From Lab to Life: Corneal Repair Goes Cellular
Long-awaited cellular therapies for corneal endothelial disease enter the clinic.

ALSO IN THIS ISSUE
Reversible
APRIL 2024 | VOLUME 29 | ISSUE 3
the Best
from the ESCRS Winter Meeting in
Best of
Highlights
Frankfurt.
Multifocality
combination offers low-risk approach to spectacle independence in presbyopes.
Two-lens
and Safety
access amid EU regulatory overhaul.
Balancing Innovation
Ensuring
Registe www.esc
er
.escrs.org
Today

2 EUROTIMES | APRIL 2024 10 Cover From Lab to Life: Corneal Repair Goes Cellular Long-awaited cellular therapies for corneal endothelial disease enter the clinic. 04 Editorial: Exciting Developments in Corneal Therapies 06 Inside ESCRS: With Eyes on Its Future, ESCRS Celebrates Its Past 07 ESCRS Update: Concerns about Health Data Regulation; ESCRS Leader Receives Honour 15 Balancing Innovation and Safety CATARACT & REFRACTIVE 16 Following the New Generation Francesco Carones MD 17 Reversible Multifocality Ramin Khoramnia MD, FEBO 18 CAPSULaser Poised to Lead Richard Packard MD, FRCS, FRCOphth and Pavel Stodůlka PhD, FEBOS-CR 20 Refocus on Multifocals Orkun Müftüoğlu MD, FEBO 21 Common Myths in Presbyopia Correction Florian Kretz MD, FEBO 22 Managing a Cataract Surgery Refractive Miss Zaina Al-Mohtaseb MD and William F Wiley MD 24 Pearls to Avoid Refractive Surprise Basak Bostanci MD, FEBO 25 Best of Frankfurt ESCRS Winter Meeting 2024 CORNEA 26 Pharmacotherapy Advances for Ocular Scarring Steven E Wilson MD 28 Cultivating Progress for LSCD Sophie X Deng MD, PhD 29 Innovative Corneal Tissue Engineering David Myung MD, PhD 30 Unleashing OCT’s Full Potential David Huang MD, PhD 32 In the Know—Iris Repair, Part 3 Soosan Jacob MS, FRCS, DNB RETINA 34 Vision Restoration for Blinding Retinal Degenerative Diseases Russell N Van Gelder MD, PhD 35 Watching a Novel Implant for RIPPLE Effects Sumit Sharma MD 36 Complement Inhibitors Promising for Geographic Atrophy Robin A Vora MD





Publisher
Filomena Ribeiro
Executive Editor
Stuart Hales
Editor-In-Chief
Sean Henahan
Senior Content Editor
Kelsey Ingram
Creative Director
Kelsy McCarthy
Graphic Designer
Jennifer Lacey
Circulation Manager
Nicola Lodge
Contributing Editors
Cheryl Guttman Krader
Howard Larkin
Dermot McGrath
Roibeárd O’hÉineacháin
Contributors
Soosan Jacob
Timothy Norris
Colour and Print
W&G Baird Printers
Advertising Sales
Roo Khan
MCI UK
Tel: +44 203 530 0100 | roo.khan@wearemci.com
EuroTimes® is registered with the European Union Intellectual Property Office and the US Patent and Trademark Office.
Published by the European Society of Cataract and Refractive Surgeons, Suite 7–9 The Hop Exchange, 24 Southwark Street, London, SE1 1TY, UK. No part of this publication may be reproduced without the permission of the executive editor. Letters to the editor and other unsolicited contributions are assumed intended for this publication and are subject to editorial review and acceptance.

The ESCRS Leadership, Business & Innovation Committee wants to hear from ophthalmologists, practice managers, and staff on what they see as the critical issues affecting medicine today and in the months ahead. Share your opinion by clicking on the link below or scanning the QR code. Completing the survey will enter you into a drawing for free registration to the 2024 Annual Congress in Barcelona (up to 3) or a 2024 Leadership, Business, and Innovation Weekend (up to 2)! (All answers are anonymous, unless respondents provide their email address to be entered into the drawing.)
https://survey.alchemer.eu/s3/90687098/ESCRS-Leadership-Business-Innovation-Survey

An earlier version of “Six Essentials to Iris Repair” (February 2024, Vol 29 Issue 2) displayed an incorrect version of the image. The image to the left represents the correct, intended image.
ESCRS EuroTimes is not responsible for statements made by any contributor. These contributions are presented for review and comment and not as a statement on the standard of care. Although all advertising material is expected to conform to ethical medical standards, acceptance does not imply endorsement by ESCRS EuroTimes. ISSN 1393-8983


3
2024 APRIL | EUROTIMES Learn more about EuroTimes or connect with ESCRS at ESCRS.org 16 41 36 26 06 ALSO IN THIS ISSUE 38 Industry News 39 JCRS Highlights 41 Upcoming Events ALSO IN THIS ISSUE
Exciting Developments in Corneal Therapies
Given the overall demographic trends and the growing incidence of corneal diseases, the demand for corneal transplantation is not expected to slow down anytime soon. The supply of donor corneas already does not meet the demand, with estimates of only one donor available for every 70 eyes requiring treatment.
In this issue, we revisit corneal endothelial cellular therapy, which we covered in our May 2023 issue. As Contributing Editor Dermot McGrath notes in the cover article, cell-based therapies carry the promise of greater sustainability and offer the possibility of a treatment accessible to general ophthalmologists with the potential to alleviate this growing demand for corneal transplantation.
Much has happened in the past 12 months. Building on the pioneering work by Shigeru Kinoshita MD, PhD of Kyoto, Japan, Aurion Biotech has already received regulatory approval in Japan for its cellular injection therapy for bullous keratopathy. AURN001—a combination cell therapy product of allogeneic human corneal endothelial cells and the Rho inhibitor Y-27632—is now in a US Phase 1 / 2 clinical trial for treating corneal oedema secondary to corneal endothelial dysfunction.
Emmecell’s EO2002 novel corneal oedema treatment is also in clinical trials. With this approach, magnetic cells are injected intracamerally and then an external magnetic patch delivers the cells to the target location. EO2002 is being investigated in a prospective, multi-centre study evaluating the safety and tolerability of this approach with and without endothelial brushing or Descemet stripping in eyes with corneal oedema secondary to corneal endothelial dysfunction that qualify for surgery involving full-thickness corneal transplantation or endothelial keratoplasty.
Meanwhile, a relatively low-tech approach reported at the recent AAO conference suggests the antihypertensive agent
EDITORIAL BOARD

Noel Alpins (Australia)
Bekir Aslan (Turkey)
Roberto Bellucci (Italy)
Hiroko Bissen-Miyajima (Japan)
John Chang (China)
Béatrice Cochener-Lamard (France)
losartan in drop form may prove useful in preventing and treating myofibroblast-induced corneal scarring. Findings from early clinical experience using topical losartan are encouraging, with case reports involving eyes with scarring as a complication of corneal surgery or microbial keratitis.
Looking a little further down the pipeline, we report on novel tissue engineering approaches capable of ushering in a revolution that will enable the management of difficult cases of corneal blindness. David Myung MD, PhD and colleagues are developing several technologies that hold promise as alternatives to human graft tissue, including next-generation keratoprostheses, xenotransplant and human donor tissue engineering products, new methods for endothelial cell delivery, 3D bioprinting and engineered donor buttons, and in situ-forming gel keratoplasty.
Research underway in Dr Myung’s laboratory encompasses several of these approaches, including 3D bioprinting, which he said could address the severe graft shortage by allowing scalable and precision tissue manufacturing.
We also update the regulatory issues in Europe that threaten to impede clinical trials of innovative agents in ophthalmology, including those developed for treating corneal diseases. While the latest cell and gene therapies have the potential to save sight and dramatically improve the lives of patients, proposed changes to the regulatory framework for advanced therapy medicinal products (ATMPs) in Europe could potentially hinder innovation and limit access to new treatments for patients, according to Mor Dickman MD, PhD, professor of ophthalmology at Maastricht University, Netherlands. He explains both sides of a debate surrounding a proposed revision of the EU general pharmaceutical legislation currently under discussion.


 Thomas Kohnen Chief Medical Editor
Thomas Kohnen Chief Medical Editor
Oliver Findl (Austria)
Nino Hirnschall (Austria)
Soosan Jacob (India)
Vikentia Katsanevaki (Greece)
Daniel Kook (Germany)
Boris Malyugin (Russia)
Marguerite McDonald (US)
Cyres Mehta (India)
Sorcha Ní Dhubhghaill (Ireland)
Rudy Nuijts (The Netherlands)
Leigh Spielberg (The Netherlands)
Sathish Srinivasan (UK)
Robert Stegmann (South Africa)
Ulf Stenevi (Sweden)
Marie-José Tassignon (Belgium)
Manfred Tetz (Germany)
Carlo Enrico Traverso (Italy)
EDITORIAL
4
José Güell Medical Editor
EUROTIMES | APRIL 2024
Paul Rosen Medical Editor
ESCRS IN A SNAPSHOT

We are a society of surgeons who specialise in improving vision and restoring clarity. Since 1991, ESCRS has promoted the education and research of implant and refractive surgery. With more than 7,500 members from 130 countries worldwide, ESCRS is a vital global platform for the field of ophthalmology.
With Eyes on Its Future, ESCRS Celebrates Its Past
Winter Meeting offers opportunities to experiment with new concepts and formats.
BY STUART HALES, EXECUTIVE EDITOR
As expected, education took centre stage at the 2024 ESCRS Winter Meeting in Frankfurt, Germany, with sessions on topics ranging from strategies and techniques for IOL exchange to future applications of femtosecond lasers. But away from the presentation halls and meeting rooms, new learning and networking concepts were being tested, ESCRS leaders were attending a workshop on implicit bias, and veteran ophthalmologists were sitting for interviews that explored the Society’s heritage and especially its roots in intraocular implantation surgery.
The new concepts were less structured and more intimate than traditional conference sessions. For example, “World Café” roundtable discussions allowed attendees to talk informally with experts about dysphotopsia and glare, making decisions during refractive surgery, and dealing with low astigmatism. “Unconference” presentations were 15-minute talks that addressed topics such as performing good and safe cataract surgery with fewer materials, the psychological nature of functional vision, understanding unconscious biases, and enhancing patient satisfaction through evidence-based strategies.
Meanwhile, EuroTimes editor-in-chief Sean Henahan sat down with former ESCRS President Thomas Neuhann, Richard Packard, and Hans-Reinhard Koch to discuss the development of the intraocular lens and their respective roles in witnessing and furthering its acceptance. The interview was part of a larger project highlighting the rich history of ESCRS and the Society’s contributions to ophthalmology.
Reaching new audiences
The juxtaposition of familiar and innovative programming created an atmosphere of calculated innovation, a sense that
ESCRS has a tried-and-true formula that has served it well but wants to explore new ideas and appeal to new audiences. Increasingly, these audiences are global in terms of both location and mindset.
“The vision of ESCRS encompasses both expansion and preservation,” said ESCRS President Filomena Ribeiro MD. “We are embracing new audiences while upholding our European heritage and fostering a global community united in scientific advancement.”
The global emphasis was evidenced by the presence of the Brazilian Association of Cataract and Refractive Surgery (BRASCRS) in the exhibition hall. Dr Ribeiro will lead an ESCRS delegation to Rio de Janeiro in late May to present a session at the 2024 BRASCRS Congress of Cataract and Refractive Surgery. Other scheduled destinations for ESCRS delegation visits in 2024 include Indonesia, China, Canada, and South Africa.
While most Winter Meeting attendees hailed from Germany—the event was held in cooperation with the German Society for Cataract & Refractive Surgery— more than 70 countries were represented, from as far away as South America (Chile and Brazil), Asia (Indonesia and China), Western Africa (Côte d’Ivoire), and Australasia. Notwithstanding their geographic and cultural diversity, attendees found common cause in their desire for professional development.
Of particular interest were hands-on training opportunities such as the wet labs, which addressed procedures such as iris reconstruction, suturing techniques, and basic phacoemulsification. The Winter Meeting’s 12 wet labs drew 120 participants, mostly trainees and residents eager to learn new techniques but also some more experienced surgeons interested in honing their skills.
Another popular attraction at the Winter Meeting was the moving




6 INSIDE ESCRS EUROTIMES | APRIL 2024
simulator, an ESCRS project launched in 2023 that sends a surgical simulator to European countries with limited access to such equipment and provides opportunities for students to schedule up to 8 hours to conduct an ESCRS-developed curriculum. The simulator was available in the exhibition hall during the Winter Meeting and was occupied continually by students wanting to develop their skills.
Engaging younger professionals
The Winter Meeting also reinforced the Society’s commitment to diversity and inclusion and to sustainable surgical practices. More than one-third (36%) of presenters and panellists were women, and ESCRS aims to increase that share to 40% at future meetings. Meanwhile, the ESCRS booth in the exhibition hall included a display promoting SIDICS (Sustainability Index for Disposables in Cataract Surgery), a tool for evaluating the sustainability of customised cataract packs used by hospitals and surgical centres. Attendees who visited the display and listened to a presentation about SIDICS received an ESCRS-branded surgical cap.
Also available in the exhibition hall were guided tours led by members of the ESCRS Executive Board. The tours allowed first-time attendees to gain insights and knowledge from veteran ophthalmologists while exploring the latest advancements from industry vendors.
With its new approaches to education and networking and its nod to sustainability, the Winter Meeting offered several opportunities to engage younger professionals and invite them to join the ESCRS community. The upcoming Annual Congress in Barcelona will allow ESCRS to try these concepts on a larger scale and perhaps debut more innovations.
“Let’s ignite curiosity with novel approaches and entice younger audiences to return eagerly,” Dr Ribeiro said. “May our endeavours beckon them to Barcelona this September.”

