Sono electro chemistry
VOL. 27, NO. 3
Fall 2018
IN THIS ISSUE
3 From the Editor: Passing the Baton (or Ball)
7 Pennington Corner: Once in a Lifetime
24 Special Meeting Section: AiMES 2018 Cancun, Mexico
33 Looking at Patent Law
39 Tech Highlights
41 A Short Introduction to Sonoelectrochemistry
43 Introduction to Ultrasound and Sonochemistry
47 Sonoelectrochemistry: Both a Tool for Investigating Mechanisms and for Accelerating Processes

53 Ultrasonic Agitation for Emerging Electrodeposition Systems
59 In Situ Ultrasonic Dispersion in Multiphase Electrolysis Systems
142 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
(
A "Sound" Technology
( ( ( ( (
ECS Institutional Members
The Electrochemical Society values the support of its institutional members. These organizations help ECS support scientific education, sustainability, and innovation. Through ongoing partnerships, ECS will continue to lead as the advocate, guardian, and facilitator of electrochemical and solid state science and technology.
2018 Leadership Circle Award Recipients
GOLD (25 years)
Central Electrochemical Research Institute
AMETEK-Scientific Instruments (37)
Bio-Logic USA/Bio-Logic SAS (10)
Duracell (61)
Gamry Instruments (11)
Gelest, Inc. (9)
3M (29)
Energizer (73)
Faraday Technology, Inc. (12)
IBM Corporation Research Center (61)
BASi (3)
Central Electrochemical Research Institute (25)

DLR-Institut für Vernetzte Energiesysteme e.V. (10)
EL-CELL GmbH (4)
Ford Motor Corporation (4)
GS Yuasa International Ltd. (38)
Honda R&D Co., Ltd. (11)
Medtronic Inc. (38)
Nissan Motor Co., Ltd. (11)
Axiall Corporation (23)
General Motors Holdings LLC (66)
Giner, Inc./GES (32)
International Lead Association (39)
Ion Power Inc. (4)
Kanto Chemical Co., Inc. (6)
Karlsruher Institut für Technologie (2)
Leclanche SA (33)
SILVER (10 years)
Bio-Logic USA/Bio-Logic SASDLR-Institut für EnergiesystemeVernetzte e.V.
Benefactor
Hydro-Québec (11)
Industrie De Nora S.p.A. (35)
Pine Research Instrumentation (12)
Saft Batteries, Specialty Batteries Group (36)
(Number in parentheses indicates years of membership)
Patron
Lawrence Berkeley National Laboratory (14)
Panasonic Corporation, AIS Company (24)
Scribner Associates, Inc. (22)
Toyota Research Institute of North America (10)
Sponsoring
Permascand AB (15)
Technic Inc. (22)
Teledyne Energy Systems, Inc. (19)
The Electrosynthesis Company, Inc. (22)
Tianjin Lishen Battery Joint-Stock Co., Ltd. (4)
TOC Capacitor Co., Ltd. (1)
Toyota Central R&D Labs., Inc. (38)
Yeager Center for Electrochemical Sciences (20)
ZSW (14)
Sustaining
Los Alamos National Laboratory (10)
Microsoft Corporation (1)
MTI Corporation (2)
Occidental Chemical Corporation (76)
Sandia National Laboratories (42)
SanDisk (4)
Targray (2)
Please help us continue the vital work of ECS by joining as an institutional member today. Contact Shannon.Reed@electrochem.org for more information.
08/03/2018



Passing the Baton (or Ball)
The dog days of summer are in full flush in Dallas as I compose this editorial, and the curtains have just come down on a most exciting edition of World Cup soccer as well. And I get to write about the third most favorite thing in my life after chemistry and music: namely, sports. There is nothing more exquisite than a perfectly thrown Tom Brady touchdown in football (i.e., the American variety!), an inside-out forehand from Roger Federer, a Lionel Messi move in soccer, or a Kobe Bryant no-look, behindthe-back pass on the hoops floor. Similarly, seamless transfer of the baton in a relay race is satisfying to all involved. Sports teaches you about life as well, the ability to face success or failure with equanimity. On this topic, Rudyard Kipling famously wrote: “If you can meet with Triumph and Disaster / And treat those two impostors just the same.”1 (I urge you to read the full poem, titled “If—.”)
I am now passing the editorial baton to the new editor of Interface, Rob Kelly. It was most pleasing to see Rob taking the reins, as I have known him a long time, also from his role as a representative of the ECS Corrosion Division on the Interface Advisory Board. I have little doubt that Rob will run with the ball and take the magazine to new heights. He has the right combination of insightfulness, vision, and humor to handle whatever editorial challenges are hurled in his direction. This includes recalcitrant authors and guest editors.
I recall Mary Yess, the erstwhile managing editor of Interface, cautioning me when I first took over the editorial duties back in 1999. She advised me to put away my tennis racket and the guitar to focus on the more urgent tasks at hand with this magazine. I will echo similar words to you, Rob; please put away those golf clubs for a while. You will not have time to improve your handicap any time soon. And stay tuned.
Krishnan Rajeshwar Interim Editor

https://orcid.org/0000-0003-4917-7790
Most importantly, Raj—on behalf of the entire ECS— thank you for your stewardship of this great baton. Your contributions will always be part of its DNA. You have left large shoes to fill, but I will do my best to follow the Hippocratic Oath, or as my father often said, “Ok, Rob, try not to screw this up.” With both hands firmly on the baton, I will run as fast as I can until it is my turn to pass the baton to the next runner.
It is an exciting time for Interface. Its evolution will continue with the help of the Interface Advisory Board (IAB), the ECS staff, and ECS members, but its bedrock purposes will remain. Its first goal is to make the work of each topical interest area (TIA) accessible to members in other TIAs as well as to scientists in other disciplines who read these articles. Showcasing the importance of the work of ECS members to the general public is of increasing importance. Finally, its role as the historical record of the Society is important to honor those who have made ECS what it is, and as a reminder to all of what is expected of the caretakers of that baton. I will keep you in suspense about the details of our nefarious plans, but hints may be found in my answers to the Five Questions in the summer 2018 issue of this magazine. That said, I look forward to my first meeting with the IAB in Cancun (I know, rough duty), as well as any and all feedback from you, the readers. I am very much looking forward to running this portion of the race. Excuse me while I go carefully stretch out my hamstrings; they have been dormant for a long time.
Published by: The Electrochemical Society (ECS) 65 South Main Street Pennington, NJ 08534-2839, USA Tel 609.737.1902, Fax 609.737.2743 www.electrochem.org

Editor: Rob Kelly, rgk6y@virginia.edu
Guest Editor: Bruno G. Pollet, bruno.g.pollet@ntnu.no

Contributing Editors: Donald Pile, donald.pile@gmail. com; Alice Suroviec, asuroviec@berry.edu
Managing Editor: Annie Goedkoop, Annie.Goedkoop@electrochem.org
Print Production Manager: Dinia Agrawala, interface@electrochem.org
Advertising Manager: Ashley Moran, Ashley.Moran@electrochem.org

Staff Contributors: Marcelle Austin, Annie Goedkoop, Ngoc Le, John Lewis, Shannon Reed, Andrew Ryan, and Mary Yess.
Advisory Board: Christopher Johnson (Battery), Masayuki Itagaki (Corrosion), Durga Misra (Dielectric Science and Technology), Philippe Vereecken (Electrodeposition), Jennifer Hite (Electronics and Photonics), A. Manivannan (Energy Technology), Sean Bishop (High-Temperature Energy, Materials, & Processes), John Weidner (Industrial Electrochemistry and Electrochemical Engineering), Uwe Happek (Luminescence and Display Materials), Slava Rotkin (Nanocarbons), Jim Burgess (Organic and Biological Electrochemistry), Andrew Hillier (Physical and Analytical Electrochemistry), Nianqiang (Nick) Wu (Sensor)
Publisher: Mary Yess, mary.yess@electrochem.org
Publications Subcommittee Chair: Stefan De Gendt Society Officers: : Yue Kuo, President; Christina Bock, Senior Vice President; Stefan De Gendt, 2nd Vice President; Eric Wachsman, 3rd Vice President; James Fenton, Secretary; Gessie Brisard, Treasurer; Christopher J. Jannuzzi, Executive Director
Statements and opinions given in The Electrochemical Society Interface are those of the contributors, and ECS assumes no responsibility for them.
Authorization to photocopy any article for internal or personal use beyond the fair use provisions of the Copyright Act of 1976 is granted by The Electrochemical Society to libraries and other users registered with the Copyright Clearance Center (CCC). Copying for other than internal or personal use without express permission of ECS is prohibited. The CCC Code for The Electrochemical Society Interface is 1064-8208/92.
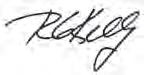
Canada Post:
Publications Mail Agreement #40612608
Canada Returns to be sent to:
Pitney Bowes International, P.O. Box 25542, London, ON N6C 6B2
ISSN : Print: 1064-8208 Online: 1944-8783
The Electrochemical Society Interface is published quarterly by The Electrochemical Society (ECS), at 65 South Main Street, Pennington, NJ 08534-2839 USA. Subscription to members as part of membership service; subscription to nonmembers is available; see the ECS website. Single copies $10.00 to members; $19.00 to nonmembers. © Copyright 2018 by The Electrochemical Society. Periodicals postage paid at Pennington, New Jersey, and at additional mailing offices. POSTMASTER: Send address changes to The Electrochemical Society, 65 South Main Street, Pennington, NJ 08534-2839.
Editor
rgk6y@virginia.edu
https://orcid.org/0000-0002-7354-0978
The Electrochemical Society is an educational, nonprofit 501(c)(3) organization with more than 8,500 scientists and engineers in over 75 countries worldwide who hold individual membership. Founded in 1902, the Society has a long tradition in advancing the theory and practice of electrochemical and solid state science by dissemination of information through its publications and international meetings.
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 3
All recycled paper. Printed in USA.
FROM THE EDITOR
1. R. Kipling, “Brother Square-Toes,” Rewards and Fairies (1910).
Rob Kelly



Rapid, High-Current Pulsing & Superior, Low-Impedance EIS www.gamry.com Reference 30K Booster Nobody is faster Distortion-free, rapid pulsing for charge and discharge 30 A Pulse on a 5 mOhm cell Nobody goes lower Up to 30A @ 20V, EIS to 300 kHz 200 nOhm Shorted Lead Curve 20 A rms, 0.9 m cell cable
by Bruno G. Pollet
by Wu Li and Muthupandian Ashokkumar
 by Mahito Atobe and
by Mahito Atobe and

142 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Vol. 27, No. 3 Fall 2018 3 From the Editor: Passing the Baton (or Ball) 7 Pennington Corner: Once in a Lifetime 9 Society News 24 Special Meeting Section: AiMES 2018 Cancun, Mexico 32 People News 33 Looking at Patent Law 39 Tech Highlights 64 Awards Program 76 New Members 80 Student News A Short Introduction to Sonoelectrochemistry
Introduction to Ultrasound and Sonochemistry
Sonoelectrochemistry: Both a Tool for Investigating Mechanisms and for Accelerating Processes
Jean-Yves
Marie-Laure Doche, Loic Hallez, Abdeslam Et Taouil, and Bruno G. Pollet Ultrasonic Agitation for Emerging Electrodeposition Systems
S. Roy and S. J. Coleman In Situ Ultrasonic Dispersion in Multiphase Electrolysis Systems
by
Hihn,
by
Frank
41 43
47 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 5
Marken
53 59
On the Cover: A cavitation bubble imploding near the electrode surface causing the formation of a highvelocity microjet of liquid hitting the surface. See article on page 41.
Cover design by Dinia Agrawala.
Research grade Potentiostat / Galvanostat Model EC301 ...
$7990
The new EC301 Potentiostat / Galvanostat meets the requirements of modern research while offering tremendous value. Hardware for electrochemical impedance spectroscopy (EIS) is built in to the instrument and included at no extra charge. The compliance, bandwidth, and polarization range of the EC301 let you handle the most demanding of electrochemical cells.

With an intuitive front panel, the EC301 gets you up and running in seconds. When you’re ready to stream data to the PC, the Ethernet and GPIB interfaces offer speed and convenience. The software is completely free; all the techniques are included. There are no modules to unlock, no add-ons to buy.
· 30 V / 1 A compliance
· ±15 V polarization range
· 1 MHz potentiostatic bandwidth
· Grounded or floating operation
· EIS & software included
Tel: (408) 744-9040 . www.thinkSRS.com/ec

Research Systems
Stanford
Once in a Lifetime
In the summer of 2009, less than a year into my time with my previous employer, IEEE, my then boss suggested I attend a meeting of an organization with which we cosponsored several technical journals. Being new to both working for a technical society and living in the state of New Jersey, I was familiar with neither the organization hosting the meeting nor the town in which it was located. But I was eager to learn all I could about my new role, and my new home state, so I agreed to attend.
Although I cannot recall specific details about that meeting, I do clearly remember how I felt after it was over. As I walked back to my car, I called my wife, Liz, and told her how impressed I was with the organization and the people working there. Even the office building, with its homely feel and comfortable, wooden library-style meeting room, appealed to me.
“I could really see myself working here,” I said.
Over the years, Liz has heard me muse about a lot of things I could see myself doing career-wise, so she did not put a lot of stock in my comment. To a certain extent neither did I—after all, I had only recently started my position with IEEE and was extremely excited about the prospects my new job held, but I never forgot about that meeting, or the people I met that day.
As you may have surmised by now, that fateful meeting I mentioned took place at ECS. The people I met with were Annie Goedkoop and Mary Yess, long-standing members of the team I so proudly joined on July 19. What an amazing thing to ponder as I sit at my desk, on day three of my new job, writing the first of (hopefully) many Pennington Corners!
When I consider the serendipity of events that led to my joining ECS, from my long-ago interest in the Society, to the alignment of my relevant skills and experience, to my strong belief in the mission of ECS and the Free the Science initiative, this is truly a once-in-a-life-time opportunity for me and one to which I am inspired to fully commit my time, talent, and resources. However, this is also bittersweet for me, for as
excited as I am to be in my new role, I will sincerely miss the opportunity to work more closely with Roque Calvo. Being his successor is as much an honor as it is a daunting task. Though I know that if I am even half as successful as Roque has been during his tenure with the Society, I will have done a great service for ECS, its members, and the community at large.
Over the coming weeks and months, my plan is to immerse myself in all I can about ECS, to develop a deep understanding of our operations and strategic plan, and to work with the volunteer leaders and senior staff to chart our future directions. Granted, there is quite a lot of work ahead of us, and we face challenges on many fronts. However, with the insightful leadership of ECS President Yue Kuo and the rest of our outstanding volunteers, coupled with the multifaceted capabilities of the Society’s professional corps, I have every confidence that together we will be successful at maintaining and advancing ECS’s preeminence in the field of electrochemical and solid state engineering.
In closing let me say what an incredible privilege it is to serve as executive director of this remarkable organization. I look forward to working closely with each and every one of you. Anytime you have questions, comments, or just want to reach out, please do! I would love to hear from you at Chris.Jannuzzi@electrochem.org.
Thanks and all the best!
Christopher J. Jannuzzi ECS Executive Director/Chief Executive Officer Chris.Jannuzzi@electrochem.org

https://orcid.org/0000-0002-7293-7404

The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 7 PENNINGTON CORNER
When I consider the serendipity of events that led to my joining ECS, from my long-ago interest in the Society, to the alignment of my relevant skills and experience, to my strong belief in the mission of ECS and the Free the Science initiative, this is truly a once-in-a-life-time opportunity for me.
Introducing Dual-Channel Functionality to the PARSTAT MC Family
PMC-200
Maximize throughput with two channels per module, each with EIS and core DC testing functionality

1-Amp of current standard allows analysis of larger batteries at higher rates
24-Bits ADC components delivering the highest quality voltage and current resolution of measured response
Installs into the PARSTAT MC Chassis in minutes with no hardware or training.
Can be operated alongside other PMC modules maximing the value of your PMC Chassis
8 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
www.ameteksi.com
ECS Appoints New Executive Director/CEO
Last year, ECS leadership set about the herculean task of finding a successor to Roque Calvo, who served as a steward of the Society for over 37 years, directing its course through periods of immense change while vastly expanding its efforts to advance electrochemical and solid state science and technology.
Now, after an intensive candidate search that spanned months of consultations, interviews, and conferences, the Society is pleased to announce that it has found its new executive director and chief executive officer in Christopher J. Jannuzzi, former executive director of two IEEE societies: the Electron Devices Society and the Photonics Society
Jannuzzi demonstrates extensive experience in all aspects of the operations of scientific societies, coupled with strategic foresight and a propulsive passion for ECS’s mission.
Prior to joining IEEE, Jannuzzi was a senior director at the College Board in New York. He received his undergraduate degree from New York University and holds an MA in organization and leadership from Teacher’s College, Columbia University. Jannuzzi lives in Shrewsbury, NJ, with his wife, Elizabeth, and their children, Michael, Raymond, and Julia.
Selecting a Candidate
The Society’s search for a new executive director and chief executive officer was conducted by a committee chaired by ECS Secretary James Fenton and consisted of past ECS presidents Larry Faulkner, Fernando Garzon, Dennis Hess, and Esther Takeuchi. With the assistance of a search firm, the committee carried out a thorough and inclusive evaluation and interview process, speaking with staff, division leaders, past presidents, and others to obtain input to guide their candidate search.
Drawing from this input, the search committee determined that the new executive director’s priorities should be to (1) expand ECS’s reach and global presence, (2) lead and organize ECS staff, (3) evaluate ECS’s financial model, (4) develop new external partnerships, and (5) strengthen governance and board relations.
“Through my work at IEEE, I was very familiar with The Electrochemical Society and had the good fortune to work with ECS staff and volunteers on several joint initiatives over the years,” says Jannuzzi. “I have long admired the Society and am honored by the incredible opportunity to serve as its next executive director.”
The Society is thrilled to welcome Jannuzzi to the position, certain that his insight, energy, and leadership will foster great success for ECS in the years to come.

About Christopher Jannuzzi
Christopher Jannuzzi joined IEEE in 2009 as the executive director of the IEEE Electron Devices Society. In his time with EDS, Jannuzzi launched several key initiatives to ensure the society maintained its preeminence in the device engineering field. From leading efforts to redesign EDS’s digital presence, to leveraging the society’s longstanding connection to the photovoltaic research community to expand IEEE’s standing in the renewable energy space, to developing EDS’s robust webinar program, he helped revitalize and reenergize the EDS community.
Building on his success with EDS, IEEE expanded Jannuzzi’s role in 2014 to include leadership of the Photonics Society, providing him with the opportunity to have impact on cross-society strategic vision in addition to managing the societies’ day-to-day operations. Among Jannuzzi’s accomplishments with the Photonics Society was the launch of the IEEE Photonics Fund to raise capital for the society’s humanitarian initiatives and to foster diversity within its membership. Launched in 2016, the fund now provides over 100 scholarships and fellowships annually to help students and traditionally underserved communities choose photonics as an educational and career path.
Beyond his work with technical societies, Jannuzzi also helped lead the IEEE Internet Initiative, which raised the institute’s influence and profile in the areas of Internet governance and cybersecurity by providing a consensus of sound technical and scientific knowledge to the technology policy development process.
The committee also concluded from this feedback that the new executive director should possess certain qualifications and personal characteristics relevant to the role, including familiarity with the significance of electrochemistry and solid state sciences as well as the abilities to work with budgets, enhance meetings, understand the scholarly publication process, build relationships with members, fundraise, and sustain commitments to transparency, open communication, diversity, and inclusivity.
Based on these priorities and sought-after qualifications and characteristics, the search committee unanimously chose Jannuzzi as the best candidate for the role. ECS President Yue Kuo then requested a motion to appoint Jannuzzi as the Society’s new executive director, a decision Kuo firmly stands by.
“According to my impression,” Kuo says, “Chris possesses years of invaluable experience managing nonprofit professional societies similar to ECS. His appointment ensures the smooth transition of this highly important position in the Society. We expect that his expertise will further extend the Society’s 116-year history of success.”
Securing the Society’s Future
Jannuzzi bears a wealth of experience that will prove vital to the pursuit of Society objectives throughout the years to come. In addition to having served as the executive director of two IEEE societies, he has successfully managed budgets of approximately $12M. He also has been conducting development work in the technical society domain for a number of years and has secured over $500K in donations.
(continued on next page)
SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 9
I have long admired the Society and am honored by the incredible opportunity to serve as its next executive director.
—Chris Jannuzzi
By endowing our publications operations through the Free the Science initiative, ECS has aligned how we fund publishing with our core mission to serve the public interest. This is precisely how nonprofits should act and one of the things that excites me most about my new role.
—Chris Jannuzzi
(continued from previous page)
Moreover, Jannuzzi has experience managing staff and working with hundreds of volunteers. He wields a collegial leadership style, interacting with members and volunteers with ease.
“Organizations such as ECS would not, and could not, exist without the dedication of its members and volunteers,” says Jannuzzi. “Therefore, the top priority of the executive director, as I see it, is to operationalize the vision of the Society’s elected leadership. ECS is blessed with a talented staff and a dedicated corps of world-class scientists and engineers to drive the organization forward.”
James Fenton, chair of the search committee, was struck by Jannuzzi’s drive to enhance collaborations between the Society’s staff, members, and volunteers.

“I feel Chris was impressed with the passion that the members felt for the Society and the family nature of the Society,” Fenton says. “Chris also has that passion and is looking forward to help lead the Society into an ever-changing future. Chris was impressed with the way ECS ‘punches above its weight class.’”
“When one considers the depth and breadth of all that ECS does—from conferences and publications, to education, awards, and member engagement, it is the staff-volunteer partnership that makes it all possible,” says Jannuzzi. “As executive director, my responsibility is to ensure that partnership continues to flourish.”
Jannuzzi was also chosen for his extensive experience in the scholarly publishing arena. IEEE has a $200M publishing enterprise; hence, Jannuzzi is quite familiar with the strains being placed upon scientific publishing. He is familiar with ECS publishing, having worked on publishing initiatives with the Society in the past, and is a staunch proponent of the Free the Science initiative.
“One of the things that most attracted me to ECS was Free the Science,” Jannuzzi says. “I have worked for many years in scientific publishing and seen firsthand the upheavals open access mandates are having on technical societies that rely on subscription-based revenue to survive. But by endowing our publications operations through the Free the Science initiative, ECS has aligned how we fund publishing with our core mission to serve the public interest. This is precisely how nonprofits should act and one of the things that excites me most about my new role.”
Jannuzzi’s appointment renders ECS optimistic for the Society’s future—for all the ways it will grow and thrive under his leadership.
“It is quite humbling to follow in the footsteps of such a storied and long-tenured predecessor as Roque Calvo,” says Jannuzzi. “Thankfully, we will have time to work together prior to his departure, so I will be sure to learn all that I can from him. His insight and perspective will be invaluable to me as I transition into my new role.”
10 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
SOCIETY NEWS
Yue Kuo Promotes ECS in Russia and Belarus
During June 2018, ECS President Yue Kuo traveled to three institutes in Russia and Belarus to meet with leading researchers, promote ECS, discuss research, and explore opportunities for future collaborations and conferences.
First, on June 11, Kuo visited the A. N. Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences in Moscow, an institute home to many famous electrochemists. There, he attended a get-together hosted by Oleg Batishchev, deputy director of science at the institute, with some Russian members of ECS.
Kuo spoke with individuals who knew V. G. Levich, winner of the ECS Olin Palladium Award in 1973, as well as Vladimir Sergeevich Bagotsky, who was an ECS fellow. Several ECS members attended the meeting, including Tatiana Lastovina of Southern Federal University, who traveled over 1,000 km to meet the Society’s president.
Next, on June 14, Kuo traveled to St. Petersburg to visit the Ioffe Institute, a Russian national laboratory that boasts several Nobel Prize winners and a reputation equivalent to that of Bell Labs or the IBM T. J. Watson Research Laboratory. His visit was hosted by Mikhail Patrov, deputy science secretary of the institute.
While there, Kuo delivered a presentation on ECS’s history and current status to members of the institute. He was given tours of laboratories focused on solid state research and discussed the potential for ECS and the Ioffe Institute to collaborate to organize conferences.
Finally, on June 21, Kuo visited the B. I. Stepanov Institute of Physics of the National Academy of Sciences of Belarus in Minsk. He met with administrative leaders at the institute and gave a presentation on ECS, fielding several questions.



Many individuals Kuo met with expressed interest in collaboration with ECS. Yurii Kurochkin, vice president of the Belarusian Physical Society, entertained the idea of organizing a conference with ECS. Another researcher, an organizer of biannual plasma conferences, voiced potential interest in collaborating with ECS plasma symposium organizers. The leading researcher in the academy’s materials center, who traditionally published papers in European journals, conveyed interested in publishing in the Journal of The Electrochemical Society.

SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 11
Attendees of the meeting at the A. N. Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences in Moscow (left to right): Alexander A. Nekrasov (A. N. Frumkin Institute), Keith Stevenson (Skoltech), Buryak Aleksey Konstantinovich (A. N. Frumkin Institute), ECS President Yue Kuo (Texas A&M University), Tatiana Lastovina (Southern Federal University of Russia), Oleg Batishchev (A. N. Frumkin Institute), and Arkady Karyakin (M. V. Lomonosov Moscow State University).
ECS President Yue Kuo met with administrative leaders at the B. I. Stepanov Institute of Physics of the National Academy of Sciences of Belarus in Minsk. From left to right: Yurii Kurochkin, Irina Nikonchuk, Yue Kuo, and Nikolai Kazak
ECS President Yue Kuo (left) with Mikhail Patrov (right) during his visit to the Ioffe Institute in St. Petersburg.
ECS President Yue Kuo delivered a presentation on ECS to members of the B. I. Stepanov Institute of Physics.
Increasing Influence of ECS Journals: 2017 Impact Factors
The journal impact factors (JIFs) for the ECS journals continue to grow, as evidenced by the data recently released by Clarivate Analytics. For the 2017 reporting year, the ECS journals continue to be among the top-ranked journals. Journal of The Electrochemical Society (JES) is in the top two for Materials Science, Coatings, and Films, and in the top ten for Electrochemistry. The JIFs are published in Journal Citation Reports (JCR), and are just one metric used to gauge the quality of a large number of scholarly journals.
For JES, the JIF rose to 3.662 for 2017 from 3.259 for 2016, an increase of over 12%. The enduring quality and usefulness of the work published in JES is significant: JES has had a cited half-life of greater than 10 years (the highest figure that JCR records) for over eight years. JES is the fourth-most-cited journal in electrochemistry, with nearly 71,000 citations; that same citation count makes JES the second most-cited journal in materials science, coatings, and films. Downloads are another important metric for scholarly journals, and for the ECS journals, there were over 2.4 million downloads for 2017.


For the ECS Journal of Solid State Science and Technology (JSS), the progress is also impressive: over 40% growth in citations for 2017 over 2016. In terms of the JIF, the growth for JSS is striking: the JIF has grown to 1.808 for 2017, from 0.917 in 2013 when JSS was launched—a 97% increase in just four years.
The increasingly influential role that the ECS journals play is due to the Society’s practice of rigorous peer review, which is based on rapid, objective, and insightful feedback, as well as evaluation
for plagiarism. The relevance of the ECS journals is due to the increasing importance of the scientific content covered by the ECS journals: batteries and energy storage, corrosion, fuel cells and energy conversion, sensors, electrochemical engineering, organic and biological electrochemistry, physical and analytical electrochemistry, electrodeposition, nanocarbons, dielectrics, electronics and photonics, luminescence and display materials, and the many subdisciplines within each.
There is a great deal of discussion about the journal impact factors, including the lagging nature of the metric (the JIF looks only at three years of published articles and comes out six months after the end of the prior, or JIF, year). Many researchers, libraries, and funding agencies are debating the validity of the metric, as well as working on developing new ways of evaluating researchers for tenure and promotion.1-3 ECS is a member of the Declaration on Research Assessment (DORA, https://sfdora.org/) which “recognizes the need to improve the ways in which the outputs of scholarly research are evaluated. It is a worldwide initiative covering all scholarly disciplines and all key stakeholders including funders, publishers, professional societies, institutions, and researchers.”
ECS is grateful for the support of its authors, reviewers, editors, and publications staff. These are the people who are responsible for the high standards to which ECS adheres, and which keep the ECS journals growing in impact every year.
2017 KEY METRICS FOR ECS JOURNALS
Journal Impact Factor*
3.662 12% increase over 2016 10% increase in citations
JSS 1.808 97% increase in just four years 40% increase in citations
Quality* 8 years in a row for the Journal of The Electrochemical Society to obtain a cited half-life of greater than 10 years.
Downloads Over 2.4 million downloads from the
Citations*
Nearly 74,000 citations for both journals
Journal of The Electrochemical Society
2nd most-cited in Materials Science, Coatings, and Films
4th most-cited in Electrochemistry
Top Rankings*
Journal of The Electrochemical Society #2 in Materials Science, Coatings, and Films
#10 in Electrochemistry
*Source: 2017 Journal Citation Reports, Clarivate Analytics
1. P. Davis, “2017 Journal Impact Factors Feature Citation Distributions,” The Scholarly Kitchen, June 27, 2018, https://scholarlykitchen.sspnet.org/2018/06/27/2017journal-impact-factors-feature-citation-distributions/
2. J. Wilsdon, “Has the Tide Turned Towards Responsible Metrics in Research?” The Guardian, July 10, 2018, www.theguardian.com/science/political-science/2018/ jul/10/has-the-tide-turned-towards-responsible-metrics-in-research?CMP=Share_iOSApp_Other
3. D. Singh Chawla, “What’s Wrong with the JIF in 5 Graphs,” Nature Index, April 2, 2018, www.natureindex.com/news-blog/whats-wrong-with-the-jif-in-five-graphs
12 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org SOCIETY NEWS
Enduring
“”
JES
ECSarXiv and the Growth of Preprints
Preprints1 are growing at a faster rate than journal articles, according to a recent study from Crossref, a nonprofit organization that makes research outputs easy to find, cite, link, and assess.2 Why are preprints becoming popular? Preprints (on ECSarXiv and most reputable services) are discoverable and have a persistent link to their location on the Internet. (ECSarXiv registers its preprints with Crossref so they receive a DOI.) A preprint can be made available immediately (for ECSarXiv preprints, there’s a
quick moderation step) for grant/hiring committees, and they actually counter scooping by serving to “plant the flag” to claim your research. Preprints enhance visibility, especially for early-career researchers. They’re highly discoverable through Google Scholar, and a growing number of other databases. Preprints can come in many forms: as a classic preprint article (short or long) or even as a slide deck. Presenting your work through a preprint can aid in reproducibility: you can post new, confirmatory, or contradictory results. Learn more about ECSarXiv and our posting policies at www.electrochem.org/ecsarxiv.3
Take a look below at a sampling of preprints from ECSarXiv
Learnings from an Open Science Effort: Virtual Project on the History of ALD
Riikka Puurunen
Aalto University, School of Chemical Engineering, Department of Chemical and Metallurgical Engineering, Finland
DOI: 10.1149/osf.io/exyv3
This work summarizes learnings from an Open Science effort “Virtual project on the History of ALD” (VPHA), started in 2013 to clarify the early history of atomic layer deposition (ALD). ALD is a multi-tool of nanotechnology and has been e.g. enabler of the continuation of Moore’s law of transistor scaling. ALD has been
developed historically through two independent routes: atomic layer epitaxy (ALE) and molecular layering (ML). Especially the details on ML have remained little known to a broader audience. In this contribution, learnings in VPHA are seen from the viewpoint of its voluntary coordinator (the author self) related to historical details of ALD as well as from an organizational viewpoint and some other viewpoints. Selected details related to ALD’s history not fully accurately described in three earlier review articles are pointed out. The work made in VPHA has resulted in journal articles, presentations and an exhibition, and VPHA has in part provided the foundation for granting the 2018 Millennium Technology Prize to Dr. Tuomo Suntola. At the time of writing this contribution, in July 2018, VPHA is still on-going, and more volunteers are welcome to join the effort.
Nonlinear Electrochemical Impedance Spectroscopy of Lithium-Ion Batteries: Experimental Approach, Analysis, and Initial Findings
Matthew D. Murbach, Victor Hu, and Daniel T. Schwartz Department of Chemical Engineering & Clean Energy Institute, University of Washington, Seattle, WA, USA
DOI: 10.1149/osf.io/t635x
Nonlinear electrochemical impedance spectroscopy (NLEIS) is a moderate-amplitude extension to linear EIS that provides a sensitive and complementary whole-battery diagnostic for charge transfer kinetics, mass transport, and thermodynamics. We present the first full-frequency, second harmonic NLEIS spectra for lithium-ion batteries using commercially available, 1.5 Ah LiNMC|C cells. The mathematical framework for NLEIS shows, and experiments confirm, that moderate-amplitude input modulations can generate a second harmonic output that does not intrinsically corrupt the linear EIS response. Experimental measurements at varied states-of-charge (SoC) and states-of-health (SoH) are used to illustrate and compare NLEIS and EIS data.
(continued on next page)
Nyquist plots of simulated second harmonic spectra, for varying positive electrode anodic transfer coefficients, αa,pos (with αc,pos = 1 αa,pos).
1. A preprint is a version of a scholarly or scientific document or other preliminary communication not yet published in a peer-reviewed outlet.
2. Preprints growth rate ten times higher than journal articles, www.crossref.org/blog/preprints-growth-rate-ten-times-higher-than-journal-articles, May 31, 2018.
3. See the summer issue of Interface for a story on the launch of ECSarXiv, Summer 2018, 27, 2, doi:10.1149/2.006182if
SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 13
Direct-Gap Photoluminescence from a Si-Ge Multilayer Super Unit Cell Grown on Si0.4Ge0.6
D. J. Lockwood,a N. L. Rowell,a L. Favre,b A. Ronda,b and I. Berbezierb
aMeasurement Science and Standards, National Research Council, Ottawa, Ontario, Canada
bCNRS, Institut Matériaux Microélectronique Nanosciences de Provence, AMU, Marseille, France
DOI: 10.1149/osf.io/rt29s
Both Si and Ge possess indirect band gaps, which makes them very inefficient light emitters. One way to overcome this limitation is through band gap engineering. In this regard, M. d’Avezac et al. [Phys. Rev. Lett., 108, 027401 (2012)] predicted that a strained SiGe2Si2Ge2SiGen super unit cell on Si0.4Ge0.6 would have a direct and dipole-allowed gap of 0.863 eV, which is ideally suited for optical fiber applications. Here we report on the epitaxial growth of such a structure and its optical properties, for which purpose two similar samples were prepared by molecular beam epitaxy and solid phase epitaxy.
Top panels give AFM images of the MBE sample (left image) and SPE sample (right image). Each full image size is 5×5 µm2. The height scale has been normalized at 20 nm. Bottom panels give the two-dimensional roughness measurements on the full scale images from which the root-meansquare roughness (RMSR) is calculated: MBE sample (left panel) RMSR = 2.07 nm and SPE sample (right panel) RMSR = 0.46 nm. The inset shows a TEM cross-section image of the Si-Ge stacking layers in the SPE sample.

Multiphysics Modeling of Surface Finishing Performance in Pulsed-Waveform Electrochemical Machining
Brian Skinn, Timothy Hall, and E. J. Taylor
Faraday Technology, Inc., Englewood, OH, USA
DOI: 10.1149/osf.io/wybx6
Electrochemical machining (ECM) is a manufacturing technology that allows metal to be precisely removed by electrochemical oxidation and dissolution into an electrolyte solution. ECM is suited for machining parts fabricated from “difficult to cut” materials and/or parts with complicated and intricate geometries. In ECM, the workpiece is the anode and the tool is the cathode in an electrochemical cell; by relative movement of the shaped tool into the workpiece, the mirror image of the tool is “copied” or machined into the workpiece. One notable difficulty with ECM is an inability to predict a priori the tool and process parameters required in order to satisfy the final specifications of the fabricated part. Accordingly, there is potential value in development of a physical phenomenonbased design platform to predict optimal ECM tool shape. Such a capability is anticipated to dramatically shorten the process/tooling development cycle.
Using Exotic Materials Like EuD4TEA and MgD4TEA to Monitor Damage and Radiation Exposure in Extreme Environments

W. A. Hollerman,a R. S. Fontenot,b P. Darby,c N. Pugh,c and J. Millera aDepartment of Physics, University of Louisiana at Lafayette, Lafayette, LA, USA
bNaval Surface Warfare Center Carderock Division, West Bethesda, MD, USA
cCajun Advanced Picosatellite Experiment (CAPE), University of Louisiana at Lafayette, Lafayette, LA, USA
DOI: 10.1149/osf.io/94ujn
Space radiation poses a significant challenge to exploration missions to the Moon, Mars, and beyond. As we consider long duration space missions, the risk from radiation exposure increases. There is no simple technique that is capable of real-time radiation detection. Past research shows that the fluorescence yield and decay time are both functions of temperature and radiation fluence. Each of these parameters need to be investigated separately in order to fully understand the physical properties for any useful phosphor.
Once the temperature dependencies are known, then the radiation fluence in the surrounding environment can be estimated. This paper will discuss recent research on europium and magnesium tetrakis dibenzoylmethide triethylammonium (EuD4TEA and MgD4TEA) that could be used to measure radiation and temperature in real time. Both of these organic materials have been shown to be sensitive to proton radiation and to changes in temperature.
14 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org SOCIETY NEWS
(continued
from previous page)
Pictures of synthesized MgD4TEA shown in standard room light (left) and ultraviolet light (right).
Focus on Focus Issues
ECS publishes focus issues of the Journal of The Electrochemical Society (JES) and the ECS Journal of Solid State Science and Technology (JSS) that highlight scientific and technological areas of current interest and future promise. These issues are handled by a prestigious group of ECS technical editors and guest editors, and all submissions undergo the same rigorous peer review as papers in the regular issues. All focus issue papers are now published open access at no cost to the authors. ECS waives the article processing charge for all authors of focus issue papers as part of the Society’s ongoing Free the Science initiative.
Recent Focus Issues
• JES Focus Issue on Proton Exchange Membrane Fuel Cell (PEMFC) Durability. [JES 165(6) 2018] Thomas Fuller, JES technical editor; Jean St-Pierre, Deborah Myers, and Rodney Borup, guest editors. The majority of the papers in this issue focus on the core PEMFC components, the membrane-electrode assembly and the majority of its constituent materials: membrane, ionomer, catalyst, catalyst support, and microporous layer. Other papers pertain to accelerated stress tests and diagnostics, which are important aspects considering the relatively large cost associated with long-duration tests to quantify degradation resistance and to diagnose degradation mechanisms. Also covered in this issue are the effects of contaminants, the reactant streams’ membrane humidifier, and the effects of operating parameters on PEMFCs.
• JES Focus Issue on Ubiquitous Sensors and Systems for IoT. [JES 165(8) 2018] Rangachary Mukundan, JES technical editor; Ajit Kholsa, Praveen Kuman Sekhar, Peter Hesketh, Charles Henry, and Luca Magagnin, guest editors. Ubiquitous sensors are becoming an integral part of IoT applications, and progress in this domain can be seen each month. The promise is that everyone and everything will be connected via wireless data collection, and services like healthcare will be brought to everyone, everywhere, anytime, for virtually any need. These devices sense the environment and provide applications in home automation, home safety and comfort, and personal health. At a macro level they provide data for smart cities, smart agriculture, water conservation, energy efficiency and Industry 4.0, and Society 5.0. Other applications include supply chain management, transportation, and logistics.
• JES Focus Issue on the Brain and Electrochemistry Honoring R. Mark Wightman and Christian Amatore. [JES 165(12) 2018] Janine Mauzeroll, JES technical editor; Lili Deligianni, Michael Wolfson, Nick Langhals, and Mekki Bayachou, guest editors. Amatore’s and Wightman’s contributions to the advancement of bioelectrochemistry, specifically the advancement of electrochemical measurements in the field of neurochemistry are impressive. Their dedication to the training of new generations of bioelectrochemists resulted in an established legacy of highly
successful faculty and researchers worldwide. Several of the articles in this issue are contributions from former students, postdocs, and colleagues exemplifying the research directions that Amatore and Wightman have molded.
• JSS Focus Issue on Semiconductor-Based Sensors for Application to Vapors, Chemicals, Biological Species, and Medical Diagnosis. [JSS 7(7) 2018] Fan Ren, JSS technical editor; Yu-Lin Wang, Ajit Khosla, Rangachary Mukundan, and Toshiya Sakata, guest editors. The papers in this issue report on the novel and advanced sensor technologies that utilize semiconductor materials to enhance device characteristics for a multitude of diverse applications. The topics addressed in this issue include biomedical sensors, gas sensors, environmental monitoring and water quality control, optoelectronics, organic semiconductor devices, packages and circuits for semiconductor sensors, and clinical diagnostics using semiconductor sensors and read-out systems.
The following focus issue is currently in production with many papers already published in the ECS Digital Library (http://ecsdl.org).
• JES Focus Issue on Electrocatalysis—In Honor of Radoslav Adzic. [JES 165(15) 2018] David Cliffel and Thomas Fuller, JES technical editors; Minhua Shao, guest editor.


The following focus issues are open for submissions. Manuscripts may be submitted at http://ecsjournals.msubmit.net:

• JES Focus Issue on Advances in Electrochemical Processes for Interconnect Fabrication in Integrated Circuits. [JES 166(1) 2019] Charles Hussey, JES technical editor; Rohan Akolkar and Peter Broekmann, guest editors.
• JES Focus Issue on Selected Papers from IMLB 2018. [JES 166(3) 2019] Doron Aurbach, JES technical editor.
• JES Focus Issue on Semiconductor Electrochemistry and Photoelectrochemistry in Honor of Krishnan Rajeshwar. [JES 166(5) 2019] David Cliffel, JES technical editor; Ajit Khosla, Nianqiang (Nick) Wu, Heli Wang, and Csaba Janáky, guest editors.
Coming soon is the call for papers for the JSS Focus Issue on Chemical Mechanical Planarization (CMP) for Sub-10 nm Technologies
SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 15
To see the calls for papers for upcoming focus issues, for links to the published issues, or if you would like to propose a future focus issue, visit
www.electrochem.org/focusissues
Christian Amatore R. Mark Wightman
IoT
Technical
Editor Charles Hussey Reappointed

Charles L. Hussey has recently been reappointed as a technical editor of the Journal of The Electrochemical Society Hussey specializes in the electrochemical/ electroless deposition topical interest area. He is the associate dean for research and graduate education in the College of Liberal Arts at the University of Mississippi, where he also serves as a professor of chemistry. Hussey is an ECS fellow and a recipient of
Institutional Member
spotlight
TOC Capacitor Co., Ltd.
ECS would like to welcome TOC Capacitor Co., Ltd. as a new sustaining member!

