Chun, CEO of Seegene. "We aspire to develop PCR diagnostic assays for all diseases so that the world free from diseases would come closer."
"We are excited about the possibility of strengthening our partnership with Seegene, and we look forward to developing assays which


LABORATORY
REFRIGERATOR
The LPR-400-PE laboratory refrigerator provides a stable, cold storage for temperature sensitive biosamples, chemicals and other substances that must be kept in an environment of between +4°C
226 LMI-07-23 COM 227 LMI-07-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The Prolisa C. difficile GDH EIA is a microwell assay for the qualitative detection of Clostridium difficile glutamate dehydrogenase (GDH) in fecal specimens. intended for use as an aid in the diagnosis of C. difficile infections
122 LMI-07-23 LINKXPRESS COM
BOOTH 3688
Quantimetrix® puts you in control of quality with the most versatile and complete line of urinalysis quality controls, designed to meet your needs from the central lab to the point-of-care.
Optimize your QC with exceptional open and closed-vial stability across all analytes, lot-to-lot consistency and friendly, dedicated customer support.

Samples of all Quantimetrix products are available absolutely free – let us help you find your best fit today!
10-0193 0523 Dipper POCT® US Pat. No. 9,835,562, US Pat. No. 10,712,283 © 2023 Quantimetrix. All rights reserved.
with us @ quantimetrix.com
Broadest
123 LMI-07-23 LINKXPRESS COM
Connect
Explore the
Range of Urinalysis QC Solutions.
RESPIRATORY DNA/RNA EXTRACTION





SAVYON DIAGNOSTICS









Early-Stage Pancreatic Cancer Screening Test



fatalities. Its survival rates are among the lowest for any cancer, primarily due to late detection and poor outcomes with conventional treatment options. Globally, the 5-year survival rate is around 9%. Nevertheless, an early diagnosis significantly improves this survival rate. Now, a first-in-class screening test based on novel microbiome markers is under development for this deadly cancer.
Mainz Biomed N.V. (Mainz, Germany; www.mainzbiomed.com) and Microba Life Sciences (Brisbane, Australia; www.microba.com)
have entered into a research collaboration for conducting a pilot study that uses Microba’s exclusive metagenomic sequencing technology and bioinformatic tools, with the aim of identifying new microbiome biomarkers for the detection of pancreatic cancer. Mainz Biomed is already progressing with an early-stage pancreatic cancer screening test, known as PancAlert. The test uses multiplex real-time PCR to identify genetic biomarkers in stool samples, and it is expected that this approach will be enhanced by incorporating microbiome biomarkers.
The project, scheduled to continue until the end of 2023, will employ Microba’s Community Profiler (MCP), a unique metagenomic platform technology. MCP has proven to be a top-tier research tool, able to generate detailed a nd precise species profiles of human gastrointestinal samples. The microbiome offers a plethora of medically relevant biomarkers that can be harnessed for therapeutic development or creating diagnostic tools. As the gut microbiome is adjustable, informed clinicians can enhance the biomarkers linked with treatment response. Microba’s biomarker discovery method employs its robust analysis platform and state-of-the-art informatic strategies to ensure superior resolution, access to new uncultured bacteria, and minimal false positives. Its artificial intelligence capabilities allow it to swiftly pinpoint diagnostic microbiome signatures from vast datasets.
“We are excited by the opportunity to collaborate with Microba as PancAlert is being developed for early-stage disease detection with the goal of being a first-in-class screening test for this deadly form of cancer,” said Guido Baechler, Chief Executive Officer of Mainz Biomed. “Given the growing understanding of the microbiome’s role in pancreatic cancer, we believe it’s of paramount importance to explore integrating diagnostic microbiome biomarkers into the test as it advances to the clinical stage of development and as such, are delighted to align with a global leader in sourcing and analyzing microbiome generated species and datasets.”

24 LabMedica International June-July/2023
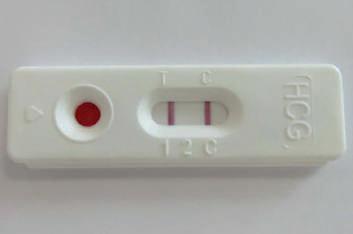
HCG WHOLE BLOOD PREGNANCY TEST
The hCG-CHECK-1 whole blood test is a qualitative rapid test for the detection of human chorionic gonadotropin (hCG) in human whole blood samples, providing results within five minutes, with a sensitivity of 25
KIT
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The Savvygen Respiratory Extraction Kit is an automatic extraction system for the isolation of high quality DNA/RNA from nasal swab, UTM. The kit is based on magnetic bead isolating nucleic acid (NA) from biological samples. 229 LMI-07-23 COM 230 LMI-07-23 LINKXPRESS COM
124 LMI-07-23 LINKXPRESS COM
Cont’d from cover
Singu20 Nucleic Acid Extractor AccuRa-32 Real-Time PCR System (32 samples) Singu20 Nucleic Acid Extractor AccuRa mini Real-Time PCR System (8-16 samples) ≤30 minutes qPCR Detection Kits (PCR fluorescence method): Respiratory Diseases: COVID-19, fluA, fluB, AdV, TB and multiplex test Blood Diseases: HBV HCV HIV and multiplex test Sexually Transmitted Diseases: HPV CT NG UU and multiplex test Viral Zoonotic Diseases: MPV Vector-borne Diseases: PF, ZIKV Genetic Diseases: MTHFR Animal Diseases: Swine/Avian/Aquatic Animal/Ruminant/Companion Animal Diseases 5-Part Hematology Analyzer Hemato ogy + CRP +SAA Joint Analyzer (Auto Sampling) ≤35 minutes
For small and medium labs, @SINGUWAY is your answer.
COME VISIT US AT AACC BOOTH #839

JULY 23-27, 2023
Automated Result Transfer to Laboratory Information Systems (LIS)
RESULT REPORTING
LIS READY Printed Results (Optional)
PATIENT INFORMATION
On-board Patient ID Bar Code Scanner


NOW CLEARED by FDA
510(k) K200754
ACCURATE DETECTION
Of Fecal Occult Blood (FIT)
STANDARDIZED ANALYSIS
Eliminate User Error
COMING SOON
• H. Pylori Fecal Antigen Test
• Rotavirus Fecal Antigen Test
HYGIENIC PATENTED
Closed Vial System
FULLY AUTOMATED
With an Easy-to-Use Touch Screen