ESCRS Update
ESCRS Voices Concerns about Health Data Regulation
ESCRS has joined 30-plus large health stakeholder organisations to share concerns about a proposed regulation governing European health data.
While they agree with the overall goals of the proposal to make European health systems function more efficiently, produce better health outcomes, and support public health research and innovation, the organisations are concerned that some fundamental issues have not been addressed properly. These issues include—
• the interaction between the European Health Data Space (EHDS) and other legal frameworks such as the GDPR;
• avoiding an opt-in mechanism and incorporating only an opt-out mechanism provided it does not lead to inconsistent implementation, increased health data disparities, or excessive administrative burdens; and
• the need to avoid excessive data localisation and international health data transfer restrictions that go beyond the requirements of the GDPR’s framework.
“The European healthcare ecosystem is concerned that the draft text of the EHDS does not provide the necessary degree of legal certainty and consistency with the existing regulatory frameworks,” the organisations stated. “Therefore, we call on the EU institutions to leverage the expertise of the healthcare ecosystem and take the necessary time to create the EHDS that makes the most of the potential of digital health to provide high-quality healthcare for all in the EU.”
The EHDS proposal currently is being negotiated by the Council of Europe, the European Commission, and the EU parliament, all of which want to finalize the regulation before the end of the current political term.
ESCRS Leader Receives Honour at Winter Meeting
Dr Burkhard Dick, chair of ophthalmology at the Ruhr University Bochum and secretary of ESCRS, was recently awarded the prestigious Science Prize of the German Society of Cataract and Refractive Surgery (DGII).
The Science Prize, supported by Hoya, honours innovators and surgeons who have contributed to progress in ophthalmology to an extraordinary degree. The prize was presented during the 2024 DGII Congress, which was held in conjunction with the ESCRS Winter Meeting in Frankfurt.
Dr Dick is widely regarded as a pioneer of femtosecond laser cataract surgery. He is the author or co-author of more than 350 peer-reviewed papers and the author/editor of several leading textbooks, including Femtosecond Laser Surgery in Ophthalmology and Cataract Surgery: Advanced Techniques for Complex and Complicated Cases. He has held the chair of ophthalmology at the Ruhr University Bochum since 2006.
The Hoya Science Prize comes with an amount of €5,000, which Dr Dick announced he will donate to Doctors without Borders.
7 2024 APRIL | EUROTIMES









8 EUROTIMES | APRIL 2024

Need a quick introduction or refresher about a surgical procedure? Have a tip to share about a technique or approach you use that makes surgery easier?
The ESCRS 100 is the place to go. It’s a library of short (roughly 100 seconds), high-quality instructional videos about all fields of cataract and refractive surgery.
More than a dozen videos have already been created, and additional videos are being uploaded each month. Current videos include the following topics:
• Secondary IOL implantation
• Extracapsular cataract extraction
• Scleral fixation
• Single-thread, single-knot sewing machine suture for iridodialysis repair
MAKE EVERY SECOND COUNT
—PUT THE ESCRS 100 VIDEO SERIES ON YOUR LIST OF MUST-WATCH EDUCATIONAL RESOURCES ! ESCRS 100
9 2024 APRIL | EUROTIMES
HTTPS://WWW.ESCRS.ORG/EDUCATION/EDUCATIONAL-RESOURCES/ESCRS-100/

10 EUROTIMES | APRIL 2024 COVER ARTICLE
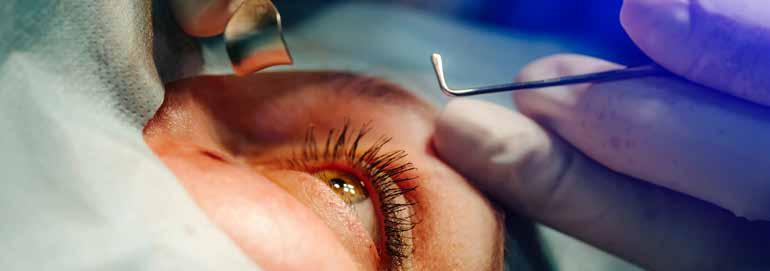
From Lab to Life: Corneal Repair Goes Cellular
Long-awaited cellular therapies for corneal endothelial disease enter the clinic.
BY DERMOT MCGRATH

Cellular therapies for corneal endothelial disease— once limited to the realm of laboratory studies and experimental research—are taking the first steps into clinical practice. Following successful initial trials, ce llbased therapies carry the promise of greater sustainability and a treatment accessible to general ophthalmologists with the potential to alleviate the growing demand for corneal transplantation.
Several companies are at the forefront of the new frontier in tissue regeneration. Aurion Biotech has already received regulatory approval in Japan for its cellular injection therapy for bullous keratopathy and is pursuing phase 1/2 studies in the United States. Emmecell has successfully completed phase 1 studies in the United States to treat corneal oedema secondary to corneal endothelial dysfunction.
Another company, Cellusion Inc, has partnered with Keio University in Tokyo, Japan, in the first trials of induced pluripotent cell-derived corneal endothelial cell substitutes for bullous keratopathy in three participants. The technology has just been granted orphan drug designation by the United States Food and Drug Administration (FDA) ahead of further clinical trials. Numerous research projects are also underway worldwide to develop cellular treatments that could mitigate or eliminate the need for corneal transplantation to treat a range of endothelial diseases.
Much of the pioneering research in utilizing cultured human corneal endothelial cells (HCEC) for transplantation has been conducted by Professor Shigeru Kinoshita’s group at Kyoto Prefectural University of Medicine, Kyoto, Japan. Although primary CECs have a limited capacity for in vitro expansion, Prof Kinoshita’s breakthrough innovation uses a Rho-kinase inhibitor to promote cell proliferation and enable the injection of mature-differentiated endothelial cells derived from young donor allogeneic cells directly into the anterior chamber.
With this technique, one donor cornea theoretically makes it possible to create enough CHCECs for 300 eyes or more. To hasten its clinical and commercial development, Prof Kinoshita licensed the cell-injection technology to US company Aurion Biotech, which subsequently carried out further trials in Japan and El Salvador, confirming the therapy’s safety and efficacy. In early 2023, Aurion obtained regulatory approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) for bullous keratopathy treatment—the first-ever regulatory approval for an allogeneic cell therapy to treat corneal endothelial disease.
Aurion also began phase 1/2 trials in the United States in autumn 2023, aiming to recruit 100 patients with corneal oedema secondary to corneal endothelial dysfunction, with study completion expected before the end of 2025. Overall, the CHCEC injection procedure has now been carried out worldwide in more than 130 patients for a variety of indications—including Fuchs’ endothelial corneal dystrophy (FECD), pseudophakic bullous keratopathy (BK), and corneal endothelial failure after penetrating keratoplasty—with patients experiencing consistent, clinically meaningful, and sustained improvements in key measures of corneal health, according to investigators associated with the trials.
11 2024 APRIL | EUROTIMES
“I have been privileged to examine the eyes of some of Professor Kinoshita’s patients, and their corneas and cell counts are amazing,” said Edward Holland MD, Director of Cornea at the Cincinnati Eye Institute and Professor of Ophthalmology at the University of Cincinnati. Dr Holland, who serves as Chair of the Medical Advisory Board at Aurion Biotech, called the therapy “a truly once-in-a-lifetime occurrence” and said he was optimistic about it becoming the standard of care and expanding the ability to treat many more patients who suffer from corneal endothelial diseases.
Core advantages
Dr Holland cited several core advantages of corneal endothelial cell therapy (CECT) compared to current endothelial keratoplasty (EK) techniques.
“This is a less invasive procedure for the patient,” he explained. “We make a small incision to ‘polish off’ diseased endothelial cells, then inject healthy culture endothelial cells into the anterior chamber. The patient lies face down for a few hours to allow the cells to settle into place and adhere to Descemet’s membrane. The patient is then able to resume normal activities of daily living. Vision begins to return quickly. This procedure eliminates the requirement of positioning the patient on their back for 24 to 48 hours, which is quite uncomfortable and sometimes not possible for many patients.”
CECT also eliminates the problem of EK detachment, which still occurs at a significant rate and results in another procedure for patients and risk of endothelial cell loss, Dr Holland pointed out.
“Studies show the postoperative endothelial cell count will be significantly higher with CECT compared to EK surgery, likely leading to a longer success rate and reduction in the need for additional endothelial replacement.”
The results also seem sustainable in the long term. In 2021, Prof Kinoshita’s group published the five-year outcomes of the first 11 patients treated in Japan for endothelial failure, reporting no serious adverse events and significant, lasting improvements in central corneal thickness and best-corrected visual acuity.
Donor shortages
One of the most compelling arguments in favour of cellular injection therapy is the implications it holds for the global shortage of donor corneas. Corneal blindness is the second most common cause of blindness worldwide, with treatment limited by the number of donors available for transplant.
The shortage of corneas results in more than 40,000 visually impaired people waiting for corneal transplants every year in Europe alone, with 10 million untreated corneal blindness patients globally and 1.5 million new cases of corneal blindness annually.
Although CECT does not completely obviate the need for donor tissue, Dr Holland believes it may still go a long way towards easing the problem associated with donor shortages.
“A recent JAMA Ophthalmology survey indicated that for every 70 diseased eyes, there is only one donor corneal available for transplant,” he said. “With corneal endothelial

cell therapy, we can potentially produce 1,000 doses or more of fully differentiated human corneal endothelial cells from a single donor. For the first time ever, we have the means to produce enough cells to treat everyone with this disease.”
Dearth of skilled surgeons
According to Ellen Koo MD, Associate Professor of Ophthalmology at Bascom Palmer Eye Institute, University of Miami, and Principal Investigator of Emmecell’s clinical trial of injected magnetic human corneal endothelial cells, “the shortage of donor corneas globally is due, in part, to lack of eye-banking access in emerging countries. Additionally, there are not enough skilled corneal transplant surgeons in many parts of the world, and effective and wide-scale skills-transfer of keratoplasty surgical techniques remains an ongoing challenge.”
Emmecell’s twist on the injection therapy is to add biocompatible magnetic nanoparticles to cultured HCECs so the cells become magnetic. The treatment includes an external magnetic eye patch worn by the patient right after magnetic HCEC injection to facilitate cell adhesion and integration into the recipient cornea.
“One donor cornea can offer treatment for hundreds of patients, and the general ophthalmologist may be able to provide this treatment without needing to take the patient to the operating room,” Dr Koo said. “Additionally, the patient may be treated at earlier disease stages.”
Emmecell’s six-month safety and tolerability outcomes for 21 patients with corneal oedema secondary to Fuchs’ endothelial corneal dystrophy were recently reported in
12 COVER ARTICLE EUROTIMES | APRIL 2024


trials at six US centres. Patients received magnetic nanoparticles in one of four doses: 50,000; 150,000; 500,000; or 1,000,000 cells via a single intracameral injection. Some patients received concurrent Descemet stripping or endothelial brushing right before injection, while others were in the injection-only group.
The patient is then able to resume normal activities of daily living. Vision begins to return quickly.
“There were no investigational treatment-related adverse events, nor did we observe issues related to intraocular inflammation or increased intraocular pressure,” said Dr Koo. “Of note, we saw a dose-dependent effect on the continued improvement in vision—up to six months after injection. None of the subjects required surgery in the six-month follow-up period. We are also the first group to show that injection of endothelial cells without surgical intervention can improve vision in subjects with Fuchs’ endothelial corneal dystrophy.”