TOC Capacitor Co., Ltd. was established in 2011 as a joint company between TPR Co., Ltd. and Okaya Electric Industries Co., Ltd. The office headquarters is located in Nagano, Japan. The company’s factory, development, and R&D are located in Osaka, Japan. Its products are electric double layer capacitors (EDLCs) with a main application of power regeneration for construction machines. The company is also performing R&D with a university to create new energy devices for IT equipment or elective vehicles.
Renewal Reminder

ECS encourages institutional members to renew their membership before the end of 2019. The institutional membership program provides academic, government, and industry partners access to research and the ECS community. Program benefits also include discounts on subscriptions, along with advertising, sponsorship, and
exhibiting discounts for ECS biannual meetings. ECS’s 50 institutional members have more than 300 member representatives. These representatives receive all ECS member benefits, including access to the ECS Digital Library and member pricing.

Contact Shannon.Reed@electrochem.org
to renew your institutional membership or inquire about the benefits of institutional membership for your organization.
the ECS Physical and Analytical Electrochemistry Division Max Bredig Award in Molten Salt and Ionic Liquid Chemistry. His current research focuses upon the electrochemistry and transport properties of a variety of room-temperature ionic liquids.
Hussey was first appointed as an ECS associate editor in 2000. Read more about his experiences as an editor—as well as his insights on peer review, the Free the Science initiative, and what distinguishes ECS journals from other journals in the field—in the spring 2018 issue of Interface.
Staff News
Ngoc Le joined ECS in April 2018 as the development associate. In this role, she is responsible for creating fundraising activities to support the Society’s goals and objectives. She will implement a comprehensive fundraising campaign for awards, grants, and the Free the Science initiative to support young scientists, scientific meetings, open access, and open science. She will work closely with the ECS executive director and leadership to engage donors and the community with ECS.

Le previously worked for the publisher Taylor and Francis Group in Philadelphia, PA, as a managing editor on the behavioral sciences portfolio. She oversaw more than 40 academic journals ensuring academic integrity, compliance, and growth. She also partnered with societies to grow their journals by developing strategic goals and budgets. She has implemented open science guidelines, resolved ethical complaints, and educated authors and editors on the concerns regarding the reproducibility crisis. Through this experience, Le found her passion for academic integrity and sustaining the future of research.
“We were attracted to Ngoc’s experience in scholarly publishing and her knowledge of nonprofit associations” says Roque Calvo, former executive director of ECS. “Her experience and knowledge will enable her to understand and effectively communicate our development message and the importance of our Free the Science initiative, which she is passionate about.”
16 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org SOCIETY NEWS
Discover
TheElectrochemicalSociety @ECSorg ecsblog.org Find out what’s trending in the field and interact with a like-minded community through the ECS social media pages.
Connect Share
SYMPOSIUM TOPICS & DEADLINES
K Organic and Bioelectrochemistry
Dallas, TX Sheraton Dallas
K01 Bioelectrochemistry: From Nature-Inspired Electrochemical Systems to Electrochemical Biosensors
K02 Electron-Transfer Activation in Organic and Biological Systems
K03 Young Investigators in Organic and Biological Electrochemistry
L Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
L01 Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry General Session and Grahame Award Symposium
L02 Impedance Technologies, Diagnostics, and Sensing Applications 5
L03 Computational Electrochemistry 5
L04 Polyoxometallates and Nanostructured Metal Oxides in Efficient Electrocatalysis, Energy Conversion, and Charge Storage
L05 Spectroelectrochemistry 4
L06 Supramolecular Materials
M Sensors
M01 Sensors, Actuators, and Microsystems General Session
M02 Semiconductor Electrochemistry and Photoelectrochemistry in Honor of Krishnan Rajeshwar - An Invited Symposium
M03 Sensors for Precision Medicine
Z General
Z01 General Student Poster Session
Z02 Sustainable Materials and Manufacturing 3
Z03 Nanoscale Electrochemical Imaging and Detection
Electrochemical Engineering
F01 Industrial Electrochemistry and Electrochemical Engineering General Session
F02 Tutorial on Industrial Electrochemistry
F03 Characterization of Porous Materials 8
F04 Multiscale Modeling, Simulation and Design 3: Enhancing Understanding, and Extracting Knowledge from Data
G Electronic Materials and Processing
G01 Silicon Compatible Emerging Materials, Processes, and Technologies for Advanced CMOS and Post-CMOS Applications 9
G02 Processes at the Semiconductor Solution Interface 8
and Devices 20
H02 Solid-state Electronics and Photonics in Biology and Medicine 6
H03 Wearable and Flexible Electronic and Photonic Technologies 2
I Fuel Cells, Electrolyzers, and Energy Conversion
I01 Hydrogen or Oxygen Evolution Catalysis for Water Electrolysis 5
I02 Materials for Low Temperature Electrochemical Systems 5
I03 Renewable Fuels via Artificial Photosynthesis or Heterocatalysis 4
I04 Energy Conversion Systems Based on Nitrogen 2
I05 Heterogeneous Functional Materials for Energy Conversion and Storage 2
I06 An Invited Symposium on Advances and Perspectives on Modern Polymer Electrolyte Fuel Cells – In Honor of Shimshon Gottesfeld
IMPORTANT DATES AND DEADLINES
Meeting Abstract submission opens ............................... August 2018
Meeting Abstracts submission deadline December 14, 2018
Notification to Corresponding Authors of abstract acceptance or rejection ...................... February 11, 2019
Technical Program published online February 2019
Meeting registration opens February 2019
ECS Transactions submission site opens February 15, 2019
Travel Grant application deadline ......................... February 25, 2019
ECS Transactions submission deadline March 15, 2019
Meeting Sponsor and Exhibitor deadline (for inclusion in printed materials) ............................ March 15, 2019
Travel Grant approval notification March 8, 2019
Hotel and Early Bird meeting registration deadlines

Release date for ECST issues
22, 2019
April
May 17, 2019 235th ECS Meeting – Dallas, TX ...............................May 26-31, 2019
Abstract Submission Now OPEN! www.electrochem.org/235 235th ECS Meeting
26-May 31, 2019
May
A Batteries and Energy Storage A01 Battery and Energy Technology Joint General Session A02 Lithium Ion Batteries and Beyond A03 Large Scale Energy Storage 10 A04 Battery Student Slam 3 A05 Battery Characterization A06 Battery Safety and Failure Modes B Carbon Nanostructures and Devices B01 Carbon Nanostructures
Energy
and Storage B02 Carbon Nanostructures
Biology
Carbon
From
Devices
Nano in Latin America
Fundamental Science to Applications B07 Light Energy Conversion with Metal Halide Perovskites, Semiconductor Nanostructures, and Inorganic/Organic Hybrid Materials B08 Porphyrins, Phthalocyanines, and Supramolecular Assemblies B09 Nano for Industry C Corrosion Science and Technology C01 Corrosion General Session D Dielectric Science and Materials D01 Chemical Mechanical Polishing 15 D02 Low Cost Photovoltaic Materials and Devices for Clean Energy E Electrochemical/Electroless Deposition E01 Electrodeposition for Advanced Node Interconnect Metallization Beyond Copper F
for
Conversion
in Medicine and
B03
Nanotubes -
Fundamentals to
B04
B05 Fullerenes - Endohedral Fullerenes and Molecular Carbon B06 2D Layered Materials from
G03 Organic Semiconductor Materials, Devices, and Processing 7 H Electronic and Photonic Devices and Systems H01 Wide Bandgap Semiconductor Materials
ECS Division Contacts
Battery
Christopher Johnson, Chair
Argonne National Laboratory
johnsoncs@cmt.anl.gov • 630.252.4787 (US)
Marca Doeff, Vice Chair Shirley Meng, Secretary
Brett Lucht, Treasurer
Doron Aurbach, Journals Editorial Board Representative
Corrosion
Sannakaisa Virtanen, Chair
Friedrich-Alexander-Universität Erlangen-Nürnberg virtanen@ww.uni-erlangen.de • +49 09131/85-27577 (DE)
Masayuki Itagaki, Vice Chair
James Nöel, Secretary/Treasurer
Gerald Frankel, Journals Editorial Board Representative
Dielectric Science and Technology
Vimal Chaitanya, Chair New Mexico State University vimalc@nmsu.edu • 575.635.1406 (US)
Peter Mascher, Vice Chair Uros Cvelbar, Secretary
Zhi David Chen, Treasurer
Peter Mascher, Journals Editorial Board Representative
Electrodeposition
Stanko Brankovic, Chair University of Houston srbrankovic@uh.edu • 713.743.4409 (US)
Philippe Vereecken, Vice Chair Natasa Vasiljevic, Secretary
Luca Magagnin, Treasurer Charles Hussey, Journals Editorial Board Representative
Electronics and Photonics
Colm O’Dwyer, Chair University College Cork c.odwyer@ucc.ie • +353 863.958373 (IE)
Junichi Murota, Vice Chair Robert Lynch, 2nd Vice Chair
Soohwan Jang, Secretary Yu-Lin Wang, Treasurer
Fan Ren, Journals Editorial Board Representative
Energy Technology
Andy Herring, Chair
Colorado School of Mines aherring@mines.edu • 303.384.2082 (US)
Vaidyanathan Subramanian, Vice Chair
William Mustain, Secretary Katherine Ayers, Treasurer
Thomas Fuller, Journals Editorial Board Representative
High-Temperature Energy, Materials, & Processes
Greg Jackson, Chair Colorado School of Mines gsjackso@mines.edu • 303.273.3609 (US)
Paul Gannon, Sr. Vice Chair Sean Bishop, Jr. Vice Chair
Cortney Kreller, Secretary/Treasurer
Raymond Gorte, Journals Editorial Board Representative
Industrial Electrochemistry and Electrochemical Engineering
John Staser, Chair Ohio University staser@ohio.edu • 740.593.1443 (US)
Shrisudersan Jayaraman, Vice Chair
Maria Inman, Secretary/Treasurer
Venkat Subramanian, Journals Editorial Board Representative
Luminescence and Display Materials
Mikhail Brik, Chair University of Tartu brik@fi.tartu.ee • + 372 737.4751 (EE)
Jakoah Brgoch, Vice Chair
Rong-Jun Xie, Secretary/Treasurer
Kailash Mishra, Journals Editorial Board Representative
Nanocarbons
Slava Rotkin Pennsylvania State University rotkin@psu.edu • 814.863.3087 (US)
Hiroshi Imahori, Vice Chair Olga Boltalina, Secretary R. Bruce Weisman, Treasurer
Francis D’Souza, Journals Editorial Board Representative
Organic and Biological Electrochemistry
Graham Cheek, Chair United States Naval Academy cheek@usna.edu • 410.293.6625 (US)
Diane Smith, Vice Chair
Sadagopan Krishnan, Secretary/Treasurer
Janine Mauzeroll, Journals Editorial Board Representative
Physical and Analytical Electrochemistry
Alice Suroviec Berry College asuroviec@berry.edu • 706.238.5869 (US)
Petr Vanýsek, Vice Chair Andrew Hillier, Secretary
Stephen Paddison, Treasurer
David Cliffel, Journals Editorial Board Representative
Sensor
Nianqiang (Nick) Wu, Chair West Virginia University nick.wu@mail.wvu.edu • 304.293.3111 (US)
Ajit Khosla, Vice Chair Jessica Koehne, Secretary
Larry Nagahara, Treasurer
Rangachary Mukundan, Journals Editorial Board Representative
18 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
NEWS
SOCIETY
New Division Officer Slates
New officers for the fall 2018–fall 2020 term have been nominated for the following divisions. All election results will be reported in the winter 2018 issue of Interface
Members-at-Large
Nick Birbilis, Monash University
Battery Division
Chair
Marca Doeff, Lawrence Berkeley National Laboratory
Vice Chair
Y. Shirley Meng, University of California, San Diego
Secretary
Brett Lucht, University of Rhode Island
Treasurer
Jie Xiao, Pacific Northwest National Laboratory
Members-at-Large (20 to be elected)
Khalil Amine, Argonne National Laboratory
Thomas Barrera, The Boeing Company
Josh Gallaway, Northeastern University
Dominique Guyomard, CNRS IEMN
Minoru Inaba, Doshisha University
Richard Jow, U.S. Army Research Laboratory
Kisuk Kang, Seoul National University
Robert Kostecki, Lawrence Berkeley National Laboratory
Prashant Kumta, University of Pittsburgh
Boryann Liaw, Idaho National Laboratory
Jun Lu, Argonne National Laboratory
Bryan McCloskey, Lawrence Berkeley National Laboratory
Tissaphern Mirfakhrai, Eaton Corp.
John Muldoon, Toyota Research Institute of North America
Jagjit Nanda, Oak Ridge National Laboratory
John Vaughey, Argonne National Laboratory
Gabriel Veith, Oak Ridge National Laboratory
Chao-Yang Wang, Pennsylvania State University
Martin Winter, Westfälische Wilhelms-Universität Münster
Kang Xu, Army Research Laboratory
Marina Yakovleva, FMC Corporation
Won-Sub Yoon, Sungkyunkwan University
Chair
Corrosion Division
Masayuki Itagaki, Tokyo University of Science
Vice Chair
James Noël, University of Western Ontario
Division Secretary/Treasurer
Dev Chidambaram, University of Nevada, Reno
Sean Brossia, INVISTA
Philippe Marcus, CNRS-ENSCP (UMR 7045)
H. Neil McMurray, Swansea University
Eiji Tada, Tokyo Institute of Technology
Chair
Sensor Division
Ajit Khosla, Yamagata University
Vice Chair
Jessica Koehne, NASA Ames Research Center
Secretary
Larry Nagahara, Johns Hopkins University
Treasurer
Praveen Sekhar, Washington State University, Vancouver
Members-at-Large
Sheikh Akbar, Ohio State University
Michael Carter, KWJ Engineering, Inc.

Pengyu Chen, Auburn University
Bryan Chin, Auburn University
Jay Grate, Pacific Northwest National Laboratory
Peter Hesketh, Georgia Institute of Technology
A. Robert Hillman, University of Leicester
Gary Hunter, NASA Glenn Research Center
Sangmin Jeon, Pohang University of Science and Technology
Mira Josowicz, Georgia Institute of Technology
Dong-Joo Kim, Auburn University
Jing Li, NASA Ames Research Center
Chung-Chiun Liu, Case Western Reserve University
Vadim Lvovich, NASA Glenn Research Center
Sushanta Mitra, University of Waterloo
Milad Navaei, Georgia Institute of Technology
Antonio Ricco, Stanford University
Michael Sailor, University of California, San Diego
Yasuhiro Shimizu, Nagasaki University
Aleksandr Simonian, National Science Foundation
Leyla Soleymani, McMaster University
Thomas Thundat, University of Alberta
Lok-kun Tsui, University of New Mexico
ECS Redcat Blog
The blog was established to keep members and nonmembers alike informed on the latest scientific research and innovations pertaining to electrochemistry and solid state science and technology. With a constant flow of information, blog visitors are able to stay on the cutting-edge of science and interface with a like-minded community.

www.electrochem.org/redcat-blog

SOCIETY
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 19
NEWS
Roque Calvo Next Generation Scholarship Fund





Without ECS support, 66% of travel grant recipients would not have been able to attend an ECS meeting.
Support student travel by making a donation today!
20 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
The Electrochemical Society and Toyota North America Announce 2018-2019 Fellowship Winners for Projects in Green Energy Technology

The ECS Toyota Young Investigator Fellowship, a partnership between The Electrochemical Society and Toyota Research Institute of North America, a division of Toyota Motor North America, is in its fourth year. The fellowship aims to encourage young professors and scholars to pursue research in green energy technology that may promote the development of nextgeneration vehicles capable of utilizing alternative fuels.
Electrochemical research has already informed the development and improvement of innovative batteries, electrocatalysts, photovoltaics, and fuel cells. Through this fellowship, ECS and Toyota hope to see further innovative and unconventional technologies borne from electrochemical research.
The ECS Toyota Young Investigator Fellowship Selection Committee has chosen two recipients to receive the 20182019 fellowship awards for projects in green energy technology. The awardees are Kimberly See, California Institute of Technology, and Iryna Zenyuk, University of California, Irvine.

The selected fellows will receive restricted grants of $50,000 to conduct the research outlined in their proposals within one year. They will also receive a one-year complimentary ECS membership as well as the opportunity to present and/or publish their research with ECS.
The ECS Toyota Young Investigator Fellowship is an annual program, and the 2019-2020 request for proposals will be released in the fall of 2018.
Kimberly See, California Institute of Technology

ECS Battery Division
“Structural Distortions in Multi-Electron Cathodes for High Capacity Batteries”
Iryna Zenyuk, University of California, Irvine
ECS Energy Technology Division
“Addressing the Activation
Overpotential in Fuel Cell Cathodes”
Special Thanks to the 2018-2019 ECS Toyota Young Investigator Fellowhip Selection Committee
John Muldoon, Toyota Research Institute of North America, Chair
Koji Suto, Toyota Research Institute of North America
Tomoyuki Nagai, Toyota Research Institute of North America
Tim Arthur, Toyota Research Institute of North America
Peter Pintauro, Vanderbilt University, ECS Energy Technology Division
Brian McCloskey, University of California, Berkeley, ECS Battery Division
John T. Vaughey, Argonne National Laboratory, ECS Battery Division
Silver/Silver Chloride

Reference Electrode
Stable Reference
0.047 Volts vs. SCE
Widely used
Non-toxic
Custom glass available
SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 21
Always in stock. Made in USA. “Fine electrochemical probes since 1966” www.koslow.com
Your article. Online. FAST! More than 141,000 articles in all areas of electrochemistry and solid state science and technology from the only nonprofit publisher in its field. ECS Proceedings Volumes Over 450 historic proceedings volumes have been added to the archival content available through the ECS Digital Library. Visit www.ecsdl.org to learn more.
websites of note
by Alice H. Suroviec
Dryad
• The Dryad Digital Repository is a curated resource that makes the data underlying scientific publications discoverable, freely reusable, and citable. Dryad provides a general-purpose home for a wide diversity of data types. Dryad’s vision is to promote a world where research data is openly available, integrated with the scholarly literature, and routinely reused to create knowledge. www.datadryad.org
CEDI University
• CEDI University is a single website that has tutorials and frequently asked questions about continuous electrodeionization (CEDI). This technology uses ion exchange membranes for water treatment. The website covers the history of the technology, how it works, as well as the possible future directions. www.cediuniversity.com

Electrochemical Education
• If you are looking for a teaching lab to use in one of your courses, this website by Pine Research provides several different exercises for use in the college labs. The exercises cover a variety of topics and would be of use in both general chemistry and advanced chemistry labs. www.pineresearch.com/shop/knowledgebase/laboratory-exercises/
About the Author
Alice Suroviec is an associate professor of bioanalytical chemistry and chair of the Department of Chemistry and Biochemistry at Berry College. She earned a BS in chemistry from Allegheny College in 2000. She received her PhD from Virginia Tech in 2005 under the direction of Mark R. Anderson. Her research focuses on enzymatically modified electrodes for use as biosensors. She is currently the chair of the ECS Physical and Analytical Electrochemistry Division and an associate editor for the physical and analytical electrochemistry, electrocatalysis, and photoelectrochemistry topical interest area of the Journal of The Electrochemical Society. She may be reached at asuroviec@berry.edu.

https://orcid.org/0000-0002-9252-2468
UPCOMING ECS SPONSORED MEETINGS
In addition to the ECS biannual meetings and ECS satellite conferences, ECS, its divisions, and its sections sponsor meetings and symposia of interest to the technical audience ECS serves. The following is a partial list of upcoming sponsored meetings. Please visit the ECS website (www.electrochem.org/upcoming-meetings/) for a list of all sponsored meetings.
2018
• 69th Meeting of the International Society of Electrochemistry; September 2-7, 2018; Bologna, Italy; http://annual69.ise-online.org/; ECS–ISE Joint Symposium: Theory: From Understanding to Optimization and Prediction
• International Conference on Solid State Devices and Materials (SSDM); September 9-13, 2018; Tokyo, Japan; www.ssdm.jp/index.html
• Redox Films for Energy Conversion –Bioelectrochemical and Molecular Systems; September 10-11, 2018; Marseille, France; https://redox-shields.org/
• III Colombian Congress of Electrochemistry; October 2-5, 2018; Cali, Colombia; https://sites.google.com/view/cceq2018/
• 7th Baltic Electrochemistry Conference: Finding New Inspiration (BEChem 2018); November 4-7, 2018; Tartu, Estonia; http://BEChem2018.ut.ee/
2019
• International Battery Association meeting (IBA 2019); March 3-8, 2019; San Diego, CA; www.international-battery-association.org/
To learn more about what an ECS sponsorship could do for your meeting, including information on publishing proceeding volumes for sponsored meetings, or to request an ECS sponsorship of your technical event, please contact ecs@electrochem.org.
22 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org SOCIETY NEWS
research
Intheissueof
• The winter 2018 issue of Interface will feature the ECS Electronics and Photonics Division. The issue will be guest edited by Jennifer Hite from the U.S. Naval Research Laboratory. The issue will be titled “Frontiers in Electronics with Focus Articles on Atomic Layer Deposition, Power Electronics, and 2D Materials,” and is scheduled to include the following articles (titles are tentative): “Emerging Technologies for Nanofabrication: MLD, SAMs, and CCD,” by Alain E. Kaloyeros, Jonathan Goff, and Barry Arkles; “GaN Power Devices – Current Status and Future Directions,” by Travis Anderson and Srabanti Chowdhury; “Cheap Ultra-Wide Bandgap Power Electronics? Gallium Oxide May Hold the Answer,” by Marko Tadjer; and “The Future of Electronics in the Flatland: 2D Semiconductors Materials in Next-Generation CMOS,” by Paul Hurley and Colm O’Dwyer.

The PAT-Chamber-16
• Highlights from AiMES 2018, the ECS and SMEQ joint international meeting. News and photos from the upcoming meeting taking place in Cancun, Mexico. (See page X of this issue for a preview of the meeting.)
• A report on the First International Conference on 4D Materials and Systems, an ECS cosponsored meeting happening August 26-30, 2018, in Yonezawa, Japan.
• The 2018 ECS Summer Fellowship Reports from the recipients of the 2018 Colin Garfield Fink Fellowship, the 2018 Edward G. Weston Fellowship, the 2018 F. M. Becket Fellowship, the 2018 Joseph W. Richards Fellowship, and the 2018 H. H. Uhlig Fellowship.
SOCIETY NEWS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 23
Temperature controlled docking station • Temperature range: 10 - 80°C • Ready for up to 16 PAT-Cells or PAT-Cell-Press for pressure monitoring • Integrated data logger • Compatible with all of today´s potentiostats and battery testers Please visit our website for more information: el-cell.com/products/docking-stations/pat-chamber-16
CANCUN MEXICO
September 30-October 4, 2018
Moon Palace Resort
The Americas International Meeting on Electrochemistry and Solid State Science (AiMES 2018), is a joint conference of the 234th Meeting of The Electrochemical Society (ECS), the XXXIII Congreso de la Sociedad Mexicana de Electroquimica (SMEQ), and the 11th Meeting of the Mexico Section of The Electrochemical Society, with the technical cosponsorship of the Sociedade Brasileira de Eletroquímica e Eletroanalítica (SBEE), the Sociedad Iberoamericana de Electroquimica (SIBAE), and the Asociación Colombiana de Electroquímica (ACEQ).

Join us at this joint international conference of ECS and SMEQ, as scientists, engineers, and researchers from academia, industry, and government laboratories come together to share results and discuss issues on related topics through a variety of formats, such as oral presentations, poster sessions, panel discussions, tutorial sessions, short courses, professional development workshops, and exhibits. The unique blend of electrochemical and solid state science and technology at an AiMES meeting provides an opportunity and forum to learn and exchange information on the latest scientific and technical developments in a variety of interdisciplinary areas.
If that isn’t enough, you will be spending your time at the beautiful Moon Palace Resort, a gigantic allinclusive luxury resort located along Cancun’s Mayan Riviera and set amidst 55 acres of lush tropical foliage. Just minutes from the Cancun International Airport, it features a full-service spa, a 27-hole golf course, a larger-than-life pool, and numerous restaurants and dining options, making for an unforgettable experience.
Highlights
• Five days of technical programming across 53 symposia
• Over 2,100 abstracts
• More than 1,700 oral presentations, with 400+ invited speakers
• 450+ posters during three evenings of poster sessions
• 14 hours of exhibit hall time over three days
• Daily morning and afternoon coffee breaks and networking breaks
• Complimentary Wi-Fi in meeting rooms
• Special program for nontechnical registrants
• All meals and drinks at the Moon Palace, including alcoholic beverages
• Special program for non-technical registrants
The ECS Lecture
Monday, October 1
“Electro- and Photo-Electro-Chemical Generation of the Fenton Reagent. Some Approaches for the Development of Electrochemical Based Advanced Oxidation Processes for Water Treatment”
Luis A. Godínez, Center of Research and Technological Development in Electrochemistry

Luis A. Godínez was born in Mexico City in 1967. After completing a chemical engineering degree and an MSc degree in physical chemistry at the National Autonomous University of Mexico, he moved to the U.S. to pursue graduate studies at the University of Miami, where he obtained a PhD in physical chemistry, and later completed a postdoctoral appointment at the University of South Florida, after which he moved back to Mexico to work as a researcher at the Center of Research and Technological Development in Electrochemistry (CIDETEQ).
The work that will be presented deals with electrochemical advanced oxidation processes that employ electro-Fenton-based reactions to treat wastewater. The explored approach employs packed activated carbon columns as cathode materials for oxygen reduction and iron-loaded resins as the source of the Fenton mixture which produces a powerful oxidant species. Using this approach, novel activated carbon regeneration processes were developed and electrochemical reactors were designed for the removal of persistent contaminants, such as helminth ova in human wastewater. The technology potential of the electro-Fenton approach is important because water management is particularly complicated in a country like Mexico, in which there are serious problems for water accessibility as well as industry-related pollution.
Award Winning Speakers
(Check the meeting app for times)
SOCIETY AWARD WINNING SPEAKERS
Tetsuya Osaka, Waseda University
ECS Edward Goodrich Acheson Award
Michael Arnold, University of Wisconsin-Madison
ECS Charles W. Tobias Young Investigator Award
DIVISION AND SECTION AWARD WINNING SPEAKERS
Yongyao Xia, Fudan University
ECS Battery Division Technology Award
Feng Pan, Peking University
ECS Battery Division Technology Award
Kang Xu, Army Research Lab & University of Maryland
ECS Battery Division Research Award
Haodong Liu, University of California San Diego
ECS Battery Division Postdoctoral Associate Research Award
Sponsored by MTI Corporation and the Jiang Family Foundation
24 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018
l eS a r ch “ ECSmobil e” in your app s t o er l GETTHE ECSAPP!
David Bock, Brookhaven National Laboratory
ECS Battery Division Postdoctoral Associate Research Award
Sponsored by MTI Corporation and the Jiang Family Foundation
Fudong Han, University of Maryland
ECS Battery Division Student Research Award Sponsored by Mercedes-Benz Research & Development
Steven Lacey, University of Maryland
ECS Battery Division Student Research Award Sponsored by Mercedes-Benz Research & Development
Rudolph Buchheit, The Ohio State University
ECS Corrosion Division H. H. Uhlig Award
Rebecca Schaller, University of British Columbia
ECS Corrosion Division Morris Cohen Graduate Student Award
Nosang Myung, University of California, Riverside
ECS Electrodeposition Division Research Award
Jon Ustarroz, Vrije Universiteit Brussel
ECS Electrodeposition Division Early Career Investigator Award
Meilin Liu, Georgia Institute of Technology
ECS High-Temperature Energy, Materials, & Processes Division
J. Bruce Wagner, Jr. Award
Pieter Dorenbos, Technische Universiteit Delft
ECS Luminescence and Display Materials Division Centennial
Outstanding Achievement Award
Robin Rogers, University of Alabama
ECS Physical and Analytical Electrochemistry Division Max Bredig
Award in Molten Salt and Ionic Liquid Chemistry
Joseph Wang, University of California San Diego
ECS Sensor Division Outstanding Achievement Award
Wolfgang Schuhmann, Ruhr-Universität Bochum
ECS Europe Section Alessandro Volta Medal
Short Courses
Sunday, September 30
ECS short courses are all-day classes designed to provide students or seasoned professionals with an in-depth education on a wide range of topics. Taught by academic and industry experts, the small classes make for excellent opportunities for personalized instruction, helping both novices and experts advance their technical expertise and knowledge.
SHORT COURSE 1
Basic Impedance Spectroscopy
Mark Orazem, Instructor
SHORT COURSE 2
Fundamentals of Electrochemistry:
Basic Theory and Kinetic Methods

Jamie Noël, Instructor
Professional Development Workshops
(Check the meeting app for times.)
Offered at each of the biannual Society meetings, the professional development workshops help to serve the career needs of our attendees. These workshops are available whether you are a student looking for some help with your resume or a mid-career researcher looking for a refresher on team management.
• Essential Elements for Employment Success
• Strategic Tools for a Successful Career
• Managing and Leading Teams
• Running an Effective Meeting
• Resume Review
Special Events
(Check the meeting app for times.)
The 8th Annual Electrochemical Energy Summit (E2S) – Sustainable and Responsible Supply of Energy Storage Materials, Components, and Devices
The ECS Electrochemical Energy Summit brings together policy makers and researchers to educate about the critical issues of energy needs and the pivotal research in electrochemical energy that can address societal needs (see page 29).
Opening Reception
Come join us for an evening of fun as we kick off what is sure to be a great week. This is an excellent opportunity to meet old and new friends, so make sure to stop by for some food, drinks, and great conversation!
ECS Data Science Showcase
The ECS Data Science Showcase will feature invited speakers from the data science and open source community and past participants of the ECS Data Sciences programs (see page 28).
Student Mixer
The mixer is a must-attend event for students! Enjoy networking with your peers and early-career professionals while light desserts and refreshments are served.
General and Student Poster Sessions
With hundreds of posters to explore, you won’t want to miss a single minute of these sessions. Grab a drink, wander the aisles, review the presentations, talk to the authors, and share some laughs. These sessions are a great way to end the day!
Cena Baile and President’s Reception
Enjoy the local tastes of Mexico and say “Hola!” to your peers as we celebrate AiMES. Drinks and food will be served in a pictureperfect setting for this memorable event featuring live music. After a vigorous week of attending technical sessions, take this opportunity to dance and drink along with your fellow attendees.
Receptions in Honor of Battery, Corrosion, and PAE/Max Bredig Division Award Winners
Join us in honor of these outstanding scientists, teachers, and friends.
Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 25
Technical Symposia
Symposium Topics, Organizers, and Sponsoring Divisions
A — Batteries and Energy Storage
A01 Battery and Energy Technology Joint General Session
M. Doeff, N. Imanishi, X. Xiao, P. Poizot, M. Manivannan, S. Narayan
Battery, Energy Technology
A02 Large-Scale Energy Storage 9
Challenges in Novel Electrolytes, Organic Materials, and Innovative Chemistries for Batteries - in Honor of Michel Armand
D. Guyomard, V. Di Noto, T. Rojo, M. Forsyth, P. Poizot, K. Zaghib
Battery, Energy Technology, Physical and Analytical Electrochemistry
A03 Fast Electrochemical Processes and Devices (Electrochemical Capacitors and Batteries) 2
D. Bélanger, J. W. Long, T. Brousse, A. Balducci, P. Simon, W. Sugimoto, N. Wu, H. Armando Mosqueda
Battery
A04 Lithium-Ion Batteries
Y. Meng, J. Allen, F. Lin, M. Inaba, G. Chen
Battery
A05 Beyond Lithium-Ion Batteries
B. D. McCloskey, D. Lu, J. Liu, M. Yakovleva, P. J. Kulesza
Battery, Energy Technology, Physical and Analytical Electrochemistry
A06 Electrolytes and Interfaces in Lithium Ion Batteries
B. L. Lucht, R. T. Jow, J. Xiao, K. Xu
Battery
A07 Battery Safety and Failure Modes
A08
T. Barrera, C. Wang, Y. Kobayashi, B. Liaw
Battery, Industrial Electrochemistry and Electrochemical Engineering
Physicochemical Modelling of Novel Components and Devices for Energy Storage: from Atomistic Level to Macroscopic Processes
P. B. Balbuena, J. G. Vázquez, A. A. Franco, S. J. Paddison, S. D. Minteer, R. Longo
Battery, Energy Technology, Physical and Analytical Electrochemistry
B Carbon Nanostructures and Devices
B01 Carbon Nanostructures: From Fundamental Studies to Applications and Devices
S. Rotkin, H. Imahori, O. V. Boltalina, D. E. Cliffel
Nanocarbons, Physical and Analytical Electrochemistry
C Corrosion Science and Technology
C01 Corrosion General Session
S. Virtanen, M. Itagaki
Corrosion
C02 Pits & Pores 8: Nanomaterials – Fabrication, Properties, and Applications
P. Granitzer, R. Boukherroub, D. J. Lockwood, H. Masuda, S. Virtanen
Corrosion, Luminescence and Display Materials

C03 Corrosion Protection
H. N. McMurray, N. Birbilis, M. Itagaki, R. Orozco-Cruz, F. J. Rodriguez-Gomez
Corrosion, SMEQ
C04 Passivity and Localized Corrosion
S. Fujimoto, J. Noel, E. Tada Corrosion
C05 Electrochemical Techniques in Corrosion Research
M. Itagaki, D. Chidambaram
Corrosion
D Dielectric Science and Materials
CD/USB
E — Electrochemical/Electroless Deposition
E01 Magnetic Materials Process and Devices 15
W. Schwarzacher, J. Leddy, S. R. Brankovic
Electrodeposition, Physical and Analytical Electrochemistry
E02 Electroless Deposition 5
S. S. Djokic, L. Magagnin
Electrodeposition
E03 Electrodeposition for Energy Application 4
N. Vasiljevic, S. Brankovic, K. Xu
Electrodeposition, Battery, Energy Technology
E04 Electroplating of Reactive Metals and Alloys for Energy Storage
P. M. Vereecken, D. A. Steingart
Electrodeposition, Battery, Energy Technology
E05 Electrodeposition for Additive Manufacturing
Q. Huang, M. Inman, T. M. Braun
Electrodeposition, Industrial Electrochemistry and Electrochemical Engineering
F Electrochemical Engineering
F01 Electrochemical Engineering General Session
J. A. Staser, V. R. Subramanian, D. P. Riemer
Industrial Electrochemistry and Electrochemical Engineering CD/USB
F02 Electrochemical Separations 2
H. Xu, J. Whitacre, A. H. Suroviec
Industrial Electrochemistry and Electrochemical Engineering, Energy Technology, Physical and Analytical Electrochemistry
F03 Electrochemical Treatment of Contaminated Water, Soil, and Air: State of the Art and Trends in Research and Technology of Environmentally Oriented Electrochemical Approaches
J. M. Peralta-Hernández, P. Atanassov
Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry, SMEQ
F04 Characterization of Electrochemical Reactors: Fluid Dynamics and Current Distribution
J. A. Staser, J. L. Nava
Industrial Electrochemistry and Electrochemical Engineering, Energy Technology, SMEQ CD/USB
G Electronic Materials and Processing
G01 Semiconductor Wafer Bonding: Science, Technology, and Applications 15
C. Tan, T. Suga, H. Baumgart, F. Fournel, M. S. Goorsky, K. D. Hobart, R. Knechtel
Electronics and Photonics
G02 Atomic Layer Deposition Applications 14
F. Roozeboom, S. De Gendt, J. Dendooven, J. W. Elam, O. van der Straten, C. Liu, A. Illiberi
CD/USB
Electronics and Photonics, Dielectric Science and Technology CD/USB
G03 SiGe, Ge, and Related Compounds: Materials, Processing, and Devices 8
D. L. Harame, Q. Liu
Electronics and Photonics
G04 Thermoelectric and Thermal Interface Materials 4
CD/USB
C. O’Dwyer, J. He, K. M. Razeeb, R. Chen, J. Lee, S. Calabrese Barton, Y. Wu
Electronics and Photonics, Dielectric Science and Technology
D01 Semiconductors, Dielectrics, and Metals for Nanoelectronics 16
D. Misra, S. De Gendt, M. Houssa, K. Kita, D. Landheer, S. Van Elshocht, S. Dayeh
Dielectric Science and Technology, Electronics and Photonics CD/USB
D02 Photovoltaics for the 21st Century 14
M. Tao, T. Druffel, J. M. Fenton, Z. Chen, V. Subramanian, J. Park, H. Hamada
Dielectric Science and Technology, Energy Technology
D04 Photovoltaics for the 21st Century 14
M. Tao, T. Druffel, J. M. Fenton, Z. Chen, V. Subramanian, J. Park, H. Hamada
Dielectric Science and Technology, Energy Technology
D06 Surface Characterization and Manipulation for Electronic Applications
C. A. Hacker, Y. S. Obeng
Dielectric Science and Technology, Electronics and Photonics
CD/USB
G05 Materials, Formulation, and Processes for Semiconductor, 2.5 and 3D Chip Packaging, and High Density Interconnection PCB
W. Dow, G. Mathad, K. Kondo, M. Hayase, M. Koyanagi, F. Roozeboom, R. Akolkar, S. Armini, Y. Takeno, L. Wei
Electronics and Photonics, Dielectric Science and Technology
H Electronic and Photonic Devices and Systems
CD/USB
H01 H01 – State-of-the-Art Program on Compound Semiconductors 61 (SOTAPOCS 61)
T. J. Anderson, J. K. Hite, E. A. Douglas, S. H. Kilgore, C. O’Dwyer
Electronics and Photonics
H02 High Purity and High Mobility Semiconductors 15
E. Simoen, O. Kononchuk, O. Nakatsuka, C. Claeys
Electronics and Photonics
H03 Thin Film Transistor Technologies 14 (TFTT 14)
Y. Kuo
Electronics and Photonics
CD/USB
CD/USB
CD/USB
CD/USB
26 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018
H04 Low-Dimensional Nanoscale Electronic and Photonic Devices 11
Y. Chueh, C. O’Dwyer, J. He, M. Suzuki, S. Jin, S. Kim, J. C. Ho, Z. Fan, Q. Li, G. W. Hunter, K. Takei, J. Wu
Electronics and Photonics
H05 Gallium Nitride and Silicon Carbide Power Technologies 8
M. Dudley, M. Bakowski, K. Shenai, N. Ohtani, B. Raghothamachar
Electronics and Photonics
H06 Fundamentals and Applications of Microfluidic and Nanofluidic Devices 4
S. W. Joo, X. Xuan, S. Qian, H. Baumgart, P. Vanýsek
CD/USB
J Luminescence and Display Materials, Devices, and Processing
J01 Luminescence and Display Materials: Fundamentals and Applications
M. Brik, M. Raukas, M. Bettinelli, A. M. Srivastava
Luminescence and Display Materials
J02 Visible and Infrared Phosphor Research and Applications
K. C. Mishra, J. Collins, A. A. Setlur, A. Piquette
CD/USB
Electronics and Photonics, Energy Technology, Physical and Analytical Electrochemistry
H07 Electronic, Thermal, and Electrochemical Properties of Metal Organic Frameworks (MOFs): Technology, Applications, and Emerging Devices 1
E. Redel, H. Baumgart, G. Wittstock, C. Wöll, J. Liu, H. Kitagawa, M. D. Allendorf
Electronics and Photonics, Energy Technology, Organic and Biological Electrochemistry, Physical and Analytical Electrochemistry
I Fuel Cells, Electrolyzers, and Energy Conversion
I01A Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Diagnostics/Characterization Methods, MEA Design/Model
F. N. Büchi, H. A. Gasteiger, A. Z. Weber, P. Shirvanian Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01B Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Cells, Stacks and Systems
K. Swider-Lyons, J. M. Fenton, T. F. Fuller, K. Shinohara Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01C Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Cation-Exchange Membrane Performance and Durability
P. N. Pintauro Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01D Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Catalyst Activity/Durability for Hydrogen (-Reformate) Acidic Fuel Cells
H. Uchida, P. Strasser, C. Coutanceau, S. Mitsushima, Y. Kim Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01E Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Materials for Alkaline Fuel Cells and Direct-Fuel Fuel Cells
T. J. Schmidt, R. A. Mantz, S. Narayan, V. Ramani Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01F Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Polymer-Electrolyte Electrolysis
B. S. Pivovar, K. E. Ayers, H. Xu Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I01Z Polymer Electrolyte Fuel Cells and Electrolyzers 18 (PEFC&E 18)Plenary Talks
D. Jones, H. A. Gasteiger, H. Uchida, T. J. Schmidt, F. N. Büchi, K. Swider-Lyons, B. S. Pivovar, P. N. Pintauro, V. Ramani, J. M. Fenton, P. Strasser, K. E. Ayers, A. Z. Weber, T. F. Fuller, R. A. Mantz, H. Xu, C. Coutanceau, S. Mitsushima, S. Narayan, P. Shirvanian, Y. Kim, Y. Gochi-Ponce
Energy Technology, Battery, Industrial Electrochemistry and Electrochemical Engineering, Physical and Analytical Electrochemistry
CD/USB
I02 Solid State Ionic Devices 12
E. Traversa, C. R. Kreller, J. Nanda
High-Temperature Energy, Materials, & Processes, Battery, Energy Technology
Luminescence and Display Materials