• Norovirus Fecal Antigen Test
• Adenovirus Fecal Antigen Test

RESULTS IN 5 MINUTES
Accu-Reader® A100 Test Cartridge & Sample Collection Tube


✓ ✓ ✓
✓ 125 LMI-07-23 LINKXPRESS COM
Synthetic Peptides to Pave Way for Testing of Inflammatory Diseases

ommon inflammatory disorders such as Crohn's disease and ulcerative colitis can be diagnosed or monitored by evaluating the protein calprotectin in fecal specimens, while serum levels of calprotectin can provide insight into inflammation status in rheumatoid arthritis. Calprotectin levels in patient samples are usually measured using antibodies that latch onto and identify the protein, such as in lateral flow assays. However, antibody-based calprotectin assays present a challenge: the outcomes can vary based on the type of antibody and test employed. This inconsistency arises because antibodies might attach to different sites on the protein or lack a uniform composition. Additionally, antibodies can become inactivated over time due to unfolding or precipitation.
A viable alternative could be employing peptides instead of antibodies to identify and quantify disease indicators like calprotectin. Peptides are sequences of up to 50 amino acids capable of binding to proteins with high specificity and affinity. Unlike antibodies,







they can be synthetically produced with high purity and uniformity. Additionally, peptides are stable over time, cost less to produce, have lower inter-batch variability, and can be fixed to a specific location on a surface, simplifying the development of diagnostic assays by enabling a more precise and controlled method for biomarker detection. The use of synthetic peptides for detecting disease markers is highly promising as they are more accurate, reliable, and cost-effective than the commonly used antibodies in diagnostic tests.


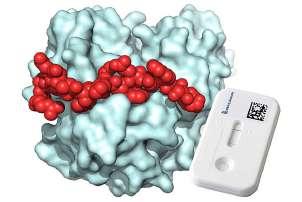
Bühlmann Laboratories AG (Schönenbuch, Switzerland; www.buhlmannlabs.ch ), in collaboration with a group of scientists at EPFL (Lausanne, Switzerland; www.epfl.ch), has created a peptide that binds to the protein calprotectin, a key marker for major inflammatory disorders, demonstrating its suitability for diagnostic tests. From a library of over 500 billion different peptides, the researchers isolated several calprotectin binders and demonstrated their effectiveness for calprotectin quantification in simplified lateral flow assays. The top-performing peptide showed a dissociation constant of 26 nM, indicating its strong bond with calprotectin, making it a potential candidate for diagnostic tests.
The peptide developed binds not only to a large surface area of calprotectin but also to a specific form of calprotectin that is the relevant species in patient samples. The peptide was also tested in professionally assembled lateral flow cassettes and was found to be suitable for the accurate detection and quantification of calprotectin. In a proof-of-concept study, researchers used this setup to quantify calprotectin concentration in blood serum derived from patient samples. The peptide developed marks the first synthetic affinity agent that could be produced against the biomarker calprotectin.
“The EPFL and Bühlmann teams are currently performing more tests with the calprotectin-specific peptide to translate the assay into a product that can bring the diagnostic power of this increasingly important biomarker to a new level to help patients suffering from inflammatory diseases,” said Professor Christian Heinis at EPFL

“This collaboration greatly benefited from Bühlmann's knowhow to produce and handle the biomarker, and expertise of the EPFL team to generate and screen large combinatorial libraries of peptides by phage display,” added Christian Gerhold, CTO of Bühlmann

Image: Lateral flow assay detecting calprotectin in blood sample (Photo courtesy of Bühlmann & EPFL)

26 LabMedica International June-July/2023
IMMUNOASSAY ANALYZER BECKMAN COULTER
The DxI 9000 immunoassay analyzer addresses the demands for speed, reliability, reproducibility, quality, and menu expansion with a throughput of up to 215 tests per hour per square meter (tests/hr/m2).
REAL-TIME PCR THERMAL CYCLER EUROIMMUN AG
232 LMI-07-23 COM 233 LMI-07-23 LINKXPRESS COM To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The Eonis Q96 is a quantitative real-time PCR thermal cycler for analyses in 96-well format with accurate results. It is operated with intuitive control software that can also be connected to the EURORealTime Analysis software
C 126 LMI-07-23 LINKXPRESS COM Rapid, simple, cost effective carbapenemase detection Contact us for more information sales@mast-group.com www.mast-group.com MAST CARBA PAcE ® Results in <10 minutes
Blood and Urine Based Biomarker Helps Identify Treatment of Acute Kidney Injury





Hospitalized patients who experience an acute kidney injury (AKI) often face unfavorable outcomes post-discharge, with limited effective treatment options. AKI can stem from various causes, such as sepsis, medication, or inadequate blood supply during cardiac bypass. Additionally, different cell types within the kidneys can sustain damage during AKI. Current AKI diagnosis relies on simple kidney function blood tests or measuring changes in urine output. These rudimentary diagnostic methods fail to identify the precise cause of injury or predict which patients are likely to respond better to treatment or recover kidney function. However, that could now change with the advent of new tests for biomarkers to identify the treatment of AKI.
Researchers at UW Medicine (Seattle, WA, USA; www.uw.edu) led a study involving retrospective analysis of 769 patients with AKI and 769 without the condition, monitoring them for five years post-hospital discharge. They identified two molecularly distinct AKI subgroups, or sub-phenotypes, linked with different risk profiles and long-term outcomes. One group had higher instances of congestive heart failure, while the other exhibited elevated rates of chronic kidney disease and sepsis. The latter group also displayed a 40% increased risk for significant adverse kidney events five years onward, compared to the first group.


Interestingly, factors like sex, diabetes rate, or major surgical procedures as the cause of AKI did not vary across AKI subgroups. This suggests that routinely measured clinical indicators may not forecast the AKI subgroups, necessitating the assessment of blood and urine biomarkers for identification. Based on the findings, the researchers have proposed a strategy to categorize AKI patient subpopulations, aiming to identify therapies tailored to specific patient groups. Similar to how unique biomarkers guide treatments for patient subgroups with cancer or asthma, blood- and urine-based biomarkers could potentially help distinguish subgroups of patients with AKI, resulting in the development of new treatment ideas.