13 2024 APRIL | EUROTIMES
BIOMECHANICS MEETS TOMOGRAPHY HEY CORVIS ST I just took a look at the tomography. These values call for caution. I don’t think I would operate. HI PENTACAM The biomechanics looks good, though. The cornea is very stable. I don’t see any problem with operating. O.K. TOGETHER NOW Tomography and corneal biomechanics together make the decision easier: Surgery could be an option. Corvis® ST meets Pentacam®: Combined measurement results for a safe decision on surgery Benefit from the combination of biomechanical data from the Corvis® ST and tomographic data from the Pentacam®. Provide surgical care to more patients safely! Follow us! www.oculus.de www.corneal-biomechanics.com Eurotimes ESCRS-Motiv Biomechanics 178x130 e 01.24.indd 1 26.01.2024 18:33:23
Internal use
Non-surgical Treatment for Corneal Endothelial Disease
In-office Injection Using Magnetic Cell Delivery





As the next step in the trial, a randomized, double-masked study has commenced at 10 centres in the United States to establish the optimal therapeutic dose.
The European angle
With Japan and the United States setting the pace in cellular therapies, the concern is that European patients may have to wait considerably longer to benefit from these groundbreaking treatments.
“It’s not particularly surprising that the companies working on primary cultured corneal endothelial cell therapies are currently mostly active in the United States,” remarked Mor Dickman MD, PhD, professor of ophthalmology at Maastricht University, Netherlands. “The United States is a big market, and the price they can expect for such a product in the United States is higher than in Europe, where the healthcare systems work differently. Also, the regulatory pathway, which was traditionally much more difficult in the United States, has somehow become more straightforward in the United States and more cumbersome in Europe.”
Dr Dickman also noted there are challenges specifically related to Europe in terms of cell therapy application.
“Most of the patients treated in Japan had bullous keratopathy, so they did not have guttae on the Descemet’s membrane. In the protocol employed, Descemet’s membrane was not removed. For those patients with Fuchs’ endothelial dystrophy treated by cell injection, the guttae were still visible on Descemet’s membrane years after the treatment,” he said. “We know the guttae themselves will cause stray light and other

cells are injected into anterior chamber

complications, [which] is particularly relevant in [a European] context, where between 70% to 80% of our transplants are for Fuchs’ disease.”
Looking further ahead, Dr Dickman said he is excited about the potential of induced pluripotent stem cells (iPSCs) for treating a range of ocular diseases, as they possess self-renewal capabilities, can be derived from adult somatic cells, and can be differentiated into all cell types including CECs.
While many technical and regulatory hurdles remain before bringing iPSC cell therapies into the clinic, Dr Dickman remains guardedly optimistic for the future.
“For iPSCs, we are still mainly in the research and development phase compared to the corneal endothelial cultured primary cells, which are more advanced in terms of technology readiness level,” he said. “The questions for these therapies are more related to regulatory approval in different markets, pricing strategies, and identifying who will benefit most from their application.”
Edward Holland MD is Director of Cornea at the Cincinnati Eye Institute and Professor of Ophthalmology at the University of Cincinnati, Cincinnati, Ohio, United States. edward.holland@uc.edu
Ellen Koo MD is Associate Professor of Ophthalmology at Bascom Palmer Eye Institute, University of Miami, Florida, United States. exk126@med.miami.edu
Mor Dickman MD, PhD is Professor of Ophthalmology at Maastricht University, Netherlands. m.dickman@maastrichtuniversity.nl
14 COVER ARTICLE EUROTIMES | APRIL 2024
1
+
Donor human corneal endothelial cells (HCECs) are combined with magnetic nanoparticles
Magnetic
Injected HCECs rapidly integrate into patient’s endothelial cell layer with application of magnetic eye patch

Balancing Innovation and Safety
Ensuring access to advanced cell therapies amid regulatory overhaul.
The latest cell and gene therapies have the potential to save sight and dramatically improve the lives of patients.
Proposed changes to the regulatory framework for advanced therapy medicinal products (ATMPs) in Europe, however, could potentially hinder innovation and limit access to new treatments for patients, according to Mor Dickman MD, PhD.
ATMPs are medicines for human use based on genes, tissues, or cells. Under the current ATMP Regulation (EC) No 1394/2007, the principle of hospital exemption (HE) allows for the use of an ATMP without a marketing authorization under certain circumstances. This only applies in a hospital setting on a non-routine basis for an individual patient and when no centrally authorized treatment or clinical trial is available.
Revision of the EU general pharmaceutical legislation is currently under discussion, and the fear is the hospital exemption will be abolished or severely restricted.
Proponents of the reform argue academic centres might abuse the hospital exemption to bypass the traditional route for marketing authorization. They further claim the exemption impacts negatively on the attractiveness of the European Union for research and innovation, as commercial partners may be reluctant to commit to Europe if hospitals are able to administer these therapies to patients anyway. They have also highlighted concerns about the availability of data to support the safety and efficacy of treatments approved under the HE, especially in the long term.
Dr Dickman believes, however, that a compromise in the form of a more clearly defined HE might satisfy all parties in the negotiations.
“Opponents of the HE raise concerns about the quality, safety, and efficacy of these treatments, and I totally agree that the HE should stay exceptional,” he said. “I also think the HE should be subjected to the same high levels of quality and
safety as other ATMPs approved in the standard way. Ideally, there also should be a clearly defined pathway to regulate ATMPs under the HE through the European Medicines Agency.”
Although well intentioned, suppressing the HE would have a negative impact on researchers, doctors, and patients.
“It would restrict patient access to treatments, especially in rare disease conditions, which are of limited interest to commercial entities,” Dr Dickman said. “And it will limit research and development activities within academia if there is ultimately no possibility of bringing these treatments to patients outside the expensive commercial domain.”
Balancing the interests of different parties will be challenging but not impossible, he observed.
“I think we can all agree how important it is to ensure that the therapies given to patients are subjected to the most stringent quality and safety standards available. However, we have seen examples of companies starting clinical trials for a promising new therapy, which is then abandoned, often for financial reasons rather than any deficiency of the product itself. And in the end, the patients are left without a treatment,” he said. “So, while we all want Europe to remain a competitive arena for companies to act and to bring new therapies to market, it should not be at the cost of blocking research and development in academia.”
Dr Dickman also highlighted the important role national and international registries could play in collecting reliable data relating to ATMPs. “There is a need to impose data collection in a way that collects long-term data and captures patient-reported outcome measures (PROMs) in an independent platform in collaboration with the industry,” he concluded.
15 2024 APRIL | EUROTIMES
Mor Dickman MD, PhD is Professor of Ophthalmology at Maastricht University, Netherlands. m.dickman@maastrichtuniversity.nl
DERMOT MCGRATH REPORTS

Following the New Generation
EDOF IOLs an option for eyes with mild comorbidities, showing potential in mini-monovision strategies.
Modern refractive extended depth of focus (EDOF) intraocular lenses (IOLs) induce fewer dysphotopsias than older diffractive IOLs and appear highly suitable for mini-monovision approaches to presbyopia treatment, said Francesco Carones MD.
The EDOF IOLs currently available include diffractive and refractive varieties: Diffractive options include the AT LARA (Zeiss) and the Symfony (Tecnis), which use echelette technology based on Fresnel principles. Refractive options such as the Clareon® Vivity® (Alcon) and the PureSee (Johnson & Johnson) use wavefront stretching and other power manipulation strategies to expand defocus. And the Mini WELL® (Sifi) provides an extended depth of focus, introducing different and controlled amounts of spherical aberrations (SAs) within 3-mm diameter in the central part of the IOL optic. The newer non-diffractive models provide around 1.75 D of defocus, Dr Carones said.
“The first generation of refractive EDOF IOLs provided more spectacle independence than monofocal with slight dysphotopsia,” Dr Carones said. “The new generation of refractive EDOF lenses provides more in the way of spectacle independence without increasing dysphotopsia.”
Optical bench testing shows the PureSee has a flatter defocus curve from distance to near compared to the Tecnis Eyhance enhanced monofocal or the Tecnis monofocal IOL. Clinical studies confirm those results and show a similar dysphotopsia profile with the Eyhance and PureSee IOLs, with more than 90% either not noticing or only slightly bothered by halos, starbursts, and glare. The two lenses also perform similarly regarding contrast sensitivity.
Because of the low degree of dysphotopsia induced and good contrast sensitivity, non-diffractive EDOF IOLs offer the potential to provide a high degree of spectacle independence to some patients for whom multifocal IOLs would normally be contraindicated. Such patients would include those for
whom night-time dysphotopsia could be a major problem and those with mild ocular comorbidities such as ocular surface abnormalities and early macular changes.
Another potential application of EDOF IOLs is in a mini-monovision strategy, Dr Carones said. He presented the results of a study he and his associates recently conducted involving three groups of 25 patients comparing two mini-monovision strategies—one with an enhanced monofocal and the other with a multifocal IOL. One group underwent bilateral implantation of the Vivity IOL, with one eye targeted to plano and -0.5 D in the other eye. Another group underwent bilateral implantation of the Impress (Hoya) enhanced monofocal IOL with one eye targeted to plano and the other eye to -0.5 D to -0.75 D. The third group underwent implantation of the diffractive hybrid Synergy (Johnson & Johnson) multifocal IOL targeted to plano in both eyes.
The study found 80% of the Vivity and Impress groups said they rarely or never needed reading glasses at 40 to 45 cm, and 100% of both groups said they never needed glasses for reading at intermediate distances (60 to 65 cm). In the multifocal group, 100% said they rarely or never needed spectacles for near, intermediate, or distance vision. Regarding dysphotopsias, the proportion reporting never or rarely seeing halos, light rings, or starbursts in night-time conditions was 80% in the Vivity group, 100% in the Impress group, and 24% in the Synergy group.
Dr Carones presented his paper at the ESCRS eConnect Webinar, “Evidence-based overview of current premium IOL technologies.”
16 EUROTIMES | APRIL 2024
Francesco Carones MD is the medical director and physician CEO of Advalia Vision, Milan, Italy. fcarones@carones.com.
CATARACT & REFRACTIVE
ROIBEÁRD O’HÉINEACHÁIN REPORTS
Reversible Multifocality
Two-lens combination offers low-risk spectacle independence for cataract patients and presbyopes.
ROIBEÁRD O’HÉINEACHÁIN REPORTS
The combination of trifocal supplementary IOL in the ciliary sulcus and monofocal capsular bag IOL is a fully reversible means of providing the same range of focus as standard trifocal IOLs for capsular bag implantation without significant loss of optical quality and no disadvantages due to additional interfaces, said Ramin Khoramnia MD.
“What I really like about the two-lens combination approach is it is currently the only available technique we have when it comes to [achieving] a reversible multifocality,” he said. “The advantage of this approach is that later on, if ever required, we can remove the supplementary IOL from the sulcus—which is certainly much easier than removing the IOL from the capsular bag.”
The two-lens approach first involves implanting a primary monofocal or toric IOL into the capsular bag, then placing a supplementary trifocal IOL in the ciliary sulcus. Unlike the piggyback lenses of the past designed for implantation into the capsular bag, supplementary IOLs don’t touch the primary lens in the capsular bag; therefore, there is no risk of interlenticular opacities or iris chafing.
The supplemental IOLs on the market include the Sulcoflex (Rayner) trifocal IOL, made of a hydrophilic material with good uveal biocompatibility, and the AddOn (1stQ). AddOn’s main advantage is four-point fixation, providing very good rotational stability—especially important with toric supplementary IOLs.
One concern regarding the use of two lenses is the possible optical and light transmission effects of the additional interfaces. Dr Khoramnia and his associates, therefore, conducted an optical bench study comparing a two-lens combination of the Sulcoflex trifocal (IOL703F) and RayOne aspheric (RAO600C) with a one-lens system using the RayOne trifocal IOL
(RAO603F). They found the modular transfer function (MTF) curves of the two-lens and one-lens optical systems matched closely, the Strehl ratios were very similar, and light transmission decreased by 1.3%.
The reversibility of supplementary trifocal IOLs means they are suitable for patients who desire multifocality but have minor ocular abnormalities or are young with a less predictable profile regarding future ocular pathology.
As an illustration, he presented his findings from a study involving 25 cataract and refractive lens exchange patients who underwent the Duet procedure. The motivations for the choice of a reversible procedure included patients aged 18 to 38 years, elevated risk of deviation from the target refraction, subtle morphologic changes without a clear contraindication for a trifocal optic, and borderline binocular function.
“Despite the fact that we had certain reasons to offer a reversible multifocality in these patients, we never had to remove a trifocal supplementary IOL in this series,” Dr Khoramnia said. “And we achieved excellent visual acuity in the far intermediate, and near 0.2 logMAR or better was achieved up to almost 3.75 D of defocus.”
Dr Khoramnia presented his paper at an ESCRS eConnect Webinar, “Evidence-based overview of current premium IOL technologies.”
Ramin Khoramnia MD, FEBO is a surgeon based at the International Vision Correction Research Centre (IVRC), a laboratory leader at The David J Apple International Laboratory for Ocular Pathology, and a professor in the department of ophthalmology, Ruprecht-Karls University of Heidelberg, Germany. Ramin.khoramnia@meduni-heidelberg.de
17 2024 APRIL | EUROTIMES

