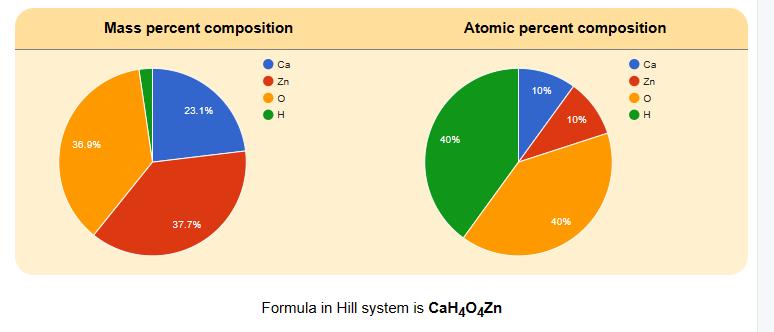
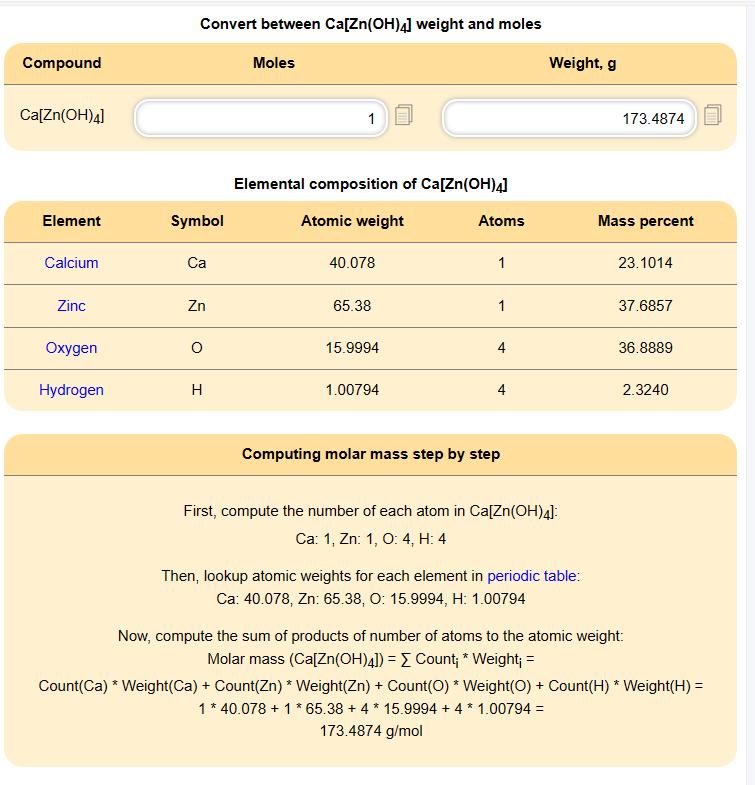
VYTIS UZA LABORANTAS

ANTIREIKI ANTIOSHO



Balance Ca(Zn(OH)4) = CaZnO2 + H2O Using the Algebraic Method
To balance the equation Ca(Zn(OH)4) = CaZnO2 + H2O using the algebraic method step-by-step, you must have experience solving systems of linear equations. The most common methods are substitution/elimination and linear algebra, but any similar method will work.
Step 1: Label Each Compound With a Variable
Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients.
a Ca(Zn(OH)4) = b CaZnO2 + c H2O
Create an equation for each element (Ca, Zn, O, H) where each term represents the number of atoms of the element in each reactant or product.
Ca: 1a = 1b + 0c
Zn: 1a = 1b + 0c
O: 4a = 2b + 1c
H: 4a = 0b + 2c
Use substitution, Gaussian elimination, or a calculator to solve for each variable.
Simplify the result to get the lowest, whole integer values.
a = 1 (Ca(Zn(OH)4))
b = 1 (CaZnO2)
c = 2 (H2O)
Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced.
Ca(Zn(OH)4) = CaZnO2 + 2 H2O
Since there is an equal number of each element in the reactants and products of Ca(Zn(OH)4) = CaZnO2 + 2H2O, the equation is balanced.
The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the beginning. To be balanced, every element in Ca(Zn(OH)4) = CaZnO2 + H2O must have the same number of atoms on each side of the equation. When using the inspection method (also known as the trial-and-error method), this principle is used to balance one element at a time until both sides are equal and the chemical equation is balanced.
Step 1: Count the number of each element on the left and right hand sides
(Left Hand Side)
(Right Hand Side)
Step 2: Multiply coefficients for compounds to balance out each element
For each element that is not equal, try to balance it by adding more of it to the side with less. Sometimes there may be multiple compounds with that element on one side, so you'll need to use your best judgement and be prepared to go back and try the other options.
1. O is not balanced. Add 1 molecule of H2O to the product (right-hand) side to balance Oxygen:
=
Since there are an equal number of atoms of each element on both sides, the equation is balanced.
Ca(Zn(OH)4) = CaZnO2 + 2H2O