“We’re attempting to better understand the clinical factors and molecular drivers of acute kidney injury so that, in the long run, we can better treat the different ways that people experience this disease process,” said Dr. Jonathan Himmelfarb, a professor of nephrology at the UW School of Medicine and the study’s senior author. “We want to better understand the individual characteristics of people who get acute kidney injury so we can establish common characteristics of subgroup populations of these patients to know whose risk is relatively higher or lower, and work toward treatments specific to their needs. The study was published on April 10, 2023 in the American Journal of Kidney Diseases.
Image: Medication, sepsis and inadequate blood supply to the kidneys are potential causes of AKI (Photo courtesy of Freepik)
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 27 LabMedica International June-July/2023
PRINT MAGAZINE INTERACTIVE DIGITAL EDITION PREMIER MULTIMEDIA PLATFORM SERVING THE WORLD’S CLINICAL LABORATORY COMMUNITY Anytime, Anywhere, On the Go... MOBILE VERSION ENGLISH • SPANISH WEB PORTAL PRINT MAGAZINE • INTERACTIVE DIGITAL EDITION WEB PORTAL • MOBILE VERSION • MOBILE APPS E-NEWSLETTER • E-MAIL MARKETING ONLINE SOLUTIONS • LABMEDICA EXPO PUBLISHED IN COOPERATION WITH BOOTH 2040
New Procalcitonin Testing Guidelines















Cont’d from cover tests, which are sometimes ordered in clinically inapplicable situations. To address this issue, new guidance offers a comprehensive analysis of procalcitonin research and its limitations, aiming to improve testing and treatment practices.
The American Association for Clinical Chemistry (AACC, Washington, DC, USA; aacc.org) has released expert guidance on procalcitonin testing, a blood marker used to detect severe bacterial infections and sepsis. This guidance aims to provide clarity for clinicians and laboratory professionals, enhancing the treatment of critically ill patients and those with specific lower respiratory infections. The document tackles key questions related to procalcitonin usage in managing adult, pediatric, and newborn patients with suspected sepsis and/or bacterial infections, especially those affecting the lower respiratory tract. It also examines the evidence for using these tests to inform antimicrobial treatment decisions and predict patient outcomes.

There is strong evidence supporting procalcitonin testing in guiding clinicians on when to discontinue antibiotic treatment in critically ill patients and those with certain lower respiratory tract infections. The AACC guidance suggests incorporating procalcitonin testing into comprehensive antimicrobial stewardship efforts, involving multidisciplinary teams. Some studies indicate that procalcitonin levels can also help determine when to stop antibiotics in newborns and pediatric patients with suspected sepsis who exhibit clinical improvement. However, the guidance authors believe these studies are insufficient in size to establish standard levels applicable across pediatric populations, given that procalcitonin reference intervals were established for adults. Consequently, if procalcitonin testing is used for these groups, it is important to define pediatric reference intervals or interpretive criteria.


Despite the FDA’s approval of procalcitonin tests for predicting 28day mortality risk in sepsis patients, the guidance does not recommend their routine use for this purpose. This is due to inconsistent procalcitonin cut-offs and clearance parameters, as well as a lack of substantial evidence demonstrating the benefit of estimating 28-day mortality risk for patients with sepsis and lower respiratory tract infections.
“Improved outcomes from [procalcitonin] implementation are more likely to be realized when the test is used in conjunction with antimicrobial stewardship programs, institutional interpretive algorithms, and clinical decision support tools,” said the guidance document authors Drs. Allison B. Chambliss, Khushbu Patel, Jessica M. Colón-Franco, Joshua Hayden, Sophie E. Katz, Emi Minejima, and Alison Woodworth. “Successful implementation of clinical [procalcitonin] requires a multidisciplinary effort among laboratorians, pharmacists, and infectious disease providers.”



28 LabMedica International June-July/2023
CARDIAC TEST YD DIAGNOSTICS
The ImmunTech Cardiac Triple Test is a rapid chromatographic immunoassay for qualitative detection of Myoglobin, CK-MB and cardiac Troponin I in whole blood, serum or plasma. It is easy-to-use and delivers results in 15 minutes.
ELECTROLYTE ANALYZER SINNOWA MEDICAL SCIENCE & TECH
The SINO-003 Electrolyte Analyzer uses the ISE Ion selective electrode assay method to test for parameters such as K+, Na+, and Cl- in samples of plasma, serum, whole blood and attenuation urine.
235 LMI-07-23 COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
236 LMI-07-23 LINKXPRESS COM
Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES Thermo-Resistant (- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS 128 LMI-07-23 LINKXPRESS COM
Edited by Katherina Psarra, MSc, PhD IFCC members
WorldLab-EuroMedLab Rome 2023: Amazing Congress in Amazing City!
 by Prof Sergio Bernardini Rome 2023 Congress President
by Prof Sergio Bernardini Rome 2023 Congress President
WorldLab-EuroMedLab Rome 2023, the 25th IFCC-EFLM Congress of Clinical Chemistry and Laboratory Medicine, together with the National Congress of the Italian Society of Clinical Chemistry and Laboratory Medicine that was held in Rome from the 21st to 25th May 2023, has been a great success!

Rome, the well-known city for its Art and Culture, hosted more than 11.500 people at the new Convention Center “The Cloud”, the stylish and futuristic Convention Center.
Additionally, two thousand people followed the Congress by streaming.
110 IVD companies participated and gave life to a dynamic exhibition floor showcasing the recent novel technologies and practical solutions for Laboratory Medicine.
Thanks to the Scientific Congress Committee work, distinguished international speakers and key opinion leaders delivered their presentations about health care, recent diagnostic technologies, scientific advances, and challenges along 5 plenary lectures, 32 accredited symposia, 10 lunch workshops, 14 educational workshops.
Satellite meetings were also planned and included a conference on Clinical Mass Spectrometry, one on Point-of-Care Testing and the 16th International Congress of Pediatric Laboratory Medicine.
An extraordinary number of posters (2140) were shown
sin-Madison titled “Forgiveness for Individuals, Family and Community Well Being”.
This is why we know there is not peace without forgiveness and scientists must be the witnesses of peace.
During the Opening Ceremony, the 2023 IFCC and the EFLM awardees were announced:
. IFCC Henry Wishinsky Award for Distinguished International Services (Sponsored by Siemens) to Dr Robert H. Christenson;
. IFCC Award for Distinguished Contributions in Education (Sponsored by Abbott Laboratories) to Dr David S. Hage;
. IFCC Robert Schaffer Award for Outstanding Achievements in the Development of Standards for Use in Laboratory Medicine (Co-Sponsored by NIST and CLSI) to Dr Anne J. Vassault;
. IFCC Young Investigator Award (Sponsored by Snibe) to Dr Joe M. El-Khoury;
. EFLM Award for Scientific Achievements in Laboratory Medicine (Sponsored by Roche) to Giuseppe Lippi;
. EFLM Award for Achievements in Advancing Laboratory Medicine in Europe (Sponsored by Roche) to Abdurrahman Coskun;
. EFLM Award for Excellence in Outcomes Research in Laboratory Medicine (Sponsored by Abbott Diagnostics) to Jain Shruti & Co-Authors;
electronically throughout the whole conference.
Moreover, at Rome 2023 Congress there have been two important innovations: first the Congress was accredited by the EFLM CPECS®, a quality assurance mechanism to provide Continuing Professional Development for participants. Second, the Congress has been transmitted by streaming worldwide with free access for mid-low income countries and all the Congress sessions will be published on the website.
It should be pointed out that the Congress Organizing Committee strictly followed the Med-Tech Ethical Code rules.
The intent of the Opening Ceremony was focused on humanity and in particular one minute of stand-up silence was dedicated to the victims of Covid 19 after the lecture of touching poetry by Sonia Giovannetti written during the Pandemic.