K Organic and Bioelectrochemistry
K02 Electron Transfer Activation in Organic and Bioorganic Systems: From Unraveling Electrode Mechanisms to Directing Synthesis of High-Value Products
D. K. Smith, M. Bayachou, G. T. Cheek, A. H. Suroviec, M. T. Oropeza-Guzman
Organic and Biological Electrochemistry, Physical and Analytical Electrochemistry, SMEQ
L Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
L01 Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry General Session
A. H. Suroviec, A. C. Hillier, M. Stoytcheva
Physical and Analytical Electrochemistry, SMEQ
L02 Molten Salts and Ionic Liquids 21
W. M. Reichert, R. A. Mantz, P. C. Trulove, L. M. Haverhals, E. Biddinger, H. C. De Long, A. H. Suroviec, M. Mizuhata, M. Ueda, A. Ispas, A. Bund
Physical and Analytical Electrochemistry, Energy Technology, Industrial Electrochemistry and Electrochemical Engineering CD/USB
L04 Photocatalysts, Photoelectrochemical Cells, and Solar Fuels 9
N. Wu, P. J. Kulesza, J. Lee, E. L. Miller, V. Subramanian, T. Tatsuma, H. Wang, M. Manivannan
Physical and Analytical Electrochemistry, Energy Technology
L05 Electroactive and Redox Active Polymers 2
J. Jiang, A. M. Herring, A. H. Suroviec, S. D. Minteer, N. Wu, D. Zhan
Physical and Analytical Electrochemistry, Energy Technology
M Sensors
M01 Chemical Sensors 14: Chemical and Biological Sensors and Analytical Systems
A. Simonian, R. Stefan-van Staden, P. Vanysek, S. Mitra, M. Bayachou, R. Mukundan, P. Chen, A. H. Suroviec
Sensor, Organic and Biological Electrochemistry, Physical and Analytical Electrochemistry
M02 Wearable Sensors and Systems 1 -and- Microfabricated and Nanofabricated Systems for MEMS/NEMS 14
A. Khosla, S. A. Akbar, J. E. Koehne, P. Hesketh, M. Navaei,
CD/USB
P. K. Sekhar, D. Kim, J. Choi, S. D. Minteer, P. Vanýsek, P. C. Trulove, R. Pratap Sensor, Organic and Biological Electrochemistry, Physical and Analytical Electrochemistry CD/USB
Z General Topics
Z01 General Student Poster Session
V. R. Subramanian, K. B. Sundaram, V. Chaitanya, P. Pharkya, A. H. Suroviec, R. Galván-Martínez
All Divisions, SMEQ
Z02 Nanotechnology General Session
O. Leonte, Z. Chen, C. Bock, J. E. Koehne
All Divisions, Interdisciplinary Science and Technology Subcommittee
Z03 Electrochemical Energy Summit: Sustainable and Responsible Supply of Energy Storage Materials, Components, and Devices
C. Bock, M. Doeff, L. A. Diaz
All Divisions, Interdisciplinary Science and Technology Subcommittee
Symposia publishing an issue of ECS Transactions are indicated with a CD/USB icon. These issues are available for purchase in CD or USB format. All preordered CD or USB editions must be picked up at the meeting. Issues of ECST are also available for purchase as an instant PDF download through the ECS Online Store
www.electrochem.org/online-store
Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 27
Monday, October 1
DATA SCIENCE SHOW CASE Cancun, Mexico
ECS Data Science Showcase
The ECS Data Science Showcase will highlight new electrochemical and solid state research and the open source software and datasets that underpin the work. Research talks will be complemented by a demonstration on how others can access, use, modify, and improve the opensource tools and data associated with the research project. The ECS Data Science Showcase is being led by the very capable and engaging team from the University of Washington—Matt Murbach, David Beck, and Dan Schwartz—who have brought you Hack Day (fall 2017) and Hack Week (spring 2018).
Because data science is a fast moving field, the Showcase will conclude with a “Late Breaking News” session to feature emerging electrochemical and solid state research that embraces open software and open data sets. Especially valued are contributions that feature earlystage research where alpha- and beta-testers can be solicited to improve new open research products. The organizers welcome direct email contact by prospective contributors to the Late Breaking News section, up to four weeks prior to the event.
Organizers
Detailed Chemistry Modeling via Cantera: A Pathway to Understanding Battery Degradation Mechanisms
Steven C. DeCaluwe,1 Daniel Korff,1 Amy LeBar,1 and Christopher
H. Lee1,2
1 Colorado School of Mines, Department of Mechanical Engineering
2 University of Colorado, Colorado Springs, Department of Computer Science
A Data Science Approach for Quantitative Analysis of Total Differential Capacity Plots
N. L. Thompson,1 S. Alamdari,1* T. A. Cohen,2,3,4* R. C. Masse,2* G. Z. Cao,2 V. C. Holmberg,1 J. Pfaendtner,1 and D. A. Beck1,5
1 Department of Chemical Engineering, University of Washington, Seattle, WA
2 Department of Materials Science and Engineering, University of Washington, Seattle, WA
Daniel Schwartz is the Boeing-Sutter Professor of Chemical Engineering and Director of the Clean Energy Institute at the University of Washington, and brings electrochemistry and modeling expertise to the team. David Beck is the director of research with the eScience Institute at the University of Washington and leads regular hackathons; he is also associate director of the NSF Data Intensive Research Enabling CleanTech PhD training program. Matthew Murbach is past president of the University of Washington ECS Student Chapter and an advanced data sciences PhD trainee; he has been leading the student section software development sessions on the UW campus and has practical experience coaching electrochemical scientists and engineers in software development.
Speakers
Open Software Tools for the Analysis of Electrochemical Impedance Spectra


Matthew D. Murbach and Daniel T. Schwartz, Department of Chemical Engineering and Clean Energy Institute, University of Washington


Tip–Surface electrode Interactions and the Effects of These Interactions on Scanning Electrochemical Microscopy Response
Alex Mirabal, Dr. Scott Calabrese Barton, Michigan State University
3 Department of Chemistry, University of Washington, Seattle, WA
4 Molecular Engineering Institute, University of Washington, Seattle, WA

5 eScience Institute, University of Washington, Seattle, WA
Using Image Recognition to Identify Platinum Surfaces with Cyclic Voltammetry Scans

Heather Baroody and Tasleem Muzaffar
Department of Chemistry, Simon Fraser University
Open Science Strategy to Accelerate Adoption of Nonlinear Electrochemical Impedance Spectroscopy as a Battery Diagnostic
V. W. Hu, M. D. Murbach, and D. T. Schwartz
Department of Chemical Engineering and Clean Energy Institute, University of Washington
What Can Electrochemistry Learn from Chess?
Neal Dawson-Elli,1 Kishalay Mitra,2 and Venkat R. Subramanian1,3
1 Department of Chemical Engineering, University of Washington, Seattle, WA
2 Indian Institute of Technology Hyderabad, Kandi, Sangareddy, Telangana, India
3 Pacific Northwest National Laboratory, Richland, WA
Open Software for Electrochemical Battery Modeling, Estimation, and Control
Scott Moura, Assistant Professor, Director of eCAL1, Hector Perez,1

Zach Gima,1 Saehong Park,1 and Dong Zhang1
1 University of California, Berkeley
28 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018
David Beck
Daniel Schwartz
Matthew Murbach
8th Annual Electrochemical Energy Summit (E2S)
SUSTAINABLE AND RESPONSIBLE SUPPLY OF ENERGY STORAGE MATERIALS, COMPONENTS, AND DEVICES – SYMPOSIUM Z03
Sunday, September 30, 2018 – Cancun, Mexico


Increasing energy demands, environmental impacts, and aging infrastructure are driving forces for technological advancements to address societal needs. The demand for energy storage is increasing due to its use in electronics and transportation, and from the increased implementation of renewable energy. While the implementation of energy storage is advancing, its increased demand poses new challenges relating to the supply of energy storage materials, components, and devices.
This summit discusses the need and solutions to achieve a sustainable and responsible supply chain for energy storage. The summit consists of invited speakers, posters, and a panel discussion featuring experts along the entire processing chain of energy storage, which includes mining as well as materials, components, and device manufacturing and recycling.
Invited Speakers
“Supply Chain Considerations for Lithium-Ion Battery Materials”
E. A. Olivetti (Massachusetts Institute of Technology) and G. Ceder (University of California, Berkeley)
“Material Criticality and Energy Storage Materials”

R. Eggert (Colorado School of Mines)



“The Advantages of the Nemaska Electrochemical Process to Directly Synthesize High Purity Lithium Hydroxide”
J. F. Magnan (Nemaska Lithium Inc.)
“Present Status and Future R&D Needs for Batteries for Vehicle and Grid Applications”

V. Srinivasan (Argonne National Laboratory)

“Direct Recycling of Lithium-Ion Batteries”
S. E. Sloop (OnTo Technology, LLC), W. Xu (Oregon State University), M. M. Lerner (Oregon State University), J. Kim (Spear Power Systems, LLC), and M. Lee (Spear Power Systems, LLC)
Panelists
E. A. Olivetti, R. Eggert, J. F. Magnan, V. Srinivasan, S. E. Sloop
Moderators: Luis A. Diaz Aldana (Idaho National Laboratory) and Marca Doeff (Lawrence Berkeley National Laboratory)
Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 29
Sociedad Mexicana de Electroquímica (SMEQ)

Due to the interest that the constant meetings of the electrochemical community in Mexico aroused, in 1980 the creation of the Mexican Electrochemistry Society was proposed, in order to group the participants of the meetings and courses. The Society was not legally registered and operated informally for two years, but on April 12, 1983, the Mexican Society of Electrochemistry (SMEQ) was established and has the general objective of promoting, stimulating the development, use and positive transcendence of Science Electrochemistry in the Country and its applications in all branches of Science.

To achieve its aims, it will group all those people, companies, groups and institutions dedicated to the tasks of research, teaching, development and application of the Electrochemistry, exchanging experiences that allow the updating and planned development of this science.
Technical Cosponsors
Sociedad Iberoamericana de Electroquímica (SIBAE)
The Sociedad Iberoamericana de Electroquímica (SIBAE) was founded in 1992 as a corollary of the tenth Ibero-American Meeting of Electrochemistry held in the city of Córdoba (Argentina). The birth of SIBAE finds its roots in the Latin American Meetings of Electrochemistry and Corrosion, which took place between 1972 and 1988 in Argentina, Chile, Venezuela, Brazil, Mexico, and Panama, and which later became the Ibero-American Meeting of Electrochemistry. From 1994 onward, this finally become the SIBAE Congress, which rotates every two years between the member countries of the society. At present, the SIBAE is made up of members from Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru, Portugal, Spain, Uruguay, and Venezuela.

The goals of SIBAE are to promote cooperation among researchers, students, technicians, and others in the Ibero-American countries; to encourage both fundamental and applied research in electrochemistry; to collaborate with the progress of electrochemistry as a science and as a technology; to contribute to the dissemination of scientific and technological knowledge; and to develop periodic meetings where the official language is the mother tongue of the member countries (Spanish or Portuguese).

30 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018
Meeting Partner
Sociedade Brasileira de Eletroquímica e Eletroanalítica
The Sociedade Brasileira de Eletroquímica e Eletroanalítica (Brazilian Society of Electrochemistry and Electroanalytical, or SBEE) was created in 2013 during the XIX Brazilian Symposium of Electrochemistry and Electroanalytical (SIBEE) at Campos do Jordão, São Paulo, Brazil. The main objectives of its creation are to promote the development of electrochemistry and electroanalytical chemistry in Brazil and to provide stimulus for the exchange of ideas, concepts and applications among the members of the electrochemical community. Thus, SBEE is configured as an academic–scientific association that seeks to integrate researchers, research centers, and companies with activities in the area of electrochemistry, electrolysis, and corrosion. On this basis, the SBEE statute was approved and the company was founded in August 2016.

The aims of our society includes the organization of a biennial national symposium, to ensure a forum of excellence for lectures and specialized presentations, the congregation of the electrochemical community, and the facilitation of the establishment and strengthening of cooperation agreements with other societies in the area of electrochemistry. The current president is Artur de Jesus Motheo (2017-2019), who, with the entire board of SBEE, is looking forward to work with SMEQ, ECS and the other technical cosponsors on promoting the AiMES meeting.
Asociación Colombiana de Electroquímica
The Asociación Colombiana de Electroquímica (Colombian Association of Electrochemistry) is made up of professors, researchers, industry professionals, and students interested in increasing the benefits derived from advances in the science and engineering of electrochemistry. The objectives of the Colombian Association of Electrochemistry are:


• To stimulate, on a national level, the research in science and engineering of electrochemistry, and to encourage the wide dissemination of the results derived from it.

• To promote friendship and cooperation among scientists and engineers of electrochemistry in all regions of Colombia.
• To provide an organization within which the scientists and engineers of electrochemistry can meet to exchange ideas, discuss the results of their studies, and publish their results for the common good.
• To call the attention of all national government agencies in all regions of the country to the importance of providing and supporting research in the sciences and engineering of electrochemistry.
• To promote the practical application of research results, in particular through the education and training of professionals and facilitating communication between specialists in electrochemistry and other disciplines.
In the pursuit of these objectives, the Association organizes periodically (every two years) the Congress of the Colombian Association of Electrochemistry.

Special Meeting Section l A i MES l Cancun, Mexico, September 30-October 4, 2018 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 31
Daniel Schwartz Receives Highest U.S. Award for STEM Mentors

Daniel Schwartz, Boeing-Sutter Professor of Chemical Engineering and director of the Clean Energy Institute at the University of Washington, received the Presidential Award for Excellence in Science, Mathematics and Engineering Mentoring from the White House Office of Science and Technology Policy and the National Science Foundation in June 2018. The award recognizes the exceptional efforts of mentors in championing the next generation of innovators in STEM.
An ECS fellow and a lead organizer of ECS Data Sciences Hack Week, Schwartz was honored with the award in recognition of his longstanding commitment to interdisciplinary graduate education, which has helped countless students connect their research to societal and market needs and driven concentrated efforts to recruit and support Native American STEM scholars at UW. Having grown up among the reservations of northern Minnesota, Schwartz witnessed firsthand the barriers faced by tribal students in their pursuit of postsecondary STEM education. Since his 1991 appointment to the faculty of UW, he has worked to make engineering programs more inclusive and accessible while fostering a thriving mentorship network.
memoriam In Memoriam
In 2007 Schwartz launched an NSF-funded interdisciplinary graduate training program that used tribal clean energy research partnerships to draw distinguished Native American students to graduate degree programs offered through UW’s College of the Environment and College of Engineering. In partnership with Washington State University and Salish Kootenai College, and with funding from the U.S. Department of Agriculture, the program has expanded to include a summer research experience program. It also prompted the formation of a tribal student-led start-up company.
As of June 2018, 26 students had earned doctoral degrees through the program. Of the PhDs bestowed, four were awarded to Native Americans, and four were awarded to other underrepresented minorities. Of the six master’s degrees also bestowed, two were awarded to Native Americans.
Schwartz was presented the award in Washington, DC, where he participated in the White House State-Federal STEM Summit, which focused on identifying educational priorities for the nation.
ECS congratulates Schwartz on his achievements and thanks him for all he contributes—to the Society and to the nation—as a proponent of innovation, accessibility, and diversity in STEM.
Pete Peterson
William M. (Pete) Peterson Jr. passed away at the age of 71 on March 23, 2018, following a brief illness. An eminent figure within the Society, Peterson attended nearly 30 ECS meetings over the years, often serving as an exhibitor—first for Gamry Instruments and later for Ivium Technologies. He was active in the ECS Corrosion Division and the ECS Pittsburgh Section and served on two Society committees: the ECS Sponsorship Committee (2007-2010) and the ECS Finance Committee (2011-2015).

Born in Lyons, Georgia, Peterson received his BS from the University of Georgia and earned his PhD in inorganic chemistry from the University of South Carolina. After finishing his doctoral studies, he completed a postdoctoral fellowship at Tulane University in New Orleans, LA. Peterson began his career at Princeton Applied Research in New Jersey, where he worked as a product manager for 10 years. He then worked in sales and marketing positions for SLM Instruments, Photon Technology International, and IMV before becoming the sales and marketing manager for Gamry Instruments in Warminster, PA, in 1998. Peterson held this position until 2011, when he joined Ivium Technologies, a potentiostat/galvanostat instruments manufacturer based in the Netherlands, as the director of sales and marketing and the manager of the company’s U.S. office in
Fernandina Beach, FL. Peterson retired in 2017, but remained active as an Ivium representative.
Peterson was renowned for his expertise in the fields of electrochemistry and corrosion, as well as his gift for stimulating conversation.
ECS fellow Gerald Frankel and former ECS Corrosion Division secretary Barbara Shaw fondly recall Peterson’s spirited lectures on the operation of a potentiostat.
“For many years Pete attended the Penn State corrosion short course as a representative of Gamry Instruments,” Frankel says. “Each year he gave a lecture on how a potentiostat works. He used the analogy of trying to control the temperature in a shower to be ‘just right.’ In his inimitable style he described how it might start out to be too cold, so you turn up the hot water. And then it is too hot, so you turn up the cold water, and then a little more hot water, but then somebody flushes the toilet (like a sudden current demand) and the system loses control, so you get scalded!”
“He had the ability to take something and describe how it works so that anyone could understand it,” says Shaw.
In his spare time, Peterson enjoyed reading, playing guitar, and spending time with his large family in Fernandina Beach, where he also volunteered at Baptist Medical Center Nassau.
Peterson is survived by his wife, Vicki, his son, William, and his daughter, Jennifer.
32 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org SOCIETYPEOPLENEWS
(1946 – 2018)
Looking at Patent Law: The Leahy-Smith America Invents
Act; Changing from a “First-to-Invent” to a “FirstInventor-to-File” U.S. Patent System and Evolving Standards in Prior Art and Public Disclosure
by E. Jennings Taylor and Maria Inman
An important focus of the legislation was the change in U.S. patent law from a “first-to-invent” to a “first-inventor-to-file” patent system. The “first-inventor-to-file” provisions of the AIA went into effect March 16, 2013, while other provisions of the AIA went into effect as early as September 16, 2011, or as late as September 16, 2015.2 In addition, there were subtle but significant changes in the definition of prior art, public disclosures, and grace periods associated with public disclosures. Based on discussions with our colleagues at the recent ECS meeting in Seattle, these changes are particularly relevant with the recent introduction of ECSarXiv and will be discussed herein. An extensive treatise regarding the AIA has been published by the American Intellectual Property Law Association (AIPLA).3
First-Inventor-to-File System
As noted above, a major change resulting from the AIA was the change from the “first-to-invent” to “first-inventor-to-file” U.S. patent system. The rationale behind this change is simply stated from the “sense of Congress” statements in the AIA: it is the sense of Congress that converting the United States patent system from “first-to-invent” to a system of “first-inventor-to-file”4
“will promote the progress of science and the useful arts by securing for limited times to inventors the exclusive rights to their discoveries and provide inventors with greater certainty regarding the scope of protection provided by the grant of exclusive rights to their discoveries.” [emphasis added]
And it is the sense of Congress that converting the United States patent system from “first-to-invent” to a system of “first-inventor-tofile”5
“will improve the United States patent system and promote harmonization of the United States patent system with the patent systems commonly used in nearly all other countries throughout the world with whom the United States conducts trade and
thereby promote greater international uniformity and certainty in the procedures used for securing the exclusive rights of inventors to their discoveries.” [emphasis added]
In summary, the rationale was basically to better define prior art and its impact on patentability as well as consistency with other patent jurisdictions. Regarding harmonization, representatives of five intellectual property jurisdictions (China, U.S., Japan, South Korea, and Europe) meet regularly in an effort to reduce the costs and burden on inventors filing patent applications in multiple jurisdictions. The IP5 collectively prosecute approximately 80% of the world’s patent applications (~2.6 M in 2017).6
Under the pre-AIA U.S. patent system, the patent of the subject invention was awarded to the “first-to-invent.” Recall, an invention is defined by “conception”7 and “reduction to practice.”8 The “inventor(s)” is/are defined as the person/persons involved in “conception.”9 So, the pre-AIA “first-to-invent” U.S. patent system awarded the subject invention to the first inventor to “conceive.” Under the “first-to-invent” system, witnessed notebook references were critical to document the inventors’ contribution to the conception of the invention and the date conception occured. Often, in determining the “first-to-invent,” a costly and time-consuming interference proceeding would be conducted to determine the first inventor to conceive the subject invention. These interference proceedings invoked considerable effort (time and money) in deciding evidentiary matters, since in order to maintain the date of conception the inventor must not have concealed, suppressed, or abandoned the invention and must have exhibited reasonable diligence while proceeding to reduction to practice. 10 These complicated legal concepts were highly case specific and added considerable uncertainty regarding the potential lack of validity of existing patents.
After the AIA, the U.S. patent system is in harmony with most of the world and awards the patent to the “first-inventor-to-file” the subject invention. In Fig. 1, we illustrate the invention timeline where Inventor A is the “first-to-invent” and Inventor B is the “first-inventor-to-file.” Inventor A conceives the invention before Inventor B, but Inventor B files for a patent first. Under AIA U.S. patent law, the earlier conception
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 33 (continued on next page)
+ -
In this installment of the “Looking at Patent Law” articles, we discuss some significant changes associated with the Leahy-Smith America Invents Act (AIA).1
of Inventor A is irrelevant, and Inventor B is awarded the patent based on the earlier filing date. The determination of the “first-inventor-tofile” is much more straightforward and does not require interference proceedings in the sense of the pre-AIA “first-to-invent” system. The witnessed notebook references are still important to identify the appropriate contributors to the conception of the invention and hence identify the correct inventors, but witnessed notebooks are no longer relevant to establishing the “first-to-conceive” the subject invention.
Novelty, Prior Art, and Public Disclosure
Recall from an earlier article that for a patent to be allowed and issued, an invention must be novel (or new), which means that it has not been anticipated by and described in publically disclosed prior art.11 Specifically, a person shall be entitled to a patent unless12,13
“(1) the claimed invention was patented, described in a printed publication, or in public use, on sale, or otherwise available to the public before the effective filing date of the claimed invention; or
(2) the claimed invention was described in a patent … or an application for a patent published …”
If there is publically disclosed prior art relevant to your claimed invention, this may cause a rejection of your patent application, based on a lack of novelty. Novelty rejections are difficult to overcome and the patent applicant generally abandons the patent application or adds limiting elements to the claim to overcome the novelty rejection. Therefore you need to know what publically disclosed prior art is and how it can affect your patent application.
Specific to The Electrochemical Society and its members and contributors, we can identify these “prior art/public disclosures” as activities at ECS meetings, publications in ECS journals, and postings on ECSarXiv. The term “printed publication” seemingly encompasses publications in the Journal of The Electrochemical Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv. The term “otherwise publically available” seemingly encompasses poster and oral presentations at ECS meetings. (Note: We will defer an explanation of the prior art terms “on-sale” and “public use” for a future article.)
Any public disclosure of elements of your claimed invention counts as prior art against your claimed invention—no matter the source, method, or type of disclosure. In other words, there is no hierarchy of the importance with regard to publically disclosed prior art. Therefore, we suggest that the same level of care regarding U.S. patent matters should be given to publications in the Journal of The Electrochemical
Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv and to posters and oral presentations at ECS meetings.
Please note that an important change with regard to timing of public disclosure of prior art from the pre-AIA system is the term “before the effective filing date” of the claimed invention, in reference to the “firstinventor-to-file” requirement for the AIA statute. The pre-AIA statute used the phrase “before-the-invention-thereof” in reference to the “firstto-invent” statutory requirement.14
Prior Art Requirement
One of the conditions for patentability is the “enablement” requirement for a patent application:15
“The specification shall contain a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art to which it pertains, or with which it is most nearly connected, to make and use the same.” [emphasis added]
However, for a prior art reference, a lower standard is applied, in that the prior art is only required to describe the invention under the following two basic requirements:16
1. Each and every element of the claimed invention must be disclosed either explicitly or inherently, and the elements must be arranged or combined in the same way as in the claim.17
2. A person of ordinary skill in the art must have been enabled to make the invention without undue experimentation.18
Therefore, while U.S. Patent Application written description must enable one skilled in the art to make and use the full scope of the invention,19 the prior art is only required to enable one skilled in the art to make a single species or embodiment of the claimed invention.20 There is no requirement that prior art meet the “how to” make and requirement of U.S. patent law. This lower standard of enablement is established by case law, which is complicated and evolving, and the reader is encouraged to consult legal counsel as to the specifics of their case.
Description of Prior Art Terms
We will now focus on the terms “patented,” “printed publication,” and “otherwise available to the public” as they relate to “disclosures” in the Journal of The Electrochemical Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv and to “disclosures” in poster and oral presentations at ECS meetings. (Note: We will return to “on-sale” and “in public use” prior art in a future column.)
34 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Taylor and Inman (continued from previous page)
Time line File 35 USC §102(b) Patent Issues Inventor A grace
public disclosure (Conception Irrelevant)
Awarded Patent Inventor A Conception Public Disclosure by Inventor A <12 Months
period after
Time line File Conception
No Patent
(Conception Irrelevant) Awarded Patent Inventor A Time line File Conception Patent Issues Inventor B
Figure 2. Timeline illustrating inventor grace period after “inventor” public disclosure post-AIA.
35 USC §102(a)
Inventor B is First-Inventor-to-File
Figure 1. Timeline illustrating preference to “first-inventor-to-file” post-AIA.
Fig. 2. Timeline illustrating inventor grace period after “inventor” public disclosure post-AIA.
Fig. 1. Timeline illustrating preference to “first-inventor-to-file” post-AIA
If the claimed invention was “patented” prior to the effective filing date of the subject claimed invention, the patent is prior art as of its issue date or publication of the patent application, whichever is earlier.21 This definition of prior art applies to both U.S. and foreign patents, and the language of which the patent is written is irrelevant from a prior art perspective. In addition, the claimed invention need only be described in a patent but not claimed in the patent. In this case, the patent “describing” the claimed invention is still prior art.
If the claimed invention was described in a “printed publication” prior to the effective filing date of the subject claimed invention, the “printed publication” constitutes prior art if it is “accessible” to the public.22 As derived from case law, the term “printed publication” is a unitary concept and the dichotomy between the words “printed” and “publication” is not valid in view of current technology.23 In the era of document duplication, data storage, and data retrieval, the meaning of “printed” is not the same as when the term was introduced into the patent statute in 1836. Rather, “printed” is more appropriately interpreted as “probability of dissemination” and “publication” is more appropriately interpreted as “publically accessible.” This court noted that a “printed publication” is “publically accessible” if the reference is sufficiently “available” such that one of ordinary skill in the art and exercising reasonable diligence could find the subject reference. Additionally, “electronic publications” are considered to be “printed publications” and constitute prior art provided they “describe” the claimed invention. Consequently, ECS journal publications and postings on ECSarXiv would seem to constitute prior art, even if they were not actually printed, provided they were “publically available” before the effective filing date and providing they “described” the claimed invention.
From above, to qualify as a “printed publication” the reference must have a “probability of dissemination” and be “publically accessible.” For example, a doctoral thesis that is indexed by subject and resides on the shelf of a university library (even with restricted access) is considered a “printed publication” assuming that the fraction of the public (scientific community) concerned with the art could find the reference.24 However, in another case, the doctoral thesis was shelved in a university library, but the indexing was alphabetically by student name and kept in a shoe box in the chemistry department library. In this case, the court reasoned that the thesis was not indexed in a “meaningful” way since the thesis could only be searched using the researcher’s name and the name was in no way related to the subject of the thesis.25 Consequently, the thesis did not constitute a “printed publication”. In addition, an oral presentation at a scientific meeting with written copies distributed to those who requested them was determined to constitute a “printed publication” for the purposes of prior art.26 An ongoing case regarding the question of whether slides and video presented at a technical conference constitute prior art “printed publication” illustrates the case specific intricacies associated with “publically accessible.” (Medtronic, Inc .v. Mark Barry Fed. Cir. June 8, 2018) Consequently, ECS oral presentations posted on ECSarXiv would seem to constitute prior art as a “printed publication” provided they were “publically available” before the effective filing date and providing they “described” the claimed invention. As noted below, even if the oral presentations are not posted on ECSarXiv, they
may constitute prior art relevant to the claimed invention as “otherwise available to the public.”
From the statute, if the claimed invention was “otherwise available to the public” before the effective filing date of the subject claimed invention, it is prior art as of the date it becomes “publically available.”27 This catch-all provision shifts the focus of the prior art inquiry to whether or not the disclosure was available to the public, rather than the means by which the claimed invention became available to the public or what constitutes a “printed publication.” The term “available” is included to describe AIA prior art and may include prior art that was not previously considered prior art under pre-AIA. For example, a poster display or oral presentation (with VuGraphs) at a scientific meeting may constitute prior art provided they “describe” the claimed invention.28 Consequently, poster presentations as well as oral presentations at ECS meetings (even without posting on ECSarXiv or otherwise distributed) would seem to constitute prior art provided they were “publically available” before the effective filing date and providing they “described” the claimed invention.
Prior Art Disclosures by the Inventor and the Grace Period
As noted above, public disclosures of prior art may result in rejection of your claimed invention, due to a lack of novelty. However, AIA U.S. patent law provides grace periods associated with “inventor originated” public disclosures. Specifically, a disclosure made one year or less before the effective filing date of a claimed invention shall not be prior art to the claimed invention if:29
A. the disclosure was made by the inventor or joint inventor or by another who obtained the subject matter disclosed directly or indirectly from the inventor or a joint inventor; or
B. the subject matter disclosed had, … been publicly disclosed by the inventor or a joint inventor or another who obtained the subject matter disclosed directly or indirectly from the inventor or a joint inventor.
An important point here is that public disclosures of prior art may be made not only by you, but also by someone to whom you have described your invention. That colleague’s public disclosure also “sets the clock” for the 12-month grace period, even if they do not inform you of said disclosure. Therefore, caution may be warranted when choosing with whom you share details of your invention, and care taken to prevent undesired and untimely public disclosure.
To summarize, if you (or someone who obtains the subject matter from you) publically discloses your invention, you have one year from the date of disclosure to file for a U.S. Patent, or you will lose your U.S. patent rights. (Note: This grace period generally does not exist in foreign jurisdictions and the loss of rights to foreign patents will generally occur immediately upon public disclosure.) In Fig. 2, we illustrate the invention timeline grace period associated with inventor or inventor originated public disclosure under AIA. Even though Inventor A publically discloses their claimed invention prior to filing a patent application, as long as they file the patent application less than one year after the public disclosure, the patent is issued.
In Fig. 3, we illustrate the invention timeline grace period associated with public disclosure by a colleague of the inventor under AIA. Inventor A privately discloses their claimed invention to their colleague, who subsequently publically discloses details of the claimed invention. This public disclosure sets the beginning of the grace period, and the patent still issues, as long as Inventor A files the patent application less than one year after their colleague’s public disclosure.
Regarding third party disclosures, the grace period only applies to the disclosure of the “same” subject matter obtained from the inventor. However, the grace period for third party disclosures does not apply to “variations” of the subject matter. For example, assume an inventor discloses an invention (eg. a television with and black and white picture tube) to a third party. Subsequently, the third party discloses an invention which includes the original invention but improves, builds on or otherwise modifies it (eg. a television with a color picture tube). Since the disclosure by the third party is not the “same” as the inventor’s
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 35 (continued on next page)
Time line Patent Filed by Inventor A 35 USC §102(b) Patent Issues Inventor A grace period after public disclosure by colleague (Conception Irrelevant) Awarded Patent Inventor A Conception Public Disclosure of Invention by Colleague <12 Months Figure 3. Timeline
Private Disclosure by Inventor A to Colleague
illustrating inventor grace period after “colleague” public disclosure post-AIA.
Fig. 3. Timeline illustrating inventor grace period after “colleague” public disclosure post-AIA.
(continued from previous page)
original idea, the disclosure can be prior art against the original inventor’s patent application. In addition, the original inventor cannot use the third party disclosure date to overcome the prior art under the AIA. Under this scenario, an inventor should seek patent protection prior to publication. If an inventor publishes before filing a patent application, the patent application should be filed as soon as possible to avoid the “inventor disclosed third party improvement” scenario.
Disqualification of Prior Art Disclosures
Another way to think about the grace period is that prior art may be disqualified if the disclosure was:30,31
1. one year or less before the effective filing date of the claimed invention; and
2. by the inventor or a joint inventor, or
3. by another who obtained the subject matter directly or indirectly from the inventor or a joint inventor.
If any claim of a patent application (or patent under reexamination) is rejected based on prior art that can be disqualified as described above, the inventor(s) or assignee32 may file a Rule 130 affidavit or declaration to disqualify the reference as prior art.33 The Rule 130 affidavits are of two general forms:34
C. Affidavit or declaration of attribution. [To establish] “that the disclosure was made by the inventor or a joint inventor, or the subject matter disclosed was obtained directly or indirectly from the inventor or a joint inventor.”
D. Affidavit or declaration of prior public disclosure. [To establish] “that the subject matter disclosed had, before such disclosure was made or before such subject matter was effectively filed, been publicly disclosed by the inventor or a joint inventor or another who obtained the subject matter disclosed directly or indirectly from the inventor or a joint inventor … must identify the subject matter publically disclosed and provide the date such subject matter was publically disclosed.”
For an affidavit or declaration to establish “public disclosure” where the disclosed subject matter was a “printed publication,” a copy of the printed publication must be included in the affidavit. If the disclosed subject matter was not a “printed publication”, the affidavit or declaration must provide a detailed description of the publically disclosed subject matter and include appropriate documentation.
Joint Research Agreements
The America Invents Act created further incentive to encourage research collaborations among universities, government, and the private sectors. The patent statute presents three conditions for the disclosed subject matter:35
1. the subject matter disclosed was developed and the claimed invention was made by, or on behalf of, 1 or more parties to a joint research agreement that was in effect on or before the effective filing date of the claimed invention;
2. the claimed invention was made as a result of activities undertaken within the scope of the joint research agreement; and
3. the application for patent for the claimed invention discloses or is amended to disclose the names of the parties to the joint research agreement.
This provision in essence removes patents and patent applications resulting from a joint R&D agreement as prior art against a subsequently filed patent application by either or all of the parties to the joint R&D agreement. Clearly these activities are managed by the research institutions, but we as electrochemical scientists, engineers, and technologists should be aware of them and seek out guidance from the appropriate responsible party at our institutions.
First-Inventor-to-Publically Disclose
In Fig 4, we illustrate the invention timeline where Inventor B is the first to publically disclose and subsequently files a patent application within the one year grace period. Under AIA U.S. patent law, Inventor B is awarded the patent even though Inventor B conceived the invention and filed the patent application after Inventor A. In Fig. 5, we illustrate the invention timeline where Inventor B publically discloses the invention prior to Inventor A. Even though Inventor A files sooner than the one year grace period and is the “first-to-file,” the patent is awarded to Inventor B based on the earlier public disclosure. Consequently, in U.S. patent law, the U.S. may be considered a “first-inventor-to-publicallydisclose” jurisdiction provided the “disclosed” invention is filed at the U.S. Patent and Trademark Office within the one year grace period.
Therefore, the public disclosures in the form of printed publications, public use, on sale, or otherwise available to the public may be used as a “stake in the ground” to provide a one year grace period in which to file a U.S. patent application. (As noted above, you will lose foreign patent rights upon public disclosure.) Consequently, public disclosure in the Journal of The Electrochemical Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv, as well as poster and oral presentations at ECS meetings can all satisfy the “firstinventor-to-publically-disclose” grace period associated with AIA U.S. patent law.
However, as noted in the scenario above, public disclosure by the inventor includes risk that a third party inventor will improve upon or modify the disclosed invention and file a patent application before the original inventor files. In this case, the third party inventor’s improved invention acts as prior art against the original inventor’s patent application.
In summary, we have described some of the changes associated with the Leahy-Smith America Invents Act as related to the shift from a “firstto-invent” to a “first-inventor-to-file” U.S. patent system. In addition, we have focused on prior art terms “patented,” “printed publication,” and “otherwise available to the public” as they relate to “public disclosure” activities at ECS meetings, publications in ECS journals, and postings on ECSarXiv. The term “printed publication” seemingly encompasses publications in the Journal of The Electrochemical Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv. The term “otherwise publically available” seemingly encompasses poster and oral presentations at ECS meetings. More importantly, we suggest that the same level of care regarding U.S. patent matters should be given to publications in the Journal of The Electrochemical Society, the ECS Journal of Solid State Science and Technology, ECS Transactions, and ECSarXiv and to posters and oral presentations at ECS meetings. In one sense, “public disclosures” may be viewed as a prior art stake-in-the-ground for novelty rejections against subsequent patent applications. In another sense, “public disclosures” may also be viewed as a stake-in-the-ground to provide an inventor a one-year grace period in which to file a U.S. patent application.
An important caveat is that this stake-in-the-ground does not provide
36 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Taylor and Inman
Time line File
No Patent
public disclosure (conception irrelevant)
Awarded Patent Inventor A Conception Time line File Patent Issues Inventor B Conception Public Disclosure by Inventor B <12 Months
35 USC §102(b)
Inventor B receives “grace period” after
Figure 4. Timeline illustrating preference to “first-inventor-to-publically disclose” and file a U.S. patent application within the grace period post-AIA.
Fig. 4. Timeline illustrating preference to “first-inventor-to-publically disclose” and file a U.S. patent application within the grace period post-AIA.
35 USC §102(b)
Inventor B is “first to publically disclose” (Conception irrelevant)
Awarded Patent
protection from third party disclosed inventions that improve upon the original disclosed invention. Generally speaking, the safest approach is to always file a patent application prior to public disclosure or as soon as possible after public disclosure.
Finally, we have attempted to introduce and provide the reader with an appreciation of the complex issues associated with patent law in general and with the nuances associated with “public disclosures” as prior in particular. With this introduction and appreciation, the reader is better prepared to engage their intellectual property counsel in a meaningful dialog regarding their publications, presentations, and postings. Please understand, the objective of this article is to provide an appreciation of complex patent law matters and is not to replace legal counsel. For specific questions related to patent law matters, the reader is encouraged to consult with legal counsel.
© The Electrochemical Society. DOI: 10.1149/2.F01183if.
About the Authors
E. Jennings Taylor is the founder of Faraday Technology, Inc., a small business focused on developing innovative electrochemical processes and technologies based on pulse and pulse reverse electrolytic principles. Taylor leads Faraday’s patent and commercialization strategy and has negotiated numerous via field of use licenses as well as patent sales. In addition to technical publications and presentations, Taylor is an inventor on 40 patents. Taylor is admitted to practice before the United States Patent & Trademark Office (USPTO) in patent cases as a patent agent (Registration No. 53,676) and is a member of the American Intellectual Property Law Association (AIPLA). Taylor has been a member of ECS for 38 years and is a fellow of ECS. He may be reached at jenningstaylor@faradaytechnology.com.
https://orcid.org/0000-0002-3410-0267
Maria Inman is the research director of Faraday Technology, Inc., where she serves as principal investigator on numerous project development activities and manages the company's pulse and pulse reverse research project portfolio. In addition to technical publications and presentations, she is competent in patent drafting and patent drawing preparation and is an inventor on seven patents. Inman is a member of ASTM and has been a member of ECS for 21 years. Inman serves ECS as a member of numerous committees. She may be reached at mariainman@ faradaytechnology.com.
https://orcid.org/0000-0003-2560-8410
References
1. Public Law No. 112-29, Leahy-Smith America Invents Act, enacted September 16, 2011.
2. U.S. Patent & Trademark Office, America Invents Act: Effective Dates (available www.uspto.gov/sites/default/files/aia_implementation/aia-effective-dates.pdf accessed May 29, 2018)
3. Alan J. Kasper et al., Patents After the AIA: Evolving Law and Practice, American Intellectual Property Law Association/ Bloomberg BNA Arlington, VA (2016).