CAPSULaser Poised to Lead
Advanced procedure may outweigh FLACS as the next choice for capsulotomy.
TIMOTHY NORRIS REPORTS
As femtosecond laser-assisted cataract surgery (FLACS) is struggling to penetrate the cataract surgery operating rooms (ORs) to match the success of traditional manual capsulotomy and ultrasound phacoemulsification, many surgeons worldwide are walking away from the procedure in favour of a more precise and less costly solution.
CAPSULaser capsulotomy—also known as selective laser capsulotomy (SLC)—is a fast, precise, and cost-effective procedure, sitting in the middle between FLACS and manual while taking the best of both worlds in precision, safety, and OR logistics.
“For this procedure, we use a device called CAPSULaser, a 590±3 nm 100% continuous wave orange laser, and proprietary 0.4% trypan blue solution for the staining of the capsule,” said Pavel Stodůlka MD, PhD.
“Using the trypan blue stained capsulotomy as a target, this laser creates a perfectly round capsulotomy that can be sized between 4.0 and 5.5 millimetres with a 0.1-millimetre increment,” said Richard Packard MD. “This procedure not only creates a circular capsulotomy, but also stronger edges and a better centration compared to femtosecond laser techniques, with less risk of early PCO.”
He added this procedure shows a high safety and efficacy profile with low downsides—the major one being the time needed for trypan blue to stain the capsule, which he said may add 30 seconds to the procedure. “On the other hand, CAPSULaser takes a quarter a fraction of a second to perform a capsulotomy,” Dr Packard observed. “And although it is a thermal laser, it uses a low amount of energy immediately absorbed by the trypan blue without any risk of damaging the retina. Moreover, it works very well with intumescent cataracts, often a problem for FLACS or manual capsulotomy.”
“Several studies also put in doubt the benefits of FLACS compared to manual capsulotomy, but as a laser guy who has annually performed thousands of FLACS in the clinical practice for more than a decade, I don’t really believe this is true,” Dr Stodůlka said. “With a proper setup at high volume, clinic femtosecond lasers do add the safety and efficacy we need. However, you can easily achieve even better results with CAPSULaser, because it is faster and easier to perform with no need for additional manipulation and better precision in terms of centration. Therefore, CAPSULaser capsulotomy became the option of choice at our clinic, and I typically start my cataract surgery day with this procedure.”
Dr Stodůlka noted this technology also comes with lesser costs per procedure, making it a sensible option. FLACS, he said, is “way too overpriced per ‘click’ fee,” as the device is expensive, with a similarly expensive service contract, and requires a dedicated OR with strict temperature and humidity control.
“It is overall a huge upfront and continuous investment, and I think this could be the main reason why many pioneers who started with FLACS eventually walked away, and some switched to CAPSULaser,” he said.
Not only is CAPSULaser more cost effective, but it is still showing some new, previously unseen features. Eighteen months ago, the renowned ophthalmologists noticed the staining of the capsule was not homogenous, instead showing a 2-to-4-millimetre dark blue central area with an enhanced staining coincident with the coaxial Purkinje reflex. “So, if you centre your capsulotomy according to this dark spot, you will have not only 360-degree coverage of the IOL optic, but it will also be completely symmetrical. This is something unique to this technology,” they said in a joint statement.
18 EUROTIMES | APRIL 2024 CATARACT & REFRACTIVE
“One of the most important steps we achieved (that I pushed hard for) was something no one, not even the company, believed in,” Dr Stodůlka said. “We spent two years trying to develop the best possible patient interface, which I always said we could totally bypass, something I was told was impossible. We achieved that, and now we can do a CAPSULaser capsulotomy without the use of an interface. You fire the laser straight on the naked eye, making it even faster, and that’s the best.”
CAPSULaser definitely has its recognition, and currently there is more demand that the company can supply.
Despite the excellent results, CAPSULaser is still in its early days, commercially speaking.
“The device has been incrementally improved, and we are at the stage where we feel it is right to be commercial ised,” Dr Packard said. “However, it is still produced by a start-up, and until a major company steps in—able to commercialise it around the world in huge numbers with marketers and trained staff involved—things will still move slowly.”
“We have early adopters, and there are more than 100 users in over 20 countries, spanning from Europe through the Middle East, South America, and all the way to Australia,” Dr Stodůlka said.
“They are happy, and the number of requests is steadily increasing. So, CAPSULaser definitely has its recognition, and currently there is more demand that the company can supply.”
Dr Packard stated it will eventually reach a broader mar ket. “It has been gradually rolled out, with a steady increase of orders. The number of devices circulating has mushroomed in a very short period of time, and I am sure someone is keep ing an eye on this progress.”
Drs Stodůlka and Packard spoke at the 2024 ESCRS Winter Meeting in Frankfurt.
Richard Packard MD, FRCS,
•
•
•
•
•
eyequack@vossnet.co.uk
Pavel Stodůlka PhD, FEBOS-CR is founder, chief surgeon, and CEO of Gemini Eye Clinics in the Czech Republic and Vienna, Austria. stodulka@lasik.cz





19 2024 APRIL | EUROTIMES
FRCOphth is a pioneer of phacoemulsification and a leader in the move to microincision cataract surgery.
Financial Disclosure: Both Packard and Stodůlka hold shares of the company CAPSULaser. Non Contact Tono/Pachymeter Non Contact Tonometer / New Design Innovations that Incorporate Operator and Patient Comfort with Gentle Measurements
Fully-automatic
measurement*1
Gentle voice guidance (available in 9 languages)*1
Reliable tono/pachymeter*2
Flexible and space-saving design
*1 Available for the NT-1p and NT-1 *2 Pachymetry is available for the NT-1p. For the NT-1, the corrected IOP is displayed by entering the patient‘s central corneal thickness. www.nidek.com ET 93 x 266mm
A variety of options to meet your needs
Refocus on Multifocals
Trifocal IOLs continue to improve as consensus grows regarding indications and contraindications.
ROIBEÁRD O’HÉINEACHÁIN REPORTS
Technological improvements in lens design have improved outcomes with multifocal intraocular lenses (IOLs) in terms of spectacle independence, but patient selection lies at the core of their successful use, Orkun Müftüoğlu MD told an ESCRS webinar.
“You need to choose the lens that serves your patient best,” he said. “Discussion is the most important part of the surgery.”
Professor Müftüoğlu noted that a meta-analysis of peer-reviewed publications of studies of multifocal intraocular lenses following cataract and refractive lens exchange involving 8,797 eyes showed 80% of patients achieved spectacle independence for distance and near vision. In more recent studies with the newer trifocal IOLs, that figure has been closer to 100%. Patient satisfaction has ranged from 60% to 100%, with most of the recent studies having close to 100% satisfaction rates.
When assessing patients’ suitability for a trifocal IOL, the surgeon must ensure their expectations are realistic and they understand they may need reading glasses, he emphasised. In addition, the surgeon must assess, based on lifestyle and profession, whether their need for quality of vision exceeds their desire for spectacle independence.
In a consensus statement, the European School for Advanced Studies in Ophthalmology (ESASO) issued key recommendations for employing a diffractive multifocal IOL. They include a potential postoperative visual acuity better than 0.5, keratometry between 40.0 D and 45.0 D, a pupil larger than 2.8 mm under photopic conditions, and root mean square (RMS) of higher order corneal aberration less than 0.5 µ for a 6.0 mm pupil. The statement also recommends considering monofocal or non-diffractive IOLs for patients with coexisting eye disorders.
Prof Müftüoğlu noted that patients tolerate ametropia less well with multifocal IOLs than with EDOF IOLs or monofocal IOLs. Therefore, biometry with modern tools such as sweptsource optical coherence tomography and fourth-generation IOL calculation formulas is essential. In addition, treatment of astigmatism when present is mandatory and should be less than 0.5 D cylinder.
Following trifocal IOL implantation, it can take several months for patients to achieve final postoperative visual acuity. They must first undergo a period of perceptual learning to adapt to the multifocal optics, after which it appears to become second nature, he noted. A functional magnetic resonance imaging study investigating neuroadaptation to multifocal IOLs showed that early in the neuroadaptation phase, patients had increased activity in the parts of the brain associated with visual attention, goal-directed behaviours, procedural learning, and effortful cognitive control. However, by the sixth month of follow-up, that activity had returned to baseline.
He noted the rate of IOL exchange due to neuroadaptation failure is less than 1%, and that rate has continued to go down with the advent of more advanced diffractive multifocal IOLs. However, IOL exchange does not always fix the problem, with 23% of patients reporting dissatisfaction with the results and most saying they would not have undergone the exchange if they had it to do over again.
Prof Müftüoğlu presented his paper at an ESCRS eConnect Webinar, “Evidence-based overview of current premium IOL technologies.”
Orkun Müftüoğlu MD, FEBO is based at Koc University School of Medicine, Istanbul, Turkey. orkun.muftuoglu@gmail.com

20 EUROTIMES | APRIL 2024 CATARACT & REFRACTIVE
Common Myths in Presbyopia Correction
Patient education key to satisfaction with refractive IOLs.
DERMOT MCGRATH REPORTS
Patient education and clear communication allied to rigorous surgery hold the key to delivering consistent visual outcomes with a high level of patient satisfaction in presbyopia-correcting intraocular lenses, according to Florian Kretz MD.
“The ESCRS clinical research survey showed that 59% of patients are not well educated or know nothing at all about refractive IOL options. Those are the patients we all need to target, and this is where many of our problems stem from,” he explained. “The key is to tailor the information we provide so the patients can understand and use it. We need to encourage them to ask questions about the possibilities of the technology and clarify any misconceptions they might have concerning refractive IOLs.”
Dr Kretz said it was vital to invest time to build empathy and develop a good relationship with the patient. “It is very important to understand the patient’s needs, lifestyle, hobbies, visual tasks, desire for spectacle independence, aversion to photic phenomena, and any comorbidities.”
Discussing some common myths in terms of patient communication regarding refractive IOLs, Dr Kretz said it was a mistake to try to present the patient with as many IOL options as possible.
“While it’s important to inform patients about their available options, do we really need to tell them about every optical design, or is it more important to discuss what their individual needs are, and then use this information to help guide them in their choice of lens?” he said.
He stressed informed consent as a critical part of the preoperative consultation. “The patient can tell you their needs, but you need to explain the risks involved in specific choices. You need to be able to make a confident recommendation to them and explain the rationale behind your recommendation,” he said.
Dr Kretz also advised against promising perfect results.
“There is no perfect result in optics because everything is a compromise. We have to set very realistic expectations for our patients—and that is dependent on the optic and the eye itself,” he said. “We can talk about depth of field, visual quality, and dysphotopsias, but the main message in lens surgery is to under-promise and try to over deliver.”
On discussing costs with the patient for the refractive IOL, Dr Kretz said matching prices with local competition is not necessarily the most effective strategy.
“It may be useful in the beginning. However, the most important thing is to give the patient a cost-benefit analysis, using simple examples from daily life where they may pay extra for particular products. And presbyopia-correcting IOLs

do give patients a real benefit, so there is justification for the co-payment to patients.”
Dr Kretz said his own policy is not to reimburse patients in the event of an unsatisfactory outcome.
“I never give refunds to my patients because we perform surgery and we have counselled the patients extensively beforehand,” he said. “I believe it is very important to understand why patients are unhappy. Most of them have visual complaints about the refractive outcomes, or some of them may have physical discomfort with dry eye or stinging sensations from eye drops. But these are all issues that can be detected in the preoperative evaluation, and the patient can be advised what to expect. And most of those issues can be resolved with further treatments.”
The essential principle is to convey to the patient they will be taken care of and there is a solution available to resolve their problem, he added.
For patients with residual refractive error after surgery, Dr Kretz said his preferred approach is to prescribe glasses in the initial phase and then consider other possible treatments.
“We also have very good options with laser correction for lower power errors, piggyback IOLs, and, in the worst case, an IOL exchange. But with presbyopia-correcting IOLs, patients may be happy to use glasses just for distance vision,” he concluded.
Dr Kretz presented at the Independent Medical Education (IME) symposium at the ESCRS Winter Meeting in Frankfurt.
21 2024 APRIL | EUROTIMES
Florian Kretz MD, FEBO is medical director of the Augentagesklinik Rheine in Rheine, Germany. Mail@Florian-Kretz.de
Managing a Cataract Surgery Refractive Miss
Weighing the pros and cons of options for intraocular intervention.
CHERYL GUTTMAN KRADER REPORTS
Despite advances in techniques and technologies used to calculate IOL power for patients undergoing cataract surgery, cases of significant residual refractive error still occur. Multiple management options exist, and multiple factors influence the decision about which to choose.
When it comes to cases involving a moderate refractive miss, Zaina Al-Mohtaseb MD believes lens exchange is preferred—particularly for US surgeons—while William F Wiley MD says there are many reasons to implant a piggyback secondary lens instead.
Dr Al-Mohtaseb acknowledged that arguments can be made favouring piggyback IOLs based on better refractive outcomes and lower risk for intraoperative complications.
“However, it is important to note that a lot of the refractive outcome data are from studies done outside the US, where there are sulcus-based IOLs that may be safer and more stable than the options available in the US,” she said.
“And there are ways to decrease the risk of complications when performing IOL exchanges, especially by making sure to place the new lens in the bag or capture the optic in the capsulorhexis. There is a reasonably high risk for IOL decentration if an IOL is placed in the sulcus without optic capture.”
To optimise success with IOL exchange, Dr Al-Mohtaseb suggested waiting three months for refractive stability, using plenty of ophthalmic viscosurgical devices, and taking extreme care manipulating the IOL to avoid iatrogenic zonular loss.
She noted iris capture, chafing, and pigment release with the potential for pupillary block and pigmentary glaucoma can occur with piggybacked IOLs. Furthermore, although studies show good refractive outcomes after piggyback IOL implantation, there can still be a refractive surprise.
“Imagine if a patient is already upset because of a refractive miss after the initial surgery and winds up with the opposite refractive error after undergoing a piggyback IOL procedure,” Dr Al-Mohtaseb said.
The case for piggybacking
Dr Wiley observed numerous study results support the safety and efficacy of implanting a piggyback IOL to manage cases of residual refractive error after cataract surgery. Perhaps the most compelling argument for choosing this approach versus performing an IOL exchange relates to the greater overall ease of implanting a secondary lens, he said.
“Anyone who has done an IOL exchange will know that they are likely to lose sleep the night before one of these cases, whereas you can expect to go home early when the surgical schedule has a piggyback IOL case because the procedure is that easy. For me, a piggyback IOL is the easiest case I can do in a month, but an IOL exchange could be the hardest case I will do all year.”
He outlined several issues to consider for guiding the approach to managing a refractive miss. The length of time
22 EUROTIMES | APRIL 2024 CATARACT & REFRACTIVE
elapsed since the initial surgery is one factor. If the primary surgery was done more than 12 months earlier, a piggyback IOL should be the obvious choice since returning to the capsular bag after such a long interval carries the risk for a variety of complications, including cystoid macular oedema, IOL decentration, and vitreous loss.
The presence of comorbidities that make IOL exchange more complicated, such as pseudoexfoliation or YAG capsulotomy, also favours a piggyback procedure.
Dr Wiley considers eyes with a history of refractive surgery as “go-to” cases for implanting a piggyback IOL.
“These eyes had an elevated risk for a refractive miss in the initial surgery, and it is hard to accurately correct any residual refractive error because they have epithelial hyperplasia,” he explained.
“Likely, they do not have significant residual astigmatism that needs to be corrected because it was already treated at the time of the laser refractive surgery. Therefore, the lack of piggyback IOLs for toric correction in the US is not an issue in these cases.”
A piggyback IOL is also ideal for patients with a refractive error and complaints of negative dysphotopsias because implanting the second lens in the sulcus will treat both problems.
One situation where Dr Wiley prefers an IOL exchange is if another doctor performs the cataract procedure and the patient complains that the surgeon put in the wrong lens. His reasoning relates to the psychological reaction a patient may have to the idea of undergoing a procedure that does not remove the “wrong lens.”
Dr Wiley’s preferred IOL for piggybacking is the threepiece acrylic AR40e (Sensar, Johnson & Johnson Vision) with a rounded edge optic that limits the risk for pigment dispersion. Addressing the potential for interlenticular opacification (ILO) between two acrylic IOLs, Dr Wiley said the problem has primarily been reported in studies of rabbits, which have a brisker inflammatory response than humans—particularly when both IOLs are placed in the capsular bag.
“Furthermore, we have debunked the myth about the risk of ILO with two acrylic IOLs in a retrospective study,” Dr Wiley said. “I think there will not be any problem if one IOL is in the sulcus and the other in the bag.”
Drs Al-Mohtaseb and Wiley spoke at AAO 2023 Refractive Surgery Subspecialty Day in San Francisco, US.































































