Characterization of two Putative Haloalkane Dehalogenases From the HLD-I subfamily Lauren
1 Carlucci ,
Ed
1 Zhou ,
Vladimir
2 Malashkevich ,
Steven C.
2 Almo and
Emily C.
1 Mundorff
1Hofstra
University, Hempstead, NY 2Albert Einstein College of Medicine, Bronx, NY
Abstract
Structural Analysis
Activity
The haloalkane dehalogenase (HLD) family consists of enzymes that catalyze the cleavage of a carbon-halogen bond, yielding a proton, halide, and an alcohol. HLDs possess broad substrate specificities, which allow them to have numerous industrial and environmental applications. Of the three subfamilies identified in the HLD phylogenetic tree, subfamily HLD-II is the best characterized. In order to fill in the gaps of sequence-structure-function knowledge in this family we have focused on characterizing enzymes from the HLD-I subfamily. We have identified two previously uncharacterized HLDs, DccA, from Caulobacter crescentus and DsaA, from Saccharomonospora azurea. DsaA has demonstrated activity and substrate specificity similar to other HLD-I enzymes, whereas, DccA is a highly active enzyme with an expanded substrate specificity profile.
800
Specific Acitivity, nmol/s/mg
700 600 500 400 300
DccA DsaA
200 100 0
Results DsaA and DccA were predicted to be HLDs based on sequence similarity with known HLDs.1 The genes were synthesized and transformed into E coli. The resultant genes were expressed and purified. A pH-based colorimetric assay was used to quantify the activity with a range of haloalkane substrates.2
Catalytic Residue
Protein Backbone
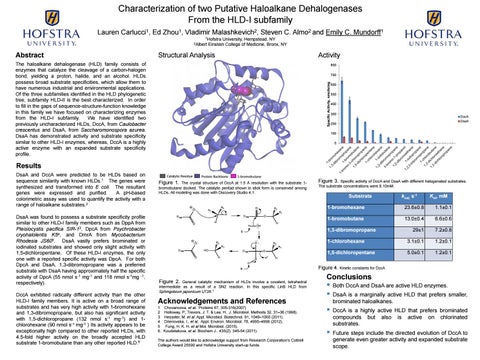
Figure 1.
The crystal structure of DccA at 1.5 Å resolution with the substrate 1bromobutane docked. The catalytic pentad shown in stick form is conserved among HLDs. All modeling was done with Discovery Studio 4.1.
O
DsaA was found to possess a substrate specificity profile similar to other HLD-I family members such as DppA from Pleisiocystis pacifica SIR-13, DpcA from Psychrobacter cryohalolentis K54, and DmrA from Mycobacterium Rhodesia JS605. DsaA vastly prefers brominated or iodinated substrates and showed only slight activity with 1,5-dichloropentane. Of these HLD-I enzymes, the only one with a reported specific activity was DpcA. For both DpcA and DsaA, 1,3-dibromopropane was a preferred substrate with DsaA having approximately half the specific activity of DpcA (55 nmol s-1 mg-1 and 118 nmol s-1mg -1, respectively). DccA exhibited radically different activity than the other HLD-I family members. It is active on a broad range of substrates and has very high activity with 1-bromohexane and 1,3-dibromopropane, but also has significant activity with 1,5-dichloropropane (132 nmol s-1 mg-1) and 1chlorohexane (90 nmol s-1 mg-1 ) Its activity appears to be exceptionally high compared to other reported HLDs, with 4.5-fold higher activity on the broadly accepted HLD substrate 1-bromobutane than any other reported HLD.6
1-bromobutane
R2
R1
O D108
O
H
O
H
OH
R2
O
Km, mM
23.6±0.8
1.1±0.1
1-bromobutane
13.0±0.4
6.6±0.6
29±1
7.2±0.8
-
R2
1-chlorohexane
3.1±0.1
1.2±0.1
1,5-dichloropentane
5.0±0.1
1.2±0.1
R1
HO O
kcat, s-1
1-bromohexane
1,3-dibromopropane
R1 D108
Substrate
H
B
O
Specific activity of DccA and DsaA with different halogenated substrates. The substrate concentrations were 8.10mM.
R1
X
D108
R2
Figure 3.
D108 H
O O
H
+
H
H B
Figure 2.
General catalytic mechanism of HLDs involve a covalent, tetrahedral intermediate as a result of a SN2 reaction. In this specific LinB HLD from Sphingobium japonicum UT26.1
Figure 4. Kinetic constants for DccA
Conclusions Both DccA and DsaA are active HLD enzymes. DsaA is a marginally active HLD that prefers smaller,
Acknowledgements and References 1 2 3 4 5 6.
Chovancova, et al. Proteins 67, 305-316(2007) Holloway, P., Trevors, J. T. & Lee, H.. J. Microbiol. Methods 32, 31–36 (1998). Hesseler, M. et al. Appl. Microbiol. Biotechnol. 91, 1049–1060 (2011). Drienovska, I., et al. Appl. Environ. Microbiol. 78, 4995–4998 (2012). Fung, H. K. H. et al Mol. Microbiol. (2015). Koudelakova, et al. Biochem J.. 435(2), 345-54 (2011).
The authors would like to acknowledge support from Research Corporation’s Cottrell College Award 25590 and Hofstra University start-up funds.
brominated haloalkanes.
DccA is a highly active HLD that prefers brominated compounds but also is active on chlorinated substrates. Future steps include the directed evolution of DccA to generate even greater activity and expanded substrate scope.