4. Public Law No. 112-29, §3(o) Leahy-Smith America Invents Act, enacted September 16, 2011.
5. Public Law No. 112-29, §3(p) Leahy-Smith America Invents Act, enacted September 16, 2011.
6. five IPoffices, www.fiveipoffices.org
7. Manual of Patent Examining Procedure (MPEP) 2138.04 Conception.
8. Manual of Patent Examining Procedure (MPEP) 2138.05 Reduction to Practice.
9. E. Jennings Taylor and Maria Inman “Looking at Patent Law: Why Is the Word ‘Right’ Mentioned Only Once in the Constitution of the United States?” Electrochem. Soc. Interface, 26 (2), 45 (2017).
10. 35 U.S.C. §102(g)(1)(2) (pre-AIA) Conditions for Patentability; Novelty and Loss of Right to Patent.
11. E. Jennings Taylor and Maria Inman “Looking at Patent Law: Patentable Inventions, Conditions for Receiving a Patent, and Claims” Electrochem. Soc. Interface, 26 (3), 39 (2017).
12. 35 U.S.C. §102(a)(1) Conditions for Patentability: Novelty.
13. 35 U.S.C. §102(a)(2) Conditions for Patentability: Novelty.
14. 35 U.S.C. §102(a) (pre-AIA) Conditions for Patentability; Novelty and Loss of Right to Patent.
15. 35 U.S.C. §112(a) In General.
16. Vas-Cath Inc. v. Mahurkar, 935 F.2d 1555, 1562, 19 USPQ2d 1111, 1115 (Fed. Cir. 1991)
17. Eli Lilly & Co. v. Zenith Goldline Pharms., Inc., 471 F.3d 1369, 1375, 81 USPQ2d 1324,1328 (Fed. Cir. 2006).
18. Impax Labs., Inc. v. Aventis Pharms. Inc., 545 F.3d 1312, 1314, 88 USPQ2d 1381, 1383 (Fed. Cir. 2008).
19. Rasmussen v. SmithKline Beecham Corp., 413 F.3d 1318, 75 USPQ2d 1297 (Fed. Cir. 2005).
20. Vas-Cath Inc. v. Mahurkar, 935 F.2d 1555, 1562, 19 USPQ2d 1111, 1115 (Fed. Cir. 1991)
21. Manual of Patent Examining Procedure (MPEP) 2152.02(a) Patented.
22. Manual of Patent Examining Procedure (MPEP) 2128(I) Printed Publications as Prior Art.
23. In re Wyer, 655 F.2d 221, 210 USPQ 790 (CCPA 1981).
24. In re Hall, 781 F.2d 897, 228 USPQ 453 (Fed. Cir. 1986).
25. In re Cronyn, 890 F.2d 1158, 13 USPQ2d 1070 (Fed. Cir. 1989).
26. Massachusetts Institute of Technology v. AB Fortia, 774 F.2d 1104, 1109, 227 USPQ 428, 432 (Fed. Cir. 1985).
27. Manual of Patent Examining Procedure (MPEP) 2152.02(e) Otherwise Available to the Public.
28. In re Klopfenstein, 380 F.3d 1345, 72 USPQ2d 1117 (Fed. Cir. 2004).
29. 35 U.S.C. §102(b)(1) Exceptions. Disclosures Made 1 Year or Less Before the Effective Filing Date of the Claimed Invention.
30. Manual of Patent Examining Procedure (MPEP) 2153.01(a) Grace Period Inventor Disclosure Exception.
31. Manual of Patent Examining Procedure (MPEP) 2153.01(a) Grace Period Inventor-Originated Disclosure Exception.
32. E. Jennings Taylor and Maria Inman “Looking at Patent Law: Why Are Patents Often Referred to as Intellectual Property?” Electrochem. Soc. Interface, 26 (1), 41 (2017).
33. Manual of Patent Examining Procedure (MPEP) 717.01) Affidavit or Declaration Under 37 CFR 1.130.
34. 37 C.F.R. Affidavit or Declaration of Attribution or Prior Public Disclosure under the Leahy-Smith America Invents Act.
35. 35 U.S.C. §102(c) Exceptions. Common Ownership under Joint Research Agreements.
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 37
Time line File Conception
Patent Issues
Inventor A Time line File Conception No Patent Inventor B Public Disclosure <12 Months <<12 Months Public Disclosure
Figure 5. Timeline illustrating preference to “first-inventor-to-publically disclose” and file a U.S. patent application within the grace period post-AIA.
Fig. 5. Timeline illustrating preference to “first-inventor-to-publically disclose” and file a U.S. patent application within the grace period post-AIA.
Aimes 2018 proceedings available for instant download
September 21, 2018
www.electrochem.org/online-store
ECS Members receive 20% off
Forthcoming publicAtions
First International Conference on 4D Materials and Systems (4DMS) Yonezawa, Yamagata, Japan — August 26-30, 2018
19th International Conference on Advanced Batteries, Accumulators and Fuel Cells (ABAF 2018) Brno, Czech Republic — August 26-29, 2018
Proceedings from the 235th ECS Meeting Dallas, TX — May 26-31, 2019
Browse all available issues: www.electrochem.org/ecst


ECS Transactions
enhance your meeting
Improving the Electrochemical Performance of Si-Based Anodes via Carbon Coating
Silicon-based nanostructures continue to attract a great deal of attention as anode materials for Li-ion batteries due to their high theoretical capacities. However, the chemical vapor deposition process, which is generally used for the fabrication of pure Si nanostructures, leads to high production costs and can limit scalability. SiO2 is a promising alternative, in terms of ease of production and cost; however, the electrical insulation properties of SiO2 result in sluggish electron transport kinetics and capacity fading issues. Researchers from the Sejong University have recently presented a novel method to prepare carbon-coated SiOx nanocomposites with significantly increased capacities and capacity retention compared to uncoated SiOx samples. The structural stability of the carbon-coated nanocomposite coupled with the electron transport paths provided by the carbon coating resulted in a significant improvement of the electrochemical performance of the SiOx composite. The carbon-coated sample delivered a capacity of ~854 mAh g−1 after the 100th discharge when cycled using an applied specific current of 0.1 A g−1, compared to ~338 mAh g−1 for the uncoated sample. This report offers valuable insight into the benefits of carbon coatings for anode materials.
From: H.-W. Yang, N. Kang, S.-T. Myung, et al., J. Electrochem. Soc., 165, A1247 (2018).
An Electrochemical Assay Based on Acid-Induced
Dissolution of Nanoparticles to Trigger Enzyme-Free Cleavage for Target Detection
Discovered in 1994, DNAzymes are single strand DNAs that can catalytically cleave their complementary nucleic acids in the presence of their metal-ion cofactors.
Researchers from the Southwest University of China recently reported a sensitive electrochemical assay of thrombin (TB) based on a Zn2+-dependent DNAzyme. The authors first prepared Fe3O4/Au and ZnO/Au nanoparticles and functionalized them with two complimentary single DNA strands, respectively—the one on ZnO/ Au being a TB-binding aptamer (TBA). Mixing the two led to the bioconjugation of the two nanoparticles. After TB was introduced, ZnO/Au was unlinked from Fe3O4/Au because of the TBA/TB binding. Magnetic separation was then applied to isolate the ZnO/Au/TBA/TB. Adding acid caused the release of Zn2+, which was then used to cleave a ferrocene-labeled DNA strand complemented with a Zn2+-dependent DNAzyme immobilized on an electrode. As a result, the ferrocene current decreased and TB could be assayed. Compared with TB, the Zn2+ was easily available with
much higher concentration in this step. The net effect was an effective amplification to the signal of the assay, which showed a detection limit of 8.6 fM and a dynamic range of 0.01 pM to 10 nM.
From: X. Zhong, J. Lv, S. Xue, et al., J. Electrochem. Soc., 165, B223 (2018).
Electrochemical Atomic Layer Etching of Copper
The increasing use of atomic layer deposition (ALD) for applications in both academia and industry for the layer-by-layer growth of thin films demonstrates the desire for fine control on material deposition. An emerging complementary technique, atomic layer etching (ALE), follows the same principles as ALD but instead focuses on layer-bylayer etching away the top layers. ALE of oxides using plasma-assisted methods are a mature process; however, ALE of metals is still in early development due to the plasma approaches having etch rates higher than 1 nm per cycle which is above that required for atomic-scale control. In a communication by Gong et al., an electrochemical-based ALE approach was developed for atomicscale layer-by-layer etching of copper films. A surface-limited sulfidization was first used to form a copper sulfide monolayer which was then selectively etched using an acid wash without damaging the underlying Cu. Through this method, an etch rate of approximately one copper monolayer per cycle was achieved. Of particular note for this electrochemical ALE process, the RMS roughness of the copper film remains consistent, demonstrating the effectiveness of the technique for conformal etching of the surface.
From: Y. Gong, K. Venkatraman, and R. Akolkar, J. Electrochem. Soc., 165, D282 (2018).
Graphene Oxide Nanoribbons Incorporated in Carbon Paste Electrodes for Supercapacitors
Though graphene nanoribbons (GNRs) have been widely studied as low-cost electrode materials, its commercialization is still limited by challenges in addressing restacking formation, lower electron mobility and nonreversible structural damages. Considering these problems, a group of researchers from Brazil incorporated graphene oxide nanoribbons (O-GNR) into carbon paste electrodes (CPE) through a modified longitudinal unzipping of MWCNTs method at various concentrations. CPE is a low-cost material with a similar composition to GNR with good electrochemical response that provides an easily renewable surface for electron exchange. The researchers found that the CPE containing 20 weight % O-GNR exhibited good performance, achieving a double layer capacitance of 353, 246, and 178.6 mF cm−2 in 1.0 M solutions of KCl, NaOH, and HCl,
respectively, which are significantly higher than those of the MWCNT electrode. This method overcomes the negative effect of oxygen functionalization as well as provides a higher potential window and eliminates metal impurities. In addition, when electrode surface renewing is established, a longer life time is allowed. Overall, a simple and effective method is reported to improve the accessibility and feasibility of O-GNR electrodes.
From: L. G. Brogliato Camargo, C. Rodrigues de Oliveira, et al., ECS J. Solid State Sci. Technol., 7, M83 (2018).
Effect of Conditioning Downforce and Pad Break-In Time on Pad Surface Micro-Texture
Chemical mechanical planarization (CMP) has been employed in integrated circuit (IC) manufacturing since the mid 1990s. To achieve consistent wafer-to-wafer results in removal of excess material deposited or grown during the previous step, a newly installed polishing pad first undergoes a breaking-in to establish the initial pad surface micro-texture. Researchers from the USA investigated as a function of time the evolution of the surfaces of two new pads, each subjected to a different downforce of the CVD-coated conditioning disc. Summit height distribution and other metrics were derived from confocal microscopy images of ~4 cm2 samples taken from the pad at 0, 5, 15, 30, and 60 minutes. The pad conditioned at 10 lbf attained stable values of mean summit height and mean summit curvature in 5 to 15 minutes, whereas the pad conditioned at 6 lbf began to approach stable values at 60 minutes of conditioning. In contrast, stable contact density and percentage contact area values were attained for both downforce conditions 15 to 30 minutes into the break-in process, with the magnitudes at the higher downforce twice those at the lower downforce. The authors suggest further experimentation using different conditioners.
From: J. McAllister, C. Stuffle, Y. Sampurno, et al., ECS J. Solid State Sci. Technol., 7, P274 (2018).
Tech Highlights was prepared by Colm Glynn of Analog Devices International, Xiaodan Cui of Louisiana State University, David McNulty of University College Cork, Ireland, Zenghe Liu of Verity Life Science, and Donald Pile of Rolled-Ribbon Battery Company. Each article highlighted here is available free online. Go to the online version of Tech Highlights in each issue of Interface, and click on the article summary to take you to the full-text version of the article.
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 39 TECH HIGHLIGHTS
CALL FOR PAPERS
Abstract Submission Opens
September 28, 2018
Abstract Submission Closes
October 31, 2018
Spring Meeting registrations include MRS Membership July 1, 2019 – June 30,
broadEr impact
BI01 High Impact Practice—Increasing Ethnic and Gender Diversification in Engineering Education
charactErization, procESSing and thEory
CP01 Advances in In Situ Experimentation Techniques Enabling Novel and Extreme Materials/Nanocomposite Design
CP02 Design and In Situ TEM Characterization of Self-Assembling Colloidal Nanosystems
CP03 Advances in In Situ Techniques for Diagnostics and Synthetic Design of Energy Materials
CP04 Interfacial Science and Engineering—Mechanics, Thermodynamics, Kinetics and Chemistry
CP05 Materials Evolution in Dry Friction—Microstructural, Chemical and Environmental Effects
CP06 Smart Materials for Multifunctional Devices and Interfaces
CP07 From Mechanical Metamaterials to Programmable Materials
CP08 Additive Manufacturing of Metals
CP09 Mathematical Aspects of Materials Science—Modeling, Analysis and Computations
ElEctronicS and photonicS
Soft Organic and Bimolecular Electronics
EP01 Liquid Crystalline Properties, Self-Assembly and Molecular Order in Organic Semiconductors
EP02 Photonic Materials and Devices for Biointerfaces
EP03 Materials Strategies and Device Fabrication for Biofriendly Electronics
EP04 Soft and Stretchable Electronics—From Fundamentals to Applications
EP05 Engineered Functional Multicellular Circuits, Devices and Systems
EP06 Organic Electronics—Materials and Devices
Semiconductor Devices, Interconnects, Plasmonic and Thermoelectric Materials
EP07 Next-Generation Interconnects—Materials, Processes and Integration
EP08 Phase-Change Materials for Memories, Photonics, Neuromorphic and Emerging Application
EP09 Devices and Materials to Extend the CMOS Roadmap for Logic and Memory Applications
EP10 Heterovalent Integration of Semiconductors and Applications to Optical Devices
EP11 Hybrid Materials and Devices for Enhanced Light-Matter Interactions
EP12 Emerging Materials for Plasmonics, Metamaterials and Metasurfaces
EP13 Thermoelectrics—Materials, Methods and Devices
www.mrs.org/spring2019
meeting chairs
yuping bao The University of Alabama
bruce dunn University of California, Los Angeles
Subodh mhaisalkar Nanyang Technological University
ruth Schwaiger Karlsruhe Institute of Technology— Institute for Applied Materials
Subhash l. Shinde University of Notre Dame
don’t miss these future mrS meetings!
2019 mrS fall meeting & Exhibit
December 1–6, 2019, Boston, Massachusetts
2020 mrS Spring meeting & Exhibit
April 13–17, 2020, Phoenix, Arizona
EnErgy and SuStainability

Energy Storage
ES01 Organic Materials in Electrochemical Energy Storage
ES02 Next-Generation Intercalation Batteries
ES03 Electrochemical Energy Materials Under Extreme Conditions
ES04 Solid-State Electrochemical Energy Storage
Catalysis, Alternative Energy and Fuels
ES05 Cooperative Catalysis for Energy and Environmental Applications
ES06 Atomic-Level Understanding of Materials in Fuel Cells and Electrolyzers
ES07 New Carbon for Energy—Materials, Chemistry and Applications
ES08 Materials Challenges in Surfaces and Coatings for Solar Thermal Technologies
ES10 Rational Designed Hierarchical Nanostructures for Photocatalytic System
ES11 Advanced Low Temperature Water-Splitting for Renewable Hydrogen Production via Electrochemical and Photoelectrochemical Processes
ES12 Redox-Active Oxides for Creating Renewable and Sustainable Energy Carriers
Water-Energy Materials and Sustainability
ES09 Advanced Materials for the Water-Energy Nexus
ES13 Materials Selection and Design—A Tool to Enable Sustainable Materials
Development and a Reduced Materials Footprint
ES14 Materials Circular Economy for Urban Sustainability
Photovoltaics and Energy Harvesting
ES15 Fundamental Understanding of the Multifaceted Optoelectronic Properties of Halide Perovskites
ES16 Perovskite Photovoltaics and Optoelectronics
ES17 Perovskite-Based Light-Emission and Frontier Phenomena— Single Crystals, Thin Films and Nanocrystals
ES18 Frontiers in Organic Photovoltaics
ES19 Excitonic Materials and Quantum Dots for Energy Conversion
ES20 Thin-Film Chalcogenide Semiconductor Photovoltaics
ES21 Nanogenerators and Piezotronics
Quantum and nanomatErialS
QN01 2D Layered Materials Beyond Graphene—Theory, Discovery and Design
QN02 Defects, Electronic and Magnetic Properties in Advanced 2D Materials Beyond Graphene
QN03 2D Materials—Tunable Physical Properties, Heterostructures and Device Applications
QN04 Nanoscale Heat Transport—Fundamentals
QN05 Emerging Thermal Materials—From Nanoscale to Multiscale Thermal Transport, Energy Conversion, Storage and Thermal Management
QN06 Emerging Materials for Quantum Information
QN07 Emergent Phenomena in Oxide Quantum Materials
QN08 Colloidal Nanoparticles—From Synthesis to Applications
Soft matErialS and biomatErialS
SM01 Materials for Biological and Medical Applications
SM02 Progress in Supramolecular Nanotheranostics
SM03 Growing Next-Generation Materials with Synthetic Biology
SM04 Translational Materials in Medicine—Prosthetics, Sensors and Smart Scaffolds
SM05 Supramolecular Biomaterials for Regenerative Medicine and Drug Delivery
SM06 Nano- and Microgels
SM07 Bioinspired Materials—From Basic Discovery to Biomimicry
Phoenix, Arizona April 22–26, 2019 | 2019 SPRING MEETING & EXHIBIT
506 Keystone Drive Warrendale, PA 15086-7573 Tel 724.779.3003 • Fax 724.779.8313 • info@mrs.org • www.mrs.org
2020
A Short Introduction to Sonoelectrochemistry
by Bruno G. Pollet
Istill vividly remember when, in 1994, I received a telephone call from Tim J. Mason (now professor emeritus, Coventry University, England, UK), director of the Coventry University Centre of Excellence for Sonochemistry offering me a PhD position under his and J. Phil Lorimer ’s supervision, in the field of sonoelectrochemistry, the subject of my PhD thesis. I was so excited that the next day I immediately went to the university’s library searching for papers on the subject! To my surprise at the time, there was a dearth of information published on the topic. Needless to say, the application of ultrasound on electrochemical systems is in fact not new—for example, it was first observed in the early 1930s by Moriguchi (1934),1 in the 1950s by Yeager and Hovorka (1953),2 and in the 1960s by Bard (1963).3 To date, over 3,500 publications4 have been published on the subject with the vast majority being published after a review paper in 1990 by Mason et al. from the Coventry group5 entitled “Sonoelectrochemistry,” highlighting the extraordinary effects of sonication in electrochemistry. It is often thought that this review paper was a precursor for reviving the area. Walton et al. (1990),6 Cataldo (1992),7 Yegnaraman and Bharathi (1992),8 Reisse et al. (1993),9 Hagan and Coury (1994),10 Compton et al. (1994),11 Klima et al. (1995),12 Lorimer, Pollet et al. (1996),13 to name but a few, were most probably the first modern researchers to investigate the effects of ultrasound on electrochemical systems; with over 100 papers on the subject published by Compton et al.14 A fairly recent book entitled Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution, which I edited in 2012,15 details the effects of ultrasound in electrochemistry (including electroanalysis, corrosion, electrochemistry in environmental applications, organic electrosynthesis, electrodeposition and electropolymerisation), but also the use of sonoelectrochemistry as a tool to investigate cavitation bubble dynamics and flow velocities16 as well as the production of nanomaterials (e.g., fuel cell and electrolyser electrocatalysts) and useful gases (e.g., hydrogen).17-19
So, what is sonoelectrochemistry? No, not SO.NO.ELECTROCHEMISTRY!
It is the study of the combination of ultrasound and electrochemical processes and its applications. This area of research covers various studies from organic syntheses, polymeric materials syntheses, production of nanomaterials, environmental (soil and water) treatments, water disinfection, corrosion of metals, analytical procedures, films, and membrane preparations to the elucidation of electrochemical mechanisms in various conventional and exotic solvents (e.g., room temperature ionic liquids20 and deep eutectic solvents21). It is well-known in the area that the main effects of ultrasound in electrochemistry are the increase in mass transfer and heat transfer induced by extreme solution mixing, a substantial thinning of the diffusion layer thickness (δ) at the electrode surface, a noticeable decrease in overall cell voltage (Vcell) and electrode overpotential (η), and an observable surface modification due to erosion and pitting (Fig. 1). These observations are mainly caused by the implosion of high-energy cavitation bubbles in turn generating high velocity microjets of liquid (up to 200 m.s−1)16

on next page)
BENEFITS OF SONOELECTROCHEMISTRY
Enhanced electrochemical diffusion processes
Improved electrodekinetics
Improved electrode surface activation
Better electrode surface cleanliness

Lower electrodeoverpotentials
Suppressed electrodefouling
Efficient degassing at the electrode surface
Efficient solution degassing
Enhanced electrochemical rates and yields
Improved electrodepositquality and properties
Better electrochemical process efficiencies
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 41
142 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Fig. 1. Damage of aluminum foil surfaces caused by acoustic cavitation at two ultrasonic frequencies: a) 26 kHz, b) 130 kHz (Quava mini; nominal power: 50 W; irradiation time: 2 mins). (With permission from Kenji Okitsu [Osaka Prefecture University].
Fig. 2. A cavitation bubble imploding near the electrode surface causing the formation of a high-velocity microjet of liquid hitting the surface.
Fig. 3. A short summary of the benefits of using ultrasound in electrochemistry. (continued
Pollet
(continued from previous page)
hitting the electrode surface (Fig. 2), and by acoustic streaming together with microstreaming at or near the electrode surface. Figure 3 shows a short summary of the benefits of using ultrasound in electrochemistry.
This special issue of Interface gathers the best recent researchers in the fields of sonochemistry and sonoelectrochemistry.

Additionally, for the first time at an ECS meeting, a sonoelectrochemistry symposium will be held at the fall 2019 meeting in Atlanta (October 13-17) and will be co-chaired by Johna Leddy, Jean-Yves Hihn, and me. Watch this space! © The Electrochemical Society. DOI: 10.1149/2.F03183if.
About the Guest Editor
Bruno G. Pollet is a full professor of renewable energy at the Norwegian University of Science and Technology. He is a fellow of the Royal Society of Chemistry, a member of the Board of Directors of the International Association for Hydrogen Energy, executive editor of the Elsevier journal Ultrasonics Sonochemistry, and a member of ECS. He was a cofounder and an associate director of the University of Birmingham Centre for Hydrogen and Fuel Cell Research (UK). He was a full professor of energy materials and systems at the University of the Western Cape (RSA) and R&D director of the Hydrogen South Africa Systems Competence Centre. He has worked for Johnson Matthey Fuel Cells Ltd (UK) and other various industries worldwide. He was awarded a diploma in chemistry and material sciences from the Université Joseph Fourier (France), a BSc (Hons) in applied chemistry from Coventry University (England, UK), and an MSc in analytical chemistry from the University of Aberdeen (Scotland, UK). He gained his PhD in physical chemistry in the field of ultrasonics electrochemistry under the supervision of J. Phil Lorimer and T. J. Mason at the Sonochemistry Centre of Excellence, Coventry University. He undertook his postdoctoral research in electrocatalysis with the Liverpool University Electrochemistry Group led by David J. Schiffrin. His research covers a wide range of areas in electrochemical engineering, electrochemical energy conversion, and sonoelectrochemistry from the development of novel materials, hydrogen and fuel cell, to water treatment/ disinfection demonstrators and prototypes. He may be reached at bruno.g.pollet@ntnu.no.
https://orcid.org/0000-0002-4928-7378


References
1. N. Moriguchi, “The Influence of Supersonic Waves on Chemical Phenomena III: The Influence on the Concentration Polarisation,” J. Chem. Soc. Jpn, 55, 349 (1934).
2. E. Yeager and F. Hovorka, “Ultrasonic Waves and Electrochemistry I: A Survey of the Electrochemical Applications of Ultrasonic Waves,” J. Acoust. Soc. Am., 25(3), 443 (1953).
3. A. Bard, “High Speed Controlled Potential Coulometry,” Anal. Chem., 35, 1125 (1963).
4. Google Scholar search performed on 19 May 2018, keyword: sonoelectrochemistry.
5. T. J. Mason, J. P. Lorimer and D. J. Walton, “Sonoelectrochemistry,” Ultrasonics, 28, 333 (1990).
6. D. J. Walton, A. Chyla, J. P. Lorimer and T. J. Mason, “Sonochemical Enhancement of Phenylacetate Electrooxidation,” Syn. Comm., 20, 1843 (1990).
7. F. Cataldo, “Effects of Ultrasound on the Yield of Hydrogen and Chlorine During Electrolysis of Aqueous Solutions of NaCl or HCl,” J. Electroanal. Chem., 332(1-2), 325 (1992).
8. V. Yegnaraman and S. Bharathi, “Sonoelectrochemistry – An Emerging Area,” Bull. Electrochem., 8, 84 (1992).


9. J. Reisse, H. Francois, J. Vandercammen, O. Fabre, A. Kirsh-De Mesmaeker, C. Maershalk, and J. L. Delplancke, “Sonoelectrochemistry in Aqueous Electrolyte: A New Type of Sonoelectroreactor,” Electrochim. Acta, 39, 37 (1994).


10. R. S. C. Hagan and L. A. Coury, “Comparison of Hydrodynamic Voltammetry Implemented by Sonication to a Rotating Disk Electrode,” Anal. Chem., 66, 399 (1994).
11. R. G. Compton, J. C. Eklund, S. D. Page, G. H. W. Sanders and J. Booth, “Voltammetry in the Presence of Ultrasound. Sonovoltammetry and Surface Effects,” J. Phys. Chem., 98, 12410 (1994).
12. J. Klima, C. Bernard and C. Degrand, “Sonoelectrochemistry: Effects of Ultrasound on Voltammetric Measurements at a Solid Electrode,” J. Electroanal. Chem., 367, 297 (1994).
13. J. P. Lorimer, B. Pollet, S. S. Phull, T. J. Mason, D. J. Walton and U. Geissler, “The Effect of Ultrasonic Frequency and Intensity upon Limiting Currents at Rotating Disc and Stationary Electrodes,” Electrochim. Acta, 41, 2737 (1996).
14. Webpage visited on 19 May 2018: http://compton.chem.ox.ac. uk/index.php?title=papers&year=pre
15. Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution, B. G. Pollet, Editor, John Wiley and Sons, Hoboken, New Jersey (2012).
16. B. G. Pollet, J.-Y. Hihn, M.-L. Doche, J. P. Lorimer, A. Mandroyan and T. J. Mason, “Transport Limited Currents Close to an Ultrasonic Horn,” J. Electrochem. Soc., 154(10), E131 (2007).
17. B. G. Pollet, “The Use of Ultrasound for the Fabrication of Fuel Cell Materials,” Int. J. Hydrogen Energy, 35(21), 11986 (2010).

18. B. G. Pollet, “A Novel Method for Preparing PEMFC Electrodes by the Ultrasonic and Sonoelectrochemical Techniques,” Electrochem. Comm., 11(7), 1445 (2009).
19. B. G. Pollet and J. T. E. Goh, “The Importance of Ultrasonic Parameters in the Preparation of Low Temperature Fuel Cell Catalyst Inks,” Electrochim. Acta, 128, 292 (2014).
20. C. Costa, J.-Y. Hihn, M. Rebetez, M.-L. Doche, I. Bisel, and P. Moisy, “Transport-Limited Current and Microsonoreactor Characterization at 3 Low Frequencies in the Presence of Water, Acetonitrile and Imidazolium-Based Ionic Liquids,” Phys. Chem. Chem. Phys., 10, 2149 (2008).
21. B. G. Pollet, J.-Y. Hihn and T. J. Mason, “Sono-electrodeposition (20 and 850 kHz) of Copper in Aqueous and Deep Eutectic Solvents,” Electrochim. Acta, 53, 4248 (2008).
42 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Get your ORCID iD today! Visit orcid.org to register.
Introduction to Ultrasound and Sonochemistry
by Wu Li and Muthupandian Ashokkumar
Sound frequencies beyond the upper audible limit of human hearing are generally categorized as ultrasound. The frequency range of ultrasound that has been explored for the purpose of processing applications and sonochemistry is in the range of 16 to 3000 kHz.1 In liquid media, the oscillation of pressure waves can give rise to a unique phenomenon, acoustic cavitation.2 During acoustic cavitation, extremely high pressures and temperatures are generated, initiating several physical and chemical effects, such as shock waves, microjetting, micro-streaming, shear forces and the generation of radicals. By implementing these effects individually or together, a wide range of applications such as food processing,3 drug encapsulation and delivery,4 material engineering,5 and electrochemistry6 (Fig. 1) have been developed.
Acoustic Cavitation
Cavitation Bubble Nucleation and Growth
The origin of cavitation bubbles includes two main sources: preexisting gas nuclei and trapped gas residues at the crevices of solid surfaces.10 When ultrasound acts upon these gas nuclei, they grow due to rectified diffusion and bubble coalescence. The coalescence of the bubbles is driven by the primary and secondary Bjerknes forces, where the main driving force for the former is associated directly with the ultrasound wave, whereas the latter one is the acoustic wave radiated by a neighbouring bubble.2 Rectified diffusion growth of bubbles can be explained by the area effect and shell effect theories.2
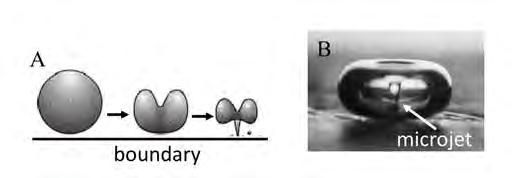
Oscillation and Collapse of Cavitation Bubbles
The Rayleigh-Plesset equation2 is one of the well-accepted theoretical models for describing bubble dynamics. On the basis of the Rayleigh-Plesset equation, parameters such as fluid compressibility, damping, condensation, and evaporation were included in the theory,
resulting in great enhancement of the theoretical modelling of cavitation bubble dynamics.11 The physical conditions generated on bubble collapse can be derived from the Rayleigh-Plesset equation. For example, Eq. 1 and 2 estimate the maximum temperature (Tmax) and pressure (Pmax) respectively, assuming the collapse is adiabatic.
where pg is the pressure of the gas inside the cavitation bubble, T0 is the solution temperature, p∞ is the hydrostatic/liquid pressure and γ is heat capacity ratio. The estimated localized temperature and pressure can reach up to 5000 K and 150 MPa, respectively.11
Chemical and Physical Effects Generated from Acoustic Cavitation
The primary extreme conditions generated by the cavitation bubble collapse are the extreme pressure and heat, which can lead to further effects from both physical and chemical aspects. The physical effects are closely related to the momentum changes from the bubble oscillation and collapse. The radial fluctuation of the bubbles gives rise to the localized liquid flow surrounding the bubbles, called microstreaming. Upon bubble collapse, two consequent effects can be observed. If a bubble undergoes a symmetric collapse, shock waves with high pressure propagate through the medium, and the pressure void formed at the collapse centre leads to extreme velocity gradient up to the order of 100 m/s at the microscale across the liquid medium. If there is an interface nearby (e.g., solid, immiscible liquid or gas phase), an asymmetric collapse will occur, where a microliquid jet,12 shown in Fig. 2, will form from, penetrating inside of the bubble and hitting interface with high speed and pressure. In addition, the localized temperature change at the collapse centre is not only drastically high, but also fast, where the cooling rates could reach >10 9 K/s.11 Such high cooling rates could lead to unique alteration between amorphous and crystalline forms as well as intermolecular hydrophobic/hydrophilic interactions, such as hydrogen bonds and π- π stacking,13 initiating the potential of highly controllable colloidal engineering.
At the microscale regime, the above-mentioned events can create high turbulence (velocity and heat), facilitating mass transfer, interparticle collision, and modifications of the surface morphology and
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 43
(1) Tp p p g g max ()11 (2) PT p p g max () 0 1
Fig. 1. A schematic representation of acoustic cavitation and its applications: sonocrystallization,7 synthesis of metal nanoparticles,2 polymerization of bio-functional materials,8 and de-emulsification using ultrasonic standing waves.9
Fig. 2. Scheme of the (A) microjet propagation and (B) image captured when a microjet was formed.12
interfacial properties. From the bulk perspective, these cumulative physical effects from the localized environment can also generate strong mechanical shear forces, leading to applications such as ultrasonic emulsification, extraction, crystallization, surface modification, and sonogelation, etc.2,13 From the general relationship between the bubble resonance radius and ultrasound frequency shown in Eq. 3, it is clear that with an increase in ultrasound frequency (f) the resonance radius of cavitation bubbles (R) decreases, leading to a less violent bubble collapse. As a consequence, the physical effects discussed above become less significant when the frequency is in the higher range.
fR × 3 (3)
Radical generation, on the other hand, is the origin of chemical reactions in ultrasonic systems. In aqueous systems, the extreme heat at the centre of the bubble when bubble collapses could break the OH bond in water molecules, generating hydrogen and hydroxyl radicals, shown in Reaction 1:
HO)))H+OH 2 ii
where, hydrogen radicals and hydroxyl radicals act as the primary reducing and oxidising agents, respectively. (Note: “)))” represents ultrasound.)
The yield of the primary radicals (H• and OH•) in general govern the rate of chemical reactions. The rate of primary radical formation is found to be strongly related to ultrasound frequency and cavitation intensity. In the general frequency range used in sonochemistry applications (20 kHz to 3 MHz), the maximum rate of radical production has been observed in the frequency range 200 to 600 kHz. A lower rate of radical production is generally observed at 20 kHz and above 1 MHz.11 The behaviour of the radical yield can be explained by the combined effects from bubble radius, collapse temperature, bubble population and life time, operating conditions (frequency and power input), and organic additives (sources of secondary radicals), etc.2
Applications of Ultrasound and Sonochemistry
Chemical reactions strongly depend upon mass transfer effects. As per the Arrhenius equation in chemical kinetics, collisions between molecules are important in many chemical reactions. Hence, the mass transfer effects generated by the physical effects of acoustic cavitation have been found useful in several processes (extraction, emulsification, etc.) and chemical reactions. The application of ultrasound technique in several fields including engineering of metallic composites, soft materials, electrochemistry, biomedical, materials, etc., are briefly discussed below, highlighting the crucial roles of chemical and physical effects, individually and combined.
Metal/Metal Oxides Nanostructures Synthesis
The fabrication of metal nanoparticles (MNPs) has drawn significant attention due to their wide applications in the areas such as biosensors, catalysis and optical storage devices. Amongst different fabrication routes, increasing interest has been shown in
sonochemical synthesis of MNPs, highlighting advantages, such as mild reaction environments, fewer reagent requirements and increased reaction kinetics.2
Sonochemical synthesis of MNPs generally involves the reduction of metal ions in aqueous solutions, where instead of externally added reducing reagents, radicals generated during acoustic cavitation act as a reducing agent. An organic additive, such as alcohols, is usually used to generate secondary reducing radicals from primary radicals as shown in Reactions 2 and 3. The reducing radicals can then be used to reduce metal ions to form metal particles (Reaction 4).
RCHOHRCHOH HO222 +H/OH)))H+/ii i
RCHO HO reducingspecies H+))) 2
Mreducingspecies M n x () 0
A great number of MNPs have been synthesized using sonochemical methods in the past two decades, including copper, iron, cobalt, and noble metals (Au, Ag, Pt, Ru, and Pd).14 Apart from offering easier synthetic routes, sonochemical approach also offers great compositional and structural tuneability for advanced engineering of material architectures.2
For instance, the frequency of ultrasound has been shown to greatly affect the size and shape of Au NPs.15 Amongst the tested frequencies (20, 213, 358, 647, and 1062 kHz), the highest reduction rate of Au(III) was found at 213 kHz, with the smallest size and narrowest size distribution in the presence of 1-propanol, which correlated well with the radical yield trend, where nucleation of the Au NPs will be promoted in a highly reducing environment (i.e., faster kinetics for reaching the critical concentration of nucleation and greater “burstnucleation”). When the radical production rate is low, the nucleation rate is slower than the growth rate of the particles, resulting in larger particles with a wider size distribution.16 The addition of surfactants, on the other hand, could also control the shape of the NPs formed. For instance, the formation of gold nanostructures of different size and shapes is shown in Fig. 3. At an applied frequency of 950 kHz in the absence of any surfactants, Au NPs were formed.17 Au nanorods were produced at 200 kHz in the presence of CTAB (cetyltrimethylammonium bromide), AgNO3 (capping agent), and ascorbic acid (reducing agent).18 Single-crystalline gold nanobelts were formed in the presence of α-D-glucose19 where the role of ultrasound (40 kHz, 100 W) was highlighted in terms of both facilitating inter-particle collision, melting of gold crystals, and the re-orientation of adsorbed α-D-glucose on the gold surface.
Fabrication methods for metal alloys and metal/metal oxide composites have also been developed.20 For example, leveraging the high heat and cooling rates provided by acoustic cavitation, the fabrication of oxides of Zn, Fe, Sn, Pg, Ti, etc. has been reported using ultrasound-assisted methods with great control over the morphology, porosity, and crystallinity,2 which are highly relevant to the areas of gas sensing,21 photocatalysis,22 and electrochemistry.23
Sonogelation
In general, the trigger of a gelation process can be either irreversible crosslinking initiated by the presence of gelators or reversible stimuli such as temperature, pH, and light absorption, etc., which affect the self-assembly and molecular orientations by the inter/intramolecular-hydrophilic/hydrophobic interactions.13 From this point of view, acoustic cavitation can be fitted in both chemical and physical aspects of the gel initiation process. Indeed, a number of studies have reported demonstrating that ultrasound could be used to trigger gelation processes in different solvent systems, forming hydrogels,24 organogels,25,26 and metallogels27 (involving metal-based coordination).
When biopolymers are involved, where both distinct intramolecular structures (e.g., secondary and tertiary structures) and various intermolecular interactions may exist, ultrasound irradiation is capable of altering the interactions from both chemical and physical aspects, causing bond creation, cleavage, and reassembly, which ultimately lead to sol-gel transitions.13 It has been reported that ultrasound irradiation greatly accelerated the secondary structural
44 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Li and Ashokkumar (continued from previous page)
Fig. 3. Examples of gold nanostructures with (A) spherical particles,17 (B) rods,18 and (C) belts.19
Fig. 4. Recreation of a schematic presentation of gelation mechanism of silk fibroins proposed by Wang et. al, 24 where the ultrasound irradiation initiates the conversion from random coils to β-sheets (step 1), and the gelation due to the physical intermolecular crosslinking between β-sheets (step 2).
change from random coil to β-sheets structures in silk fibroin, which was correlated by the increase in ellipticity at 217 nm in circular dichroism analysis, leading to the formation of the hydrogels (Fig. 4).24

Sonoelectrochemistry
The last two decades have witnessed great developments in the area of sonoelectrochemistry due to the awareness of the benefits provided by interdisciplinary topics.23 Meanwhile, improvements have been continuously reported in the well-developed research areas, such as metal deposition and plating,2 fuel cells,28 and electrodes fabrication29 when combining theories from sonochemistry and electrochemistry. The radicals created by the sonolysis during the bubble collapse provide a “clean” source of reactants that initiate redox reactions, which could vary from polymerization, electrodeposition, degradation of organic compounds, nano-structured material synthesis, enhancement of the performance of electrodes, etc.,2 without introducing harmful external chemicals. In addition, the physical effects of acoustic cavitation could drastically facilitate the reaction kinetics, providing extreme shear, heat and pressure at the centre of the cavitation bubbles, acting as micro-reactors.
Summary
The basics of ultrasound and sonochemistry were introduced in this article, linking them to selected applications that include the engineering of metallic composites, sonogelation and sonoelectrochemistry. The basic concepts and applications discussed in this article may provide necessary background knowledge for the rest of the articles covered in this issue. © The Electrochemical Society. DOI: 10.1149/2.F04183if.
About the Authors
Wu Li is a PhD candidate at the University of Melbourne working in the Sonochemistry Research Group within the School of Chemistry. During his MSc studies, his work on ultrasoundassisted fabrication of mesoporous metal frameworks helped him to develop a deeper understanding of sonochemistry. He is now in his final year of PhD coursework, and his research topic is ultrasonic emulsification and its application in dairy systems. His research is part of the Australian Research Council-funded Dairy Innovation Hub. The main focus of his work is to develop in-depth understanding of the fundamental aspects of ultrasonic emulsification and the effects of ultrasound on protein interfacial properties in emulsion/colloidal systems. He may be reached at wul3@student.unimelb.edu.au.
https://orcid.org/0000-0002-1815-4038
Muthupandian (Ashok) Ashokkumar is a physical chemist who specializes in sonochemistry, teaches undergraduate and postgraduate chemistry, and is a senior academic staff member of the School of Chemistry, University of Melbourne. He is a renowned sonochemist, with more than 20 years of experience in the field, has developed a number of novel techniques to characterize acoustic cavitation bubbles, and has made major contributions of applied sonochemistry to the materials, food, and dairy industry. He is the editor in chief of Ultrasonics Sonochemistry, an international journal devoted to sonochemistry research with a journal impact factor of 4.3. He has edited/coedited several books and special issues for journals and has published ~360 refereed papers (H-Index: 50) in high-impact international journals and books. He may be reached at masho@unimelb.edu.au.
https://orcid.org/0000-0002-8442-1499


References
1. T. S. H. Leong, S. Manickam, G. J. Martin, W. Li, and M. Ashokkumar, Ultrasonic Production of Nano-emulsions for Bioactive Delivery in Drug and Food Applications, Springer (2018).
2. M. Ashokkumar, Theoretical and Experimental Sonochemistry Involving Inorganic Systems, Springer Science & Business Media (2010).

3. W. Li, T. S. Leong, M. Ashokkumar, and G. J. Martin, “A Study of the Effectiveness and Energy Efficiency of Ultrasonic Emulsification,” Phys. Chem. Chem. Phys, 20, 86 (2018).
4. F. Cavalieri, M. Ashokkumar, F. Grieser, and F. Caruso, “Ultrasonic Synthesis of Stable, Functional Lysozyme Microbubbles,” Langmuir, 24, 10078 (2008)
5. B. Neppolian, A. Bruno, C. L. Bianchi, and M. Ashokkumar, “Graphene Oxide Based Pt–TiO2 Photocatalyst: Ultrasound Assisted Synthesis, Characterization and Catalytic Efficiency,” Ultrason. Sonochem., 19, 9 (2012).
6. J. Madhavan, F. Grieser, and M. Ashokkumar, “Degradation of Orange-G by Advanced Oxidation Processes,” Ultrason. Sonochem., 17, 338 (2010).
7. J. Lee, M. Ashokkumar, and S. E. Kentish, “Influence of Mixing and Ultrasound Frequency on Antisolvent Crystallisation of Sodium Chloride,” Ultrason. Sonochem., 21, 60 (2014).
8. F. Cavalieri, E. Colombo, E. Nicolai, N. Rosato, and M. Ashokkumar, “Sono-assembly of Nanostructures via Tyrosine–Tyrosine Coupling Reactions at the Interface of Acoustic Cavitation Bubbles,” Mater. Horizons, 3, 563 (2016).
9. L Johansson, T. Singh, T. Leong, R. Mawson, S. McArthur, R. Manasseh, and P. Juliano, “Cavitation and Non-cavitation Regime for Large-Scale Ultrasonic Standing Wave Particle Separation Systems – In Situ Gentle Cavitation Threshold Determination and Free Radical Related Oxidation,” Ultrason. Sonochem., 28, 356 (2016)
10. F. R. Young, Cavitation, World Scientific (1999).
11. S. K. Bhangu and M. Ashokkumar, “Theory of Sonochemistry,” Top. Curr. Chem., 374, 56 (2016).
12. B. Liu, J. Cai, X. Huai, and F. Li, “Cavitation bubble collapse near a heated wall and its effect on the heat transfer,” J. Heat Transfer, 136, 022901 (2014).
13. G. Cravotto and P. Cintas, “Molecular Self-Assembly and Patterning Induced by Sound Waves. The Case of Gelation,” Chem. Soc. Rev., 38, 2684 (2009).
14. J. H. Bang and K. S. Suslick, “Applications of Ultrasound to the Synthesis of Nanostructured Materials,” Adv. Mater., 22, 1039 (2010).