Zaina Al-Mohtaseb MD is a cataract, refractive, and corneal surgeon and director of research at Whitsett Vision Group and a clinical associate professor of ophthalmology at Baylor College of Medicine, both of Houston, Texas, US. zaina1225@gmail.com
William F Wiley MD is medical director at the Cleveland Eye Clinic, Cleveland, Ohio, US. DrWiley@ClevelandEyeClinic.com

23 2024 APRIL | EUROTIMES
Pearls to Avoid Refractive Surprise
Modern power formulas and keratometry facilitate better outcomes.
DERMOT MCGRATH REPORTS
The risk of refractive surprise after cataract surgery can be significantly reduced by employing a number of clear strategies to hit the target refraction and deliver on patient expectations, according to Basak Bostanci MD.
“There are steps we can take to reduce the risk of refractive error,” she said. “We need to diagnose and manage ocular surface disease before surgery. We need to validate our biometric data and avoid transcription and technical errors. We have to take care of astigmatism and use modern IOL power calculation formulas. For complex eyes, we have to use the IOL power formula best adapted to that particular eye, manage patient expectations, and try to learn from the first eye with staged surgeries.”
Although biometric measurements have advanced considerably in recent years, ocular surface disease (OSD) remains a common cause of incorrect power calculations.
“In cataract surgery, we know 60% of routine cataract patients are asymptomatic dry eye patients, and 50% have central corneal staining,” Dr Bostanci said. “OSD and corneal pathology can lead to errors in keratometry, incorrect identification of the astigmatism axis, and inaccurate IOL power calculations. If a patient has moderate dry eye, epithelial basement membrane dystrophy, or pterygium, these should be addressed not only before surgery, but before any preoperative measurements.”
The surgeon should check the patient’s biometric data carefully before surgery. “Always check the patient data yourself,” she said. “If the patient is hyperopic with an axial length more than 25 millimetres, this should raise a red flag. Some biometers are capable of detecting if the patient is fixating during the measurements, and you can also verify the quality of the measurements by using quality indexes and standard deviations.”
Comparing measurements from different instruments is also useful to confirm data validity, particularly if the two eyes are not highly symmetric, she added.
“If there is a 1.0 D difference between eyes in average K, the cornea is very steep or very flat, or in the presence of a very shallow or very deep anterior chamber, we always recheck the data.”
Correcting the astigmatism is also important to optimise the visual outcome.
“Some studies have shown that more than 42% of the eyes undergoing cataract surgery have more than 1.0 D of astigmatism,” Dr Bostanci noted. “Monofocal IOLs can tolerate up to 1.0 D of cylinder, but for multifocal IOL patients, this threshold is as low as 0.5 D of astigmatism.”
She advised using a combination of devices, if possible, to measure astigmatism.
“The 2023 ESCRS clinical research survey showed that 82% of surgeons use optical biometry as the primary preoperative
measurement for astigmatism. In our clinic, we use keratometry, topography, and optical biometry to get a more complete picture of the astigmatism.”
For the best IOL power calculation formulas to use, Dr Bostanci said surgeons have a wide variety of tools available.
“There is no room for regression formulas anymore. We have to use modern formulas that add new variables, which are better for non-standard eyes,” she said. “It has been shown that many of the recent formulas—such as Barrett Universal II, EVO 2.0, Kane, and Castrop-Rauxel—perform well for most standard eyes. But for eyes with short axial length, the use of Barrett Universal II should be reconsidered.”
Dr Bostanci also advised using tools such as the ESCRS online calculator, which allow surgeons to compare results of many formulas for each patient and then select the optimal IOL power.
For more complex eyes, Dr Bostanci said it was important to counsel the patient fully and avoid bilateral sequential surgery for such cases.
“It is always better to perform two separate surgeries for these complex eyes and to use the result from the first eye to improve the refractive outcome for the second eye,” she concluded.
Dr Bostanci presented at the Independent Medical Education (IME) symposium at the ESCRS Winter Meeting in Frankfurt.
Basak Bostanci MD, FEBO is an ophthalmologist at Bahcesehir University School of Medicine, World Eye Hospital, Istanbul, Turkey. drbbostanci@gmail.com



24 EUROTIMES | APRIL 2024 CATARACT & REFRACTIVE
Our IOL Calculator is now live on the ESCRS website!


Best of Frankfurt ESCRS Winter Meeting 2024
BY SEAN HENAHAN, EDITOR IN CHIEF
The two-hour long “Best of the Best” session on the final day of the ESCRS Winter Meeting in Frankfurt highlighted the wide array of innovative therapies and technical controversies in current cataract, cornea, and refractive surgery practice.
Drs Burkhard Dick, Suphi Taneri, Sorcha Ní Dhubhghaill, and Filomena Ribeiro oversaw 17 panel discussions. The all-star panels included Drs Oliver Findl, Joaquín Fernández, José Güell, Marcus Blum, Gerd Auffarth, Farhad Hafezi, Maya Mueller, Sigfried Priglinger, Claudette Abela-Formanek, Béatrice Cochener-Lamard, Thomas Kohnen, Pavel Stodůlka, Paolo Vinciguerra, Nic Reus, Ramin Khoramnia, Christopher Wirbelauer, and Durval Carvalho Jr.
One panel discussed the continuing evolution of the digital OR, including the use of digital microscopes and 3D systems for cataract surgery. Panellists discussed the potential advantages of these technologies, ranging from the back-saving ergonomic benefits of not having to stoop over the traditional operating microscope to the significant reduction in light required, a positive benefit for patients.
The 3D headset systems now becoming available offer an increasing amount of integration with various imaging systems and patient data. These systems also have incredible potential as teaching tools, where many students can don 3D goggles and watch the surgery as it happens.
Another panel on high tech discussed the current and future state of robotic cataract surgery, with a consensus that robotic-enhanced cataract surgery is not a matter of if but rather when it will become part of everyday practice. The many potential benefits include movement scaling and tremor filtering for enhanced precision, and preplanned paths to help lower the risk of “collateral damage.” Robotic systems also
offer the potential for remote surgery and enable the development of new surgical instruments and methods that are not practical otherwise.
Robotics researcher Marvin Bende described how a remote system might work. Telemetry data extracted from a 3D image, supplemented with additional data sources such as OCT, would be used to adapt a digital representation of the surgical scene to the real-world situation. Inputs from the operator wearing a 3D headset would translate to robotic arm movement. Additional haptic feedback could also be provided to the operator throughout the surgery.
“I expect that in five to ten years, we will have robot-assisted cataract surgery. There will be resistance, but change is coming,” predicted Farhad Hafezi MD.
On the cornea front, panellists discussed various cell therapies now entering the clinic for treating corneal endothelial disease, including Emmecell, a novel way to treat corneal oedema now in the advanced stage of clinical trials. Magnetic cells are injected intracamerally, and then an external magnetic patch delivers the cells to the target location.
Attendees also learned about mitomycin intravascular chemoembolization (MICE), a potential treatment of visually significant corneal neovascularization. This technique is in the very early clinical stages, with promising results reported for a handful of patients. Panellists were interested, but there was some concern about quantity of mitomycin leaking into the circulation.
Other topics featured in the panel discussions included topical losartan for the inhibition of myofibroblasts, AI and keratoconus detection, and AI and phakic IOL implantation. EuroTimes will provide in-depth coverage from the ESCRS Winter Meeting in Frankfurt on these and related topics.
25 2024 APRIL | EUROTIMES
Pharmacotherapy Advances for Ocular Scarring
Early results call for clinical studies investigating topical losartan.
CHERYL GUTTMAN KRADER REPORTS
The antihypertensive agent losartan may prove useful in the prevention and treatment of myofibroblast-induced corneal scarring, animal and early clinical studies suggest.
Steven E Wilson MD has spearheaded research in this area. His interest in investigating the effects of losartan on corneal scarring was sparked by an article he read several years earlier in which researchers studying prevention of aortic aneurysm in a mouse model of Marfan syndrome reported that the angiotensin II receptor blocker blocked ERK-modulated transforming growth factor-β (TGF-β) signalling.
In 2018, with funding from a US Department of Defense grant, Dr Wilson and colleagues conducted a study in rabbits showing that topical losartan (not orally administered) decreased scarring after descemetorhexis without endothelial transplantation. Subsequent studies showed topical losartan was effective for clearing post-PRK late haze, scarring after blast injury, and scarring after alkali burn injury when used with a topical corticosteroid.
“Findings from early clinical experience using topical losartan are very encouraging, as illustrated by case reports involving eyes with scarring as a complication of corneal surgery or microbial keratitis,” he said.
In addition to the above-mentioned conditions, Dr Wilson proposed topical losartan might be effective for preventing corneal scarring following thermal injury or in combination with a topical antimicrobial agent to treat active corneal infections. Other potential uses include treatment of persistent epithelial defects and late haze that can develop after a complicated corneal laser refractive or corneal cross-linking procedure and treating posterior stromal fibrosis after descemetorhexis, DMEK, DSAEK, or herpes simplex endotheliitis.
Dr Wilson emphasised the potential applications involve only myofibroblast-mediated fibrosis.
“Losartan would not be effective for treating corneal haze caused by corneal fibroblasts, such as mild, normal haze after PRK or corneal cross-linking,” he clarified.
Because losartan can penetrate through the entire cornea, Dr Wilson suggested it might also penetrate the conjunctiva and be effective for treating conjunctival fibrotic diseases and improving the long-term success of glaucoma surgeries by treating scarred blebs and encapsulated glaucoma shunts. Incorporated in a sustained release intraocular delivery system, losartan might also be used to treat fibrotic conditions involving the retina (proliferative vitreoretinopathy), choroid, vitreous, and optic nerve.