The famous poetry “No Man is an Island” by John Donne, introduced the Opening Lecture of Prof Robert Enright, Full Professor of Educational Psychology, University of Wiscon-
. EFLM Award for Excellence in Performance Specifications Research (Sponsored by Abbott Diagnostics) to Fernando Marques Garcia & Co-authors;
. EFLM Cardiac Marker Award (Sponsored by HyTest) to Rami Aalto & Co-Authors;
. EFLM Academy Award 2021 to Evgenija Homsak;
. EFLM Academy Award 2022 to Sedef Yenice.
Such a high level of attendance at the WorldLab/EuroMedLab Congress in Rome is a testament to the value and importance of the IFCC Congresses. The significant number of attendees, both in person and online, indicates a strong interest in the topics and discussions presented at the Rome Congress. This success opens the way for future IFCC Congresses to continue growing and achieving even greater success.
Thanks to the COC (Congress Organizing Committee), to the SPC (Scientific Programme Committee) and MZ Events (Organizing Secretariat) for their tremendous effort that made possible this successful Congress.
29 LabMedica International June-July/2023 NEWS
send news to Email:
may
enews@ifcc.org
Photo: Congress leadership at the closing ceremony (L-R): Prof. Bernardini, Congress President; Prof. Ozben, EFLM President and IFCC President-Elect; Dr. Haliassos, IFCC Treasurer; Prof. Adeli, IFCC President; Dr. Trenti, SIBioC President.
Photo: Congress President Prof Bernardini addressing opening ceremony
EDITORIAL
By Katherina Psarra, MSc, PhD
Dear Colleagues,
The successful atmosphere of the WorldLab-EuroMedLab 2023 is still lingering with us. I am thinking of the antithesis between the wonderful ancient masterpieces of Rome and the really modern congress centre and its surroundings, we kept moving between the past, the present and the future.
The future prevailed in the interesting presentations, full of innovations, and especially in the immense exhibition of this meeting, which was a suscessful congress in all respects. In this issue you will find a lot about the congress and the satellite meetings.
Starting with the Congress President, Prof. Sergio Bernardini, all the contributors in this issue write with great enthusiasm about their experience. I can assure you that they don’t exaggerate at all. It is true.
Everything was wonderful and, most of all, the attendees were wonderful people – full of life, full of energy! In this issue there are fewer contributions from the IFCC national societies (perhaps everybody’s thoughts are still in Rome). Go through this issue, dear friends, to keep the Rome atmosphere alive, but don’t forget your contributions for the next issue. We are still missing updates from the divisions, the committees, the Working Groups, the Task Forces’ meeting reports. Thank you very much to you all! We wish you all the best. Keep up the good work!
2022 UNIVANTS of Healthcare Excellence Awards: 11 Teams Recognized for Transformational Care
It is with excitement and honor that IFCC, in partnership with Abbott and 6 other prestigious partner organizations, celebrate 11 UNIVANTS of Healthcare Excellence award winning teams. The 2022 winning teams and best practices are recognized for achieving measurably better health outcomes by “UNIFYING” across disciplines to enable the development and implementation of “AVANTGARDE” processes.
Suffolk and North Essex NHS Foundation Trust
Gerry Rayman Emma Page Rachel Allen Ruth Deroy Alison Czarnota
« UNIVANTS OF HEALTHCARE EXCELLENCE RECOGNITION OF DISTINCTION

Early Diagnosis of Maternal Cytomegalovirus for Improved Management and Reduced Risk of Fetal Transmission and Complications
National Reference Center for Herpesvirus, University Hospital Center
Sébastien Hantz | Perrine Coste-Mazeau Sophie Alain Elodie Ribot Melissa Mayeras
Enhanced Resource Utilization, Reduced Waste, and Expedited Transplantation Through Real-Time Donor Screening for Infectious Disease
Mid America Transplant, St. Louis, Missouri
Amber Carriker | Linda Martin Erica Hinterser Lindsey Speir Kevin Lee
Getting to Zero Harm in Controlled Substance Prescribing: Increasing the Accuracy of Prescription Compliance Monitoring Through Enhanced Drug Testing Support University Hospitals Cleveland
Jaime Noguez Christine Schmotzer Sean Hoynes Heidi DelVecchio Jeanne Lackamp

«
Across the 11 impressive and diverse best practices recognized this year, these teams tackled varying disease states such as cervical cancer, diabetes, COVID-19, traumatic brain injury, addiction and more. Blindly reviewed through rigorous and comprehensive scoring, these teams were selected for their impact on patients, payors, clinicians and entire health systems. Outcomes include positively affecting patient experiences and patient safety, improving care delivery, enhancing utilization of resources, all while mitigating risk and health care costs. While many applications were initiated, the final outcomes for the 2022 UNIVANTS of Healthcare Excellence awards revealed 3 top global winners, 3 teams of distinction, and 5 teams of achievement.
« UNIVANTS OF HEALTHCARE EXCELLENCE RECOGNITION OF ACHIEVEMENT «
Enhancing Resource Utilization and Improving Patient Experience Through Strategic Laboratory Stewardship
Ain-Shams University - Emergency Hospital
Wessam EL Sayed Saad Essam Fakhery Ebied Rawan Mahmoud Mohamed | Ashraf Hassan Abdelmobdy Nouran Mahmoud Bahig
Enhanced Staff Satisfaction and Resource Utilization During the COVID-19 Pandemic
Associação Fundo de Incentivo a Pesquisa - AFIP
Debora Ribeiro Ramadan Tatiane Rodrigues dos Santos Josué Augusto do Amaral Rocha | Cristiane Franca Ferreira Paulo Eduardo de Andrade Souza
Improving Emergency Department Flow and Decreasing Risk Through Development and Implementation of Molecular Diagnostics Guided Triage
Clinical Hospital Center Rijeka
Martina Pavletic Vanda Juranic Lisnic | Mate Lerga | Mario Franic | Jennifer Babic
Improved and Accelerated Diagnostic Pathway for Patients That Present to the Emergency Department with Suspected Mild Traumatic Brain Injury Hospital Universitario Virgen de las Nieves
Gemma Álverez Corral Maria Molina Zayas Francisco Ruiz-Cabello Osuna Maria Isabel Romero Manjon Eva Gutierrez Pérez
A Noninvasive Serologic Model Using an Intelligent Informatic Solution to Enhance Clinical Decision-Making and Improve Patient Safety
The Second Norman Bethune Hospital of Jilin University
Yinlong Zhao Zhenjing Jin | Yongsheng Yang Chunmei Hu | Yan Zhao
ADD-144294-GBL-EN 04/23
