15. K. Okitsu, M. Ashokkumar, and F. Grieser, “Sonochemical Synthesis of Gold Nanoparticles: Effects of Ultrasound Frequency,” J. Phys. Chem. B, 109, 20673 (2005).
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 45
What’s Your L egacy ?
IRA Charitable Rollover
U.S. donors age 70½ years or older may transfer distributions from their IRA.
Li and Ashokkumar (continued from previous page)
16. N. T. K. Thanh, N. Maclean, and S. Mahiddine, “Mechanisms of Nucleation and Growth of Nanoparticles in Solution,” Chem. Rev., 114, 7610 (2014).
17. T. Sakai, H. Enomoto, K. Torigoe, H. Sakai, and M. Abe, “Surfactant- and Reducer-Free Synthesis of Gold Nanoparticles in Aqueous Solutions,” Colloids Surf. A, Physicochem. Eng. Asp., 347, 18 (2009).
18. K. Okitsu, K. Sharyo, and R. Nishimura, “One-Pot Synthesis of Gold Nanorods by Ultrasonic Irradiation: The Effect of pH on the Shape of the Gold Nanorods and Nanoparticles,” Langmuir, 25, 7786 (2009).
19. J. Zhang, J. Du, B. Han, Z. Liu, T. Jiang, and Z. Zhang, “Sonochemical Formation of Single‐Crystalline Gold Nanobelts,” Angew. Chem., 118, 1134 (2006).
20. K. S. Suslick, T. Hyeon, and M. Fang, “Nanostructured Materials Generated by High-Intensity Ultrasound: Sonochemical Synthesis and Catalytic Studies,” Chem. Mater., 8, 2172 (1996).
21. I. Ray, S. Chakraborty, A. Chowdhury, S. Majumdar, A. Prakash, R. Pyare, and A. Sen, “Room Temperature Synthesis of γ-Fe2O3 by Sonochemical Route and Its Response Towards Butane,” Sens. Actuators B Chem., 130, 882 (2008).
22. J. C. Colmenares, W. Ouyang, M. Ojeda, E. Kuna, O. Chernyayeva, D. Lisovytskiy, S. De, R. Luque, and A. M. Balu, “Mild Ultrasound-Assisted Synthesis of TiO2 Supported on Magnetic Nanocomposites for Selective Photo-oxidation of Benzyl Alcohol,” Appl. Catal., B, 183, 107 (2016).
23. B. G. Pollet, Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution, John Wiley & Sons (2012).
Planned Giving
Support ECS during your lifetime or through your estate—gifts can benefit you or loved ones and ECS!
24. X. Wang, J. A. Kluge, G. G. Leisk, and D. L. Kaplan, “SonicationInduced Gelation of Silk Fibroin for Cell Encapsulation,” Biomaterials, 29, 1054 (2008).
25. T. Naota and H. Koori, “Molecules That Assemble by Sound: An Application to the Instant Gelation of Stable Organic Fluids,” J. Am. Chem. Soc., 127, 9324 (2005).
26. D. Bardelang, “Ultrasound Induced Gelation: A Paradigm Shift,” Soft Matter, 5, 1969 (2009).
27. F. Fages, “Metal Coordination to Assist Molecular Gelation,” Angew. Chem., 45, 1680 (2006).
28. B. G. Pollet, “The Use of Ultrasound for the Fabrication of Fuel Cell Materials,” Int. J. Hydrogen Energy, 35, 11986 (2010).
Leadership Collection
Help preserve our scientific legacy while supporting the future of ECS publications.
29. B. G. Pollet, “A Novel Method for Preparing PEMFC Electrodes by the Ultrasonic and Sonoelectrochemical Techniques,” Electrochem. Commun., 11, 1445 (2009).
Sponsored Collection
Create a collection which honors an author’s significant contributions. Make it free for all to read.
46 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
a free preprint service for electrochemistry and solid state science and technology
powered by OSF Preprints
www.electrochem.org/ecsarxiv
Sonoelectrochemistry: Both a Tool for Investigating Mechanisms and for Accelerating Processes
by Jean-Yves Hihn, Marie-Laure Doche, Loic Hallez, Abdeslam Et Taouil, and Bruno G. Pollet
Surface Treatment and Surface Coating
The process of irradiating surfaces with power ultrasound is now widespread, especially in industrial cleaning operations. The principle is very simple: the ultrasonic transducers, vibrating between 20 kHz and up to 400 kHz, are fixed on a tank wall which can contain up to several hundred litres of a solution, in which the ultrasonic wave propagates. In addition to the convective flow generated by the ultrasonic wave absorption, cavitation is induced by the local variation of pressure in the liquid and acts on the contaminants adhering to the metal, plastic, glass, etc. Millions of very tiny bubbles (1 µm to 100 µm), perfectly distributed in the liquid, grow before collapsing violently, penetrating every crevice of the parts requiring cleaning, detaching the stains in a matter of a few seconds.
It is therefore natural to extend this process to the coating process itself, thanks to the increase in mass transfer, due to the agitation induced by convection and cavitation bubble collapse. Moreover, if the bubble collapse occurs in close vicinity of the surface, the surface may either (i) be affected by the ultrasonic shock waves and thus undergo important mechanical effects or (ii) cause a deformation of the bubble and therefore the collapse becomes asymmetric.1 It is interesting to note that the electronic transfer can also be strongly affected by ultrasound, in turns keeping the substrate surface continuously clean by preventing the formation of “parasite” layers able to inhibit important reactions.2 Moreover, the numerous bubbles collapsing at the surface may act as nucleation sites that influence directly nucleation mechanisms, resulting in more random growth.3 Walker et al.4 were the first to report the benefits of ultrasonic irradiation for metallic coating elaboration. Prasad et al.5 showed evidence that several properties may be greatly modified and improved, such as brightness and hardness, better adhesion to substrates, finer grains, a reduced porosity and less internal stress.
The use of ultrasound has also been extended to electroless coating on nonconductive substrates, as for example copper deposition on epoxy resins under high frequency ultrasound.6 Ultrasound not only leads to a considerable increase in the deposition rate, but also in the adhesion (up to 4 N with an increase of almost 40%), whereas the internal stress decreased drastically (down to 50% of the value measured in the coatings obtained under silent conditions). The study of corrosion phenomenon in the presence of ultrasound is an “old challenge,” with the main objective of minimizing the synergetic effect termed corrosion-cavitation frequently observed at industrial scale for fluid circulation (e.g., tube walls, turbine or pump blades, etc.) or on ships’ propellers.7 Various research studies on a large number of substrates (e.g., copper alloys, aluminum, stainless steels, etc.) give strong evidence that ultrasound may increase many types of corrosion. Finally, these results have led to the creation of an international standard (ASTM G32-10)6 allowing the prediction of metals’ behavior in such environments.
A Need for Cavitation Activity Quantification and Characterization
Although power ultrasound offers many advantages at laboratory scale, and thus has attracted a lot of interest since the mid-1990s, only a few cases of “technology transfer” have taken place in industry. This limited translation to practice is due to the lack of fundamental knowledge and data related to ultrasonic systems’ operations. As the intensity of cavitation bubble production modifies drastically the ultrasonic wave propagation and absorption, non-linearity behavior occurs, which in turns prevents the use of simple mechanical laws for accurate reactor modeling.
However, it is possible to use electrochemistry as a tool to study phenomena occurring at an electrode surface and at a given location in a sono-reactor. From a process accelerated by ultrasound, electrochemistry becomes a useful tool to quantify the ultrasonic energy scattered at the immediate electrode surface vicinity. The electro-diffusional method will be of great help, consisting in the measurement of limiting currents during linear sweep voltammetry with quasi-reversible redox couples such as Fe2+/Fe3+ at low active species concentration in solution. This approach has turned out to be extremely efficient for the determination of acoustic intensity at different locations in the sono-reactor.8 By systematically moving an electrode in an ultrasonic field, it is possible to map the acoustic activity, especially the zone close to the transducer where the most intense cavitation activity takes place. Then, to demonstrate the “portability” of our results (i.e., to allow relevant comparisons between experiments performed with different ultrasonic and electrochemical equipment) we proposed to convert the raw electrochemical values into equivalent velocities U, corresponding to normal flows directed toward the electrode surface resulting in the same electrochemical signal than in the presence of ultrasound.9 Subsequent use of the so-called Pollet-Hihn equation,9 makes it possible to obtain a physical quantity characteristic of the ultrasonic activity at each measured point.
Pollet-Hihn Equation
U nF C Dr j 1 045 2 43 13 2 (. *) // lim
where n is the number of electrons transferred, F is the Faraday constant (C.mol−1), C* is the bulk concentration of the electroanalyte (mol.m−3), D is the diffusion coefficient of the electronalyte (m2.s−1), υ is the kinematic viscosity (m2.s−1), r is the electrode radius (m), and jlim is the limiting current density (A.m−2).
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 47
(continued from previous page)
Comparisons with “real fluid motion” at a given location become possible thanks to Particle Image Velocimetry (PIV) (measurement using a high-speed camera and a chemical tracer, allowing tracing the displacement into a 2D laser sheet of light). The systematic comparison allows separation of the respective contributions of cavitation events occurring at the electrode surface to the convection flow called ultrasonic wind (Fig. 1).10
The main result is that the range of magnitude of the “real fluid motion” at 20 kHz is about 10 times lower than the calculated equivalent flow. This finding clearly indicates that the main contributor
to the extremely high agitation at the sonicated electrode surface is ca. 90% due to cavitation bubble collapse (including shock waves as well as microjets). A systematic experimental study was gathered together in a phenomenological model and in correlations linking dimensionless Sherwood (representative of agitation) to Reynolds numbers (representing flow) and Schmidt number (representative of the solution electrochemical properties).1
This need for characterization was extended to unconventional solvents such as room temperature ionic liquids. These solutions present physical and chemical properties particularly interesting for numerous applications in electrochemistry (e.g., actinides separation, electrodeposition of metals, etc.).11 Their high viscosities are definitively problematic, but their detrimental effects may be counterbalanced by the strong agitation provided by ultrasound. Thus, the study of the behavior of redox couples adapted to this type of solvents has been undertaken in a micro-sono-reactor designed for this purpose12 (Fig. 2). Depending upon the solvent nature, it was found that the limiting current density (j) decreased in the following order: CH3CN, water, [Bmim] [Tf2N], in good agreement with the variations observed for the electroactive species diffusion coefficient (D). However, while regrouping the electrochemical measurements in Sherwood dimensionless criteria, a single behavior of the [Bmim] [Tf2N] was observed. At similar ultrasonic intensity transmitted to the media (1 W.cm−2), ultrasonic agitation was found to be more efficient in ionic liquids comparatively to water or acetonitrile. Then, the sonoelectrochemical responses of the redox couples in four ionic liquids ([Bmim] [Tf2N], [Omim] [Tf2N], [N1113] [Tf2N], and [Bmpyr] [Tf2N]) as well as in various mixtures of given viscosities (water/PEG, acetonitrile/PEG, and acetonitrile/[Omim] [Tf2N]) were generated and were compared to those obtained under silent conditions at a rotating disc electrode (ω = 5,000 rpm). The data showed that there were two groups of solvents behaving distinctively under sonication: one group for which the interaction “ultrasonic wave/electrolyte” was comparable to that of water only, and another one for which the liquid structuration was different, translated by an eight-fold increase in mass-transfer under ultrasonic conditions.13
This control of the ultrasonic parameters was found to be a major milestone for the design of sono-reactors and their applications. For example, the determination of an “equivalent” tangential equivalent flow was crucial in the design of boats’ hull cleaning tools (Navy Clean company).14 The intense activity of ultrasound on a surface can be used to develop and implement accelerated corrosion tests and to obtain faster responses to corrosion protection level of coatings while complying with the natural corrosion mechanisms and therefore remaining close to natural conditions. Examples of such an application of ultrasound include studies of zinc-coated steels under different electrolytes with the view of reproducing atmospheric conditions15 or on stainless steels.16 For example, the rate of corrosion in 0.5 M Na2SO4 electrolyte is directly proportional to the distribution of the cavitation activity in the sono-reactor (Fig. 3). The figure shows agitation maxima, the positions of which depend upon the ultrasonic frequency and the distance between the electrode and the ultrasonic horn in a “face-to-face” configuration. The position that increases uniform corrosion the most was at a sample-ultrasonic horn distance directly proportional to the quarter of a wavelength, λ/4. By modifying the ultrasonic frequency and the signal amplitude, it becomes possible to modulate the corrosion “intensity.” Alternatively, ultrasound pulses can induce a switch on/switch off effect, corresponding to passivation/depassivation conditions of the 304L stainless steel in sulfuric acid enriched in chlorides.17 In addition, ultrasound has been shown to greatly accelerate the pitting corrosion generated in halide media.16
The use of sonoelectrochemistry in “anodic mode” is very rare for metal treatment, but an increasing number of studies have been carried out in electropolymerisation processes.18 Cavitation bubble collapse seems to be at the origin of a better distribution of nuclei at the surface, and a better solubility of the monomers under ultrasound.18 Thus, coatings with a finer and more homogeneous topography can be obtained as shown in Fig. 4, allowing a high number of applications possibilities where the surface morphology plays a key role (e.g., sensors, corrosion protection etc.).18 Films formed under

48 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Hihn et al.
Fig. 2. Sono-reactor set up for the electrochemical measurements with RTILs. (© Georges Pannetton – University of Franche-Comté)
0 10 20 30 40 50 60 0 10 20 30 40 50 60 70 p Vaxis VR VT Electrode Electrode
shadow
Fig. 1 Example of a flow velocity vector field in a sono-reactor, close to an electrode at 20 kHz with a 25 mm horn, VR: recirculating average velocity; VT: tangential flow average velocity; and Vaxis: axial flow average velocity.
a reflective surface), an ultrasonic standing wave is established and gives rise to a cluster of cavitation bubbles that erode the surface. By optimizing all these parameters in collaboration with C & K Components, a reel-to-reel process was designed for the removal of a masking resin,21,22 with a view to obtaining selective deposits. The resin deposited on connectors in continuous scrolling was melted and expelled within a few milliseconds from the chosen zone. The band covered with partially ablated resin underwent an electrolytic coating of precious metals which was selectively deposited on the areas denuded by the HIFU. Finally, this need to control cavitation (i.e., to amplify or completely quench cavitation events) led us to study the frequency modulation of ultrasound.23 This phenomenon uses the ability for the bubbles to oscillate in resonance with the acoustic wave. For a positive frequency sweep (towards high frequencies), the bubbles become too large to resonate in phase with the ultrasonic wave whereas for a negative sweep, a larger number of bubbles can be activated depending upon the sweep rate, opening the doors to an effective control of the acoustic activity in a very wide range.
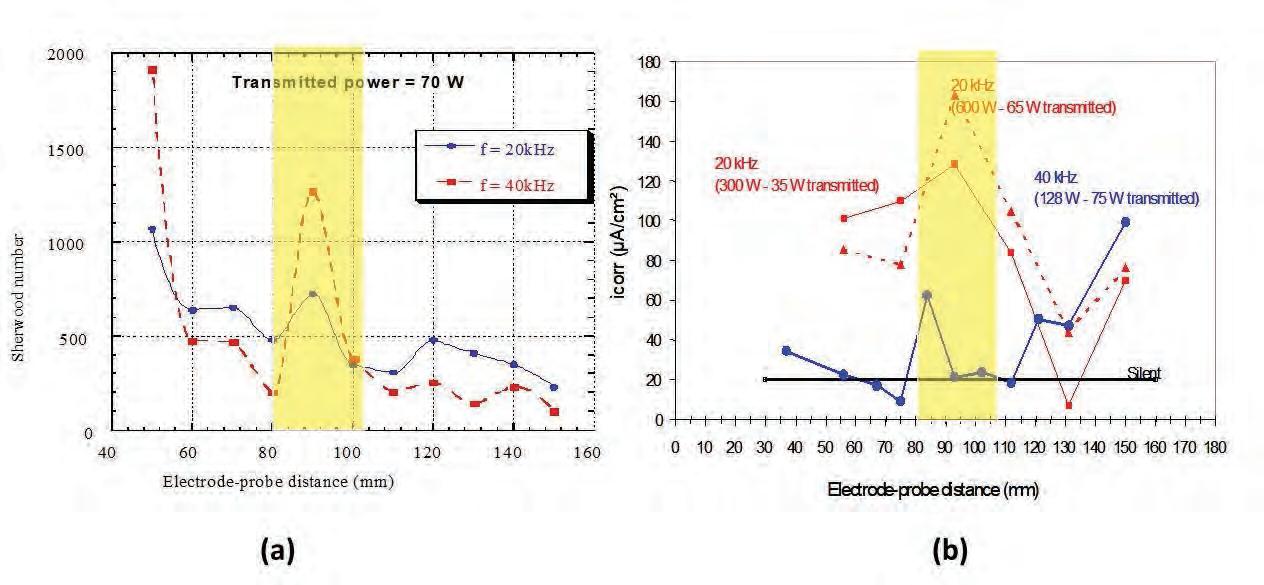
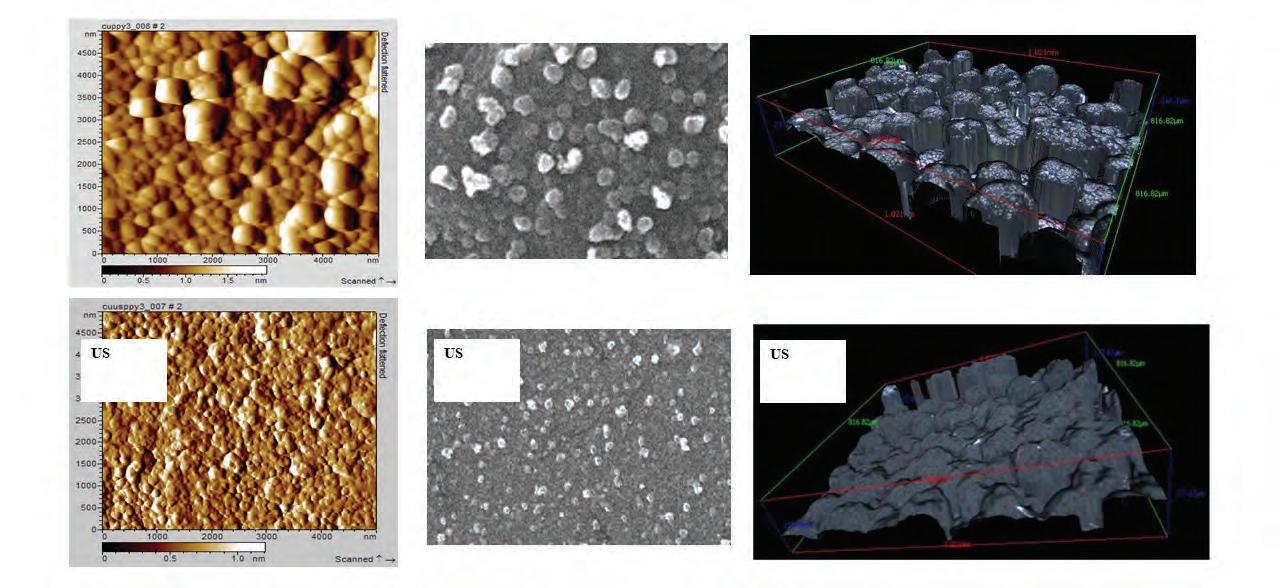
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 49
Fig. 4. Characterization of surface morphology by AFM, SEM, and optical microscopy of conducting polymer films elaborated under silent and high frequency ultrasonic conditions.
(continued on next page)
- 65 W transmitted)
Fig. 3. (a) Variation of the mass transfer in a corrosion cell under sonication from 20 kHz and 40 kHz and various horn to electrode distances; (b) Corrosion rates of zinc coated steel plates measured in the same conditions. 20 kHz (300 W - 35 W transmitted) 40 kHz (128 W - 75 W transmitted) Silent
Electrode-probe distance (mm)
Electrode-probe distance (mm)
irradiation are much more compact in texture leading to a reduced mobility of the ions within the films, and the possibility of controlling the movement of the charged species within the organic matrix. It should be noted that the electrical conductivity of organic coatings is slightly reduced under sonication, due to the partial degradation of the polymer chains.19 For example, the conductivity of perchlorate-doped polypyrrole was found to decrease from 0.3 × 104 to 0.2 × 104 S/m. That said, a selective modification of the surface at a strictly delimited zone is also possible with High Intensity Focused Ultrasound (HIFU) transducers, which possess a concave emitting surface that allows the focusing of the high frequency acoustic wave over an area of a few mm2 20 The acoustic intensity generated by this type of technology can exceed the performance of flat transducers, reaching several kW/cm2 with fluid velocity up to 10 cm/s at the focal length. Their integration into electrochemical processes was conducted in conjunction with IMASONIC company (Fig. 5). For polymers layers with acoustic impedance close to that of water, the acoustic energy is converted into heat by viscous friction, at the opposite (i.e.,
(continued from previous page)
Conclusions
With a good knowledge of the cavitation activity mechanisms and of their specific influence on electrochemical processes, it is possible to define the best conditions of the use of power ultrasound in many applications, from accelerated corrosion to cleaning and coating elaboration. This is of considerable importance, by considering both favorable added value/energetic cost ratio and scale-up ability of sonoelectrochemical systems.

© The Electrochemical Society. DOI: 10.1149/2.F05183if
About the Authors
Jean-Yves Hihn is a professor at the Université of Franche-Comté, Besançon, France. He received his PhD from the Université de Savoie in 1992 and his habilitation in 2001 from the National Polytechnic Institute of Grenoble in materials and chemical engineering. He is currently leading the Surface Reactivity and Sonochemistry Group (30 people) of the Institute UTINAM UMR 6213 CNRS UBFC dedicated to academic and applied research in surface treatment. The group is involved in several projects related to main industrial sectors such as aeronautic, automotive, electronics, and watchmaking. All works concern electrochemistry, metallic plating, and anodic reaction, and are dedicated to electrolytes and chemical system modifications as well as processes management (pulsed currents, ultrasound, etc.). He has published over 80 papers, three patents, and seven books chapters, and has given more than 200 communications. He is a member of Swiss and French Societies of Surface Treatment (A3TS board), ISE, and ECS, and is president of the European Society of Sonochemistry. He may be reached at jeanyves.hihn@univ-fcomte.fr.



https://orcid.org/0000-0002-7857-2098
Marie-Laure Doche is a senior lecturer at the Université of Bourgogne Franche-Comté, Besançon, France. Holder of a degree in engineering from the National Polytechnic Institute of Grenoble, she received a PhD in electrochemistry from the same institute in 1997. She joined the Surface Reactivity and Sonochemistry Group of the Institute UTINAM (UBFC) in 1998, where she is currently conducting research on electrochemistry and surface treatment. She is involved in several academic and industrial projects, particularly in the field of electropolishing and material recovery. She has published over 30 papers and is a member of ECS. She may be reached at mldoche@univ-fcomte.fr.

https://orcid.org/0000-0003-3490-7412
Loïc Hallez is an associate professor at the University of Burgundy-Franche-Comté, Besançon, France. He graduated from the National School of Mechanics and Microtechnology and received his PhD from the University of Burgundy-Franche-Comté in chemical engineering in 2009. Since 2010, he has worked as a researcher in materials chemistry and sonochemistry at the UTINAM Institute UMR 6213 CNRS UBFC. He is specialized in the study of acoustic cavitation and the development of materials under ultrasonic irradiation. Hallez has published 14 papers, two patents, and one book chapter, and has given about 25 oral communications in international congresses. He is member of the European Society of Sonochemistry and the French Society of Surface Treatment. He may be reached at loic.hallez@univ-fcomte.fr.
Abdeslam Et Taouil has been an associate professor in chemistry of materials at the University of Bourgogne Franche-Comté in Besançon (France) since September 2012. He received his PhD from the same university in September 2011, after which he carried out a postdoctoral period at the Laboratoire Interfaces et Systèmes Electrochimiques (Pierre et Marie Curie University, Paris). His research is focused on organic electrochemistry and surface modification under ultrasound irradiation. Et Taouil has published about 20 papers, one patent, and one book chapter. He is a member of the International Society of Electrochemistry and the European Society of Sonochemistry. He may be reached at abdeslam.et_taouil@ univ-fcomte.fr.
Bruno G. Pollet is a full professor of renewable energy at the Norwegian University of Science and Technology in Trondheim. He is a fellow of the Royal Society of Chemistry, a member of the Board of Directors of the International Association for Hydrogen Energy, executive editor of the Elsevier journal Ultrasonics Sonochemistry, and a member of ECS. He was a visiting professor at the University of Ulster, in Vladimir Molkov’s HySAFER (UK), and at the University of Yamanashi, in Masahiro Watanabe’s labs (Japan). His research covers a wide range of areas in electrochemical engineering, electrochemical energy conversion, and sonoelectrochemistry (power ultrasound in electrochemistry), from the development of novel materials, hydrogen and fuel cell, to water treatment/disinfection demonstrators and prototypes. He may be reached at bruno.g.pollet@ntnu.no.
https://orcid.org/0000-0002-4928-7378




50 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
et al.
Hihn
Fig. 5. Ultrasonic irradiation of a metallic target by HIFU. (© Georges Pannetton – University of Franche-Comté)
References
1. J.-Y. Hihn, M. L. Doche, A. Mandroyan, L. Hallez, and B. G. Pollet, in Handbook on Applications of Ultrasound: Sonochemistry for Sustainibility, p. 599-622, CRC Press Boca Raton, FL (2011).
2. J. Y. Hihn, F. Touyeras, M. L. Doche, A. Mandroyan, C. Costa, and B. G. Pollet, in Power Ultrasound in Electrochemistry: From Versatile Tool to Engineering Solution, p. 169-214, John Wiley & Sons Ltd, Chichester, UK (2012).
3. A. Nevers, L. Hallez, F. Touyeras, and J-Y Hihn, “Effect of Ultrasound on Silver Electrodeposition: Crystalline Structure Modification,” Ultrason. Sonochem., 40B, 60 (2018).
4. C. T. Walker and R. Walker, “Effect of Ultrasonic Agitation on Some Properties of Electrodeposits,” Electrodeposition Surf. Treat., 1, 457 (1973).
5. R. Prasad, P. Vasudevan, and S. K. Seshadri, “Ultrasonic Agitation During Electrodeposition,” Trans. Indian Inst. Met., 46, 247 (1993).
6. F. Touyeras, J. Y. Hihn, M.-L. Doche, and X. Roizard, “Electroless Copper Coating of Epoxide Plates in an Ultrasonic Field,” Ultrason. Sonochem., 8, 285 (2001).
7. J. Steller, “International Cavitation- Erosion Test and Quantitative Assessment of Material Resistance to Cavitation,” Wear, 51-64, 233 (1999).
8. A. Mandroyan, M. L. Doche, J. Y. Hihn, R. Viennet, Y. Bailly, and L. Simonin, “Mapping Flow Velocities in an Ultrasonic Reactor Working at 3 Frequencies: 20, 40 and 60 kHz,” Ultrason. Sonochem., 16, 97 (2009).
9. B. G. Pollet, J.-Y. Hihn, M. L. Doche, A. Mandroyan, J. P. Lorimer, and T. J. Mason, “Transport Limited Currents Close to an Ultrasonic Horn: Equivalent Flow Velocity Determination,” J. Electrochem. Soc., 154, E131 (2007).
10. J. Y. Hihn, M. L. Doche, A. Mandroyan, L. Hallez, and B. G. Pollet, “Respective Contribution of Cavitation and Convective Flow to Local Stirring in Sonoreactors,” Ultrason. Sonochem., 18, 881 (2011).
11. F. Endres and A. Schweizer, “The Electrodeposition of Copper on Au(111) and on HOPG from the 66/34 mol% Aluminium Chloride/1-butyl-3-methylimidazolium Chloride Room Temperature Molten Salt: An EC-STM Study,” Phys. Chem. Chem. Phys., 2, 5455 (2000).
12. C. Costa, J. Y. Hihn, M. Rebetez, M. L. Doche, I. Bisel, and P. Moisy, “Transport Limited Current and Microsonoreactor Characterization at 3 Low Frequencies in Presence of Water, Acetonitrile and Imidazolium Based Ionic Liquids ([BuMIm] [(CF3SO2)2N]),” Phys. Chem. Chem. Phys., 10, 2149 (2008).
13. C. Costa, M.-L. Doche, J.-Y. Hihn, I. Bisel, P. Moisy, and J.M. Lévêque, “Hydrodynamic Sono-voltammetry of Ferrocene in [Tf2N] - Based Ionic Liquid Media,” Ultrasonics, 50, 323 (2010).
14. G. Mazue, R. Viennet, J.-Y.Hihn, L. Carpentier, P. Devidal, and I. Albaïna, “Large-Scale Ultrasonic Cleaning System: Design of a Multi-transducer Device for Boat Cleaning (20 kHz),” Ultrason. Sonochem., 18, 895 (2011).
15. V. Ligier, J.-Y.Hihn, M. Wery, and M. Tachez, “The Effects of 20 kHz and 500 kHz Ultrasound on the Corrosion of Zinc Precoated Steels in [Cl ][SO42−][HCO3 ][H2O2] Electrolytes,” J. Appl. Electrochem., 31, 213 (2001).
16. M.-L. Doche and J.-Y. Hihn, in Power Ultrasound in Electrochemistry: From Versatile Tool to Engineering Solution, p. 215-247, John Wiley & Sons Ltd Chichester, UK (2012).
17. G. O. H. Whillock and B. F. Harvey, “Preliminary Investigation on the Ultrasonically Enhanced Corrosion of Stainless Steel in the Nitric Acid/Chloride System,” Ultrason. Sonochem., 3, 111 (1996).
18. F. Lallemand, J.-Y. Hihn, M. Atobe, and A. Et Taouil, in Power Ultrasound in Electrochemistry: From Versatile Tool to Engineering Solution, p. 249-281, John Wiley & Sons Ltd Chichester, UK (2012).
19. A. Et Taouil, F. Lallemand, J-Y Hihn, L. Hallez, and V. BlondeauPatissier, “Effects of High Frequency Ultrasound Irradiation on Doping Level and Electroactivity of Conducting Polymers: Influence of OH Center Dot Radicals,” Polym. Degrad. Stab., 98, 1413 (2013).
20. L. Hallez, F. Touyeras, J.-Y. Hihn, J. Klima, J.-L. Guey, M. Spajer, and Y. Bailly, “Characterization of HIFU Transducers Designed for Sonochemistry Application: Cavitation Distribution,” Ultrasonics, 50, 310 (2010).
21. L. Hallez, F. Touyeras, J.-Y. Hihn, and Y. Bailly, “Interactions H.I.F.U. / Polymer Films,” Phys. Procedia, 3, 179 (2010).
22. S. Rochon, L. Hallez, J.-Y. Hihn, and F. Touyeras, Internstional Patent Application PCT/EP2009/056803 (2009).
23. L. Hallez, J. Lee, F. Touyeras, A. Nevers, M. Ashokkumar, and J.-Y. Hihn, “Enhancement and Quenching of HIFU Cavitation Activity via Short Frequency Sweep Gaps,” Ultrason. Sonochem., 29, 194 (2016).

The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 51
“ECS has been my primary technical home. The Society has a great network, the best symposia, and an outstanding journal. I’ve been very involved with the Society for many years and will continue down that path.”
Gerald Frankel, Member since 1990 Technical Editor & ECS Fellow H. H. Uhlig Award and Ernest B. Yeager Award Winner
Meet Our Members
www.electrochem.org/membership
Gerald Frankel
Join a Powerful Partnership!
The ECS institutional membership program provides content access, member-related discounts, as well as advertising, exhibit, and sponsorship opportunities.
Memberships are available for all types of organizations!
GovernmentIndustry
Standard Package Benefits
• Profile on the ECS website featured on the Institutional Members webpage and in the ECS Organizational Member Directory
• Listing in the quarterly Interface and biannual meeting publications
• Eligible for annual Leadership Circle Awards recognition
Most-valued customizable options:
• Up to 50% off Exhibits and General Meeting Sponsorships
• 5-30% off ECS Subscriptions
• Advertising Discounts
• 25-50% discounts on Career Expo packages
• Member representatives that receive full member benefits
• Open access publishing article credits
• Complimentary meeting registrations Contact Shannon.Reed@electrochem.org to customize a package to meet your organization’s needs!
Academic
Institutional Membership Tiers
Visionary
Cultivating Endorsing
Benefactor
Patron Sponsoring
Sustaining
Institutional membership packages start as low as $1,250 per year!
Available standard package and customizable options are dependent on the level of institutional membership.
52 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Ultrasonic Agitation for Emerging Electrodeposition Systems
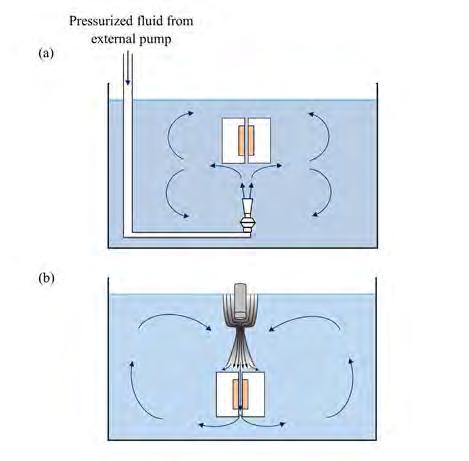 by S. Roy and S. J. Coleman
by S. Roy and S. J. Coleman
Despite the growing importance of electrodeposition for the development of new materials for energy applications,1 sensor fabrication,2 micro and nanofabrication,3,4 the technologies used in these processes remain reliant mostly on traditional methods as rack and barrel plating. In the first half of the twentieth century, agitation in these systems was achieved by cathode movement or air sparging.5 The systematic introduction of eductors,5 the paddle cell and fountain flow for wafer plating,6 and a jet plating system to selectively plate metal at a particular location7 were major innovations of the final quarter of the past century.
While these changes and other academic studies5-12 testify to the importance of agitation during an electrodeposition process, there have been fewer explorations of agitation in the current century. Notwithstanding, over the past two decades there have been new developments in electrodeposition, such as electrochemical printing13,14 and mask-less microfabrication,15,16 which required unconventional agitation schemes as a core need for the process to work. The electrochemical printing method used new jetting technology to deliver fluids, which required precise control over jet placement, fluid viscosity and surface tension.13,14 Electrochemical mask-less microfabrication required that the anode and cathode be placed within 500 μm17 while maintaining good agitation. These developments required new thought for agitating fluids during electrodeposition and understanding them sufficiently to enable the design of new systems. Ionic liquids,18,19 which have been proposed as the next generation of electrolytes for metal deposition, which are highly viscous18,19 and corrosive,20 will also require new approaches for agitation. If conventional methods were used to pump the electrolyte, pump ratings would need to increase by at least one order of magnitude, and pump materials of construction would need to be corrosion proof.
Ultrasonic (US) agitation can provide a way forward for agitation of electrochemical systems.21,22 For example, it is possible that viscous fluids could be displaced using US, thereby eliminating mechanical pumps and contact with corrosive liquids. US agitation using miniature probes could be used to pulsate fluids and through small channels enabling jet flow. For the case where the space between electrodes is constrained, there is the possibility of focusing the flow within the gap. Figure 1 illustrates the last idea: (a) traditional pumping versus (b) US probe focusing flow within the gap.
In this paper we describe the deployment of US to increase agitation in constrained spaces. In particular, here we describe the adaptation and changes needed for measurement of mass transfer rates. The use of US via horns (or probes) as well as tanks for electrodeposition has been described. Specifically the two systems correspond to laboratory scale measurement and industrial scale apparatus. By constrained space we have limited ourselves to a narrow gap between two electrodes; this is generally in the mm scale [23].
Ultrasonic Agitation for Electrodeposition
Ultrasonics has already been used in electrodeposition of metals to improve deposits, current efficiency and improve material distribution.24-26 Enhancement of mass transfer has been detected while studying the effect of US streaming on oxide films27 while others have decoupled mass transfer enhancements caused by US 22,28,29 There have also been attempts to analyse agitation using probes in a face-on29 and side-on systems,30 and mass transfer boundary layer thickness based on these measurements have been quantified.29,30
Here we explain the approach taken by the authors to address an important question: Can US be employed to increase material transport within constrained spaces, i.e., a narrow gap?16,23 At this point, it is unclear to what extent agitation could be improved; furthermore, it was not even evident if experimental techniques, such as the limiting current method, could be applied in these circumstances. In fact, other researchers had already found that there are distortions in potential distribution due to the presence of an US probe.31
Mass Transfer Measurement in a Laboratory Scale System
Since the effect of material transport within a narrow gap using US was not known, the starting point for our analysis was to examine if an experimental system, which could accommodate closely placed anode, cathode, reference electrodes and US horn could be designed. Figure 2 shows a laboratory scale system used by our group, where two electrodes were placed in close proximity within a cylindrical reservoir. The distance between the two electrodes could be controlled via a screw system, and the system was calibrated so that we could adjust the gap reasonably accurately. The US probe was placed above it to focus the US agitation directly within the gap. The US system (SONICS Vibra-Cell VC505 Processor) with a titanium alloy tip was operated at a fixed frequency of 20 kHz, and the power was varied between 9-29 W/cm2 (25-75 W L−1).
The detail for the placement of the reference is shown in Fig. 3. A reference probe could not be placed within the inter-electrode gap, because this could cause a disturbance in the electric field between
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 53
Fig. 1. Schematic of electrolyte flow around narrow electrode gap using (a) eductor agitation and (b) ultrasonic agitation. In (a) the high velocities bypass the interior of the gap, whereas pressure waves could travel through the gap in (b). (Adapted from Ref. 33.)
the two electrodes. Therefore, a reference probe was placed 5 mm above and to the side of the gap. The design of the electrochemical cell allowed one to assess the effect of two different parameters: (1) the inter-electrode gap which defined the constrained space, and (2) probe-to-electrode distance which controlled the power delivered into that space. The placement of the reference above the plane of the anode and cathode, as well as the insertion of the US tip (or horn), which can serve as a third electrode, is a departure from a standard three-electrode set-up used for limiting current measurement.
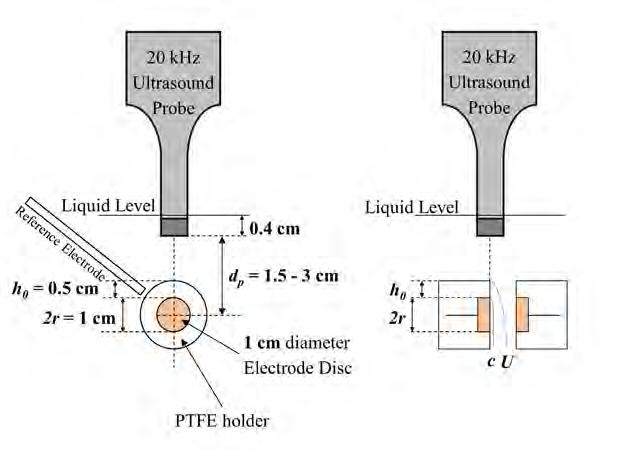
The methodology in the limiting current technique employs the collection of current vs. potential data. When an electrochemical reaction is kinetically controlled, the current density increases with applied potential. As mass transfer limitations are encountered, the current density remains nearly constant with increasing potential, and is called the limiting current plateau. The mass transfer boundary layer, δ, is related to the plateau current, called the limiting current, by the following equation (1)
Here, iL is the value of the limiting current, n is the number of electrons transferred, F is Faraday constant, D is the diffusion coefficient, and Cb is the bulk concentration of reacting species in solution.
Figure 4 shows the limiting current data obtained for a 0.1 M CuSO4/0.1 M H2SO4 electrolyte using this apparatus. The conditions for electrode gap, horn-to-electrode distance, dp, and measurement conditions are provided in the caption. The oscillations in current are due to cavitation activity near the electrode surface. As the horn was brought closer to the inter-electrode gap, the current potential data show distortions, some of which show up as apparent hydrogen evolution commencing at a low overpotential. This distortion is caused by a third metallic, grounded electrode (horn) placed within the electrochemical cell.32 However, limiting current plateaux were still identifiable, and they were used to determine the mass transfer boundary layer.
The limiting current data were used to develop mass transfer correlations within the constrained volume. Sherwood-SchmidtReynolds number correlations for different electrode gaps and hornto-gap distances are listed below:32,33
In these equations Sh, Re and Sc correspond to Sherwood, Schmidt, and Reynolds numbers respectively, and de and L are the equivalent diameter and length, respectively. Equations 2a and 2b correspond to electrode gaps of 10 mm and 1.5 mm, respectively. These correlations show that for a wider gap, developing turbulence controls mass transfer. For the narrower gap, one obtains a fully turbulent condition, presumably due to eddies generated within the gap.
Mass Transfer Measurement in a Large Scale 18 Litre Tank System
Measurement of limiting current within an industrial scale tank system is more complicated. There are two major issues— tank systems have lower power, and the power is very evenly distributed.34 In addition, much of the earlier work has focused on high power to influence reaction chemistry, and studies related to microfabrication have shown that lower power may be more useful for electrodeposition.23,24
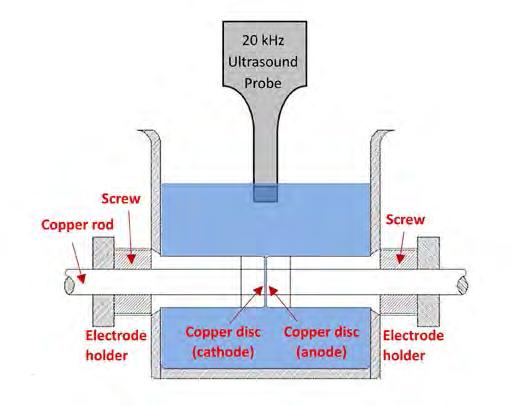
Figure 5 shows the design for the electrodes with a narrow gap within a tank system. The electrodes are placed at the center of the tank, and connectors to the electrodes as well as the inter-electrode gap are maintained via the design of an appropriate holder which is described in detail elsewhere.33,35 The gap between the electrodes was set at 1.5 mm, achieved by placement of spacers in the corners and one in the center of the plate. The tank was 18 L, and the electrode size was 74 mm × 105 mm (A7 size), much larger than the lab scale system (which was of 10 mm diameter). The tank operated at 30 kHz and limiting current experiments were performed at 30, 40 and 60 WL−1
Figure 5 also shows the detail of anode and cathode used in these experiments. The cathode was a Perspex plate with a copper contact at the back. Two square slots 10 mm × 10 mm, near the center and edge, were made where copper inserts were placed, depending on the position where the limiting current had to be measured. The anode was an A7 size copper plate, the same size as the Perspex plate. Limiting currents were measured via galvanostatic means. Although limiting currents would normally be measured potentiostatically, when such large electrodes are used, there can be significant potential distribution, which makes data difficult to interpret. Therefore,
54 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
and Coleman (continued
previous page)
Roy
from
||inFD C L b
(2a) Sh kReSc d L e 1 082 (2b) Sh kReSc d L e 2 138
Fig. 2. Experimental set-up of the electrochemical cell used with ultrasound probe placed directly above a narrow electrode gap. (Adapted from Ref. 32.)
Fig. 3. Dimensions of the probe and the placement of a reference electrode. The placement of the probe and the development of the momentum and concentration boundary layers along the length of the electrode is indicated. Here c is concentration and U is flow velocity. (Adapted from Ref. 32.)
Fig. 4. Linear Potential scans with a 0.1 M CuSO4 + 0.1 M H2SO4 electrolyte; scan rate = 5 mV/s. (a) he = 0.5 cm; at varying dp of 3 cm (light orange), 2 cm (orange), and 1.5 cm (dark orange) at fixed p of 18 W/cm2 (b) he = 0.15 cm, with same ultrasound conditions as for ‘a’ and varying dp at distances of 3 cm (light blue), 2 cm (blue), and 1.5 cm (dark blue). Dotted line denotes the iL under silent conditions, he = 0.5 cm. (Adapted from Ref. 32.)
measuring an average current and cell potential to locate a plateaux current provides a practical method to determine limiting current in a tank-type reactor.
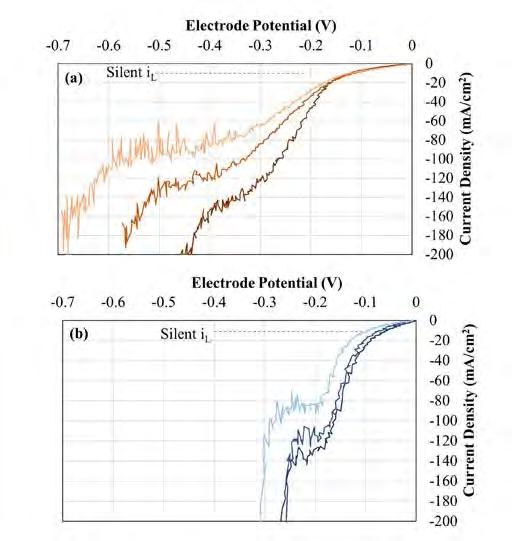
The limiting currents at locations (A) and (B) were determined to be similar; at location A, δ was found to vary between 15 μm and 19 μm, and it was 17 μm at location B, indicating that the variation in mass transfer at the edges and center are not significant. The Sherwood-Schmidt-Reynolds number for this system was found to be:35
These analyses show that while US tanks are capable of significantly increasing agitation within the narrow gap between electrodes of A7 size, the effect of US streaming is less pronounced. This tank system was used to carry out electrodeposition of copper structures at the micron scale, which required photolithographed substrates. It was found that powers higher than 10 WL−1 caused de-adhesion of the photomasks from substrates.23,35 It was found that low powers were favourable for microfabrication due to increased longevity of masks. Therefore, increasing mass transfer is not the sole criterion for optimisation of microfabrication systems.
Imaging
While mass transfer correlations are useful chemical engineering tools for design, a closer inspection of mechanistic processes during US application is also necessary. Imaging techniques have previously been used to visualise cavitation and the phenomenon of micro-jetting using special photography.36 The experiments managed to capture the precise moment of a cavitation bubble collapse and the formation of a micro-jet. There is also work using fluorescence microscopy to detect microbubbles which have propagated from an ultrasound probe,37 studying the effect of collapsing bubbles on the surface of a cell.
Although it is crucial to quantify cavitation activity as a function of the process parameters, there are a limited amount of studies. In some very interesting work, the laser phase-Doppler technique was used to study bubble velocity, size distributions, and volumetric flow of ultrasonic cavitation bubbles propagating from a 20 kHz ultrasound probe38 in a 1.7 L tank and a free-flowing geometry. The experiments demonstrated that the jet from the probe had similar hydrodynamic properties to turbulent circular jet flows. The work also showed that ultrasound power increases bubble velocity quasi-linearly, and changes bubble diameter which was dependent on distances from the probe tip.
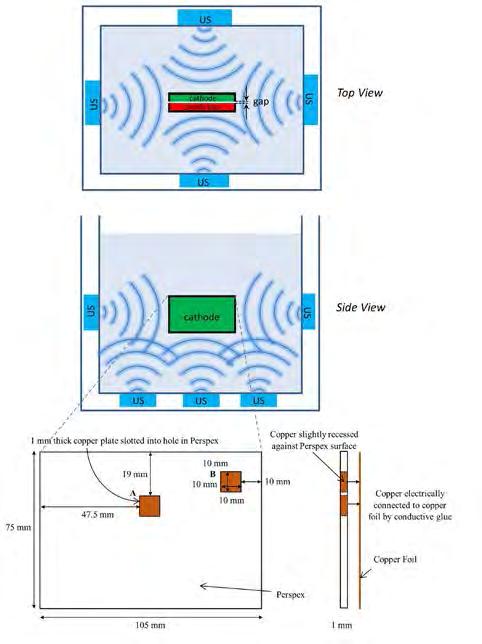
The authors considered the feasibility of using optical techniques to visually capture the movement of cavitation bubbles within a narrow gap of <1 mm. An imaging technique was deemed feasible for a thin gap since light could be transmitted through the electrolyte in the gap, and be interpreted from transmitted images. Therefore a glass cell was constructed with borosilicate windows positioned (almost exactly) parallel to each other with a gap of <1 mm. A 20 kHz ultrasound probe was used as the ultrasonic source, and was placed 3 cm above the gap. The glass cell was filled with 500 ml of electrolyte, and the probe tip was dipped into the solution to generate US agitation. Although the full details of the imaging experiment are beyond the scope of this paper, other additional components required were a light source, a diffuser, slit, iris, two achromatic lenses, two mirrors, a razor blade, and a Nikon D50100 camera.
Both Schlieren and Shadow Graph imaging were tested with the assistance of Patrick Bunton (visiting professor from Vanderbilt University), of which Shadow Graph appeared to provide reasonable images. One frame from the videos during the first few seconds of applying ultrasound at 23 W L−1 is shown in Fig. 6. Although the camera used in this experiment operated at only 25 frames per second, the tracks of the streaming bubbles are discernible, shown by the dark vertical lines indicated by the arrows. It is known that ultrasonic cavitation effects can be captured using a synchronized high-speed stroboscopic Schlieren imaging technique,39 using a
Fig. 5. Electrode arrangement used for limiting current experiments in the US tank. Ultrasound transducers, indicated by US, show the arrangement in the 18 L tank. The limiting current was measured at location (A) and (B), i.e., at the center and edge of the plate to detect differences in agitation. (Adapted from Ref. 23 and 33.) (continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 55
(3) Sh kReSc d L e 3 041
Roy and Coleman
from previous page)
cavitational trigger, such as sono-luminesence and cavitation noise. If a similar technique were used, it is likely that bubble movement within narrow gaps could be observed.
Outlook
Overall, the work shows how US can enhance agitation greatly, especially in constricted spaces. Our work discussed the need of deeper understanding of mass transfer due to US agitation within constricted spaces. The logical development of electrochemical apparatus and adaptation of experimental techniques to quantify mass transfer and develop correlations from these measurements and the challenges faced during the measurements have been outlined. Going further, the use of imaging techniques, which can help in visualisation of cavitation bubbles and elucidate agitation mechanisms, have been discussed.
© The Electrochemical Society. DOI: 10.1149/2.F06183if.
Acknowledgment
The authors acknowledge the help of Patrick Bunton, University of Vanderbilt, for imaging experiments.
About the Authors
Sudipta Roy is the chair professor and head of the Department of Chemical and Process Engineering at the University of Strathclyde, Glasgow, UK (2015-present). She was born in New Delhi, India, in 1963, and completed her bachelor’s degree at the Indian Institute of Technology, Delhi, in chemical engineering (1985). She obtained her PhD from Tulane University (1991), and carried out postdoctoral studies at the Swiss Federal Institute of Technology in Lausanne (1991-1994). She proceeded to take up her lectureship at Newcastle University (1994) and built a research group, which earned her full professorship (2005). She received the Queen’s Reception Invitation for Scientific Achievement (2006) and Royal Academy Industrial Secondment (2001-2002) to JDS Uniphase. She has led a spin-out company (2010) on mask-less electrochemical microfabrication and authored a book on pulse plating (2012). She may be reached at sudipta.roy@strath.ac.uk.