Recommendations for clinical investigators
Current clinical studies are being conducted using losartan 0.8 mg/mL in BSS—Dr Wilson cautioned higher concentrations should not be investigated until there is preclinical evidence documenting their safety because TGF-β also regulates normal functions in the cornea. He also stated the treatment should be applied six times a day, and it can take four to six months before seeing improvement at the slit lamp, but patients can notice vision improvement earlier. After the fibrosis is cleared, patients should continue losartan for at least four months to allow complete epithelial basement membrane repair and prevent recurrence.
“If used to treat posterior cornea scarring, surgical replacement of Descemet’s membrane will probably be necessary,” Dr Wilson said.
He observed that pharmaceutical companies are showing little interest in developing topical losartan because it can be readily formulated by compounding pharmacies.
“My hope is this situation will change if a patent is awarded. For now, investigators should beware of fake losartan because it is out there, according to the DEA.”
Dr Wilson spoke at AAO 2023 in San Francisco, US.
Steven E Wilson MD is professor, staff refractive and corneal surgeon, and director of corneal research at the Cole Eye Institute at Cleveland Clinic, Cleveland, Ohio, US. wilsons4@ccf.org
CORNEA 26 EUROTIMES | APRIL 2024

27 2024 APRIL | EUROTIMES YOUNG OPHTHALMOLOGISTS ESCRS
https://escrs.org/special-interest-groups/yos/

Cultivating Progress for LSCD
Clinical trials now underway could further support promise shown by cultivated limbal stem cells.
CHERYL GUTTMAN KRADER REPORTS
Research advances are reshaping the diagnosis and treatment of limbal stem cell deficiency (LSCD), according to Sophie X Deng MD, PhD.
Stage classification is part of the foundation for management decisions, so in diagnosis, Dr Deng said recent evidence indicates biomarkers of LSC function identified by in vivo confocal microscopy may provide more accurate information for staging than clinical examination.
She illustrated her point by presenting a case of a patient misdiagnosed with stage III LSCD based on clinical exam but found to have minimal LSCD when assessed with confocal microscopy. Dr Deng also noted using the in vivo imaging tool revealed hidden normal epithelial cells in eyes staged with total LSCD by clinical exam.
“These existing limbal epithelial cells can be retrieved by targeted biopsy and expanded in culture to achieve autologous stem cell transplantation that is favoured over the use of allogeneic cells whenever possible,” she said.
“Although both simple limbal epithelial transplantation (SLET) and cultivated limbal epithelial transplantation (CLET) can provide excellent results, cultivated LSCs might be the preferred choice for treating total LSCD, while SLET might be sufficient in eyes with less severe LSCD. Randomised controlled clinical trials using a set of standardised disease staging criteria are necessary to compare the efficacy of different therapies.”
New methods for rejuvenation
Cases of stage I LSCD require observation and optimisation of the ocular surface and might provide enough improvement of the corneal surface to avoid limbal stem cell transplantation in eyes with moderate LSCD—although Dr Deng noted promising results obtained in ongoing research indicate the possibility of treating the latter eyes with mesenchymal stem cells or growth factors that will promote the growth of residual LSCs in the future.
“It has been shown that mesenchymal stem cells and their secretome have anti-scarring properties and can promote epithelial wound healing,” she said. “Perhaps in the future, topical medications made from the extracellular vesicles/ secretome of the mesenchymal stem cells might replace or reduce the need for corneal transplantation to treat corneal scarring and epithelial defects.”
Progress in LSC cultivation
CLET is not available in the United States because the cultivation process involves xenogeneic murine feeder cells and foetal bovine serum, making it more challenging to meet the FDA regulatory requirement. Research in the field of LSC transplantation also includes efforts to improve the safety of manufacturing LSCs by removing all animal products.
Dr Deng identified Dr Ula Jurkunas and colleagues as one of the teams in the US actively working on this area and cited their development of a xenobiotic- and feeder-free LSC manufacturing process that would meet FDA requirements. Dr Deng and colleagues have also been working on a new process for manufacturing LSCs and have been successful in devising a robust method with a favourable safety profile.
Recruitment is now underway for patients with severe to total LSCD for participation in a phase 1 study she is conducting investigating the safety and feasibility of expanding cultivated autologous LSCs using their manufacturing process. The preliminary data show promising safety and efficacy results. Dr Deng provided the clinicaltrials.gov ID number (NCT03957954) and asked colleagues to refer potential candidates.
Dr Deng spoke at AAO 2023 in San Francisco, US.
CORNEA 28 EUROTIMES | APRIL 2024
Sophie X Deng MD, PhD is Professor of Ophthalmology and Co-Chief Cornea Division, Stein Eye Institute, UCLA, Los Angeles, California, US. deng@jsei.ucla.edu
Innovative Corneal Tissue Engineering
Range of approaches hold promise as alternatives to conventional keratoplasty.
CHERYL GUTTMAN KRADER REPORTS
Just as advances in corneal cross-linking and endothelial keratoplasty previously revolutionised the management of corneal disease, a variety of novel tissue engineering approaches could usher in another revolution that enables management of difficult corneal blindness cases, reports David Myung MD, PhD.
“Corneal blindness is a global problem affecting millions of people worldwide, and its impact has not changed much because graft shortage is a persisting issue,” Dr Myung said.
Technologies that hold promise as alternatives to human graft tissue include next-generation keratoprostheses, xenotransplant and human donor tissue engineering products, new methods for endothelial cell delivery, 3D bioprinting and engineered donor buttons, and in situ forming gel keratoplasty.
Research underway in Dr Myung’s laboratory encompasses several of those approaches, including 3D bioprinting he said could address the severe graft shortage by allowing scalable and precision tissue manufacturing. He noted 3D bioprinting development has lagged behind commercial bioprinting because of challenges faced in cultivating suitable bioinks.
“Bioinks, which are the matrix materials used to carry cells for 3D bioprinting, must strike a balance between being biocompatible and having the necessary structural integrity,” Dr Myung explained. “Achieving both of those goals simultaneously can be difficult.”
Working in collaboration with Professor Sarah Heilshorn and students from his university’s material science and engineering department, Dr Myung and colleagues developed an

engineered bioink platform—UNION (UNIversal Orthogonal Network)—used to print engineered corneal stromal substitutes laden with viable corneal stromal stem cells. The group designed UNION bioink using click chemistry as a cross-linking strategy. Unlike other cross-linking techniques, click chemistry is a “clean and gentle” process because it avoids cross-reactions with both encapsulated cells and surrounding host cells and proteins and does not use a light energy source, require a catalyst, or generate potentially harmful side products. Funding from the US National Institutes of Health (NIH) allows Dr Myung’s lab to study its use in corneal wound healing applications—how cross-linking chemistry and the presence or absence of embedded stromal stem cells impact how wounded corneas respond to these materials.
“This technology is ideally suited to treat corneal blindness through suture-free ‘gel keratoplasty’, a procedure where a stromal scar is removed, followed by placement of a biomaterial that serves as a substrate for epithelialisation and, hopefully, transparent remodelling,” he said.
In one such approach Dr Myung’s lab is pursuing, the same bio-orthogonal cross-linking strategy employed in UNION bioinks creates in situ-forming corneal stromal tissue substitutes that start as a viscous liquid, fill a stromal wound bed, and then form a solid gel that supports epithelialisation.
Other strategies reported to be under development include light-curable biomaterials as well as 3D printed and in situ-forming transplant materials created by pulverising and milling decellularised animal corneas reformulated into a pourable or printable gel. A similar process uses human donor corneal tissues unsuitable for transplantation, with this latter material already being investigated in clinical trials.
Promising phase 1 data in work from other investigators are available for corneal endothelial cell injection, including magnetic cell delivery, which makes in-office treatments possible. This approach involves delivering a solution containing donor human corneal endothelial cells and magnetic nanoparticles into the anterior chamber. The injected cells are attracted to the posterior corneal surface by a magnetic patch placed over the eye and have been shown to integrate into the host’s endothelial cell layer.
Dr Myung spoke during AAO 2023 in San Francisco, US.
David Myung MD, PhD is Associate Professor of Ophthalmology and, by courtesy of Chemical Engineering, Stanford University, Stanford, California, US. david. myung@stanford.edu
29 2024 APRIL | EUROTIMES
Unleashing OCT’s Full Potential
Performance of newest tool for corneal evaluation meets or beats older standard technologies.
CHERYL GUTTMAN KRADER REPORTS
Optical coherence tomography (OCT) corneal imaging is a unique and powerful diagnostic tool with utility already documented in several applications. Its capabilities are expected to enhance in the near future, pending approval of more advanced features and new platforms offering even higher performance, according to David Huang MD, PhD, who co-invented OCT technology in 1991.
“Corneal OCT is a relatively new tool that is now hitting the mainstream after several devices received marketing clearance from the US FDA,” Dr Huang said. “My hope is more clinicians will begin using this technology that gives a comprehensive evaluation of the cornea and is the only noncontact modality for corneal imaging that can resolve the epithelium.”
Functions and applications
OCT corneal mapping generates four types of maps—anterior topography, posterior topography, epithelial thickness, and pachymetry. Its current applications include measurement of total (net) corneal astigmatism through its ability to measure astigmatism of the cornea’s anterior and posterior surfaces.
Dr Huang noted the possibility of mapping corneal topography with OCT became a reality with the advent of higher-speed imaging. Following implementation of a motion detection algorithm to remove motion error and improve repeatability, research he conducted with co-workers showed the maps generated by corneal OCT agreed well with those provided by current standards (e.g., Scheimpflug imaging).
Additional research evaluating corneal OCT for measuring net corneal astigmatism showed it had greater repeatability and accuracy than Scheimpflug imaging when the two techniques were used in patients with keratoconus and post-LASIK pseudophakic patients implanted with a non-toric IOL and compared to the patients’ refraction as the reference standard.
Differential diagnosis of corneal steepening represents another application of corneal OCT, where its ability to detect ectasia and differentiate it from other corneal irregularities is done based on the topography, pachymetry, and epithelial thickness maps. Dr Huang explained that the epithelium is generally thinner at sites of focal steepening in eyes with ectasia but tends to be thicker at sites of focal steepening related to other causes, such as dry eye disease, basement epithelial membrane dystrophy, or contact lens-related warpage.
“This concept relates to the fact that there is secondary modulation of epithelial thickness along with coincident thinning at the site of thickening in ectasia or keratoconus. In contrast, steepening related to primary epithelial deformation (i.e., warpage) is caused by focal epithelial thickening,” he said.
“In addition, the steepening in keratoconus is inferotemporal and associated with a concentric pattern of epithelial thinning.”
Anterior Axial Power
Epithelial Thickness
Pachymetry Posterior Mean Curvature

Figure: Inferior steepening on axial power maps is often used to identify patients who are likely to have keratoconus, but several conditions can cause this appearance. Corneal OCT can help distinguish between them. In forme fruste keratoconus (FFK), the site of focal steepening (red circle) is accompanied by focal epithelial and pachymetric thinning (red circles), as well as posterior steepening (red circle). In dry eye and contact lens-related warpage, focal epithelial thickening (dotted circles) causes focal steepening (red circles).
Dr Huang reported that by using the pattern recognition features he described along with other quantitative parameters, corneal OCT performed at a very high level of accuracy, provided near-perfect recognition of subclinical keratoconus, and showed good sensitivity for detecting forme fruste keratoconus.
Case examples involving patients being screened for refractive surgery or having a corneal irregularity post-LASIK demonstrated how the corneal OCT’s algorithm could determine if steepening was associated with ectasia, a steep central island, or epithelial deformation.
Dr Huang spoke at AAO 2023 in San Francisco, US.
David Huang MD, PhD is Professor of Ophthalmology and Biomedical Engineering, OHSU School of Medicine and Wold Family Chair in Ophthalmic Imaging, OHSU Casey Eye Institute, Portland, Oregon, US. huangd@ohsu.edu
CORNEA 30 EUROTIMES | APRIL 2024
Forme Fruste Keratoconus
Dry Eye
Warpage
Apply for the
John Henahan Writing Prize
Burnout is a chronic issue in ophthalmology, leading a growing number to abandon the field early in their careers. What should be done to reduce unnecessary stress in training and practice, allowing for a successful long-term career?
Young ophthalmologists are invited to submit their answer to that question in an 800-word essay for the John Henahan Writing Prize. The author of the winning essay will receive a €500 bursary and a specially commissioned trophy, awarded during the 2024 ESCRS Congress in Barcelona, Spain. The winning essay will be published in EuroTimes.
The competition is open to ESCRS members (including the free membership available to trainees) age 40 or younger on 1 January 2024.

For details, please see the dedicated page on our website https://www.escrs.org/eurotimes/john-henahan-writing-prize
In the Know—Iris Repair, Part 3
The third of this multi-part series reveals key techniques no surgeon should pass.
DR SOOSAN JACOB MS, FRCS, DNB
Iridodialysis refers to a separation of the iris from the ciliary body at the iris root. It can be post-traumatic or iatrogenic.
Small iridodialysis, especially if covered by the upper lid, may be ignored, but larger dialysis can cause visual symptoms such as glare, photophobia, and monocular polyopia. It also causes polycoria and often results in a displaced pupil. In light-coloured irides, it causes cosmetic concerns as well.
Contemporary iridodialysis techniques
When suturing the iris back, the first step is to make a peritomy and create a scleral groove about 1.5 mm behind the limbus. Next, the anterior chamber (AC) is filled with viscoelastic, and the iris straightened out with all folds removed and brought to lie in the final intended position, using a double-armed 10-0 prolene suture with two long, thin, and preferably curved needles (CIF-4, Ethicon) on either side. A paracentesis is made on the side opposite to iridodialysis, and one of the needles is passed carefully through the paracentesis into the iris near the dialyzed root and docked into a 30-gauge needle passed through the scleral groove, then railroaded out through the sclera. The second needle is then passed through the iris stroma about one clock hour away from the previous pass and again railroaded into a needle passed through the scleral groove radial to it and a short distance away from the first one.
Care should be taken to ensure a free loop between the two needles. Once both needles have been externalized, they
are pulled, drawing the loop into the AC and against the iris. The externalized sutures are then cut and tied down to appose the iris against the scleral wall. Overtightening the prolene suture can occur easily, so the knots should be tightened carefully. Multiple such passes of double-armed prolene suture are required for a large dialysis area. The knots are pushed deep into the scleral groove, and the conjunctiva is closed. A partial thickness scleral flap is sometimes used instead of a scleral groove to avoid exposed knots.
Hoffman pocket
The scleral groove technique can result in exposed knots— leading to the introduction of the Hoffman pocket to avoid this problem. A tunnel is created backwards from the limbus into the sclera, and the two needles of the double-armed suture exit through the roof of this pocket. These are then cut and withdrawn from within the pocket by pulling them out with a rod or Sinskey hook. Tying the two ends of the suture buries the knot within the tunnel. This technique has the added advantage of not requiring any conjunctival peritomy.
Scleral tunnel
A technique similar to the Hoffman pocket, this follows a conjunctival peritomy with the creation of a scleral tunnel starting about 2.0 mm posterior to the limbus. The needles are externalized from the roof, and the cut suture ends are pulled out through the scleral pocket and tied within it. This method has the disadvantage of needing a conjunctival peritomy. If a scleral tunnel is already created for some other purpose—e.g., rigid IOL explantation or manual small incision cataract surgery—an underlying iridodialysis can be sutured to the scleral tunnel floor.