More details on these best practices and/or the award program itself can be found at www.UnivantsHCE.com. Also available are valuable links, tips and tricks, as well as access to an application portal for all healthcare teams who wish to apply for a UNIVANTS of Healthcare Excellence award in 2023 – the deadline for 2023 applications is Nov. 15, 2023. New this year, is the inaugural ELX (Executive Leadership Exchange) forum, a prestigious educational program for healthcare professionals who are seeking to learn more about best practices and healthcare excellence. Don’t miss out
register today.
The UNIVANTS of Healthcare Excellence award program proudly includes the following founding program partners: International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), AACC, EHMA (European Health Management Association), Modern Healthcare, Healthcare Information and Management Systems Society (HIMSS), National Association of Healthcare Quality (NAHQ), and the Institute of Health Economics (IHE); each
partnership with
News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
–
in
Abbott. 30 LabMedica International June-July/2023 « UNIVANTS OF HEALTHCARE EXCELLENCE GLOBAL WINNERS « Program ROSE (Removing Obstacles to cervical ScrEening) - Empowering Women to Eliminate Cervical Cancer ROSE Foundation Yin Ling Woo Marion Saville | Yit Lee Choo Adeeba Kamarulzaman Mun Li Yam The “Bubble”: Safe and Informed Population Health Management Based on Strategic, Novel Laboratory Testing to Restart a Global Sports League, Stimulate the Economy and Foster Normalcy During the COVID-19 Pandemic National Basketball Association Christina Mack Jim Weisberger David Weiss Yonatan Grad | David Ho Improving the Peri-Operative Pathway of People with Diabetes Undergoing Elective Surgery: the IP3D Project Ipswich Hospital, East
2nd IFCC Young Scientists’ Forum Held Successfully at WorldLab-EuroMedLab 2023

Following the successful inaugural “Young Scientists’ Forum” in Seoul, the second forum was conducted with more young scientist participation as a satellite meeting at the WorldLab-EuroMedLab 2023, on 21 May in Rome, Italy.
The opening of the second Young Scientists’ Forum got off to a great start with distinguished guests namely Prof. Khosrow Adeli, Prof. Tomris Ozben, Dr. Santiago Fares Taie, Dr. Tommaso Trenti, and Prof. Rajiv Erasmus delivering motivational speeches to the young scientists. Prof. Adeli also mentioned that the number of scholarships for young scientists for this meeting has increased. Dr Fares Taie, Chair of the TF-YS, addressed the young scientists reiterating the activities and objectives of the Young Scientist’s Task Force urging young scientists to continue their activities across the world.
The forum commenced with the session on “Digital technologies”, moderated by Aleksei Tikhonov (France) and Marie Lenski (France), featuring four presentations. “Evaluating A Patient for A Monoclonal Gammopathy: The MG-Testing Shiny App” by John Gabriel Bautista Abcede (Australia). Arnel Christian King Dy (Philippines) presented on “Enzymatic Correction of Jaffe Derived Serum Creatinine Interferences: A Machine Learning Approach”. Ronald Khunga (Malawi) presented a “Social Network Strategy: An Innovative Way of Identifying and Testing High-Risk Men”. The last presentation on “Digital Competence in Laboratory Medicine: Results Of A Survey Among Young Scientists” was by Marie Lenski (France). This session was a great opportunity to showcase the innovative work of young scientists in laboratory medicine and to foster networking and collaboration among them.
The second session of the forum was on “Chronic Diseases” coordinated by Dr. Sean Campbell, (United States) and Dr. Tamar Ramishvili (Georgia). This session commenced with a great presentation by Mohammed Yassine Kaabar, (Tunisia) on “Evaluation of GPR score for non-invasive assessment of Liver Fibrosis in Chronic Hepatitis B Tunisian patients”. Next was Don Makwakiwe Matshaz, (South Africa), who discussed an excellent study on “HHEX And MTR1B Gene Polymorphisms Associated with The Risk of Type 2
Diabetes Mellitus in A Mixed Ancestry South African Urban Population”. Sushant Pokhrel (Nepal), shared with us a study on the “Associated Non-Invasive Biomarkers for Risk of Liver Disease in Type 2 Diabetes Mellitus Patients”. Shruti Gupta (India) presented: “Association of Interleukin-22 Transmembrane Receptor and Binding Protein with its Levels in Tuberculosis”. The presentations of these studies generated great interest from the audience and the interaction between the speakers and YS from the audience facilitated sharing experiences and opinions about the research processes with each other.
The third session in the forum was on “Laboratory Management and Quality Control” chaired by Claudia Imperiali (Spain) and Udara Senarathne (Sri Lanka). Serafeim Karathanos (Greece) discussed “Education and Training of Young Scientists in EQA Schemes Operation and Management”. Next talk was by Josep Miquel Bauça (Spain): “Past, present and future of the reference intervals in medicine”, followed by a talk on the “Establishment of Age-Specific Reference Interval for Amino Acids in Dried Blood Spot by Tandem Mass Spectrometry” by Babu Vinodh Kumar (India). The last talk by Hamideh Ghazizadeh (Iran), discussed “Comparison of Biochemical and Hematological Markers Reference Intervals Derived by Direct and Indirect Procedures Based on The Isfahan Cohort Study”. The session was interactive with many questions arising from the audience, allowing a better understanding of the concepts of reference interval establishment and laboratory quality management among young scientists.
The 4th session: “Biomarkers of cardiovascular risk”, was moderated by Dr. Marco Alfonso Perrone (Italy) and Dr. Giulia Sancesario (Head of Italian YS and past member IFCC-YS). The first speaker was Dr. Marco Alfonso Perrone (Italy): “Cardiac Biomarkers during Exercise: from Patient to Professional Athlete”. Dr. Judit Gonda (Hungary) followed with: “The Increase of Soluble Urokinase Plasminogen Activator Receptor in Heart Failure is Related to Disease Severity and to Cardiac Mortality”. Dr. Dharmsheel Shrivastav (India) presented: “Risk factors influencing left ventricular ejection fraction in patients
31 LabMedica International June-July/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
Cont’d on page 32
A group photo of participants at the 2nd IFCC Young Scientists’ Forum held during WorldLab-EuroMedLab 2023 in Rome.
16th International Congress in Paediatric Laboratory Medicine: A Report
By Dr Tim Lang Congress Chair, IFCC C-ETPLM Chair
The 16th International Congress in Paediatric Laboratory Medicine Satellite meeting was successfully held on Saturday 20th May in the Rome Convention Centre “The Cloud”. It was attended in person and virtually by scientists, clinicians including a large contingent of local paediatricians in training and corporate members. The Congress is a unique IFCC event that brought together clinical and laboratory practitioners who have an interest in paediatric laboratory medicine and provided important networking opportunities and a forum for discussion.
The Congress began with the opening plenary delivered by Prof Carlo Caffarelli, a Paediatrician from Parma, Italy, describing the impact of COVID-19 on Italian children from the initial outbreaks observed in Northern Italy, through the following 2 years and other related social and health impacts.
The first symposium, prepared in collaboration with the International Society for Neonatal Screening, focused on the implementation of emerging technologies in supporting newborn blood spot screening. Speakers from the Netherlands and Italy described the progression from specific analyte identification to metabolomics to proteomics to genomics and how these techniques would support short- and long-term follow-up.
The second symposium focused on the use of biomarkers in renal and cardiac disease. Prof Adeli, the current IFCC President, delivered the first presentation describing the benefits of high sensitivity troponin and NT-proBNP when investigating different cardiac presentations. This was then followed by Dr Papassotiriou, Athens, Greece, presenting data on the use of cardiac and renal biomarkers to assess the toxicity of chemotherapy. In the final presentation Dr Marzuillo, a Paediatrian from Naples, Italy, described the use of established and novel biomarkers to assess acute kidney injury.
The last symposium of the day brought together examples of algorithms in paediatric laboratory medicine. Dr Zierk, the current chair of the TF-GRID described the current approaches used to prepare reference ranges using real-world data. This was followed by Prof Martin Stocker, a Consultant Neonatalo-
gist from Switzerland who discussed what were the best current markers for neonatal sepsis and how they could be used in the developing world. Finally, Dr Fawkner-Corbett from Oxford described the use of AI in predicting which were the best tests to decide whether a child with suspected appendicitis needed urgent review and those that could be managed conservatively.
Concluding the Congress with the closing plenary Dr Mai from Vietnam described her own experiences in establishing a GCMS service to introduce newborn screening for a number of inherited metabolic conditions. Introducing this service, she faced many challenges which her team overcame resulting in a fully accredited service with support from her regional IFCC federation the Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine (APFCB).
The Organising Committee would like to thank the support of the IFCC, Congress Organisers MZ Events and the German Society for Clinical Chemistry and Laboratory Medicine (DGKL) for their sponsorship of the event. We look forward to presenting another exciting ICPLM programme in Dubai next year.
2nd IFCC Young Scientists’ Forum Held Successfully at WorldLab-EuroMedLab 2023