https://orcid.org/0000-0002-3399-035X


Simon Coleman is currently a research associate at the University of Strathclyde, Glasgow. He earned a master’s degree in chemical engineering at Newcastle University, where he also received his PhD in electrochemical engineering in 2015. His work focused on the scale-up of a mask-less electrodeposition process that incorporated ultrasound agitation. He worked as a research engineer with Royenface, which was part of a collaborative European consortium for an EUfunded project, Mesmoproc. He was the postgraduate student representative for the SCI Electrochemical Technology Group, and was the chairman/organizer for the first SCI Electrochem Postgraduate Conference in 2015. He is currently a KTP research associate based at Alconbury Weston Ltd., which specializes in continuous processing solutions. He may be reached at simon.j.coleman@strath.ac.uk.

https://orcid.org/0000-0001-8617-4420
References
1. T. Osaka and M. Datta, Energy storage systems in electronics. CRC Press. (2014).
2. M. Datta, T. Osaka, and J. W. Schulze, Microelectronic Packaging. CRC Press: Boca Raton. (2005).
3. M. J. Madou, Fundamentals of microfabrication: the science of miniaturization. (eds.) CRC press: Boca Raton. (2002).
4. M. Datta and D. Landolt, Electrochimica Acta, 45(15-16), pp. 2535-2558. (2000).
5. D. R. Gabe, Trans. Inst. Metal Finishing, 84(2), pp. 67-78. (2006).
6. T. L. Ritzdorf, G. J. Wilson, P. R. Mchugh, D. J. Woodruff, K. M. Hanson, and D. Fulton, IBM J. Res: Dev, 49(1), pp. 6577. (2005).
7. D. T. Chin, J. Electrochem. Soc., 138(9), pp. 2643 – 2650. (2017).
8. J. R. Selman and C. W. Tobias, Advances in Chemical Engineering, 10(1) 1-318. (1978).
9. A. A. Wragg and T. K. Ross, Electrochimica Acta, 12(10), pp. 1421-1428. (1967).
10. J. C. Bazán and A. J. Arvia, Electrochim Acta, 9(5), pp. 667-684. (1964).
11. B. Levich, Discuss. Faraday Soc., 1, pp. 37-49. (1947).
12. J. Newman, Electroanalytical Chemistry, 6, pp. 279-297. (1973).
13. D. Whitaker, J. B. Nelson, and D. T. Schwartz, J. Micromech, Microeng, 15, pp. 1498. (2005).
14. J. B. Nelson, Z. Wisecarver, and D. T. Schwartz, J. Micromech, Microen., 17, pp. 1192. (2007).
15. I. Schoenenberger and S. Roy, Electrochim Acta, 51(1) pp. 809819. (2005).
16. Q.-B. Wu, T. A. Green, and S. Roy, Electrochem. Comm, 13(11), pp. 1229-1232. (2011).
17. S. Nouraei and S. Roy, J. Electrochem. Soc., 155, pp. D97-D103. (2008).
18. T. Tsuda and C. L. Hussey, Electrochemical Applications of Room-Temperature Ionic Liquids. Interface. (2007).
19. F. Endres, D. MacFarlane, and A. Abbott, Electrodeposition from Ionic Liquids. (eds.), Wiley-VCH: Weinheim. (2017).
20. P. Valverde-Armas, T. Green, and S. Roy, J. Electrochem. Soc., 165(9), pp. D313-320. (2018).
21. B. G. Pollet, Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution. Chichester: John Wiley & Sons. (2012).
22. R. G. Compton, J. C. Eklund, S. D. Page, T. J. Mason, and D. J. Walton, J. Appl. Electrochem, 26, pp. 775-784. (1996).
23. A. Serra, S. J. Coleman, E. Gomez, T. A. Green, E. Valles, J. Vilana, and S. Roy, Electrochimica Acta, 207, pp. 207-217. (2016).
56 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Fig. 6. Frame from video of shadow graph experiment showing traces of bubbles within a narrow gap of 1 mm with 23 W L−1 ultrasound power.
(continued
24. J. A. D. Jensen, P. Pocwiardowski, P. O. Å Persson, L. Hultmann, and P. Møller, Chemical Physics Letters, 368, pp. 732-737. (2003).
25. T. Ohsaka, Y. Goto, K. Sakamoto, M. Isaka, S. Imabayashi, and K. Hirano, Trans. Institute of Metal Finishing, 88(4), pp. 204208. (2010).
26. G. Cravotto and P. Cintas, Chemical Science, 3, pp. 295-307. (2012).
27. P. R. Birkin, R. O’Connor, C. Rapple, and S. S. Martinez, Journal of the Chemical Society, Faraday Transactions, 94(22), pp. 3365-3371. (1998).
28. J. P. Lorimer, B. Pollet, S. S. Phull, T. T. Mason, D. J. Walton, and U. Geissler, Electrochim Acta, 41 (17), pp. 2737-2741. (1996).
29. B. G. Pollet, J. Y. Hihn, M. L. Doche, J. P. Lorimer, A. Mandroyan, and T. J. Mason, J. Electrochem. Soc., 154(10), pp. E131-E138. (2007).
30. J. C. Eklund, F. Marken, D. N. Waller, and R. G. Compton, Electrochim Acta, 41(9), pp. 1541-1547. (1996).
31. F. Marken and R. G. Compton, Ultrasonics Sonochemistry, 3(2), pp. S131-S134. (1996).
32. S. J. Coleman and S. Roy, Chemical Engineering Science, 113, pp. 35-44. (2014).
33. S. J. Coleman, Thesis: Scale-Up of Enface Electrochemical Reactor Systems. Newcastle University. (2015).

34. L. Csoka, S. N. Katekhaye, and P. R. Gogate, Chemical Engineering Journal, 178, pp. 384-390. (2011).
35. S. J. Coleman and S. Roy, Ultrasonics–Sonochemistry, 42, pp. 445-451. (2018).
36. T. B. Benjamin and A. T. Ellis, Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences. 260, pp. 1110. (1966).
37. N. Kudo, K. Okada, and K. Yamamoto, Biophysical Journal, 96(12), pp. 4866–4876. (2009).
38. N. A. Tsochatzidis, P. Guiraud, A. M. Wilhelm, and H. Delmas. Chemical Engineering Science, 56, pp. 1831-1840. (2001).
39. H. Struyf, P. Mertens, M. Heyns, S. De Gendt, C. Glorieux, and S. Brems, Ultrasonics – Sonochemistry, 20, pp. 77-88. (2013).
To help Free the Science visit www.freethescience.org. Research
of publishing,
is meant to be shared. Not sold. Support the costs
make a donation today.

More than 141,000 articles and abstracts in all areas of electrochemistry and solid state science and technology from one of the leading nonprofit publishers in the field. Get your subscription today ! Visit www.ecsdl.org for more information. Flexible subscription options are available to academic and corporate libraries and other institutions.
In Situ Ultrasonic Dispersion in Multiphase Electrolysis Systems
 by Mahito Atobe and Frank Marken
by Mahito Atobe and Frank Marken
Water is generally regarded as the most ideal solvent for many types of organic electrosyntheses because of good conductivity and polarity, stabilizing effects on radical intermediates, and straightforward product isolation by extraction or filtration.1 However, a large number of organic materials cannot be efficiently electrolyzed in aqueous solutions due to their low aqueous solubility. Hence, in most cases, the electrolysis of water-insoluble materials has been carried out in organic polar solvents. However, the electrolysis in organic solvents is practically disadvantageous based on aspects such as conductivity, operational costs, and safety. Therefore, the extension of the range of systems undergoing an electrochemical synthesis in water is of considerable interest and systems employing surfactant-stabilized emulsions and suspensions have been investigated.2 The presence of surfactants or “carrier molecules” may cause other problems such as difficulty post-synthesis in separation of products from the reaction mixtures and additional production of wastes. Hence, it is desirable to conduct emulsion or suspension electrosynthetic processes in the absence of surfactants or stabilizers in order to fulfill the potential of electrosynthesis as a green methodology. Ultrasonic dispersion in situ during electrolysis can be employed to create conditions (see sidebar) of enhanced multiphase reactivity and enhanced mass transport towards the electrode.
Electrochemical Reaction of Water-Insoluble Organic Droplets in Aqueous Electrolytes Using Acoustic Emulsification
It is well known that ultrasound allows multi-phase systems (liquid/liquid, liquid/solid, or liquid/gas) to be efficiently homogenized (given the right choice of frequency, power, and conditions). For example, ultrasonication provides stable emulsions without stabilizing agents simply by mechanical dispersion forces, which arise at the liquid/liquid phase boundaries.3 This has been termed as “acoustic emulsification.”4 Furthermore, it is also known that emulsions prepared by acoustic emulsification have the characteristic of giving narrow droplet size distributions in the submicrometer range.5 Hence, electrochemical systems can be designed for these droplets to be electrolyzed smoothly at electrodes in the presence of power ultrasound. Practically, electrochemical cells can be immersed into ultrasonic bath systems, or sonic horn probes can be immersed directly into the electrochemical systems (with precautions to avoid shunt currents). Figure 1 shows a typical reactor system with a horn emitter pointing at the working electrode surface. The distance between horn and electrode as well as the power of sound emission affect the diffusion layer at the electrode (as well as causing emulsification).
In situ emulsification of an oil phase into the aqueous electrolyte induces practically useful reaction rates even without any added emulsifying reagents. Compton and coworkers reported that apparent diffusion rates of water-insoluble molecules (diethylmaleate, diethylfumarate, and diethylacetylene dicarboxylate) in emulsion systems under ultrasonication (voltammetrically estimated transport rates) were large enough to perform preparative electrolyses.6 Also, Kolbe electrolyses at both platinum and at boron-doped diamond working electrodes were reported.7 The highly water insoluble C10,
Effects of Power Ultrasound During Electrolysis
• vigorous agitation or macro-streaming due to sound induced convection
• micro-streaming at the electrode surface creating high shear forces, cleaning, or even cavitation and erosion
• fast mass transport as expressed by a Nernst diffusion layer thickness down to 1 μm
• turbulent conditions with impacts of microdroplets and particles
• heating of the solution due to energy dissipation (this effect can be used to calibrate the energy flow from sound emitter to electrolysis cell)
• some chemical reactions (radical formation) due to cavitational processes can occur but usually at a rate much lower than that of the electrolytic process
C12, and C22 alkane products (3) as well as some ester products (4) were ultrasonically dispersed and removed from the electrode surface (Fig. 2).
Atobe et al. reported that electrochemical oxidation of waterinsoluble amines (noctylamine and ndecylamine) was accomplished in aqueous electrolytes using acoustic emulsification and gave much higher current efficiencies compared to those obtained under conditions of mechanical stirring (see Table I).8 In addition, they also
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 59
Illustration for Fig. 1 (Atobe and Marken)
Fig. 1. Schematic drawing of an electrosynthesis cell (undivided) with reference (R), counter (C), and working (W) electrodes, a steel cooling coil, and a horn emitter inducing macro-streaming and micro-streaming.
proposed that the smooth electrochemical reaction in the emulsions prepared by ultrasonication took place via direct electron transfer between the electrode and the water-insoluble organic droplets (see Table I).
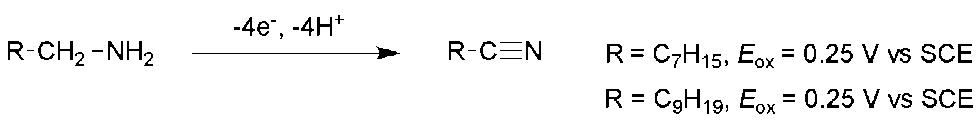
A novel electrolytic system for anodic substitution reactions using acoustic emulsification was developed by Atobe and coworkers.9 This system involves the generation of a carbocation by anodic oxidation of substrate, and then its reaction with nucleophile droplet formed by ultrasonication (Fig. 3). In this system, even if the oxidation potential of nucleophile is lower than that of substrate, the substrate was predominantly oxidized to give the corresponding cation intermediate because the nucleophile phase which was insoluble in an electrolytic medium was electro-inactive. In addition, the overoxidation of the desired products was considerably suppressed by the extraction of products from electrolyte solution into the nucleophile phase. As a result, anodic substitution reaction of several carbamates with allyltrimethylsilane was carried out to provide the corresponding products in good to moderate yields (see Table II).

A related area of application of ultrasound in multi-phase electrosynthesis is in the formation of polymer films.10 Dualfrequency methods were developed to achieve the synthesis of very densely packed polymer colloidal films.11
Ultrasonic Dispersion in Electrosynthetic Processes at Suspended Particle-Electrodes and Slurry-Electrodes
Suspended particle-electrodes or slurry-electrodes can be used to achieve a high space time-yield since these can play a role as an electron transfer mediator and possess a large surface. Therefore, an application of suspended particle-electrodes to electrosynthetic processes has been studied by many researchers.12-15 However, the particle-electrode is usually aggregated during electrolysis in the absence of stabilizer and the total surface area of the electrode is reduced. To overcome this problem, ultrasonic dispersion is very useful for suppression of agglomeration of particle-electrode. Atobe et al. examined ultrasonic effect on the electroreduction of acrylonitrile at suspended lead particle-electrode (see Fig. 4).16 The product selectivity for adiponitrile 1, which was formed as the corresponding hydrodimeric product along with propionitrile 2 as the hydromonomeric one in the cathodic reduction of acrylonitrile, was increased by addition of lead particles as a particle-electrode, and moreover the selectivity was further increased under ultrasonication. It was concluded that ultrasonic effects were observed not only for the mass transport of lead particles to the feeder cathode but also as

60 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org Atobe and Marken (continued from previous page)
Illustration for Fig. 2 (Atobe and Marken)
Fig. 2. Proposed reaction sequence in biphasic Kolbe electrolysis under sonication.7
Fig. 3. Schematic illustration of the anodic substitution reaction system using acoustic emulsification.9
Illustration
n-octylamine 500 - 2.5 n-octylamine 1000 - 31 n-octylamine 1400 - 33 n-octylamine - 106 44 n-octylamine - 177 68 n-octylamine - 248 71 n-decylamine 1400 - 17 n-decylamine - 248 46
Table I. Current efficiency in the anodic oxidation of emulsified amines.
for Table 1(Atobe and Marken)
yield (%) 70 50 43 66 0 N COOMe N COOMe N COMe substrate product N COOMe N COOMe N COMe entry 1 2 3 4 6 7 N COOMe N COOMe electricity (F mol-1) 2 2 3 4 (1 mmol) (10 eqiv.) substrate SiMe3 -2e-H+ ))) EMIM BF4 0.2 mA cm-2 , Pt-Pt product nucleophile
Table II. Anodic substitution reactions of various substrates with allyltrimethylsilane using acoustic emulsification.
Table 2 Anodicsubstitutionreactionsofvarioussubstrates withallyltrimethylsilane usingacousticemulsification Illustration for Fig. 3 (Atobe and Marken)
an increase in the effective surface area of the particle-electrode by ultrasonic dispersion.
Direct interaction or “impact” of microparticles17 and nanoparticles18 can be enhanced and studied in the presence of ultrasound. Sono-electrolytic solid state reactions have been reported for example for Fe2O319 and for graphite powders with and without chemical surface modification (see Fig. 5).20 The characteristic “spike” signal recorded for impacts can be related to the point of zero charge (PZC), the capacitance, and the radius of particles. The impact frequency was found to be related to the dispersed mass of carbon particles.
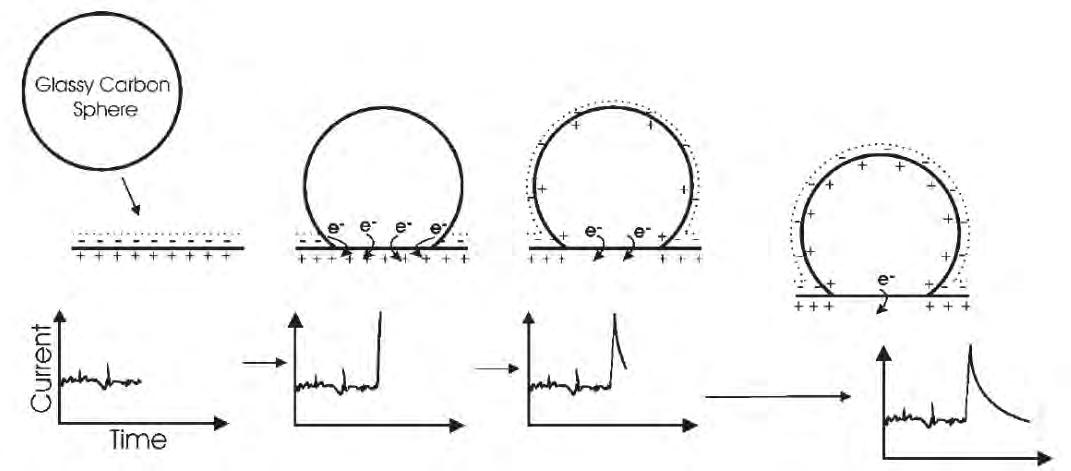
For these solid particles collision rates with the electrode and transport rates are enhanced. Time-resolved information about the individual particles is obtained. However, in future also quantitative conversion may be feasible with sono-electrosynthesis providing a new way for clean solid-solid or electro-catalytic transformations.
Conclusions and Outlook
Given the fact that power ultrasound substantially increases mass transport, it is now possible to electrolyse faster even for slowly diffusing droplets, clusters, or particles.21 Therefore processes with “slow diffusers” such as colloids or nanoparticles are most affected by the application of ultrasound due to overwhelming mass transport effects. A new development is the application of “impact processes” where interaction with solid particles are ultrasonically enhanced
or particles are accelerated onto the electrode surface to undergo electrolysis. This could lead to entirely new types of electrode reactions based on nanoparticles and nanomaterials as long as the underlying principles of ultrasound – multi-phase interactions are better understood. Particle impacts have been shown to be sometimes very energetic—even leading to partial melting of metal particles.22 We believe that, although electrosynthesis needs to be performed with simple tools and practical inexpensive reactor systems, there is still a need to enhance reactions and to trigger unusual surface conditions and processes by power ultrasound. In future ultrasound-enhanced multi-phase electrosynthesis with slow diffusers, microdroplets, or particles will be developed further and could be applied to electrosynthetic reactions that are useful for organic syntheses. © The Electrochemical Society. DOI: 10.1149/2.F07183if.

About the Authors
Mahito Atobe is a professor at Yokohama National University. He was born in Yamanashi, Japan. He received his PhD degree from Tokyo Institute of Technology in 1998. He has held positions at Tokyo Institute of Technology (1996-2011) and Yokohama National University (2011-current). He has been awarded the Young Researcher Award of The Electrochemical Society of Japan (2003), the Commendation for (continued on next page)

The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 61
Illustration for Fig. 4 (Atobe and Marken)
Fig. 5. Schematic drawing of an impacting particle with capacitive charge transfer during interaction with the electrode surface.20
Fig. 4. Schematic representation of the electroreduction of acrylonitrile at suspended lead particle-electrode.16
Atobe and Marken
(continued from previous page)
Science and Technology by the Minister of Education, Culture, Sports, Science, and Technology: The Prize for Young Scientists (2009), and other awards. He may be reached at atobe@ynu.ac.jp.
https://orcid.org/0000-0002-3173-3608


Frank Marken worked as a lecturer at Loughborough University, was appointed to a senior lecturer position in September 2004, and in 2011 was promoted to a personal chair in physical chemistry at the Department of Chemistry, University of Bath. His research is focused on materials electrochemistry and innovative methods in electrosynthesis and electroanalysis. Marken was awarded the RSC Theophilus Redwood Award in 2008 and the RSC Geoffrey Barker Medal in 2018. He may be reached at f. marken@bath.ac.uk.

https://orcid.org/0000-0003-3177-4562
References
1. H. Feess and H. Wendt, in Technique of Electroorganic Synthesis. Part III, N. L. Weinberg and B. V. Tilak, Editors, p. 81-178, John Wiley & Sons, New York (1982).
2. R. A. Mackay and J. Texter, Electrochemistry in Colloids and Dispersions, Wiley VCH, Weinheim (1992).
3. C. E. Banks, N. V. Rees, and R. G. Compton, “Sonoelectrochemistry in Acoustically Emulsified Media,” J. Electroanal. Chem., 535, 41 (2002).
4. S. R. Reddy and H. S. Fogler, “Emulsion Stability of Acoustically Formed Emulsions,” J. Phys. Chem., 84, 1570 (1980).
5. K. Kamogawa, G. Okudaira, M. Matsumoto, T. Sakai, H. Sakai, and M. Abe, “Preparation of Oleic Acid/Water Emulsions in Surfactant-Free Condition by Sequential Processing Using Midsonic-Megasonic Waves,” Langmuir, 20, 2043 (2004).
6. F. Marken, R. G. Compton, S. D. Bull, and S. G. Davies, “Ultrasound-assisted Electrochemical Reduction of Emulsions in Aqueous Media,” Chem. Commun., 33, 995 (1997).
7. J. D. Wadhawan, F. J. Del Campo, R. G. Compton, J. S. Foord, F. Marken, S. D. Bull, S. G. Davies, D. J. Walton, S. Ryley, “Emulsion Electrosynthesis in the Presence of Power Ultrasound Biphasic Kolbe Coupling Processes at Platinum and Boron-doped Diamond Electrodes,” J. Electroanal. Chem., 507, 135 (2001).
8. M. Atobe, S. Ikari, K. Nakabayashi, F. Amemiya, and T. Fuchigami, “Electrochemical Reaction of Water-Insoluble Organic Droplets in Aqueous Electrolytes Using Acoustic Emulsification,” Langmuir, 26, 9111 (2010).
9. R. Asami, T. Fuchigami, and M. Atobe, “Development of an Anodic Substitution Reaction System Using Acoustic Emulsification,” Chem. Commun., 44, 244 (2008).
10. R. Asami, M. Atobe, and T. Fuchigami, “Electropolymerization of an Immiscible Monomer in Aqueous Electrolytes Using Acoustic Emulsification,” J. Am. Chem. Soc., 127, 13160 (2005).
11. K. Nakabayashi, T. Fuchigami, and M. Atobe, “Tandem Acoustic Emulsion, an Effective Tool for the Electrosynthesis of Highly Transparent and Conductive Polymer Films,” Electrochim. Acta, 110, 593 (2013).
12. D. N. Baria and H. M. Hulburt, “Reduction of Crotonic Acid with Hydrogen on a Slurry Electrode,” J. Electrochem. Soc., 120, 1333 (1973).
13. E. Keren and A. Soffer, “Hydrogen Adsorption Rate and Discharge Mechanism on Palladium-Carbon Suspension Electrode,” J. Electroanal. Chem., 44, 53 (1973).
14. H. Kametani, “Potential Curves in the Suspension Electrode Electrolysis of Copper,” Denki Kagaku (presently Electrochemistry) 40, 449 (1972).
15. S. Yoshizawa, Z. Takehara, Z. Ogumi, M. Matsubara, and T. Tsuji, “Application of a Circulating Suspended-particle Electrode to Electrochemical Hydrodimerization of Acrylonitrile,” J. Appl. Electrochem., 6, 403 (1976).
16. R. Asami, M. Atobe, and T. Fuchigami, “Ultrasonic Effects on Electroorganic processes. Part 27. Electroreduction of Acrylonitrile at Suspended Lead Particle-Electrode,” Ultrasonics Sonochem., 13, 19 (2006).
17. N. V. Rees, C. E. Banks, and R. G. Compton, “Ultrafast Chronoamperometry of Acoustically Agitated Solid Particulate Suspensions: Nonfaradaic and Faradaic Processes at a Polycrystalline Gold Electrode,” J. Phys. Chem. B, 108, 18391 (2004).
18. K. J. McKenzie and F. Marken, “Direct Electrochemistry of Nanoparticulate Fe2O3 in Aqueous Solution and Adsorbed onto Tin-doped Indium Oxide,” Pure Appl. Chem., 73, 1885 (2001).
19. M. A. Murphy, F. Marken, and J. Mocak, “Sonoelectrochemistry of Molecular and Colloidal Redox Systems at Carbon Nanofiberceramic Composite Electrodes,” Electrochim. Acta, 48, 3411 (2003).
20. A. D. Clegg, N. V. Rees, C. E. Banks, and R. G. Compton, “Ultrafast Chronoamperometry of Single Impact Events in Acoustically Agitated Solid Particulate Suspensions,” ChemPhysChem, 7, 807 (2006).
21. A. J. Bard, “High Speed Controlled Potential Coulometry,” Anal. Chem., 35, 1125 (1963).
22. N. A. Madigan, C. R. S. Hagan, H. H. Zhang, and L. A. Coury, Ultrason. Sonochem., 3, S239 (1996).
62 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
PLUS www.electrochem.org Join the movement!
GETINVOLVED WITH ECS SECTIONS
ECS sections serve an integral role within the Society. Many sections offer opportunities for members and student members to connect with their regional peers and host local symposia/meetings. Like our divisions, the sections help to serve the mission of ECS.
The Society provides services for sections to assist with marketing, meeting registration, and member recruitment. The services available are:
• Online meeting registration
• Provide quarterly financial statements
• Process reimbursements from section accounts
• Marketing through branded emails, weekly eNews, ECS Redcat Blog, and social media
• Assist with recruitment/retention of section members
Sections are also encouraged to publish updates about their activity in the Society’s quarterly magazine, Interface. Publishing section news promotes the activities of section members to the Society.
Scott Smith from the ECS Canada Section used many of the services offered when planning the Canada Section’s spring 2018 meeting.
We encourage our members to get involved with our sections. There are 23 ECS sections around the world:
Arizona Section – Candace Kay Chan, Chair
Brazil Section – Ernesto Pereira, Chair
Canada Section – Bradley Easton, Chair
Chicago Section – Alan Zdunek, Chair
Chile Section – Jose H. Zagal, Chair
China Section – Yong Yao Xia, Chair
Cleveland Section – Heidi B. Martin, Chair
Detroit Section - Dennis Corrigan, Chair
Europe Section – Petr Vanýsek, Chair
Georgia Section – Seung Woo Lee, Chair
India Section – Vijayamohanan K. Pillai, Chair
Israel Section – Daniel Mandler, Chair
Japan Section – Hiroshi Iwai, Chair
Korea Section – Won-Sub Yoon, Chair
Mexico Section – Carlos Frontana Vázquez, Chair
National Capital Section – Eric D. Wachsman, Chair
New England Section – Sanjeev Mukerjee, Chair
Pittsburgh Section – Clifford W. Walton, Chair
San Francisco Section – Yarik Syzdek, Chair
Singapore Section – Qingui Yan, Chair
Taiwan Section – Nick Wu, Chair
Texas Section – Jeremy P. Meyers, Chair
Twin Cities Section – Peter Zhang, Chair
“ECS staff were always available and quick to offer support for any issues that arose. From setting the event’s registration to collecting the registration fees, everything was done with the utmost ease. I have continued to receive positive feedback from the meeting regarding the simplicity for all attendees to register for the event as well as the ease of completing multiple reimbursements, including student award distribution, after the event. I have and will continue to highly recommend utilizing all of these services to all future ECS organizers.”
— Scott Smith
customerservice@electrochem.org
23
AWARDS PROGRAM
Awards, Fellowships, Grants




ECS distinguishes outstanding technical achievements in electrochemistry, solid state science and technology, and recognizes exceptional service to the Society through the Honors & Awards Program. Recognition opportunities exist in the following categories: Society Awards, Division Awards, Student Awards, and Section Awards.


ECS recognizes that today’s emerging scientists are the next generation of leaders in our field and offers competitive Fellowships and Grants to allow students and young professionals to make discoveries and shape our science long into the future. See
Society Awards
The ECS Carl Wagner Memorial Award was established in 1980 to recognize mid-career achievement and excellence in research areas of interest of the Society, and significant contributions in the teaching or guidance of students or colleagues in education, industry, or government. The award consists of a silver medal, a wall plaque, Society life membership, complimentary meeting registration, and travel assistance of up to $1,000.
Materials are due by October 1, 2018.
The ECS Olin Palladium Award was established in 1950 to recognize distinguished contributions to the fields of electrochemical or corrosion science. The award consists of a palladium medal, a wall plaque, a $7,500 prize, Society life membership, and complimentary meeting registration. Materials are due by October 1, 2018.





























Division Awards
The ECS Energy Technology Division Research Award was established in 1992 to encourage excellence in energy-related research. The award consists of a framed certificate, a $2,000 prize, and membership in the Energy Technology Division for as long as the recipient is an ECS member.

Materials are due by September 1, 2018.
The ECS Energy Technology Division Supramaniam Srinivasan Young Investigator Award was established in 2011 to recognize and reward an outstanding young researcher in the field of energy technology. The award consists of a framed certificate, a $1,000 prize, and complimentary meeting registration.
Materials are due by September 1, 2018.
The ECS Nanocarbons Division Richard E. Smalley Research Award was established in 2006 to encourage excellence in fullerenes, nanotubes, and carbon nanostructures research. The award is intended to recognize, in a broad sense, those persons who have made outstanding contributions to the understanding and applications of fullerenes. The award consists of a framed certificate, a $1,000 prize, and assistance up to a maximum of $1,500 to facilitate attendance of the meeting at which the award is to be presented.
Materials are due by September 1, 2018.
The ECS Physical and Analytical Electrochemistry Division David C. Grahame Award was established in 1981 to encourage excellence in physical electrochemistry research and to stimulate publication of high-quality research papers in ECS journals. The award consists of a framed certificate and a $1,500 prize.
Materials are due by October 1, 2018.
The ECS Corrosion Division Herbert H. Uhlig Award was established in 1972 to recognize excellence in corrosion research and outstanding technical contributions to the field of corrosion science and technology. The award consists of a framed certificate, a $1,500 prize, and possible travel assistance.
Materials are due by December 15, 2018.
The ECS High-Temperature Energy, Materials, & Processes Division J. Bruce Wagner, Jr. Award was established in 1998 to recognize a young Society member who has demonstrated exceptional promise for a successful career in science and/or technology in the field of high temperature materials. The award consists of an appropriately worded scroll and the sum of $1,000. The recipient may receive (if required) complimentary registration and up to $1,000 in financial assistance toward travel expenses for attendance of the Society meeting at which the award is to be presented.
Materials are due by January 1, 2019.
highlights below and visit www.electrochem.org for further information. NEW MEMBERS 64 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
AWARDS PROGRAM
The ECS Organic and Biological Electrochemistry Division Manuel M. Baizer Award was established in 1992 to recognize individuals for their outstanding scientific achievements in the electrochemistry of organics and organometallic compounds, carbon-based polymers and biomass, whether fundamental or applied, and including but not limited to synthesis, mechanistic studies, engineering of processes, electrocatalysis, devices such as sensors, pollution control, and separation/recovery. The award consists of an appropriately worded scroll and the sum of $1,000.
Materials are due by January 15, 2019.
Section Awards
The ECS Europe Section Heinz Gerischer Award was established in 2001 to recognize an individual or a small group of individuals (no more than three) who have made an outstanding contribution to the science of semiconductor electrochemistry and photoelectrochemistry including the underlying areas of physical and materials chemistry of significance to this field. The award consists of a framed certificate, a EUR 2,000 prize, and if required, financial assistance for unreimbursed travel expenses incurred to receive the award, not to exceed EUR 1,000.
Materials are due by September 30, 2018.
Student Awards
The ECS Corrosion Division Morris Cohen Graduate Student Award was established in 1991 to recognize and reward outstanding graduate research in the field of corrosion science and/or engineering. The award consists of a certificate and the sum of $1,000. The award, for outstanding master’s or PhD work, is open to graduate students who have successfully completed all the requirements for their degrees, as testified to by the students’ advisers, within a period of two years prior to the nomination submission deadline.
Materials are due by December 15, 2018.
The ECS Energy Technology Division Graduate Student Award sponsored by Bio-Logic was established in 2012 to recognize promising young engineers and scientists in fields pertaining to this division. The award consists of a framed certificate, a $1,000 prize, complimentary student meeting registration, and complimentary admission to the Energy Technology Division business meeting.
Materials are due by September 1, 2018.
The ECS Georgia Section Outstanding Student Achievement Award was established in 2011 to recognize academic accomplishments in any area of science or engineering in which electrochemical and/or solid state science and technology is the central consideration. The award consists of a $500 prize.




Materials are due by August 15, 2018.
The ECS Industrial Electrochemistry and Electrochemical Engineering Division H. H. Dow Memorial Student Achievement Award was established in 1990 to recognize promising young engineers and scientists in the field of electrochemical engineering and applied electrochemistry. The award consists of a framed certificate and a $1,000 prize to be used for expenses associated with the recipient’s education or research project.
Materials are due by September 15, 2018.
The ECS Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award was established in 1989 to recognize promising young engineers and scientists in the field of electrochemical engineering and to encourage the recipients to initiate careers in this field. The award consists of a framed certificate and a $1,000 prize.
Materials are due by September 15, 2018.
The ECS India Section S. K. Rangarajan Graduate Student Award was established in 2017 to assist a deserving student in India to pursue a career in disciplines related to electrochemistry and solid state science and technology. The award consists of a $500 (USD) prize and is presented to the recipient in person at a meeting of the India Section.
Materials are due by September 30, 2018.
The ECS Korea Section Student Award was established in 2005 to recognize academic accomplishments in any area of science or engineering in which electrochemical and/or solid state science and technology is the central consideration. The award consists of a $500 prize and is presented at a designated Korea Section meeting. At that time, the recipient may be requested to speak on a subject of major interest to him/her in the field of electrochemical and/or solid state science and technology.
Materials are due by September 30, 2018.