Hang-back technique
An overtightened knot can result in an updrawn pupil. In the hang-back technique, the double-armed sutures are tied down with enough slack
Figure 1, Trocar-assisted iridodialysis repair: A) Irdodialysis during Descemet’s membrane stripping in endothelial keratoplasty; B–C) Double-armed 10-0 prolene sutures passed through a trocar inserted into the paracentesis and out through the iris root and the floor of a scleral groove; D) Tying down the sutures results in iridodialysis repair. Resultant knot is pushed into the pocket.
CORNEA 32 EUROTIMES | APRIL 2024
to give a cosmetically and visually ideal outcome. The non-appositional closure results in an iris literally hung back from the sclera.
Trocar-assisted iridodialysis repair
Take care passing the needles into the AC to avoid snagging onto corneal fibres at the paracentesis—which results in the prolene suture catching at the incision and not being able to be pulled freely into the AC. Such a situation necessitates cutting the suture and restarting. A simple modification, described by Dr Amar Agarwal, uses a trocar passed through the paracentesis. Passing the double-armed needles through the trocar removes any chance of the needle accidentally engaging corneal tissue during its passage. The trocar can be removed once the loop lies free within the AC.
Cobbler’s technique
This technique allows easy and rapid repair of the entire iridodialysis through a small incision and without repeated entry and exit or AC shallowing. One end of a 10-0 prolene suture is threaded through a 26- or 27-gauge needle, which then passes from a diametrically opposite paracentesis into the AC to take a bite of the iris periphery and exit through a slanting scleral groove. The cut end of the suture is retrieved outside the eye while the needle is again internalized, only to be passed through an adjacent part of the iris and scleral groove. This time, a loop of suture is retrieved, and the needle reinternalized. This repeats as many times as required to cover the entire dialyzed iris, creating multiple loops. The initial free end is then passed through all loops except the last one and tied down to the cut end of the last loop. Cobbler’s technique requires only one knot instead of multiple knots.
Rivet technique
The cut end of a 6-0 prolene suture is flanged using a low-temp cautery. A 26-gauge needle then passes through the sclera and the dialyzed iris periphery. The free end of the suture passes into the needle, and the needle withdrawn. The flanged end apposes against the iris and pulls it towards the scleral wall. The other end is trimmed sufficiently and also flanged to ensure iris apposition and the flange burial in the sclera.
Flanged repair
Two ends of a 6-0 prolene suture are passed through the peripheral iris and out through the sclera. Both ends are drawn so the internal loop brings the iris against the sclera, then flanged with a low-temperature cautery and buried in the sclera.

Research Education Innovation
ESCRS’s vision is to educate and help our peers excel in our field. Together, we are driving the field of ophthalmology forward.
Soosan Jacob MS, FRCS, DNB is Director and Chief of Dr Agarwal’s Refractive and Cornea Foundation at Dr Agarwal’s Eye Hospital, Chennai, India, and can be reached at dr_soosanj@hotmail.com.
33 2024 APRIL | EUROTIMES
Vision Restoration for Blinding Retinal Degenerative Diseases
Small molecule photoswitch shows encouraging activity in first in-human trial.
CHERYL GUTTMAN KRADER REPORTS
Aphase 1b clinical study provided proof of concept that intravitreal injection of BENAQ (aka KIO301), a small molecule photoswitch, can potentially restore or improve vision in patients with retinal degenerative disease. A phase 2 study is expected to launch this year.
BENAQ is a light-isomerizable potassium channel blocker taken up selectively by viable retinal ganglion cells (RGC) downstream of degenerated photoreceptors and induces RGC firing upon exposure to light.
KIO-301-2101: Phase 2 Study Design (ABACUS-2)
“In a preclinical model of endstage retinal degenerative disease performed in animals with no rods or cones, BENAQ induced robust firing of RGCs, suggesting that it offers potential to restore vision in individuals who have suffered even near-complete loss of photoreceptors in the outer retina,” said Russell N Van Gelder MD, PhD.
ABACUS is an open-label, single-ascending dose trial conducted at two centres in Australia. It enrolled two patient cohorts, each consisting of three patients with end-stage retinitis pigmentosa. Patients in the first cohort had visual acuity ranging from no to bare light perception. Patients in the second cohort had low vision ranging from counting fingers to hand motion.
BENAQ doses tested in the study ranged from 7.5 to 30 μg. Patients in each cohort received a low dose in one eye and returned for four weekly visits for safety and exploratory efficacy evaluations. Then, after a 30-day washout period, patients were treated with a higher dose of BENAQ in the contralateral eye.
The study met its primary endpoint, showing BENAQ was safe and well-tolerated. There were no serious adverse events, and only three recorded adverse events, including one case of transient ocular hypertension and two reports of post-injection discomfort.
“Importantly, we saw no sign of uveitis or retinal toxicity,” Dr Van Gelder said.
Efficacy signals
At day 14 post-injection, patients in cohort one showed improved light perception, and the benefit remained stable at day 28. Visual acuity in cohort two, estimated using the
Berkeley Rudimentary Vision Test, improved after treatment with the higher BENAQ dose from logMAR 2.1 at baseline to logMAR 1.8 at day 14, with the benefit maintained over the next 14 days.
Visual field testing used a modified Goldmann perimetry test with a blue stimulus because BENAQ is primarily sensitive to blue light. The results showed a statistically significant improvement of approximately 20 degrees in total visual field that occurred by day 7 post-injection and maintained through day 29. Functional magnetic resonance imaging showed new activity in the primary visual cortex in cohort one patients and enhanced cortical activity in cohort two patients, Dr Van Gelder reported.
A three-point change in the mean National Eye Institute Visual Function Questionnaire-25 score documented quality of life improvement, and real-world assays of visual function showed improved performance in motion detection and ambulation testing. Exit interviews eliciting patients’ subjective experiences also revealed benefits for improving visual function in daily living.
Dr Van Gelder spoke at AAO 2023 in San Francisco, US.
Russell N Van Gelder MD, PhD is Professor and Chair, Department of Ophthalmology, University of Washington School of Medicine, Seattle, US, and Director of the Roger and Angie Karalis Johnson Retina Center. russvg@uw.edu
RETINA 34 EUROTIMES | APRIL 2024
24
Sham
Multiple
–
(Australia) 5 RP pts with NLP randomized to receive 50μg KIO-301 or sham (3:2) OU monthly for 3 months 5 RP pts with LP randomized to receive 50μg KIO-301 or sham (3:2) OU monthly for 3 months Patients randomized to control will be eligible to receive 50μg KIO-301 OU monthly for 3 months Cohort 2A Cohort 2B Open Label Extension *Subject to ongoing nonclinical tox results Study Design ABACUS-2 ▪ Controlled ▪ N=20 patients ▪ Includes higher dose* (100µg) Key Elements Cohort 1A Cohort 1B Open Label Extension 5 RP pts with NLP randomized to receive 100μg KIO-301 or sham (3:2) OU monthly for 3 months 5 RP pts with LP randomized to receive 100μg KIO-301 or sham (3:2) OU monthly for 3 months Patients randomized to control will be eligible to receive 100μg KIO-301 OU monthly for 3 months ▪ Multiple injections over 3 months ▪ Binocular injections ▪ Open label extension for controls ▪ Endpoints incorporating US FDA feedback
Controlled, Masked, Randomized,
Ascending Dose Trial
4 Sites
Watching a Novel Implant for RIPPLE Effects
First in-human trial investigates treatment for DME and RVO.
CHERYL GUTTMAN KRADER REPORTS
Follow-up data from the phase 2 RIPPLE-1 study showed lasting benefits in eyes treated for diabetic macular oedema (DMO) or macular oedema due to retinal vein occlusion (RVO) with IBE-814, a novel intravitreal sustained-release dexamethasone implant.
Treated eyes had reduced retinal thickening and stable or improved vision. In addition, eyes that had been receiving other intravitreal therapy benefited from a reduction in treatment burden, according to Sumit Sharma MD.
“This first in-human study of IBE-814 has a planned duration of 18 months, and long-term follow-up is continuing.”
IBE-814 is a 6 mm polymer-free implant fabricated solely with dexamethasone dimers. Compared to polymeric sustained-release devices, the polymer-free system has several advantages that include higher drug-loading capacity, a smoother drug release curve resulting from surface-based versus bulk erosion, longer durability (6 to 9 months), absence of pro-inflammatory degradation products, and the ability to be implanted with a smaller gauge needle (22G instead of 30G), Dr Sharma said.
RIPPLE-1 enrolled treatment-naïve and previously treated patients with DMO or RVO, BCVA 20/40 to 20/320, and central subfield thickness (CST) greater than or equal to 325 μm. Patients were randomised to receive low-dose treatment with a 70 μg implant or high-dose with two 70 μg implants. Monthly follow-up visits began after 1 month, and from month 5 to month 12, retreatment was allowed based on prespecified criteria. Monthly visits for safety evaluations are continuing to month 18.
In all four subgroups defined by IBE-814 dose and diagnosis, implantation resulted in a significant CST reduction by month 1 that sustained through month 6. BCVA improved in both RVO dose groups at month 6, but the changes in CST
and BCVA were numerically better in the low-dose group. Two of 10 low-dose eyes and 1 of 15 high-dose eyes were retreated; rescue treatment was given before month 5 to two low-dose eyes and three high-dose eyes.
The anatomic and functional outcomes showed a dose response in the DMO cohort, with the low-dose group having less improvement in CST at month 6 than the high-dose group. And despite a modest initial BCVA gain, the low-dose group had a mean loss of 1.9 letters at month 6 versus a gain of 8.7 letters in the high-dose group. No DMO eyes were retreated, but 8 of 23 low-dose and 1 of 12 high-dose eyes received rescue therapy.
Twenty-two patients had been receiving intravitreal therapy with an anti-VEGF agent or steroid before RIPPLE-1. Across the four study subgroups, treatment burden was reduced between 57% and 88% following implantation of IBE814 compared to the preceding 12-month period.
“More importantly, the percentage of subjects with both a decreased treatment burden and increase in visual acuity throughout the study ranged from 67% to 100%,” Dr Sharma said.
The safety review showed there were no serious ocular adverse events. Four of 47 eyes that were phakic at baseline developed cataract. IOP elevation occurred in 11 eyes and was managed with topical therapy.
Dr Sharma spoke at the 2023 AAO Retina Surgery Subspecialty Day in San Francisco, US.
Sumit Sharma MD is an Assistant Professor of Ophthalmology at Cole Eye Institute, Cleveland Clinic, Ohio, US. sumitsharma.md@gmail.com
ESCRS iLearn is an online learning platform, free for ESCRS members.
Visit elearning.escrs.org to access over 30 hours of interactive, assessed, and accredited e-learning content, including surgical videos, diagrams, animations, quizzes, and forums.