Cont’d from page 31
with coronary artery disease: a tertiary care center experience in North India”, and Dr. Marlena Aginieszka Olejnik (Poland) presented: “Klotho And Fgf23 as Potential Biomarkers for Myocardial Infarction In Patients With Acute Coronary Syndrome”.
The session aroused great attention from the audience with subsequent questions and a rich discussion. Cardiovascular diseases (CVD) remain the leading cause of death in the world, but, thanks to clinical and biochemical innovations, today CVD also represent one of the fields in which there are major innovations available to the health of cardiac patients. Thanks to the IFCC and the YS for this beautiful session!
The closing remarks were delivered by Dr. Ashlin Rampul (South Africa), who commended the highly scientific program at the second Young Scientists’ Forum. He urged the young scientists to continue activities and become part of the IFCC TF-YS activities. Prof. Damian Gruson also a former member of the Young Scientist Task Force, stated: “You will have good
times in your career and not so good times but the most important fact is that as a young scientist you should continue to work hard without letting failures hinder the progress in your career”. The young scientists were all thanked for their contribution to the forum and special thanks were noted to the Italian Society, especially Giulia Sancesario and Marco Perrone who were excellent hosts and invited the young scientists to a spectacular get-together.
IFCC OFFICE
Via Carlo Farini 81, 20159 Milan, ITALY Web: www.ifcc.org
Tel: (39) 02-6680-9912 • E-mail: ifcc@ifcc.org
Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi, Smeralda Skenderaj
32 LabMedica International June-July/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
ICPLM speakers and organizers: Dr. Tran Thi Chi Mai (Vietnam), Dr. Tim Lang (UK), Dr. Tze Ping Loh (Hong Kong), Dr. Jolanda de Rijke (the Netherlands), Dr. Lianna Kyriakopoulou (Canada), Dr. Ronda Greaves (Australia), Dr. Klaus Kohse (Germany)
The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers.
MEDICA 2023 to Serve as Showcase for Medtech Start-Ups