NEW
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 65
AWARDSMEMBERS
Interested in funding an ECS award? Your organization can help support the remarkable work of leading researchers in the field. Learn more by contacting Ngoc Le in the ECS Development Office, development@electrochem.org.

NEW MEMBERS 66 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org AWARDS PROGRAM ECS FELLOWS 2019 Call for Nominations Deadline: February 1, 2019
AWARDS PROGRAM
Award Winners
Join ECS as it extends congratulations to the following award winners who will be recognized during AiMES in Cancun.
Society Awards
Charles W. Tobias Young Investigator Award
Michael S. Arnold is currently a Professor of Materials Science and Engineering at the University of Wisconsin-Madison. There, he has directed the Advanced Materials for Energy and Electronics Group since 2008.


Arnold graduated summa cum laude from the University of Illinois at UrbanaChampaign with a BS in Electrical Engineering in 2001. He earned his PhD in 2006 from Northwestern University in Materials Science and Engineering under Professors Mark Hersam and Sam Stupp.
Arnold conducted post-doctoral research at the University of Michigan at Ann Arbor from 2006-2008 with Stephen Forrest. Arnold has been a recipient of the ECS Nanocarbons SES Research Young Investigator Award (2018); the National Science Foundation CAREER award (2014); the American Chemical Society Arthur K. Doolittle Award in Polymeric Materials Science and Engineering (2012); the Presidential Early Career Award for Scientists and Engineers (PECASE) – nominated by the U.S. Department of Defense, Army Research Office (2011); the U.S. Department of Energy (DOE) Early Career Research Award (2011); and a 3M NonTenured Faculty Award (2011, 2012, 2013). He was also a runner-up in the U.S. Department of State ASPIRE Competition (2017).
Arnold’s work addresses fundamental challenges – in controlling the growth, processing, ordering, and heterogeneity of nanomaterials and in understanding phenomena beyond the scale of single nanostructures – that must be overcome to exploit nanomaterials in technology. Arnold’s research has resulted in 100 journal publications and 15 patents/patent applications.
Edward Goodrich Acheson Award
Tetsuya Osaka is Senior Research Professor and Emeritus Director of the Institute for Research Organization for Nano & Life Innovation, and Professor Emeritus of the Faculty of Science and Engineering, Waseda University, Tokyo, Japan.

Osaka is a past President of The Electrochemical Society. He has also served as president of the Magnetics Society of Japan, president of the Electrochemical Society of Japan, president of Japan Institute of Electronic Packaging, vice-president of the Surface Finishing Society of Japan, vice president of the International Society of Electrochemistry.
His research field is electrochemical technology, and his recent work is focused on electrochemical nanotechnology, including electro- and electroless-deposition/surface finishing, electronic packaging materials, magnetic storage and energy storage devices, and chemical- and bio-sensors.
Osaka currently focuses on batteries and battery energy managing system. He has contributed as an author and/or editor to more than 90 books and published more than 1,020 original and review papers in these fields. He has been identified as one of the highly cited researchers in the materials science category in Thomson ISI’s ISIHighlyCited.com.
His technical contributions have been recognized by many awards including the Medal with Purple Ribbon bestowed from the Decoration Bureau of the Cabinet Office, Japan, in 2010, IBA Yeager Award of International Battery Association in 2017, Electrochimica Acta Gold Medal of the International Society of Electrochemistry in 1999.
Norman Hackerman Young Author Award
Raymond Smith earned his PhD in chemical engineering from MIT in 2017 with a minor in computational materials science.
While a graduate student, Smith worked with Martin Bazant on various projects involving mathematical modeling of electrochemical systems. His primary focus involved developing models for battery electrode materials with phase separation behavior, coupling them to full porous electrode battery models, and validating those models with the help of experimental collaborations.
Smith also participated in the MIT Chemical Engineering Practice School program, both as a student and as a station director. In this program, he was involved in high-intensity, short-term industrial consulting collaborations on a widely diverse set of projects at Corning, BP, MedImmune, and Emirates Global Aluminum.
Smith earned his undergraduate degree from Clemson University. For the past year, he has been working as an engineer at Tesla, focused on battery performance and lifetime modeling.
Bruce Deal & Andy Grove Young Author Award
Shihyun Ahn received his BS and MS degrees in chemical engineering from Carnegie Mellon University and earned his PhD degree from the University of Florida in May 2017.

His research focused on the effect of irradiation damage on GaN-based metaloxide semiconductor high-electron mobility transistors and β-Ga2O3. He is also interested in the processing and characterization of III-V compound semiconductors.
He authored or coauthored over 20 papers during his PhD studies. Ahn is currently working in the Ultraviolet Product Development Department at Seoul Viosys in Korea.
(continued on next page)
NEWAWARDSMEMBERS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 67
AWARDS PROGRAM
(continued from previous page)
Fellow of The Electrochemical Society –Class of 2018
Sheikh A. Akbar is a Professor of Materials Science and Engineering and Founder of the National Science Foundation Center for Industrial Sensors and Measurements at The Ohio State University in Columbus, OH.
His research deals with synthesismicrostructure-property relations of ceramic bulk, thin-film and nanostructures for electrochemical devices such as sensors and biomedical applications. Akbar was the chair of the 12th International Conference on Chemical Sensors held in 2008. His sensors received three R&D 100 Awards as part of the 100 best inventions of 2007 and 2005 as selected by R&D Magazine, and 2005 NASA TGIR (turning Goal into Reality) award.
Akbar is the recipient of the 2012 The Electrochemical Society Sensor Division Outstanding Achievement Award, the 2002 Tan Chin Tuan Fellow of Nanyang Technologi-cal University in Singapore, and the 2001 Fulrath Award and the 2002 W.E. Cramer Award of the American Ceramic Society.

He was elected a Fellow of the American Ceramic Society in 2001. He also received the 1993 B.F. Goodrich Collegiate Inventors Award for the development of a rugged and durable CO/H2 sensor; one of three national awards. Akbar is on the editorial boards of the Journal of Nanoengineering and Nanomanufacturing, Materials Focus, Sensors, Ceramics International, Journal of Nanomaterials, and Sensor Letters and Frontiers in Materials.
He has published more than 220 technical papers and holds eight patents. Akbar received the Mars G Fontana Outstanding Teacher Award in Materials Science and Engi-neering at OSU for both 2016 and 2017.
Yi Cui is a Professor in the Department of Materials Science and Engineering at Stanford University.

He received BS in Chemistry in 1998 from the University of Science and Technology of China (USTC) and his PhD in 2002 from Harvard University. After that, he went on to work as a Miller Postdoctoral Fellow at University of California, Berkeley.
In 2005 he became an assistant professor in the Department of Materials Science and Engineering at Stanford University. He was promoted with tenure in 2010 and became a full professor in 2016. His current research is on nanomaterials for energy storage, photovotalics, topological insulators, biology and environment.
He has founded three companies to commercialize technologies from his group: Amprius Inc., 4C Air Inc. and EEnovate Technology Inc. He is a Fellow of Materials Research Society and a Fellow of Royal Society of Chemistry. He is an associate editor of Nano Letters He is a co-director of the Bay Area Photovoltaics Consortium and a co-director of Battery 500 Consortium.
He is a highly proliferate materials scientist and has published ~390 research papers with an H-index of 162 (Google). In 2014, he was ranked No. 1 in materials science by Thomson Reuters as “The World’s Most Influential Scientific Minds.” His selected awards include: Blavatnik National Laureate (2017), MRS Kavli Distinguished Lectureship in Nanoscience (2015), the Sloan Research Fellowship (2010), KAUST Investigator Award (2008), ONR Young Investigator Award (2008), and the Technology Review World Top Young Innovator Award (2004).
Jan Fransaer is a professor of Materials Engineering at the KU Leuven. He received his engineering degree in metallurgy from the UGent in 1985, and his PhD in materials engineering from KU Leuven in 1994.

He received the NSF Graduate Student Award in 1994 for his work on developing models and sensors for suspension plating. He was a Postdoctoral Research Fellow at Harvard University where he studied the nucleation of metals during solidification with Frans Spaepen’s group.

At the age of 7, his older brother steered him towards electrochemistry by electrolyzing water using a 4.5 V battery, car acid and two carbon electrodes from spent alkaline batteries. Since then, his group has made significant contributions to the practical and theoretical understanding of the electrodeposition of particles together with metals, and was one of the first groups to study this process using non-aqueous electrolytes. This resulted a few years later in the study of ionic liquids for electrochemical applications, mainly focused on electrodeposition, but now extended to batteries, fuel cells and supercapacitors.
Together with Koen Binnemans, he has explored the properties of a class of ionic liquids, called liquid metal salts, which are metal salts that are liquid below 100 °C. With his wife, Dominguez-Benetton, Fransaer is exploring the electrochemical synthesis of nanoparticles.
His current research focuses on experimental and computational electrochemistry, the electrochemistry of metal ions in ionic liquids and the electrodeposition of metals, alloys, semiconductors and MOFs. He has published over 200 papers in this area and has filed 14 patents, of which four have been licensed to industry.
Turgut M. Gür is a retired adjunct professor of Materials Science and Engineering at Stanford University.
He spent nearly four decades in research, education and mentoring, as well as in providing leadership for major research centers on advanced materials and energy, including the NSF-MRSEC Center for Materials Research, Geballe Laboratory for Advanced Materials, and the DOE-EFRC Center on Nanostructuring for Efficient Energy Conversion. He also holds a visiting professor appointment from the Chinese University of Mining and Technology-Beijing in China.
Gür is the former chair of the ECS High-Temperature Energy, Materials, & Processes Division and served on several ECS committees as well as on the board of directors. He was a board member of the International Society for Solid State Ionics for ten years.
His primary research interest is high temperature electrochemical energy conversion and storage materials and technologies. He is recognized for his contributions in the development of carbon fuel cells for clean power generation; hydrogen and electricity coproduction; high temperature electrocatalysis of NOx reduction, CO2-to-fuels conversion, and Fischer-Tropsch electrosynthesis; surface and interface engineered thin film SOFCs; and solid state ionic tools for fundamental research on functionally active oxides.
Gür has BS and MS degrees in chemical engineering from the Middle East Technical University in Ankara (Turkey), and a PhD in materials science and engineering from Stanford University. He holds 11 U.S. patents, and has authored or co-authored more than 150 technical publications related in large part to high temperature electrochemical energy sciences and materials.
NEW MEMBERS 68 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
AWARDS PROGRAM
Pawel J. Kulesza received his PhD from Southern Illinois University at Carbondale and DSc from University of Warsaw, Poland.

After postdoctoral appointments at University of Illinois at Urbana-Champaign, University of Texas at Austin, Fritz-HaberInstitut of Max-Planck-Gesselshaft in Berlin, EPFL – Swiss Federal University of Technology in Lausanne and University of North Carolina at Chapel Hill, he took a position of a professor of chemistry at University of Warsaw, Poland also held the position of dean there for many years.

In The Electrochemical Society, he served on many committees as well as chair of the Physical Analytical Electrochemistry Division and chair of the European Section. He is a member of Polish Academy of Sciences and, currently, a member of editorial boards of Electrocatalysis, Journal of Solid State Electrochemistry, Russian Journal of Electrochemistry and Electrochimica Acta (associate editor).
Kulesza has significantly contributed to description of mechanisms and dynamics of charge propagation in solid or semisolid materials characterized by reversible redox transitions, fast charge propagation, and storage. His research has also led to the development of new electroanalytical methodology and advances in diagnostic measurements based on ultramicroelectrodes. He proposed new functional materials composed of networks of noble metal nanoparticles, carbon nanostructures, metal oxides, polyoxometallates, cyanometallates, or their hybrids with polymers.
More recently, his attention has centered on the development of hybrid materials for electrocatalysis (fuel cell fundamental research) and photoelectrochemistry (energy conversion, water splitting, CO2conversion, and generation of fuels). He has published more than 250 refereed papers and has h-index of 55 (Google Scholar).
Stuart Licht is an environmentalist and electrochemist. He is a Dean’s Research Professor in the Department of Chemistry at George Washington University, and with his group has contributed over 400 papers and patents, focused on sustainability or fundamental, physical chemistry, and is the Leader of Team C2CNT, one of five finalist teams competing in the international Carbon XPrize to produce the most valuable product from the CO2 emissions of a gas power plant.
Stuart Licht served as a program director in the National Science Foundation. An early pioneer in the field of photoelectrochemistry, Licht helped establish basic principles of the field as well as highest efficiency photoelectrochemical solar cells (cover article, Nature, 1987). His principals of STEP (solar thermal electrochemical processes) have led to the demonstration of CO2free syntheses of ammonia, iron & steel, cement, highest efficiency solar fuels, and organics. His research group has helped established the principals of multiple electron per molecule charge storage, including the 11 electron VB2, the hexavalent “super-iron,” and molten air batteries.
Licht has broadened the foundation of understanding of a wide range of electrochemical phenomena ranging from generation/ collection microelectrochemistry, to fundamentals of speciation, and water purification, and recently introduced a process to remove the greenhouse gas CO2 to mitigate climate change by its transformation to carbon nanotubes.
Recognitions include Licht’s Hilldebrand Award, BASF 150th Anniversary Electrochemical Storage Award, the ECS Energy Technology Division Research Award, the Alcoa Research Award, GWU’s Trachtenberg Scholarship Award, the Technion Gustella Award, and Clark’s Carlson Endowed Chair in Chemistry.
Y. Shirley Meng received her PhD in advance materials for micro and nano systems from the Singapore-MIT Alliance in 2005, after which she worked as a postdoc research fellow and became a research scientist at MIT.
Meng currently holds the Zable Chair Professor in Energy Technologies and professor in Materials Science & NanoEngineering at University of California San Diego. She is the principal investigator of the research group – Laboratory for Energy Storage and Conversion. The LESC research focuses on the direct integration of experimental techniques with first principles computation modeling for developing new materials and architectures for electrochemical energy storage.
She is the founding director of Sustainable Power and Energy Center, consisting faculty members from interdisciplinary fields, who all focus on making breakthroughs in distributed energy generation, storage, and the accompanying integration-management systems.
She received several prestigious awards including American Chemical Society ACS Applied Materials & Interfaces Young Investigator Award (2018), International Coalition for Energy Storage and Innovation Inaugural Young Career Award (2018), IUMRS-Singapore Young Scientist Research Award (2017), C.W. Tobias Young Investigator Award of The Electrochemical Society (2016), BASF Volkswagen Electrochemistry Science Award (2015) and NSF CAREER Award (2011).

Meng sits on the executive board of the International Battery Association as one of the associate directors. She is the author and co-author of more than 160 peer-reviewed journal articles, one book chapter and four issued patents.
Junichiro Mizusaki has been active in the field of solid state ionics for nearly 50 years. Most of his works are on the properties of perovskite-type mixed conductors or on the gas-electrode kinetics of solid electrolyte cells.

He also performed several characteristic researches on such topics as the extended DC polarization techniques to determine the electronic properties of silver halides, electrochemical degradation of ceramic electrodes, halide ion conduction of perovskite-type halides, electrochemical determination of copper and sulfur nonstoichiometry in copper-chevrel compounds, lithium ion conduction of lithium carbonate and its application to CO2 sensors, O– and F– emission from solid anion conductors, and application of acoustic emission analysis for the study of mechanical stability of solid oxide fuel cells.
He received his BS, Master’s, and PhD degrees from Faculty of Engineering, the University of Tokyo. He was a research associate there from 1975 until 1986. In 1977-1978, he was with Department of Materials Science and Engineering at the Massachusetts Institute of Technology.
In 1986, he moved to Yokohama National University as an associate professor. In 1994, he was became a professor at Tohoku University and led a laboratory in Research Institute of Scientific Measurements, later reorganized into the Institute of Multidisciplinary Research for Advanced Materials. He retired from Tohoku University in 2012 and received the title of a professor emeritus. He was a chairperson of the SOFC Society Japan (2004-2011) and of the Solid State Ionics Society of Japan (2006-2010). He also served as a councilor of International Society of Solid State Ionics (1997-2001).
(continued on next page)
NEWAWARDSMEMBERS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 69
AWARDS PROGRAM
(continued from previous page)
Venkat R. Subramanian received his Bachelor of Technology degree in chemical and electrochemical engineering from the Central Electrochemical Research Institute, Karaikudi, India, in 1997 and his PhD degree in chemical engineering from the University of South Carolina, Columbia, SC in 2001. He is currently a Washington Research Foundation Innovation Professor of Chemical Engineering & Clean Energy and an adjunct professor of Electrical Engineering, at the University of Washington, Seattle. He holds a joint appointment at Pacific Northwest National Laboratory as a Chief Scientist.
His research interests include multiscale simulation of electrochemical systems, electrochemical engineering, energysystems engineering, real-time state-of-charge and state-of-health estimation of lithium-ion batteries, model-based battery management systems for electric transportation, and renewable microgrids and application of nonlinear model predictive control methods.
Subramanian’s group has the fastest algorithm reported in the literature for the simulation of battery models.
Subramanian is a past elected chair of IE&EE division of The Electrochemical Society. He was awarded the Dean's award for excellence in graduate study in 2001 for his doctoral research. Het acknowledges the influence of his undergraduate and graduate advisors/teachers, his past and current students, and many researchers/ well-wishers in the ECS community.
Jay Switzer is the Donald L. Castleman Distinguished Professor of Chemistry at Missouri University of Science and Technology. He also is a senior investigator at the Materials Research Center.

Switzer received his BS degree in chemistry from the University of Cincinnati, and his PhD degree in inorganic chemistry from Wayne State University under Professor John F. Endicott. After receiving his PhD degree, he joined Union Oil Company of California as a senior research chemist. His research at UNOCAL was on photoelectrochemistry and the electrochemical processing of photovoltaic cells.
In 1986, Switzer joined the Materials Science and Engineering Department of the University of Pittsburgh as an associate professor. In 1990, he moved to the University of Missouri, Rolla as a professor of chemistry. Switzer has spent most of his career working on the electrodeposition of nanostructured metal oxide semiconductors, magnetic materials, and catalysts.
He is best known for his work on the electrodeposition of epitaxial metal oxides, oxide superlattices, chiral surfaces, and freestanding single-crystal metal foils. Switzer received the ECS Electrodeposition Division Research Award in 2003, and he presently serves as the faculty advisor to the ECS Missouri S&T Student Chapter.
Tomás Torres is a full professor and director of the Institute of Advanced Research in Chemical Sciences, at Autonoma University of Madrid and associate senior scientist of the IMDEA-Nanoscience. His research has focused on the preparation of molecular materials based on phthalocyanines and on the study of their properties for applications in organic solar cells and photodynamic therapy. He has published more than 500 articles and reviews and 43
patents, has advised 50 doctoral theses, and has an H-Index of 77. He has been co-founder, and is a partner of NanoInnova Technologies.

Torres belongs to the editorial board of Chemical Society Reviews (RSC, United Kingdom), and is a member of the International Advisory Board of Chemical Communications (RSC), among others.
In 2001 and 2017, he was distinguished as Visiting Fellow of the Japan Society for the Promotion of Science. In 2009, he was invested Doctor Honoris Causa by the Ivanovo State University, Russia, and 2016 by the Miguel Hernández University of Elche. In 2013 he was awarded the Research Prize and the Gold Medal of the Royal Spanish Society of Chemistry.
In 2016, he received the Linstead Career Award in Phthalocyanine Chemistry by the Society of Porphyrins and Phthalocyanines. In 2017, Torres was elected member of the European Academy of Sciences and received the “Miguel Catalán” Research Prize of the Community of Madrid. In 2018, he was awarded the Elhuyar-Goldschmidt Prize of the Gesellschaft Deutscher Chemiker of Germany.

Hiroyuki Uchida is currently a professor and director of the Clean Energy Research Center at the University of Yamanashi.
For the past 25 years, Uchida has devoted himself to the research and development of highly active, durable electrocatalysts for polymer electrolyte fuel cells and solid oxide fuel cells. He has co-authored more than 240 research papers.

In order to establish clear strategies for the design of electrocatalysts for PEFCs, Uchida has analyzed fuel cell reactions and degradation by use of a manifold of instrumental techniques. The use of monodisperse Pt or Pt-alloy nanocatalysts, with uniform composition and uniform dispersion over the surface of carbon supports by use of the original “nanocapsule” method, has contributed greatly to clarifying the effects of various properties (particle size, composition, and surface structure, etc.) on the activity and durability of the cathode and anode catalysts. He has demonstrated several important concepts for developing highperformance hydrogen and oxygen electrodes with high durability for a reversible solid oxide cell, operating as both SOFC and electrolysis cell.
Uchida has received the Award of the Electrochemical Society of Japan, Science and Technology Award of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Scientific Achievement Award of the ECSJ. He has served as the editor-inchief (2015–2018) of Electrochemistry (ECSJ), lead organizer of Electrocatalysts for PEFCs in the Fuel Cell Subcommittee of ECS, co-chair of the PRiME 2016 organizing committee, and vice-chair of Division 3 of the ISE (2012–present).
Sannakaisa Virtanen is professor for Surface Science and Corrosion at the Department of Materials Science of the Friedrich-Alexander University ErlangenNürnberg, Germany.

She studied metallurgy at the Helsinki University of Technology, Finland (MSc), and then joined the Swiss Federal Institute of Technology, ETH-Zurich, where she carried out her PhD research. After receiving her PhD, she was senior scientist at the ETHZürich, with research stays at the Brookhaven National Laboratory, USA and at the McMaster University, Canada.
In 1997, she was elected assistant professor at the ETH-Zürich, Department of Materials, and then joined FAU Erlangen as professor in 2003. In 2006 she was visiting professor at the Medical Faculty of the University of Helsinki, and in 2008 visiting professor at the Institute of Advanced Energy of the Kyoto University.
NEW
70 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
MEMBERS
AWARDS PROGRAM
Virtanen is author of >200 peer-reviewed publications with an h-index of 36. She is the 2008 recipient of the H.H. Uhlig Award of the National Association of Corrosion Engineers. She was elected chair of the Gordon Research Conference on Aqueous Corrosion in 2016.
Virtanen’s research interests are on elucidation of reaction mechanisms at solid/liquid- and solid/gas-interfaces: passivity, oxidation, corrosion and degradation behavior of advanced metallic materials as well as their surface modification and functionalization. For this, advanced electrochemical and surface analytical tools are being employed. A strong focus of the research activities is on the corrosion behavior of metallic materials used in biomedical devices, including biodegradable metals.
As an active member of The Electrochemical Society, Virtanen is currently Chair of the Corrosion Division.

Adam Z. Weber holds BS and MS degrees from Tufts University, and a PhD from University of California, Berkeley in chemical engineering under the guidance of John Newman. His dissertation work focused on the fundamental investigation and mathematical modeling of water management in polymer-electrolyte fuel cells.
Weber continued his study of water and thermal management in polymer-electrolyte fuel cells at Lawrence Berkeley National Laboratory where he is now a staff scientist and program manager for Hydrogen and Fuel Cell Technologies and deputy director for both the Fuel-Cell Performance and Durability (FC-PAD) and HydroGEN consortia. His current research involves understanding and optimizing fuel-cell performance and lifetime using advanced modeling and diagnostics, understanding flow batteries for grid-scale energy storage, and analysis of solar-fuel generators, where he is a Thrust coordinator at the Joint Center for Artificial Photosynthesis.
Weber has coauthored over 110 peer-reviewed articles and 10 book chapters and has been invited to present his work at various international and national meetings. He is the recipient of a number of awards including a Fulbright scholarship to Australia, the 2008 Oronzio and Niccolò De Nora Foundation Prize on Applied Electrochemistry of the International Society of Electrochemistry, the 2012 ECS Supramaniam Srinivasan Young Investigator Award of the Energy Technology Division, a 2012 Presidential Early Career Award for Scientists and Engineers, the 2014 ECS Charles W. Tobias Young Investigator Award, Kavli Fellow in 2014, and the 2016 Sir William Grove Award from the International Association for Hydrogen Energy.
ECS Division & Section Awards
Battery Division Research Award
Kang Xu has been doing research on electrolyte materials for 30 years. His interests encompass materials synthesis and interphasial mechanisms in electrochemical energy storage devices. He has been recognized 20 times for numerous awards. In the electrochemical community he is mainly credited for contributions to new electrolyte materials and fundamental understanding of interphasial chemistries. His work on high voltage aqueous electrolytes was considered disruptive, which opens new horizons to next generation of aqueous batteries and brings many unprecedented features including safety, tolerance against abuse, and form-factor flexibility.
He has published 180+ papers, four chapters/books, and currently holds 20+ patents. Among those he is best known in the field for the two comprehensive reviews written for Chem. Rev. (2004 and 2014), which have been extensively regarded as desk-references by the community. He is co-founder of Center of Research on Extreme Batteries, advisory board member of the ACS Applied Materials and Interfaces, and associate editor of Energy & Environmental Materials and Electrochemistry.
Kang Xu has been an ECS member since 1994. He served various duties in the Society and Battery Division, and has been active as session chairs/symposium organizers in Society meetings.
Battery Division Technology Award
Feng Pan, with more than a decade of experience in large international corporations, has been engaged in fundamental research and product development of novel optoelectronic and energy storage materials and devices.
As chief scientist, Pan led eight entities in Shenzhen to win the $150 million RMB grant for the national EV-battery innovation project in 2013-16. Pan also led 12 entities to win the National Key project of Material Genomic Engineering for Solid State Li-Ion Battery in China in 2016.
He has led his group to achieve outstanding research vs. MGI on electrochemistry, battery, and materials by a combination of comprehensive experimental tests and theoretical calculations, e.g. developing novel cathode materials and methods to study related electrochemical mechanism, and exploring new generation of solid state batteries.


Pan has published more than 250 peer-reviewed papers in international journals and book chapters and 80 patents for inventions. He has been selected as one of the 2016 winners of the Outstanding Research Award of Advanced Lithium Batteries for Automobile Applications. He is the Founding Dean of School of Advanced Materials at the Peking University Shenzhen Graduate School and Director of National Center of Electric Vehicle Power Battery and Materials for International Research.
(continued on next page)
NEW
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 71
AWARDSMEMBERS
AWARDS PROGRAM
(continued from previous page)
Battery Division Technology Award
Yongyao Xia is a professor in the Department of Chemistry and Institute of New Energy of Fudan University, China. His research interests involve advanced materials and technologies for energy storage and conversion devices, e.g., lithium-ion batteries, electrochemical supercapacitors, lithium-air, lithium-sulfur batteries, etc. He has worked in these areas for nearly 28 years.
Throughout his career, he has published more than 280 papers in peer-reviewed scientific journals, contributed to three book chapters, and holds 40 Japanese and Chinese patents on energy conversion and storage devices. His research work has been highly cited over 16,000 times.
He received the Outstanding Young Scientist Award of the Natural Science Foundation of China in 2009, Contribution Award in Electrochemistry of the Chinese Society of Electrochemistry in 2015, and the first prize of Natural Science Award of Ministry of Education in 2015.
He serves as the president of the Chinese Society of Electrochemistry, the China representive of the International Society of Electrochemistry, and the chair of The Electrochemical Society China Section. He is also the editor of Journal of Power Sources.
Battery Division Postdoctoral Associate Research Award
Sponsored by MTI Corporation and the Jiang Family Foundation
Haodong Liu received his bachelor’s degree in chemistry from Xiamen University in 2011. During this period, his research focused on fluoride cathode materials for lithium primary battery under the guidance of Yong Yang.
Haodong received his PhD in 2016 from the University of California San Diego under Ying Shirley Meng’s guidance, after which he worked as a postdoc research fellow at UC San Diego with Ping Liu. He is now managing the MTI-UCSD battery fabrication lab, and Arbin-UCSD battery testing lab.

He is the founder of the ECS UCSD Student Chapter, which promotes the interest and advancement of electrochemistry science and technology among both graduate and undergraduate students. His research focuses on novel materials for energy storage and conversion. His recent research topics include the design, synthesis, processing, and operando characterization of advanced electrode materials for Li ion batteries; new intercalation materials for high energy density sodium ion batteries; and novel strategies for high coulombic efficiency dendrite free Li metal anode.
Haodong is the author and co-author of more than 24 peerreviewed journal articles and three U.S. patents.

Battery Division Postdoctoral Associate Research Award
Sponsored by MTI Corporation and the Jiang Family Foundation
David Bock is currently an assistant scientist in the Energy Sciences Directorate at Brookhaven National Laboratory.

He received his BS in chemistry from the State University of New York at Geneseo in 2008. After graduating, he was employed as an analytical chemist at Inficon Inc., where he worked on development of person-portable gas chromatograph/mass spectrometer systems. He then went to graduate school and received a PhD from Stony Brook University in 2015. His PhD research concentrated on understanding and preventing parasitic cathode dissolution reactions in the battery system used to power implantable cardioverter defibrillators.
After his PhD, he joined Brookhaven National Laboratory as a postdoctoral research associate. While in that position, his research focused on using advanced X-ray characterization techniques, including X-ray absorption spectroscopy and energy dispersive X-ray diffraction, to provide mechanistic insight into Li-ion battery materials. He then joined Brookhaven National Laboratory staff as an assistant scientist.
David’s main research interests are in energy storage applications. He has coauthored over 30 publications in the field. His research emphasizes using state of the art tools, including synchrotron techniques, to probe battery systems. In his spare time, he enjoys outdoor activities and spending time with his wife and daughter.
David would like to thank his PhD and postdoctoral advisors Esther Takeuchi, Kenneth Takeuchi, and Amy Marschilok for their exceptional mentorship and for nominating him for this award.
Battery Division Student Research Award sponsored by Mercedes-Benz Research & Development
Fudong Han began his academic career at Shandong University in China, where he obtained his BS degree (2009) and MS degree (2012) in Materials Science and Engineering. During this time, he had been working on synthesizing and characterizing carbon-based anodes for lithium-ion batteries.

Han started his PhD in 2012 under the supervision of Chunsheng Wang at the Department of Chemical and Biomolecular Engineering at University of Maryland College Park. His PhD work focuses on the development of safe, high-performance all-solid-state batteries through understanding and addressing the key challenges in electrodes, solid electrolyte and their interfaces.
As one of Han’s main findings, he shows the electrochemical stability windows of solid electrolytes are overestimated from the conventional measurement, and the decompositions of solid electrolytes occur and need to be seriously considered when developing high-performance all-solid-state batteries.
Han has published 49 peer-reviewed papers and co-authored two U.S. patents. Han is a recipient of Materials Research Society Graduate Student Gold Award.
NEW MEMBERS 72 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Battery Division Student Research Award sponsored by Mercedes-Benz Research & Development
Steven David Lacey’s passion for electrochemistry and engineering culminated at a young age, when he realized the need for a more fuel-efficient means of transportation while restoring classic cars with his father.
As an undergraduate at the University of Maryland, he began collaborative energy storage research with Kang Xu and Arthur v. Cresce at the U.S. Army Research Laboratory, where he developed a planar device platform to study alkali-metal-ion battery operation at the nanoscale. Over several DoD internships, his research efforts began to expand into additional topics including optoelectronics, plasmonics, and material development for defense-related interests (body armor, smoke obscurants). After obtaining his BS in Materials Science and Engineering, he started his PhD at his alma mater under the continued supervision of Liangbing Hu.
Lacey is a recipient of the 2015 National Defense Science and Engineering Graduate Fellowship and President (2017-2018) of the ECS Student Chapter at UMD. He has published 30 peer-reviewed papers.
His PhD thesis research is devoted to nanomaterial synthesis, understanding battery fundamentals, and the development of alternative electrode manufacturing processes for advanced electrochemical energy storage systems. His current research focuses on the development of novel multimetallic electrocatalysts for metalair batteries using a synthesis method described in his recently published work in Science.

Corrosion Division H. H. Uhlig Award
Rudolph G. Buchheit is professor of chemical and materials engineering and dean of the College of Engineering at the University of Kentucky.
Prior to his appointment at Kentucky, he was professor and chair in the Department of Materials Science and Engineering, and later, associate dean in the College of Engineering at Ohio State. Before that he was a senior member of the technical staff at Sandia National Laboratories.

Buchheit earned a BS at Loyola University Maryland, and MS and PhD in materials science from the University of Virginia.
His research focuses on corrosion science and engineering with emphasis on corrosion, corrosion protection, and corrosion prediction of light metals. He has published over 270 technical articles on these subjects with students and colleagues, and holds 18 patents and patent disclosures related to surface treatments and coatings.
He has contributed seven chapters to books, edited three technical proceedings and co-authored two books. He is a Fellow of The Electrochemical Society, and NACE International and has served in committee and division leadership positions within each organization. He is also a past chair of the University Materials Council. He is the recipient of the H.H. Uhlig Educator’s Award from NACE, and the Morris Cohen Graduate Student Award from the Corrosion Division of The Electrochemical Society. He is also the recipient of the Stanley E. Harrison Faculty Award from the College of Engineering at Ohio State, and is a two-time recipient of the college’s Charles Ellison MacQuigg Award for outstanding teaching.
Corrosion Division Morris Cohen Graduate Student Award
Rebecca Schaller is currently an assistant professor in the Department of Materials Engineering at the University of British Columbia in Vancouver, Canada.
She earned a BS degree in Spanish in 2005 from the University of Oregon and an MS degree in Physics from Portland State University in 2010. Rebecca received her PhD in Materials Science and Engineering from the University of Virginia in 2016 under the direction of John R. Scully. Her work focused on developing techniques to probe local scale hydrogen concentrations and interactions in ultra-high strength steels, specifically those produced under atmospheric exposure.
During her graduate studies Rebecca received an Endeavour Research Fellowship, which was carried out in collaboration with Monash University and the Commonwealth Scientific and Industrial Research Organization in Clayton, Australia.

Since her time at UVA, Rebecca has worked as a postdoctoral assistant at Sandia National Laboratories in the Materials Aging and Reliability group, where her research focused on the local chemical and morphological effects of atmospheric corrosion of aluminum and corrosion of additively manufactured materials. She has recently begun her position as assistant professor at UBC where her current work will incorporate an emphasis on understanding corrosion at the local, microstructural level to better predict overall corrosion properties and rates.
Electrodeposition Division Research Award
Nosang Vincent Myung received his BS, MS, and PhD degree in chemical engineering from the University of California, Los Angeles in 1994, 1997, and 1998, respectively. He spent three years as a research engineer at the same institution.

From 2001-2003, he was an engineer in the micro electromechanical systems group at NASA’s Jet Propulsion Laboratory. In 2003, he joined the Department of Chemical and Environmental Engineering at University of California-Riverside. He served as the Department Chair from 20112016.
Currently, he is serving as the founding director for UC-KIMS Center for Innovative Materials for Energy and Environment and codirector for Winston Chung Global Energy Center.
During his career he has received the KIChE President Award, Brainpool Fellow from Korean Government, University of California Regent Fellowship, Jet Propulsion Laboratory Spot Award, and the Abner Brenner gold medal award from American Electroplaters and Surface Finishers Society.
Myung’s research interests are focused on the synthesis of nanoengineered materials and applying these materials in various advanced applications including spintronics, sensors, electronics, optoelectronics, energy harvesting, and environmental remediation. Myung’s group objective is to control nanoscale sized features to enhance material properties and device functions beyond those that we currently know.
He has published over 200 peer-reviewed journal papers and his h-index is 54 with the total citation of over 10,000.
(continued on next page)
NEWAWARDSMEMBERS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 73 AWARDS PROGRAM
AWARDS PROGRAM
(continued from previous page)
Electrodeposition Division Early Career Investigator Award
Jon Ustarroz graduated summa cum laude in materials engineering from the University of Navarra (Spain) and in innovation and technology management from the University of Deusto (Spain).
He obtained his PhD in engineering summa cum laude with congratulations from the jury from the Vrije Universiteit Brussel in 2013, supervised by Herman Terryn and Annick Hubin. While studying the early stages of electrodeposition by using transmission electron microscopy grid electrodes, he developed a novel theory related to electrochemical growth by nanocluster aggregation, which sets the bases for revisiting the Volmer-Weber growth mechanism.
For his postdoctoral research, he worked on the synthesis of nanomaterials by electrochemical routes in non-aqueous solvents and on the electrocatalytic properties of electrodeposited nanostructures. He earned his postdoctoral grant, ranked first, from the Research Foundation – Flanders. In 2016, he conducted research in the group of Patrick R. Unwin at the University of Warwick (U.K.) on the dynamics between single nanoparticles and polarized surfaces by scanning electrochemical cell microscopy.
Ustarroz is author of 30 publications and he is currently supervisor of four PhD students in the Electrochemical and Surface Engineering group of the Vrije Universiteit Brussel. There, he continues his research on non-traditional electrochemical nucleation and growth pathways from aqueous solutions and deep eutectic solvents. He is also involved in developing a multi-scale numerical modelling platform for investigating elementary electrodeposition processes.
High-Temperature Energy, Materials, & Processes Division
Outstanding Achievement Award
Meilin Liu is the B. Mifflin Hood Chair, Regents’ Professor, and Associate Chair of the School of Materials Science and Engineering at Georgia Institute of Technology.
He received his BS from South China University of Technology and his MS and PhD from University of California at Berkeley, all in MSE. His research interests include point defects and transport in solids, electrochemical behavior of thin films and interfaces, solid state ionics, and electroceramics.

His current research activities include in situ/operando characterization and multi-scale modeling of charge and mass transfer along surfaces, across interfaces, and in membranes, thin films, and nanostructured electrodes, aiming at achieving rational design of materials and structures with unique functionalities for efficient energy storage and conversion.
He has supervised 32 postdoctoral fellows, 38 PhD students, 25 joint PhD students, 13 MS students, and 28 visiting scholars. He holds 27 U.S. patents, published ~430 refereed journal papers, coedited 7 proceedings volumes, and co-organized 11 international symposia/workshops.
He is a Fellow of The Electrochemical Society and American Ceramic Society. He was the winner of the Charles Hatchett Award, Kolon Faculty Fellow, Outstanding Faculty Research Author Award
and Outstanding Achievement in Research Program Development Award from Georgia Tech, Ross Coffin Purdy Award, NASA Tech Brief Award, Crystal Flame Innovation Award in Research, Sustained Research Award and Best Faculty Paper Award from Sigma Xi, among others.
Luminescence and Display Materials Division Centennial Outstanding Achievement Award
Pieter Dorenbos received his physics education and did his PhD research at the University of Groningen, where he was appointed in July 1988 as assistant professor at the Technical University of Delft, The Netherlands.

He remained employed at TUD, where he is now heading, as full professor, the section Luminescence Materials in the Faculty of Applied Sciences. He started with projects on the development of new lanthanide activated scintillation crystals for detection of ionizing radiation. This ongoing research has resulted in various new patented scintillators that led to successful commercial products. Notably, LaBr3:Ce3+;Sr2+ is the best scintillator available to detect the energy of gamma photons.
Dorenbos’ interest broadened to all types of lanthanide activated luminescence inorganic compounds, and he became particularly interested in where lanthanide energy levels are located within the bandgap, and how that affects luminescence properties. This work resulted in various empirical models to construct diagrams showing all lanthanide levels within the band gap.
In 2012, he presented the chemical shift model that enables to relate energy levels of host bands and lanthanide impurity states in different compounds to a common energy reference, i.e., the vacuum level. It is a unique approach that is being adopted in the field.

He is currently interested in the energy level locations of transition metal elements and 6s2-elements like Bi3+. His mission is to develop new luminescence materials by rational design instead of serendipity.
He (co)authored >400 papers that appeared in peer reviewed scientific journals and that are cited about 17,000 times.
Physical and Analytical Electrochemistry Division Max Bredig Award in Molten Salt and Ionic Liquid Chemistry
Robin D. Rogers is a research professor at The University of Alabama and president, owner, and founder of 525 Solutions, Inc., in Tuscaloosa, AL.

He obtained both his BS in chemistry and his PhD in chemistry from The University of Alabama. Rogers is the founding editorin-chief of the American Chemical Society journal Crystal Growth & Design and is an advisory board member to eight other international peer-reviewed journals.
Rogers holds 27 issued patents and has published over 820 papers, with over 40,000 citations, a Hirsch index of 92, and an i10 index of 535.
Rogers was named in the 2014 and 2015 Thomson Reuters Highly Cited Researchers Lists ranking among the top 1% most cited in chemistry. His research interests cover the use of ionic liquids and Green Chemistry for sustainable technology through innovation.
NEW MEMBERS 74 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
AWARDS PROGRAM
Most recently he has embarked on an effort to commercialize the use of biorenewable polymers such as chitin in an effort to eliminate the need for synthetic plastics.
He had an influential role in the expansion of interest and research in ILs, his initial paper on IL/aqueous partitioning (Chem. Comm. 1998, 1765) effectively kick-started interest in applying ILs to clean separations. He was awarded the U.S. Presidential Green Chemistry Challenge Award for work related to the use of ILs in sustainable technology.
Sensor Division Outstanding Achievement Award
Joseph Wang is Distinguished Professor, SAIC Endowed Chair and Chair in the Department of Nanoengineering at University of California, San Diego. He is also the director of the UCSD Center of Wearable Sensors.

He received his PhD from the Technion in Israel. He was a member of the Chemistry department at NMSU where he held a Regents Professor and a Manasse, and served as the director of Center for Bioelectronics and Biosensors of Arizona State University before joining UCSD in 2008. Wang has published more than 1,050 papers, 11 books and he holds 25 patents (H Index=151, >95,000 citations).
He received two American Chemical Society National Awards, one in 1999 (Instrumentation) and the other in 2006 (Electrochemistry) and Honorary Professors from Spain, Argentina, Czech Republic, Romania, China, Slovakia, and Slovenia. Wang has been the founder and editor-in-chief of Electroanalysis (Wiley) for 30 years.
His scientific interests are concentrated in the areas of biosensors, bioelectronics, wearable sensors, bionanotechnology, nanomachines, and electroanalytical chemistry.
Europe Section Allessandro Volta Medal
Wolfgang Schuhmann is from the Center for Electrochemical Sciences. He obtained his degree in chemistry from the University in Karlsruhe, Germany and his PhD from the Technical University of Munich. After finishing his habilitation thesis at the Technical University of Munich, he was appointed professor for analytical chemistry at the Ruhr-Universität Bochum.
His research interest addresses the development of reagentless amperometric biosensors, enzymatic biofuel cells and biocapacitors, micro- and nanoelectrochemistry, scanning electrochemical microscopy, localized corrosion, combinatorial microelectrochemistry, electrocatalysis for energy conversion and storage, new materials for electrocatalysis and photoelectrocatalysis as well as the in-depth understanding of electrified interfaces.
He is coauthor of more than 620 scientific publications. He has received the Biosensors & Bioelectronics Award (2000), became a Fellow of the Royal Society of Chemistry (2008), received the Julius-von-Haast Fellowship Award of the Royal Society of New Zealand and the Humboldt Foundation Germany (2008), the Katsumi-Niki-Award of the International Society of Electrochemistry (2011), was appointed to be Fellow of the International Society of Electrochemistry (2012), received the Howard Fellowship of the University of New South Wales, Sydney (2014), was appointed to be a Distinguished Visiting Professor, University Iuliu Hatieganu, Cluj-Napoca, Romania, and was awarded the Schulich Visiting Professor Lectureship for 2018-19 of the Technion – Israel Institute of Technology; Haifa.