35 2024 APRIL | EUROTIMES
iLEARN

Complement Inhibitors Promising for Geographic Atrophy
Expert urges caution when choosing appropriate patients for these first-ever treatments.
CHERYL GUTTMAN KRADER REPORTS
The US FDA approval of the complement inhibitors pegcetacoplan (Syfovre, Apellis) and avacincaptad pegol (Izervay) was a major development in the long search for a therapy for geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Approval of both agents was based on the demonstration of efficacy for slowing GA lesion growth, and their anatomical benefit appears to increase as treatment continues. However, these drugs do not stop GA progression, nor have they shown any benefits on visual function endpoints compared to sham. In addition, there are safety issues to consider, cautioned Robin A Vora MD.
Against this background, the million-dollar question becomes, who should be treated for GA with a complement inhibitor?
Dr Vora addressed this issue with a few specific yes and no scenarios but suggested most patients probably fall within a grey area where the answer is less clear. His final advice was more general.
“My best recommendation, as for all treatments, is to think about the risks and benefits for each patient.”
Patient selection
The first consideration is confirming the patient has GA
36 EUROTIMES | APRIL 2024
RETINA
related to AMD and not some other condition. Then, reasonable candidates include patients with vision lost to GA in one eye and foveal-sparing GA in the fellow eye or with foveal threatening or clearly progressing GA, assuming they are willing to commit to extended and indefinite treatment.
However, many patients with GA secondary to AMD probably fall into the category of having early disease where the risk of long-term therapy might outweigh the benefit or already have such significant vision loss that intervention would not be worthwhile.
Safety questions
In clinical trials, both complement inhibitors were associated with a two- to fourfold higher incidence of choroidal neovascularisation (CNV) development than sham. These patients then may require treatment with both anti-VEGF and anti-complement therapy. How best to manage these patients’ dual injection needs is unclear.
Also concerning are cases of retinal vasculitis occurring in patients treated with pegcetacoplan post-marketing. As of August 2023, retinal vasculitis ranging from mild to severe was reported in 10 eyes of 9 patients; vision loss corresponded to vasculitis severity and ranged from 20/200 to no light perception in 7 eyes. The cases presented about 10 to 14 days post-injection, differing from the typical onset of bacterial endophthalmitis. They usually occurred after the first injection of pegcetacoplan, and many affected patients were also receiving anti-VEGF treatment for CNV.
Dr Vora credited pegcetacoplan manufacturer Apellis for undertaking an intensive investigation to understand the cause or risk factors for the retinal vasculitis not seen in any clinical trial participants. The search determined that the mesh filters in the needles provided with the injection kits could—potentially—allow particulate matter to pass into the suspension for injection.
“However, a definite causal relationship could not be established, and I don’t think the needle is related to the retinal vasculitis,” Dr Vora said.
The US FDA approved the pegcetacoplan injection for GA treatment secondary to age-related macular degeneration in February 2023. However, the European Medicines Agency recommended the refusal of the marketing authorisation for pegcetacoplan in January 2024, questioning whether the risks outweighed the potential benefits of treatment. Apellis Europe B.V. may appeal the decision.
Dr Vora spoke at AAO 2023 in San Francisco, US.
GLOBAL REACH
As a renowned authority in the field of cataract and refractive surgery, ESCRS facilitates global connections amongst ophthalmic professionals, fostering collaboration and the exchange of knowledge.
Our events span across continents, providing a platform for pioneering research, advanced surgical techniques, and continuous professional development.
Using the interactive map on our website, we invite you to explore our global presence by viewing upcoming events and academies. Join us to network with esteemed experts, access the latest advancements, and contribute to the enhancement of eye care on a worldwide scale.
escrs.org/meetings-and-events/global-reach/
Robin A Vora MD is a medical retina specialist and serves as Chair of Ophthalmology, Kaiser Permanente Northern California, Oakland, US. robin.vora@kp.org
37 2024 APRIL | EUROTIMES
Direct SLT Eagle granted FDA clearance
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Belkin’s direct selective laser trabeculoplasty (DSLT) Eagle™ device for treating primary open-angle glaucoma and ocular hypertension glaucoma. Use of the Q-switched, 532 nm-wavelength, frequency-doubled Nd:YAG laser does not require gonioscopy or direct contact with the eye. Instead, the Eagle uses a proprietary algorithm and eye-tracking technology to automatically administer 120 laser shots through the limbus across 360 degrees of the trabecular meshwork. The technique therefore simplifies and shortens the procedure, potentially increasing accessibility to SLT as an alternative to eye drops as a first line of treatment for glaucoma. The Eagle device received its CE mark in May 2022.
https://belkin.com
Rayner acquiring Sophi phaco technology
Rayner has announced its entry into a definitive agreement to acquire Sophi (Swiss Ophthalmology Innovation), the Swiss-based inventor, developer, and manufacturer of Sophi phacoemulsification machines. The acquisition is Rayner’s first venture into the phacoemulsification machine market. The company notes Sophi’s phaco technology has advanced features to enhance mobility, simplicity, and safety within the operating theatre. They include triple pump fluidics, active IOP control, clean venturi and day cassette, a cable-free power supply, and wireless communication.
https://rayner.com
Sponsored diabetic retinopathy screening programme in Vietnam
NIDEK has announced it donated six specialised fundus cameras to Orbis International in support of Orbis’s artificial intelligence (AI)-based diabetic retinopathy screening services in Vietnam. The addition will expand Orbis’s programme to six high-volume screening health facilities in three provinces across Vietnam. The cameras will support the screening of an estimated 72,000 patients with diabetes over the next three years. Images from NIDEK’s desktop fundus cameras will be uploaded to and graded by Cybersight AI, Orbis’s state-of-the-art technology, determining within seconds if diabetic retinopathy is detected, allowing immediate referral for treatment when necessary.
https://www.nidek.com
Optogenetic therapy regulatory discussion update
Nanoscope Therapeutics provided an update following recent regulatory discussions with the US Food and Drug Administration (FDA) and the Icelandic Medicines Agency (IMA) regarding the regulatory path of MCO-010 (sonpiretigene isteparvovec, suspension for intravitreal injection) for the treatment of advanced retinitis pigmentosa (RP). MCO-010 is an optogenetic gene therapy which encodes retinal neurons with an opsin gene, enabling them to act as photoreceptors. The FDA stated a change from baseline in visual acuity measurement in low vision patients could be an appropriate primary efficacy assessment. The IMA endorsed a 0.3 logMAR change in visual acuity as clinically meaningful for RP patients with severe vision loss. In the phase 1/2 and ongoing phase 2b RESTORE trials, MCO-010 demonstrated favourable safety and nominal dose-dependent improvement in visual acuity in advanced RP patients. MCO-010 has received FDA fast-track and orphan drug designations for retinitis pigmentosa and Stargardt disease.
https://nanostherapeutics.com
38 INDUSTRY NEWS EUROTIMES | APRIL 2024

POSITION PAPER CALLS FOR WORLDWIDE ADOPTION OF ELECTRONIC INSTRUCTIONS FOR USE IN OPHTHALMIC SURGICAL PRODUCT PACKAGING
A multisociety position paper called for replacing paper instructions for use (IFU) pamphlets with electronic versions (e-IFU) whenever possible for ophthalmic surgical prod ucts. The authors, representing EyeSustain—a coalition sponsored by ASCRS, ESCRS, and AAO—noted surgeons perform almost 30 million cataract procedures worldwide. Ophthalmic surgery is therefore a major contributor to healthcare-related waste and greenhouse gases. Ophthal mic surgical supplies are packaged with IFUs printed in multiple languages. In the case of IOLs, the IFUs significantly increase the size and weight of the packaging. For example, a 2013 analysis of carbon emissions from cataract surgery in the UK’s National Health Service (NHS) showed that the IOL packaging—which included a 70-page IFU booklet translated into 11 languages—weighed 64 grams; the IOLs themselves weighed less than 1 gram. In contrast, e-IFUs consist of a single page with a QR code to access a complete multilingual IFU online. Therefore, transitioning to e-IFU could reduce greenhouse gases from IOL packaging by 67%, saving approximately 3,500 metric tons of CO2 emissions annually, equivalent to the annual greenhouse gas emissions from 753 passenger cars or the energy use of 425 US homes. Eliminating the excess weight of IOL packaging might also reduce costs for waste treatment and product shipment.

OBSTACLES TO WIDER ADOPTION OF E-IFUS
The position paper authors noted that a survey of American instrument and supply manufacturers across multiple surgical specialties showed 95% believed e-IFUs were an acceptable alternative to paper IFUs. However, e-IFUs were currently used for all products by only 20%, most products by 10%, many products by 25%, a few products by 25%, and no products by 20%. Varying and inconsistent IFU requirements between different countries are a barrier to wider e-IFU adoption by the ophthalmic surgical industry. Yet there is no evidence that e-IFUs pose a danger to patient care. On the contrary, manufacturers can update safety information much faster and more effectively. Both the European Union (EU) Medical Device Regulation (MDR) and the US Food and Drug Administration (FDA) permit e-IFU, although for MDR, this is limited to certain products. Currently, the MDR allows e-IFU for implants such as IOLs across EU member states. However, the MDR does not permit e-IFU for other products used in cataract surgery, such as instruments and phacoemulsification tubing and machines. “We recommend the ophthalmic surgical manufacturing industry moves exclusively to e-IFU, initially prioritizing those products routinely used in high volumes, such as IOLs, IOL cartridges, and OVD. We request that every global government and regulatory agency facilitate these efforts,” the authors concluded. E M Schehlein, et al. “Reducing ophthalmic surgical waste through electronic instructions for use: a multisociety position paper,” 50(3): 197–200.
39 JCRS HIGHLIGHTS 2024 APRIL | EUROTIMES
escrs.org/eurotimes/ supplements/ Explore IOL solutions and demystify common misunderstandings with insights into a refractive future that offers more improved postoperative outcomes—exclusively on the ESCRS website. SUPPLEMENT YOUR IOL KNOWLEDGE

Educational Resources Advanced FEBOS-CR Exam/Diploma (Cataract and Refractive Surgery) ESCRS iLearn • ESCRS Research Portals • Important Publications ESCRS EuroTimes Podcasts • ESCRS on Demand • JCRS Online Case Reports The Video Journal of Cataract, Refractive, and Glaucoma Surgery escrs.org/education/educational-resources
Upcoming Events
April 5–8
2024 ASCRS Annual Meeting
Boston, United States
May 5–9
2024 ARVO Annual Meeting Seattle, United States
May 24–26
2024 EuCornea Annual Congress Paris, France
June 1–4
2024 European Glaucoma Society Congress Dublin, Ireland
June 5–8
2024 Retina International World Congress Dublin, Ireland
September 6–10
2024 ESCRS Congress Barcelona, Spain

April
May

Sep

41 2024 APRIL | EUROTIMES
5
24
6
STOP!

Glaucoma Disease Progression
iStent ® technologies have been proven to stabilize visual fields loss and glaucoma disease progression with a favourable risk-benefit ratio1

+3 YEARS FOLLOW-UP
15 STUDIES ON ISTENT TECHNOLOGIES
1,115 EYES

26.6 % MEAN IOP REDUCTION WAS SUFFICIENT TO ACHIEVE THIS FUNCTIONAL EFFECT
-0.024
DBS/YEAR MEAN RATE OF PROGRESSION SIMILAR TO THAT REPORTED IN NONGLAUCOMATOUS EYES
INTERVENE EARLIER WITH ISTENT INJECT ® W TO PRESERVE PATIENTS’ FUNCTIONAL VISION AND QUALITY OF LIFE
1. Gillmann K, Hombeak DM. BMJ Open Ophth 2024;9:e001575.doi:10.1136/bmjophth-2023-001575.
iStent inject® W IMPORTANT SAFETY INFORMATION
INDICATION FOR USE: The iStent inject ® W, is intended to reduce intraocular pressure safely and effectively in patients diagnosed with primary open-angle glaucoma, pseudo-exfoliative glaucoma or pigmentary glaucoma. The iStent inject ® W, can deliver two (2) stents on a single pass, through a single incision. The implant is designed to stent open a passage through the trabecular meshwork to allow for an increase in the facility of outflow and a subsequent reduction in intraocular pressure. The device is safe and effective when implanted in combination with cataract surgery in those subjects who require intraocular pressure reduction and/or would benefit from glaucoma medication reduction. The device may also be implanted in patients who continue to have elevated intraocular pressure despite prior treatment with glaucoma medications and conventional glaucoma surgery.
CONTRAINDICATIONS: The iStent inject ® W System is contraindicated under the following circumstances or conditions: • In eyes with primary angle closure glaucoma, or secondary angle-closure glaucoma, including neovascular glaucoma, because the device would not be expected to work in such situations. • In patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
WARNINGS/PRECAUTIONS: • For prescription use only. • This device has not been studied in patients with uveitic glaucoma. • Do not use the device if the Tyvek® lid has been opened or the packaging appears damaged. In such cases, the sterility of the device may be compromised. • Due to the sharpness of certain injector components (i.e. the insertion sleeve and trocar), care should be exercised to grasp the injector body. Dispose of device in a sharps container. • iStent inject ® W is MR-Conditional; see MRI Information below. • Physician training is required prior to use of the iStent inject ® W System. • Do not re-use the stent(s) or injector, as this may result in infection and/or intraocular inflammation, as well as occurrence of potential postoperative adverse events as shown below under “Potential Complications.” • There are no known compatibility issues with the iStent inject ® W and other intraoperative devices. (e.g., viscoelastics) or glaucoma medications. • Unused product & packaging may be disposed of in accordance with facility procedures. Implanted medical devices and contaminated products must be disposed of as medical waste. • The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. If intraocular pressure is not adequately maintained after surgery, the surgeon should consider an appropriate treatment regimen to reduce intraocular pressure. • Patients should be informed that placement of the stents, without concomitant cataract surgery in phakic patients, can enhance the formation or progression of cataract. ADVERSE EVENTS: Please refer to Directions For Use for additional adverse event information. CAUTION: Please reference the Directions For Use labelling for a complete list of contraindications, warnings and adverse events. © 2024 Glaukos Corporation. Glaukos, iStent®, iStent inject ® and iStent inject ® W are registered trademarks of Glaukos Corporation. PM-EU-0294















 Thomas Kohnen Chief Medical Editor
Thomas Kohnen Chief Medical Editor



































