The medical trade fair MEDICA (Düsseldorf, Germany; www.medica.de) has long been recognized as a global platform for health sector startups. At the forthcoming MEDICA 2023, which will be running concurrently with the professional suppliers’ trade fair COMPAMED 2023 from 13-16 November, hundreds of new developer teams will be present among more than 5,000 exhibiting companies to seek collaborations related to funding, production, product approval, marketing, and sales. The event boasts a number of program highlights geared towards startups, providing them with an optimal platform for introducing their innovative solutions to the international professional healthcare community. Notable program highlights include the 12th MEDICA Startup Competition, the 15th Healthcare Innovation World Cup, the MEDICA START-UP PARK, and numerous startup exhibitions at the MEDICA CONNECTED HEALTHCARE FORUM.
The MEDICA Startup Competition places emphasis on a wide array of healthcare innovations, ranging from artificial intelligence (AI) and health apps to laboratory diagnostics and medical robotics. This year, “Sustainability” is being introduced as a new category. The 2022 winner of the competition was a Spanish startup, Idoven, which has developed an AIbased platform for cardiology-as-a-service. Idoven’s proprietary AI enhances the accuracy and consistency of electrocardiograph (ECG) interpretations. Their participation in the competition offered the company immense global
Avacta Acquires Rapid Test Maker in Belgium
Avacta Group plc (London, UK; www. avacta.com), a life sciences company developing oncology drugs and diagnostics, has acquired Coris Bioconcept SRL (Gembloux, Belgium; www.corisbio.com) for an upfront cash consideration of GBP 7.4 million plus a contingent earnout.
Established in 1996, Coris specializes in the development, manufacturing, and marketing of rapid diagnostic test kits, mainly lateral flow tests, for healthcare professionals. Coris' range of products includes diagnostic tests for respiratory, gastrointestinal, and bloodborne pathogens (including bacteria, viruses, and parasites) as well as for the detection of markers indicating antibiotic resistance. “The acquisition of Coris provides the Group with a broad, professional-use rapid test product portfolio. Complementing the acquisition of Launch Diagnostics last year, the Acquisition represents an important step in establishing a full-spectrum in-vitro diagnostics business covering centralized, pathology laboratory diagnostics, as well as decentralized, point-ofcare testing solutions outside the hospital setting,” said Dr. Alastair Smith, Chief Executive Officer, Avacta Group plc.
visibility and attracted around USD 20 million in funding for future projects and developments.
The 15th Healthcare Innovation World Cup could be another interesting opportunity for startups, scale-ups, and smaller mid-level companies. It focuses on smart Internet of Medical Things (IoMT) solutions, such as digital biomarkers, smart band-aids, or wearables with network connectivity. The 12 selected finalists will be invited to showcase their businesses at MEDICA 2023 on the MEDICA CONNECTED HEALTHCARE FORUM stage. Last year’s winner was ViewMind Inc., a company specializing in management of neurodegenerative diseases.
The MEDICA START-UP PARK has become a central hub for startup networking, already attracting a record 40 early registrations this year. A repeat participant is the Ukrainian start-up initiative “Up To Future,” promoting startups such as HandyUsound, which developed a portable ultrasound system. Also participating is Korea’s
Megnosis, the creator of EEG helmets designed to detect dementia early and stimulate brain cells and neurons. Among German participants is AssistMe, which will introduce a smart sensor system for use in underwear for incontinence at MEDICA 2023.
“I was very surprised at how global this trade fair is!” said Rika Christanto, COO at Idoven, who is pleased with the effect of participating at MEDICA and the competition finals on the stage of the MEDICA CONNECTED HEALTHCARE FORUM.
33 LabMedica International June-July/2023 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
For a free listing of your event or a paid advertisement contact: LabMedica International Calendar
E-mail: info@globetech.net
2023
JULY
Analytica Lab Africa 2023. Jul 5-7; Johannesburg, South Africa; analytica-africa.com
APCCMI 2023 – 19th Asia Pacific Congress of Clinical Microbiology and Infection. Jul 6-8; Seoul, Korea; apccmi2023.com
FEBS 2023 – 47th Congress of the Federation of European Biochemical Societies. Jul 8-12; Tours, France; febs.org
FEMS 2023 – 10th Congress of European Microbiologists. Jul 9-13; Hamburg, Germany; fems2023.org Analytica China. Jul 11-13; Shanghai, China; analyticachina.com
2023 AACC Annual Scientific Meeting & Clinical Lab Expo. Jul 23-27; Anaheim, CA, USA; meeting. aacc.org
AUGUST
MedLab Asia 2023. Aug 16-18; Bangkok, Thailand; medlabasia.com
12th International Palestinian Conference for Laboratory Medicine (IPCLM). Aug 24-26; Ramallah, Palestine; ipclm.ps
SEPTEMBER
33rd MACB Annual Scientific Conference – Malaysian Association of Clinical Biochemists. Sep 5-6; Kuala Lumpur, Malaysia; macb.org.my
32nd WASPaLM Congress – World Association of Societies of Pathology and Laboratory Medicine. Sep 5-8; Sao Paulo, Brazil; waspalm.com
Thailand LAB International 2023. Sep 6-8; Bangkok, Thailand; thailandlab.com
ECP 2023 – 35th Congress of the European Society of Pathology. Sep 9-13; Dublin, Ireland; esp-congress.org
EUROTOX 2023 – 57th Congress of the European Societies of Toxicology. Sep 10-13; Ljubljana, Slovenia; eurotox2023.com
45th Mexican National Congress of Clinical Chemists and Expoquím 2023. Sep 11-16; Mazatlán, Mexico; conaquic.com
ACBICON 2023 – 48th Annual Conference of Association of Clinical Biochemists of India. Sep 13-16; Thiruvananthapuram, India; acbindia.org.in
India Lab Expo & Analytica Anacon India. Sep 1416; Hyderabad, India; analyticaindia.com
17th National Congress of the Czech Society of Clinical Biochemistry. Sep 17-19; Hradec Králové, Czech Republic; sjezdcskb2023.cz
Arab Lab 2023. Sep 19-21; Dubai, UAE; arablab.com
31st Congress of the Latin American Society of Cytopathology (SLAC). Sep 20-23; San Miguel de Allende, Mexico; congresoslac2023.com
Analitica Latin America 2023. Sep 26-28; Sao Paulo, Brazil; analiticanet.com.br
BCLF 2023 – 30th Meeting of the Balkan Clinical Laboratory Federation. Sep 27-30; Herceg Novi, Montenegro; bclf2023.org
92nd Annual Meeting of the American Thyroid Association (ATA). Oct 29 - Sep 1; Washington, DC, USA; thyroid.org
OCTOBER
ECC 2023 – 44th European Congress of Cytology. Oct 1-4; Budapest, Hungary; cytology2023.eu
AACC Middle East 2023. Oct 7-8; Dubai, UAE; aaccme.com
CAP23 – Annual Meeting of the College of American Pathologists. Oct 7-10; Chicago, IL, USA; cap.org
DKLM 2023 – Annual Congress of the German Society for Clinical Medicine and Laboratory Medicine (DGKL). Oct 12-13; Mannheim, Germany; dgkl.de
CELME 2023 5th Symposium: Cutting Edge of Laboratory Medicine in Europe. Oct 12-13; Prague, Czech Republic; celme2023.cz
JFBM 2023 – Journées Francophones de Biologie Médicale. Oct 11-13; France; jfbm.fr
Advertising Index
85th Annual Meeting of the Japanese Society of Hematology. Oct 13-15; Tokyo, Japan; jshem.org.jp
60th Annual Scientific Conference of the Australasian Association of Clinical Biochemistry and Laboratory Medicine (AACB). Oct 16-19; Brisbane, Australia; aacb.asn.au
ASHI 2023 – 49th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 16-20; San Antonio, TX, USA; ashi-hla.org
MedLab Africa 2023. Oct 17-19; Johannesburg, South Africa; africahealthexhibition.com
ASCP 2023 – Annual Meeting of the American Society for Clinical Pathology. Oct 18-20; Long Beach, CA, USA; ascp.org
LABCLIN 2023 – 17th National Congress of the Spanish Societies for Clinical Laboratory (AEBMML, AEFA & SEQCML). Oct 18-20; Zaragoza, Spain; seqc.es
EndoBridge 2023. Oct 19-22; Antalya, Turkey; endobridge.org
26th Congress of the Society of Medical Laboratory Technology of South Africa (SMLTSA). Oct 19-22; Johannesburg, South Africa; smltsa.org.za
7th ESPT Congress – European Society for Pharmacogenomics and Personalised Therapy. Oct 25-27; Copenhagen, Denmark; esptcongress.org
63rd Annual Academic Assembly of the Japan Society of Clinical Chemistry (JSCC). Oct 27-29; Toyama, Japan; jscc-jp.gr.jp
34th National Congress of the Turkish Biochemical Society. Oct 29 - Nov 1; Fethiye, Turkey; biyokimyakongresi.org
NOVEMBER
ASHG 2023 – Annual Meeting of the American Society of Human Genetics. Nov 1-5; Washington, DC, USA; ashg.org
44th Annual Meeting of the American College of Toxicology (ACT). Nov 12-15; Orlando, FL, USA; actox.org
MEDICA 2023. Nov 13-16; Dusseldorf, Germany; medica-tradefair.com
Events Calendar
Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions.
Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 40 No. 4 6-7/2023 LabMedica International – AACC 21 116 Alcor Scientific 16 112 Awareness Technology, Inc 12 114 B&E Bio-technology 14 105 DiaSys 5 110 Dymind 10 108 DxGen 8 122 GBC 22 118 Goldsite 18 109 Greiner Bio-One 9 125 Hemosure 25 – IFCC WorldLab 2024 33 – LabMedica 27 – LabMedica EXPO 35 126 Mast Group 26 107 Nova Biomedical 7 123 Quantimetrix 23 120 Puritan 20 102 Randox 2 115 Sekisui 15 124 Singuway 24 103 Snibe 3 117 Vedalab 17 128 Vicotex 28 119 Vircell 19 113 YHLO 13 111 Werfen 11 136 Werfen 36 ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LMI-07-23 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information
WSPID 2023 – 13th Congress of the World Society for Pediatric Infectious Diseases. Nov 14-17; Durban, South Africa; wspid2023.com
AMP 2023 – Annual Meeting & Expo of the Association for Molecular Pathology. Nov 16-18; Salt Lake City, UT, USA; amp.org
71st Annual Scientific Meeting of the American Society of Cytopathology (ASC). Nov 16-19; Austin, TX, USA; cytopathology.org
2023 Annual RBSLM Meeting - Royal Belgian Society of Laboratory Medicine. Nov 17; Brussels, Belgium; rbslm.be
JIB 2023 – Journées de l’innovation en biologie. 17-18; Paris, France; jib-innovation.com
34th Regional Congress of the International Society of Blood Transfusion (ISBT). Nov 18-21; Cape Town, South Africa; isbtweb.org
IUIS 2023 – International Union of Immunological Societies. Nov 27 - Dec 2; Cape Town, South Africa; iuis2023.org
DECEMBER
ASI 2023 – 51st Annual Scientific Meeting of the Australian and New Zealand Society for Immunol ogy. Dec 4-8; Auckland, New Zealand; asi2023.org
65th Annual Meeting & Exposition of the Amer ican Society of Hematology (ASH). Dec 9-12; San Diego, CA, USA; hematology.org
2024
FEBRUARY
SLAS 2024 – International Conference & Exhibition of the Society of Laboratory Automation and Screening. Feb 3-7; Boston, MA, USA; slas.org
Medlab Middle East 2024. Feb 5-8; Dubai, UAE; medlabme.com
Labquality Days 2024 – International Congress on Quality in Laboratory Medicine. Feb 8-9; Hel sinki, Finland; labqualitydays.fi
3rd International Congress of Laboratory Diagno sis 2024. Feb 15-18; Virtual; ldcongress.com
Pittcon 2024. Feb 26-28; Philadelphia, PA, USA; pittcon.org
MARCH
ICE 2024 – 21st International Congress of Endo crinology. Mar 1-3; Dubai, UAE; isendo.org
China Lab 2024. Mar 5-7; Guangzhou, China; chinalabexpo.com
MASCL 2024 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Mar 17-22; Monterey, CA, USA; msacl.org
USCAP 113th Annual Meeting – United States & Canadian Academy of Pathology. Mar 23-28; Baltimore, MD, USA; uscap.org
APRIL
Analytica 2024. Apr 9-12; Munich, Germany; analytica.de
Korea Lab 2024. Apr 23-26; Seoul, Korea; korealab.org
ExpoMED Eurasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com