NEWAWARDSMEMBERS The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 75
Visit ecsdl.org.
©RUB, Kramer
NEW MEMBERS
ECS is proud to announce the following new members for April, May, and June 2018.
Members
Younes Abghoui, Reykjavik, Iceland
Nicole Adelstein, San Francisco, CA, USA
Shams Tania Afroza Islam, Oxford, EN,, UK
Rasin Ahmed, Charlottesville, VA, USA
Wook Ahn, Asan, South Korea
Erik Anderson, Troy, MI, USA
Thomas Anthopoulos, Thuwal, Saudi Arabia
Nicholas Apollo, Philadelphia, PA, USA
Demetrius Ashlion, Heverlee-Haasrode, Belgium
Tristan Asset, Albuquerque, NM, USA
Changkeun Back, Daejeon, South Korea
Thiruvelu Bhuvana, Kanpur, UP, India
Andrea Bourke, Limerick, Ireland
Michael Brothers, Dayton, OH, USA
Mingwei Chen, Baltimore, MD, USA
Shutang Chen, Columbus, OH, USA
Sekar Chinnathambi, Karaikkudi, TN, India
Wen-Yaw Chung, Chung Li, Taiwan
Whitney Colella, Arlington, VA, USA
Eric Coleman, Lemont, IL, USA
Mike Cooke, Bristol, EN, UK
Desiree Czapski, Azle, TX, USA
Mohsen Dadfarnia, Redmond, WA, USA
Rajib Das, Gainesville, FL, USA
Kristin Di Bona, Laramie, WY, USA
Nan Ding, Kings Mountain, NC, USA
Fabio Dionigi, Berlin, BE, Germany
Thomas Doneux, Bruxelles, Belgium
Edward Duranty, Mobile, Al, USA
Assma El Kaddouri, Vandoeuvre les Nancy, France
Balazs Endrodi, Stockholm, Sweden
Imasuen Eweka, Southampton, EN, UK
Hugh Fan, Gainesville, FL, USA
Jiemin Feng, Shanghai, China
Aaron Franklin, Durham, NC, USA
Michael Fusco, Raleigh, NC, USA
Ping Gao, Hong Kong, Hong Kong
Jerzy Gazda, Austin, TX, USA
Ryan Gebhardt, Ames, IA, USA
Oleg Gluschenkov, Poughkeepsie, NY, USA
Roberto Gonzalez Rodriguez, Fort Worth, TX, USA
Warren Gray, Portland, OR, USA
Zhansheng Guo, Shanghai, China
Su Ha, Pullman, WA, USA
Qingli Hao, Nanjing, China
Karen Heinselman, Lakewood, CO, USA
Danielle Henckel, Seattle, WA, USA
Lindsay Hernandez Munoz, Ozumba. Mexico
Andy Hessler, Grove City, PA, USA
Bruce Hinds, Seattle, WA, USA
Rachel Hjelm, Alexandria, VA, USA
Aaron Hollas, Richland, WA, USA
Tatsuo Horiba, Fukaya, Japan
Hamed Hosseinzadeh, Glassboro, NJ, USA
Boxun Hu, Storrs, CT, USA
Yuanyuan Hu, Changsha, China
Binbin Huang, Changsha, China
Yun Jeong Hwang, Seoul, South Korea
Hicham Idriss, Thuwal, Saudi Arabia
Hiroshi Imoto, Utsunomiya, Japan
Akimitsu Ishihara, Yokohama, Japan
Ankur Jain, Arlington, TX, USA
Jasna Jankovic, Storrs, CT, USA
Ali Javey, Berkeley, CA, USA
Wei Jin, Wuxi, China
Niko Jovicevic, Ann Arbor, MI, USA
Zachary Kaiser, Lockport, IL, USA
Hyun Suk Kang, Lakewood, CO, USA
SeungYeon Kang, Plainsboro, NJ, USA
Honorata Kazimierczak, Krakow, Poland
Bongchull Kim, San Jose, CA, USA
Dong Ha Kim, Seodaemun-gu, Seoul, South Korea
Hansu Kim, Seoul, South Korea
Hyungtak Kim, Seoul, South Korea
Ki Jae Kim, Seoul, South Korea
Myong Hwan Kim, Cheonan-si, South Korea
Youn Sang Kim, Seoul, South Korea
Hyunhyub Ko, Ulsan, South Korea
Jason Koeller, Berkeley, CA, USA
Praveen Kolla, Boston, MA, USA
Douglas Kushner, El Cerrito, CA, USA
Gary Leach, Burnaby, Canada
Jinwoo Lee, Pohang, South Korea
Jung Woo Lee, Busan, South Korea
Soo Yeol Lee, Daejeon, South Korea
Yueh-Lin Lee, Pittsburgh, PA, USA
Christophe Leger, Marseille, France
Guanchen Li, Oxford, UK
Juan Li, Xi’an, China
Xiaojing Li, Hopewell Junction, NY, USA
Tianquan Lian, Atlanta, GA, USA
Larry Lien, Escondido, CA, USA
Yi Lin, Yorktown, VA, USA
Brian Litt, Philadelphia, PA, USA
Chaofeng Liu, Seattle, WA, USA
Dongren Liu, Shenzhen, China
Fupin Liu, Dresden, Sachsen, Germany
Gang Liu, Shenyang, China
Hong Liu, Corvallis, OR, USA
Xinhua Liu, London, EN, UK
Qi Lu, Beijing, China
Julia Mainka, Vandoeuvre les Nancy, France
Rahul Malik, Cambridge, MA, USA
Rebeca Marcilla, Móstoles, Spain
Ayumu Matsumoto, Himeji, Japan
Hiroshige Matsumoto, Fukuoka, Japan
Rachel McKerracher, Southampton, UK
Brendan Meany, Hyattsville, MD, USA
Vitalii Mishchenchuk, Chernivtsi, Ukraine
Gary Moore, Tempe, AZ, USA
Mahendra More, Pune, MH, India
Yutaka Moritomo, Tsukuba,, Japan
Fengwen Mu, Bunkyo-ku, Tokyo, Japan
Samuel Mugo, Edmonton, Canada
Catherine Murphy, Urbana, IL, USA
Parimaladevi Muthusamy, Erode, TN, India
Zahra Nazemi, Tempe, AZ, USA
Bich-Yen Nguyen, Austin, TX, USA
Richard Oleksak, Albany, OR, USA
Sehmus Ozden, Los Alamos, NM, USA
Kailash Patil, Newton, MA, USA
Colin Pelletier, Dallas, TX, USA
Remi Petibon, Fremont, CA, USA
Michael Pope, Waterloo, Canada
Denis Proshlyakov, East Lansing, USA
Margaret Renaud-Young, Calgary, Canada
Raghunathan Rengasamy, Chennai, TN, India
Katy Roodenko, Plano, TX, USA
Ben Roth, Bloomington, IN, USA
Saman Sadeghi, Los Angeles, CA, USA
Madhumita Sahoo, Manchester, UK
Sanjiv Sambandan, Bangalore, KA, India
A. Fatih Sarioglu, Atlanta, GA, USA
Klaus Schepers, Braunfels, Germany
Nathan Schuh, Broomfield, CO, USA
Manuel Sellier, Grenoble, France
Ashwini Sharma, Patna, BR, India
Alan Shipley, New Haven, CT, USA
Burton Simpson, Pasadena, CA, USA
Martin Sjödin, Uppsala, Sweden
Hanna Sopha, Pardubice, Czech Republic
Corey Stephenson, Ann Arbor, MI, USA
Tyler Stevens, Albuquerque, NM, USA
Masayoshi Takase, Matsuyama, Japan
Chaou Tan, Sheffield, S Yorkshire, UK
Fujian Tang, Dalian, China
William Tarpeh, Stanford, CA, USA
Katsuya Teshima, Matsumoto, Japan
Ana Torres, Ciudad de Mexico, DF, Mexico
Quang Tuyen Tran, San Diego, CA, USA
Stephen Trask, Lemont, IL, USA
Hiroyuki Tsubota, Shibata, Japan
Andrew Udit, Los Angeles, CA, USA
Xavier Vendrell, Sheffield, S Yorkshire, UK
Judith Vidal, Lakewood, CO, USA
Flavia Vitale, Philadelphia, PA, USA
Haihua Wang, Evanston, IL, USA
Haotian Wang, Cambridge, USA
Kaixue Wang, Shanghai, China
Yang Wang, Tianjin, China
Yuxing Wang, Richland, WA, USA
76 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
Qi-Huo Wei, Kent, OH, USA
Valerie Wiesner, Kennedy Space Center, FL, USA
Adam Willard, Cambridge, MA, USA
Da Hye Won, Seoul, South Korea
Sean Wood, Idaho Falls, ID, USA
Sheng Xu, La Jolla, CA, USA
Yeming Xu, Shenzhen, China
Tao Yang, Morgantown, WV, USA
Ping-Hung Yeh, Taipei County, Taiwan
Seung-Eul Yoo, Cheonan-si, Chungnam, South Korea
Choongho Yu, College Station, TX, USA
Ki Jun Yu, Seoul, South Korea
Qichun Zhang, Singapore, China
Sonia Zhang, Cambridge, MA, USA
Xuelin Zhang, Harbin, China
Yingying Zhang, Beijing, China
Yu Zhang, New Taipei City, Taiwan, Taiwan
Wengao Zhao, Richland, WA, USA
Renjie Zhou, Alhambra, CA, USA
Hanyu Zhu, Berkeley, CA, USA
Lauren Zucker, Indianapolis, IN, USA
Student Members
Orrsam Abubaker, Calgary, Canada
Pashupati Adhikari, Denton, TX, USA
Santosh Adhikari, Stillwater, OK, USA
Mohammadali Ahmadi, Calgary, Canada
Yong Nam Ahn, Seoul, South Korea
Laura Almar, Karlsuhe, Germany
Miguel Angel Alvarez, Guadalajara, Mexico
Hunter Andrews, Richmond, VA, USA
Gabriel Armas, Iowa City, IA, USA
Seong Hwa Bae, Daegu, South Korea
Yeong Jo Baek, Asan, South Korea
Sterling Baird, Provo, UT, USA
Matthew Bamidele, Stillwater, OK, USA
Adam Bass, Calgary, AB, Canada
Ayaulym Belgibayeva, Koto, Tokyo, Japan
Ahmed Ben Sahil, Pullman, WA, USA
Wenchao Bi, Seattle, WA, USA
Kamila Bladek, Calgary, AB, Canada
Daniela Blanco, Brooklyn, NY, USA
Tim Bobrowski, Bochum, NW, Germany
Katie Browning, Oak Ridge, TN, USA
Jiyu Cai, Fayetteville, AR, USA
Rong Cai, Salt Lake City, UT, USA
Oliver Calderon, Calgary, AB, Canada
Hayden Cameron, Birmingham Gardens, Australia
Esdras Canto Aguilar, Mérida, Yucatan, Mexico
Annika Carlson, Stockholm, Sweden
Gabriel Carrillo, Clover, SC, USA
Hiram Manuel Castro-Cruz, Mexico City, DF, Mexico
Maira Ceron, Livermore, CA, USA
NEW MEMBERS
Younghwan Cha, Pullman, WA, USA
Valérie Charbonneau, Sherbrooke, QC, Canada
Binyu Chen, St. John’s, NL, Canada
Feng-Ju Chung, Hsinchu, Taiwan, Taiwan
Sunki Chung, Gwangju, South Korea
Theodore Cohen, Seattle, WA, USA
Paul Cooper, University Park, MD, USA
Oscar Cornejo, Guanajuato, GTO, Mexico
Federico Cuneo, Milano, Italy
Kaushalya Dahal Sharma, Stillwater, OK, USA
Sidney DeBie, Iowa City, IA, USA
Camden DeBruler, Logan, UT, USA
Felix Deku, Dallas, TX, USA
Jim Dickinson, Stillwater, OK, USA
Aniruddha Dive, Pullman, WA, USA
Sharon Dong, Calgary, AB, Canada
Mohamed Ali Elharati, Pullman, WA, USA
Rubi Enciso Perez, San Luis Potosi, Mexico
Ricardo Escalona, Sanfandila, Pedro Escobedo, QT, Mexico
Roozbeh Falah Ramezani, Kaohsiung, Taiwan, Taiwan
Lijun Fan, Tianjin, China
Sabrina Farias, Ponca City, OK, USA
John Feather, Tucson, AZ, USA
Horacio Antonio Figueredo, Southampton, EN, UK
Abraham Samuel Finny, Potsdam, NY, USA
Margaret Fitzgerald, Lakewood, CO, USA
Ariel Friedman, Ramat Gan, Israel
Xuewei Fu, Pullman, WA, USA
Yuya Fukuzumi, Tsukuba, Japan
Vicente Galvan, Los Angeles, CA, USA
Diego Galvez, Bryan, TX, USA
Tian Gan, Tianjin, China
Yifan Gao, Rochester, NY, USA
José García Béjar, Pedro Escobedo Querétaro, Mexico
Ruijing Ge, Austin, TX, USA
Leila Ghadbeigi, Salt Lake City, UT, USA
Monalisa Ghosh, Bangalore, KA, India
Eugenio Gibertini, Milano, Italy
Wubshet Girma, Taipei City, Taiwan
Benjamin Godwin, Olds, AB, Canada
Zachary Gossage, Urbana, IL, USA
Akshay Gowda, Potsdam, NY, USA
Hamsa Gowda, Irvine, CA, USA
Alex Hall, Benicia, CA, USA
Kahyun Ham, Gwangju, South Korea
Iqra Hamdani, New Delhi, UP, India
Ross Harnden, Stockholm, Sweden
Dennis Haupt, Clausthal-Zellerfeld, NI, Germany
Shawn Headley, Davis, CA, USA
Robert Hein, Oxford, Oxfordshire, UK
Isaac Hernandez, Pedro Escobedo, Mexico
Nicolas Hernandez Sanchez, Southampton, EN, UK
Sanaz Hesamedini, Ilmenau, TH, Germany
Zahra Heydarzadeh Sheykhangafsheh, Irvine, CA, USA
Zack Hollingworth, Mt Vernon, NY, USA
Sujik Hong, Gwangju, South Korea
Zhong-Jie Hong, New Taipei City, Taiwan, Taiwan
Seyed Pouya Hosseini Yazdeli, Oldenburg, NI, Germany
Nianjun Hou, Tianjin, China
Yang Hu, Seattle, WA, USA
Racheal Huynh, Calgary, AB, Canada
Hongsun Hwang, Buk-gu, Gwangju, South Korea
Kang In Hye, Asan-si, Chungnam, South Korea
Muhammad Naoshad Islam, Calgary, AB, Canada
Solomon Isu, Fayetteville, AR, USA
Yeon JeongSeok, Suwon, South Korea
Aadithya Jeyaranjan, Orlando, FL, USA
Kun Jiang, Cambridge, MA, USA
Hossein Jiriyaeisharahi, Calgary, Canada
Taigyu Joo, Yelm, WA, USA
Siddharth Joshi, Troy, NY, USA
Mingyu Jung, Seoul, South Korea
Muhammad Salahuddin Kabir, Rochester, NY, USA
Esra Karakaya, Ankara, Turkey
Devin Keck, Clemson, SC, USA
Juyeon Keum, Changwon, South Korea
Iman Khakpour, Miami, FL, USA
Jayoung Kim, La Jolla, CA, USA
Michael Kindle, Pullman, WA, USA
Eugene Kong, Aliso Viejo, CA, USA
Junmo Koo, Seoul, South Korea
Aslan Kosakian, Edmonton, AB, Canada
Bianca Kovalenko, Franklin Park, NJ, USA
Matthias Kuenzel, EggensteinLeopoldshafen, BW, Germany
Ankit Kumar, Bangalore, KA, India
Rakesh Kumar, Manchester, EN, UK
Ilia Kuznetcov, Calgary, AB, Canada
Francis Kwofie, Stillwater, USA
Pierre Lannelongue, Montpellier, France
Rachel Lau, Singapore, Singapore
Doohee Lee, Auburn, Al. USA
Kaylyn Leung, Vancouver, BC, Canada
Elina Levchenko, Calgary, AB, Canada
Benjamin Levitas, Boston, MA, USA
Xiaomeng Li, Suzhou, Jiangsu, China
Zairui Li, Dayton, OH, USA
Jianneng Liang, London, ON, Canada
Aaron Liu, Halifax, NS, Canada
Panpan Liu, Pullman, WA, USA
Pingwei Liu, Cambridge, MA, USA
Xu Liu, Vancouver, BC, Canada
Yulong Liu, Halifax, NS, Canada
Yulong Liu, London, ON, Canada
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 77
(continued from previous page)
Wipula Liyanage, Rolla, MO, USA
Christian Loza, Woodstock, IL, USA
Tianxiao Ma, Vancouver, BC, Canada
Xuetian Ma, Atlanta, GA, USA
Armando Marenco, Calgary, AB, Canada
Isaac Martens, Vancouver, BC, Canada
Nirul Masurkar, Detroit, MI, USA
Austin McKee, Congerville, IL, USA
Matt McPhail, Berkeley, CA, USA
Danea Medina, Bochum, NW, Germany
Samantha Medina, Golden, CO, USA
Marcela Mendez Tovar, Ciudad de Mexico, DF, Mexico
Daniel Molina, Pullman, WA, USA
Elena Montoto, Urbana, IL, USA
Dulce Maria Morales Hernandez, Bochum, NW, Germany
Thorben Muddemann, Clausthal-Zellerfeld, NI, Germany
María Murrieta, Guanajuato, GTO, Mexico
Shingo Nagahara, Koganei, Tokyo, Japan
Arpita Nandy, Calgary, AB, Canada
Praveen Narangoda, Mülheim a.d. Ruhr, NW, Germany
Dina Nashed, Romeoville, IL, USA
Shova Neupane, Hasselt, Belgium
Benjamin Ng, Columbia, SC, USA
Freddy Nguyen, Cambridge, MA, USA
Michelle Nguyen, Calgary, AB, Canada
Danika Nicoletti, Calgary, AB, Canada
Huilong Ning, Changsha, Hunan Province, China
Funeka Nkosi, Boksburg,Gauteng, South Africa
Fumihiro Nomura, Yokohama, Kanagawa, Japan
Glenn Packard, Williamson, NY, USA
Pin-Chun Pan, Taipei City, Taiwan
Kavita Pandey, Oxford, Oxfordshire, UK
Krishna Pandey, Fayetteville, AR, USA
Nilushi Paranamana, Stillwater, OK, USA
Jeonghee Park, Suwon, Kyunggi, South Korea
Kangjoon Park, Seoul, South Korea
Sangwook Park, Stanford, CA, USA
Yohan Parsa, Saint Martin d’Heres, France
Fathima Pary, Stillwater, OK, USA
Alexander Pearse, Berwyn Heights, MD, USA
Saul Perez Beltran, Bryan, TX, USA
Nathan Phillip, Knoxville, TN, USA
Stefan Piontek, Philadelphia, PA, USA
Alexandra Ploner, Roskilde, Denmark
NEW MEMBERS
Supriya Pulipaka, Sangareddy, TG, India
Andrea Quintero Colmenares, Grenoble, Isere, France
Josephine Quirino Gutiérrez, Mineral de la Reforma, Hidalgo, Mexico
Letch Radomirov, Fairview Park, OH, USA
Abidur Rahaman, Seoul, South Korea
Duraichelvan Raju, Montreal, QC, Canada
Daniel Ramírez Chan, Ciudad de México, DF, Mexico
Nusrat Rashid, Pulwama, JK, India
Ali Rashti, Auburn, AL, USA
Muhammad Rauf, Shenzhen, China
Erik Reale, Urbana, IL, USA
Stuart Robertson, Salt Lake City, UT, USA
Carlos Alberto Rodriguez Garcia, Guadalajara, DF, Mexico
María Romero, Ciudad de México, DF, Mexico
Mohammadreza Rostami, Athens, OH, USA
Sohini RoyChoudhury, Miami, FL, USA
José Gabriel Ruiz-Montoya, Ciudad de Mexico, DF, Mexico
Waqas Saddique, Clausthal-Zellerfeld, NI, Germany
Yatir Sadia, Dvira, Israel
Daniel Salem, Cambridge, MA, USA
Heythem Salhi, Daxi, Taiwan, Taiwan
Nileshi Saraf, Orlando, FK, USA
Ananta Sarkar, Bombay, MH, India
Paran Sarma, Faridaibad, HR, India
Theresa Schoetz, Elsterheide, Germany
Derek Schwanz, West Lafayette, IN, USA
Luis Selis, Bryan, TX, USA
Khyati Shah, La Jolla, CA, USA
Suman Shahim, East District, Hsinchu City, Taiwan
Dlshad Shaikhah, Leeds, W Yorkshire, UK
Ehsan Shamloo, Irvine, CA. USA
Samaneh Sharifi Golru, New York, NY, USA
Aida Sheibani, Fayetteville, AR, USA
Meng Shi, Idaho Falls, ID, USA
Alfonso Shin, Irvine, CA, USA
Subeom Shin, Seoul, South Korea
Aniruddh Shrivastava, Urbana, IL, USA
Raunak Singh, Calgary, AB, Canada
Rochan Sinha, Eindhoven, Netherlands
Raymond Smith, Cambridge, MA, USA
Luis Soenksen Martinez, Boston, MA, USA
Liang Song, Upton, NY, USA
Hidetoshi Sonoki, Tsu, Mie, Japan
Isio Sota-Uba, Stillwater, OK, USA
Natalie Stubb, Seattle, WA, USA
Rathod Suman, Bangalore, KA, India
Qian Sun, Fayetteville, AR, USA
Sathish Swaminathan, Chennai, TN, India
Jessica Tabert, Downers Grove, IL, USA
Shin-Yi Tang, Hsinchu City, Taiwan, Taiwan
Patrick Teppor, Tartu, Estonia
Akila Thenuwara, Philadelphia, PA, USA
Pavlina Theodosiou, Bristol, EN, UK
Denis Therien, London, ON, Canada
Nicole Thompson, Great Falls, MT, USA
Gretchen Tibbits, Pullman, WA, USA
Christian Tooley, Newmarket, NH, USA
Luis Torres Pacheco, Queretaro, Mexico
Mai Tran, Houston, TX, USA
An-Ni Tsai, Taipei City, Taiwan, Taiwan
Chia-Jung Tsai, Hsinchu, Taiwan, Taiwan
Liang-Feng Tsai, Taipei, Taiwan, Taiwan
Secil Tutar, Pullman, WA, USA
Sasan V. Grayli, Burnaby, BC, Canada
Ganesh Kumar Veerasubramani, Seongdong-gu, South Korea
Katrina Vuong, San Marcos, CA, USA
Yijia Wang, Pullman, WA, USA
Zhi-Hao Wang, Taipei, Taiwan, Taiwan
Philipp Weber, Oldenburg, NI, Germany
Huang Wei, New Taipei City, Taiwan, Taiwan
Ding Jhou Wu, Hsinchu, Taiwan, Taiwan
Shu-Chi Wu, Hsinchu, Taiwan, Taiwan
Nengneng Xu, Lafayette, LA, USA
Kavita Yadav, Raipur, CT, India
Fengxia Yang, Tianjin, China
Li-Heng Yang, Hsinchu City, Taiwan
Song Yang, Pullman, WA, USA
Wei Yang, Suzhou, Jiangsu, China
Wenli Yang, Houston, TX, USA
Miad Yarali, Houston, TX, USA
Ziwei Ye, Toronto, ON, Canada
Han Yelin, Asan-si, Chungnam, South Korea
Lin-Chen Yen, Taoyuan City, Taiwan, Taiwan
Rowena Yew, Acton, ACT, Australia
Yuan Yue, Berkeley, CA, USA
Michelle Zaleski, Romeoville, IL, USA
Zheyu Zhang, Calgary, AB, Canada
Xinyi Zhao, West Columbia, SC, USA
Yan Zhao, London, London, UK
Yiwei Zheng, Columbia, SC, USA
Chengtian Zhou, Calgary, AB, Canada
Xuemei Zhou, Erlangen, BY, Germany
Hannah Zmuda, Pullman, WA, USA
Laura Zurauskaite, Kista, Sweden
78 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
MEMBER BENEFITS
Involved members get noticed! Join a committee and enhance your leadership skills.
It’s not just who you know, it’s who others know! Networking is powerful!
Educational programming is growing to support our membership.
Formed in 1902 – become a part of this highly respected community.

Members receive exclusive pricing on meeting registrations, publications, and professional development opportunities.


Utilize the ECS network to advance your career!














































 –
of
–
of







































The Electrochemical Society
VISIBIlITy GRowTh
cREDIBIlITy NETwoRk
DIScouNTS caREER
“Our community is more than just academics and subject matter. It’s family.”
$ Renew online: www.electrochem.org/renew
Jim Fenton, Secretary
The Electrochemical Society
STUDENT
Six New Student Chapters
While in Seattle, WA, at the 233rd ECS Meeting, the ECS Board of Directors approved the chartering of six new student chapters. The student chapter program continues to grow as students harness the benefits of student chapter membership. ECS now has 75 student chapters around the world!
The six new student chapters are:
1 Complutense University of Madrid (Spain)
2 Florida Institutional University (USA)
3 Helmholtz-Institute Ulm (Germany)
ECS student chapter membership provides a number of benefits:
• Engage with fellow students and peers
• Opportunities to organize technical meeting programs and scholarly activities
• Collaborate with members to present posters at ECS biannual meetings
• A network of 8,000 international ECS members
• Access to career resources
• Impressive extracurricular activity for resume
• Funding to support chapter activities
• Partnership opportunities with local ECS section on activities and technical programs
• Recognition on the ECS website and in quarterly publication, Interface
Contact Education@electrochem.org if you are interested in starting a student chapter at your institution.

4 University of Guelph (Canada)
5 Virginia Polytechnic Institute and State University (USA)
6 Yamagata University (Japan)
Calgary Student Chapter
A new executive committee for the ECS Calgary Student Chapter was officially announced for 2018, involving Behzad Fuladpanjeh Hojaghan (president), Annie Hoang (vice president), Yalda Zamani Keteklahijani (secretary), and Katelynn Daly (treasurer), as well as members-at-large Marwa Atwa, Tianpei Shu, Hamideh Eskandari, and Umer Farooq. Atwa will also serve as the membership coordinator for the chapter. In addition, the chapter launched a new profile on Twitter (@ecs_calgary) to announce upcoming events and reach out to the Calgary community through social media.
The first event of 2018 involved the chapter’s participation in the Graduate Students’ Association’s Reverse Science Fair on the afternoon of March 3 at the University of Calgary. Traditional science fairs allow young students to present a project or experiment to a panel of judges, whereas a reverse science fair involves scientists and engineers presenting a demonstration to young students. The purpose of the event was to promote interest in science and engineering among young children. The chapter decided to set up an electrochemistry booth to demonstrate fruit and vegetable operated batteries using lemons, limes, apples, bananas, and potatoes as the electrolyte. Nearly 100 participants attended the event, including grade school children and their parents. Attendees were initially asked to guess at which fruit/vegetable battery they believed would work, and then completed the circuit to light up an LED bulb. For each option, the children also measured the cell potential with a voltmeter to determine which fruit/ vegetable was the best battery. The chapter plans to organize more science outreach events during the year to increase interest in science and promote electrochemistry and its many practical applications.

80 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
NEWS
Students attending the student mixer, a perennial favorite at meetings, here at the 233rd ECS Meeting in Seattle.
The ECS Calgary Student Chapter at the University of Calgary’s Reverse Science Fair (left to right): Katelynn Daly, treasurer; Annie Hoang, vice president; Marwa Atwa, membership coordinator; Yalda Zamani Keteklahijani, secretary; and Behzad Fuladpanjeh Hojaghan, president.
Two chapter members, Atwa and Hoang, traveled to the University of Alberta in Edmonton for the ECS Canada Section’s spring meeting on May 26, where they presented their work on the corrosion resistance of carbon supports in PEM fuel cells, and core@shell nanoparticles as catalysts for direct alcohol fuel cells, respectively. The chapter’s faculty advisor (Dr. Viola Birss) was an invited speaker
at the meeting, where she presented her research group’s work on nanoscale templates and scaffolds for electrochemical applications. This meeting occurred one day before the 101st Canadian Chemistry Conference and Exhibition, also held in Edmonton, where Hoang, Daly, and Atwa gave oral presentations in the Materials for Renewable Energy Technologies symposium while gaining valuable knowledge about advances in electrochemistry and materials science research.
Norwegian University of Science and Technology Student Chapter
On a beautiful day in May 2018, the ECS Norwegian University of Science and Technology Student Chapter hosted its annual group seminar at Lian Restaurant in the leafy suburbs of Trondheim. Around 40 students, professors, and chapter members attended to hear about the research being done in the group.

After an introduction from the chapter’s president, Kristian Thorbjørnsen, a number of master’s students, PhDs, and postdocs presented their work. The talks were on topics including reverse
electrodialysis, aluminium production, and catalysts for water electrolysis. The chapter also got an in-depth introduction to a newly purchased Raman spectrometer from Professor Andreas Erbe, selfconfessed Raman enthusiast.
Afterwards, the chapter headed down to the beautiful Lianvatnet Lake, where the lively discussion continued over a few rounds of volleyball and a dip or two in the lake, with more events for the group planned for the future.
University of Washington Student Chapter
The ECS University of Washington Student Chapter continued its dedication to two core missions: aiding members in their career development and positively affecting its community through outreach. Achieving these goals required ECS@UW to organize activities that prepared its members for conferences, provided opportunities for professional networking, taught elementary school students about electrochemistry, and offered educational seminars for its members.
The chapter proudly reports that 22 of its members participated in the 233rd ECS Meeting held this May in Seattle, WA. Prior to the conference, ECS@UW organized several events to position its members for success. Specifically, ECS@UW arranged multiple sessions for members to practice oral presentations and receive feedback from their peers prior to the conference. Moreover, Matthew Murbach, an ECS@UW member, planned eight Python tutorials held on a weekly basis leading up to the conference. These tutorials served to prepare students with minimal prior data science experience to fully participate in ECS Data Sciences Hack Week at the meeting.
On May 24, ECS@UW hosted its second annual industry panel. This year’s panel was selected according to the themes of clean energy and data science, thus embracing the recent boom in electrochemical publications at this intersection. Accordingly, ECS@UW partnered with the local student-led organization Diversity in Clean Energy to
invite six panelists from organizations ranging from local start-ups to international corporations. Represented companies included Hive Battery, E8, Distributed Energy Management, DVN-GL, KPMG, and Doosan GridTech. Representatives were separated into two panels, the first fielding questions regarding start-ups, and the second fielding questions on the transition from academia to industry. These panels were complemented by a networking session enabling students to interact with the local professionals. In total, the event spanned four hours. ECS@UW gratefully acknowledges sponsorship of this event by the Clean Energy Institute and eScience Institute.
On April 19 and 20, ECS@UW participated in the University of Washington’s Engineering Discovery Days. At this event, UW students and faculty facilitated numerous hands-on experiments oriented toward fourth to eighth grade students. This afforded elementary school students a fun environment for learning and the opportunity to witness the exciting nature of STEM research. Here, ECS@UW featured its popular electrodeposition experiment, Enginearrings, which involved the anodization of titanium wires to produce colorful patterns. These wires were then bent into earrings and distributed as keepsakes to attendees. In total, Engineering Discovery Days reached over 10,000 attendees and benefited from the civic involvement of over 1,200 UW students.
(continued on next page)
The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 81 STUDENT NEWS
The participants in the ECS Norwegian University of Science and Technology Student Chapter’s annual group seminar in Trondheim in 2018.
(continued from previous page)
Finally, ECS@UW continued its theme of educational seminars. This included a series of student-led, biweekly presentations on characterization techniques that concluded with presentations by Elena Pandres and Evan Jahrman on electron microscopies and X-ray
absorption spectroscopies, respectively. This summer, ECS@UW will continue its Coffee & Electrochemistry book series with weekly discussions focused on the chapters of Electrochemical Methods by Bard and Faulkner.
Improving its community and aiding the career development of its members remain the central foci of ECS@UW.
Drexel University Student Chapter
On May 3, 2018, electrochemistry-focused students and professors at Drexel University got together to participate in the annual gathering of the ECS Drexel University Student Chapter. The event was organized by the Drexel University Student Chapter’s board, led by Mallory Clites, chapter president, and Ekaterina Pomerantseva, chapter faculty advisor.


This year, for the first time, the chapter hosted a Family Feudstyle game, called Electrochemistry Feud, in which students from the Chemical & Biological Engineering and Materials Science & Engineering Departments at Drexel competed on opposite teams to answer opinion-based questions that electrochemists across the
country had taken a survey on. The surveys themselves had been electronically distributed to university professors, postdocs, graduate students, national laboratory scientists, and industry professionals working in the field of electrochemistry. A total of 57 responses had been collected and analyzed to determine the top six answers to each of the 10 survey questions. The survey questions focused on electrochemical energy storage and general electrochemical research. The surveys included such questions as “Who do you believe to be the most influential electrochemist of all time?” to which the most common answers were Michael Faraday and John Goodenough. Another question asked, “What material do you believe
82 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
NEWS
STUDENT
Members of the ECS University of Washington Student Chapter and Diversity in Clean Energy with representatives from Hive Battery, E8, Distributed Energy Management, DVN-GL, KPMG, and Doosan GridTech following the chapter’s second annual industry panel.
Attendees and organizers of the ECS Drexel University Student Chapter’s first Electrochemistry Feud event.
can revolutionize the field of energy storage?” and the common answers were MXenes, a versatile family of transition metal carbides and nitrides that offer capacitive energy storage and high electron conductivity (discovered at Drexel in 2011 by students working under Dr. Yury Gogotsi and Dr. Michel Barsoum) and metal organic frameworks, stable structures that possess a great degree of open space where significant amounts of large beyond Li-ion charge carriers can insert with minimal strain. For the question “What is your favorite electrochemistry-related journal?” the most common answer was the Journal of The Electrochemical Society
Team MSE was composed of five PhD and MS students, Varun Natu, Asya Sarychevaa, Samantha Buczek, Yongwei Li, and Patrick West, while Team CBE included students Sophia Lee, Luis Rebollar, Yawei Li, Swarnendu Chatterjee, and Rahul Pai. Dr. Katie Van Aken, a postdoctoral researcher at Drexel University, acted as the game show host.
The teams battled it out over the course of the 10 survey questions. Team MSE led for the first seven rounds, correctly guessing the most common answers to questions such as “What is the most important battery ever discovered?” and “What application do supercapacitors have the capability to outperform batteries in?” However, Team CBE managed to pull ahead in the eighth round, correctly guessing the top four most popular electrochemistry-related journal responses, and ultimately was crowned the winner. More than 40 students and professors from Drexel University, as well as guest visitors from the

University of Pennsylvania, attended the event. The Electrochemistry Feud game was followed with lunch and a poster session in which the students were able to discuss their ongoing research. The chapter would like to thank everyone who took the time to fill out the survey that made its event a success. The chapter is also very grateful to The Electrochemical Society for sponsoring its event.

The Electrochemical Society Interface • Fall 2018 • www.electrochem.org 83 STUDENT NEWS
Team CBE (left) and Team MSE (right) prepared to compete in the Electrochemistry Feud game.
Dr. Katie Van Aken, host of the game, revealed the answers to the question “What is your favorite electrochemistry-related journal?” Pictured on right: Team MSE contestant Patrick West
AMETEK........................................................................... 8 Bio-Logic back cover Call for Papers MRS ....................................................... 40 Conference Direct 10 ECST Cancun .................................................................. 38 El-Cell 23 Gamry ................................................................................ 4 Ion Power 20 Koslow .............................................................................. 21 Pine Research 2 Stanford Research Systems .............................................. 6 Scribner 1 Advertisers Index
ECS STUDENT PROGRAMS
Awarded Student Membership
Our divisions offer free memberships to full-time students.You can re-apply to receive an awarded student membership for up to four years!
Check
Biannual Meeting Travel Grants
Many ECS divisions offer funding to undergraduates, graduate students, postdocs, and young professionals that are presenting research at ECS biannual meetings.
Visit www.electrochem.org/travel-grants to learn more!
Summer Fellowships
Apply for a $5,000 summer fellowship with ECS! The annual deadline for applications is January 15.

Review candidate qualifications at www.electrochem.org/summer-fellowships
Student Chapters
There are more than 75 student chapters worldwide. ECS offers funding to support chapter events!
Find the guidelines for starting a student chapter at www.electrochem.org/student-center
Student Chapter Membership
Apply for a free student membership for those involved in active ECS student chapters.You must apply or re-apply each year for a student chapter membership.
Make the Connection
The ECS Career Expo gives students the opportunity to meet with interested employers and advance their job search with various career services.
More information at www.electrochem.org/career-expo
Enhance Your Resume
ECS equips our student members to be successful when starting their careers. The professional development workshops provide attendees with skills not often learned in the classroom.
View offerings on www.electrochem.org/education.
84 The Electrochemical Society Interface • Fall 2018 • www.electrochem.org
out www.electrochem.org/student-center for qualifications!





www.electrochem.org/meetings 235th ECS Meeting Dallas, TX May 26-May 31, 2019 Sheraton Dallas 236th ECS Meeting aTlanTa, Ga October 13-17, 2019 Hilton Atlanta 2019 2019 FuturE ECS MEEtingS ECEE 2019 Electrochemical Conference on Energy and the Environment: Bioelectrochemistry and Energy Storage GlasGOw, scOTlanD July 21-26, 2019 237th ECS Meeting with the 18th International Meeting on Chemical Sensors (IMCS 2020) MOnTréal, canaDa May 10-15, 2020 Palais des congress de Montréal PriME 2020 HOnOlulu, HI October 4-9, 2020 Hawaii Convention Center & Hilton Hawaiian Village 2020 2020
BECAUSE LINEARITY MATTERS
Bio-Logic introduces EIS quality indicators*
Detect non-linearity : Total Harmonic Distortion - THD
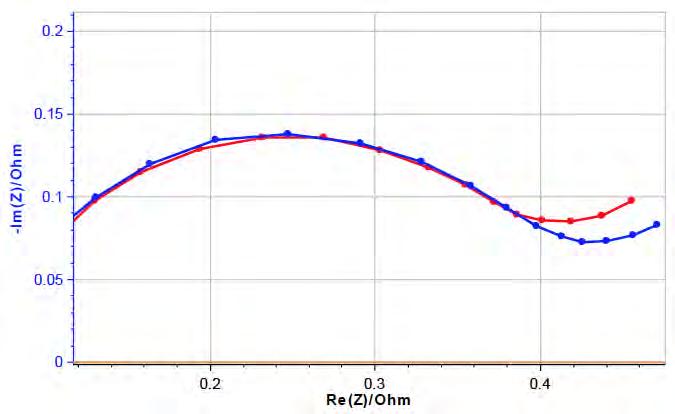
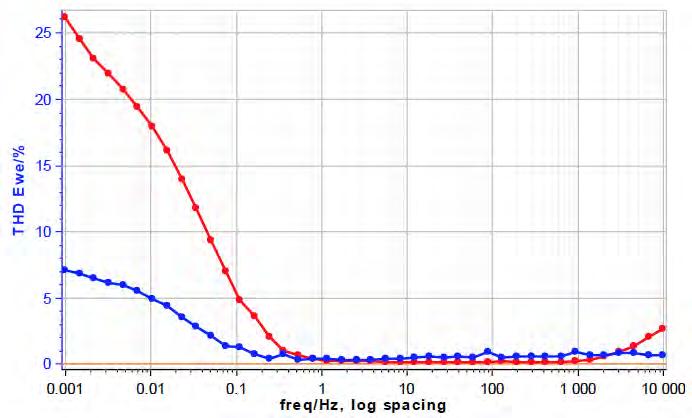



Detect non-stationarity : Non-Stationary Distortion - NSD

Detect noisy response : Noise to Signal Ratio - NSR
Electrochemical impedance spectroscopy (EIS) measurements at two different AC amplitudes (50 mA & 400 mA) are performed on the same Li-ion battery cell.

The non-linearity of the cell at low frequency is evident in the Nyquist plot to the right.
After an equivalent circuit fit analysis, the charge transfer resistance is over-estimated by 8% with the 400 mA AC amplitude measurement.
The THD indicator can be used to detect this non-linearity and help optimize the stimulation amplitude.
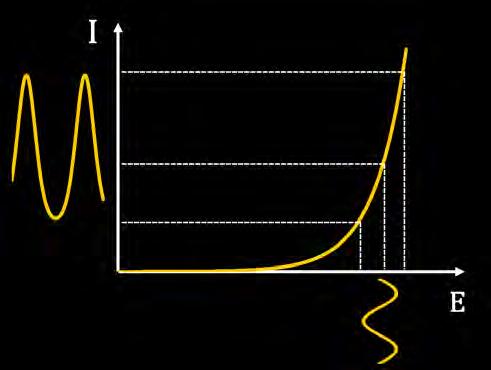
*Only for xP-300 technology
www.bio-logic.net
la = 400 mA la = 50 mA la = 50 mA la = 400 mA








































































































 by S. Roy and S. J. Coleman
by S. Roy and S. J. Coleman









 by Mahito Atobe and Frank Marken
by Mahito Atobe and Frank Marken