ECCMID 2024 – 34th European Congress of Clinical Microbiology and Infectious Diseases. Apr 27-30; Copenhagen, Denmark; eccmid.org

MAY
Immunology 2024 – Annual Meeting of the American Association of Immunologists (AAI).
May 3-7; Chicago, IL, USA; aai.org
AACE Annual Meeting 2024 – American Association of Clinical Endocrinology. May 9-11; New Orleans, LA USA; pro.aace.com
Hospitalar 2024. May 21-24; Sao Paulo, Brazil; hospitalar.com
107th Annual Meeting of the German Society for Pathology. May 23-25; Munich, Germany; pathologie-dgp.de
IFCC WorldLab 2024 – 26th International Congress
JUNE
ESHG 2024 – European Human Genetics Conference. Jun 1-4; Berlin, Germany; eshg.org
ASM Microbe 2024 – American Society for Microbiology. Jun 13-17; Atlanta, GA, USA; asm.org
FOCIS 2024 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 18-21; San Francisco, CA, USA; focisnet.org
FIME 2024 – Florida International Medical Expo. Jun 19-21; Miami, FL, USA; fimeshow.com
LabMedica

Events Calendar
35 LabMedica International June-July/2023
Send inquiries directly to suppliers... Receive latest product alerts... Chat live with suppliers, and more...
MARKETPLACE BOOTH 2040
EXPO provides a sophisticated yet easy-to-use global B2B platform for sourcing clinical laboratory equipment. LabMedica EXPO connects buyers and sellers worldwide through a safe, secure and dynamic network, regardless of size or budget.
WORLD’S CLINICAL DIAGNOSTICS
The Intelligent Analyzer
GEM Premier 5000 blood gas testing system provides automated quality assurance with every whole-blood* sample. Now with next-generation Intelligent Quality Management (iQM2), featuring IntraSpect™ technology, potential errors are detected not only before and after, but also during sample analysis, along with real-time correction and documentation. Plus, it’s simple—just change the all-in-one GEM PAK once a month. So regardless of testing location or point-of-care operator, quality results and compliance are assured with every sample.
Real-time assurance and advanced simplicity. Now
that’s intelligent.
For more information, contact your local Werfen representative.
werfen.com
*Heparinized.
132 LMI-4-23 LINKXPRESS COM 124 LMI-05-23 LINKXPRESS COM
Acute Care Diagnostics
GEM, Premier and iQM are trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 136 LMI-07-23 LINKXPRESS COM BOOTH 1447































































































































































































 by Prof Sergio Bernardini Rome 2023 Congress President
by Prof Sergio Bernardini Rome 2023 Congress President















