
9 minute read
Biorefinery-Derived Long Chain Dibasic Complexing Agents for Lithium Thickened Lubricating Grease
Paul A. Bertin,† and Paul A. Bessette‡
†Elevance Renewable Sciences, Woodridge, IL 60517 USA ‡Triboscience and Engineering, Inc., Fall River, MA 02740 USA
Abstract
Elevance Renewable Sciences, through recent commercialization of Nobel Prize-winning catalytic olefin metathesis of natural oils, can access novel long chain dibasic chemical building blocks from a joint world-scale biorefinery with Wilmar International Limited. In this paper, the authors report the feasibility of using specific long chain dibasic derivatives as complexing agent alternatives to azelaic acid in synthetic lithium complex grease.
Introduction
In 2013, Elevance Renewable Sciences (ERS) announced the startup and shipment of commercial products from a joint world-scale biorefinery with Wilmar International Limited located in Gresik, Indonesia. The biorefinery was constructed based on proprietary Nobel Prize-winning olefin metathesis technology capable of converting renewable natural oils (e.g. palm, soybean, canola, mustard, algal, etc.) into high-value specialty difunctional molecules, olefins, and oleochemicals with a capacity of 180 kMT. As shown in Figure 1, select ERS biorefinery products with potential lubricant applications include PAO precursor 1-decene and dimethyl octadecanedioate (DM-C18), a long chain α,ω-functionalized linear diester. The objective of this work was to examine the feasibility of using biorefinery-derived DM-C18 as a complexing agent in lithium thickened grease. Neat polyalphaolefin (PAO) was selected as a highly nonpolar base oil matrix to determine if inherent physicochemical differences, such as reduced water solubility, between DM-C18 and industry standard azelaic acid (C9) resulted in manifest differentiated base grease performance.

Figure 1. (Left) Elevance joint biorefinery. (Right) Select ERS biorefinery products and potential downstream bio-derived lubricant applications.
Lithium complex greases represent nearly 40% of the North American market and a minor yet emerging proportion of global grease production.1 High operating temperatures accompanying modern transportation and industrial machinery often demand multipurpose lithium complex greases to function due to higher dropping points than simple soap alternatives. Complex grease properties depend on the nature of the base oil, thickener, and additive components with thickener chemistry and process predominantly dictating high temperature performance. Excluding borate ester technology,2 thickener systems typically comprise lithium carboxylate salts of 12-HSA and organic dibasic acids or esters. The salts are commonly formed in situ via neutralization or saponification of the corresponding acids or esters with lithium hydroxide during thickener kettle processing. Since lithium is an alkali metal that yields monovalent cations, it is generally accepted that “complexing” is not contingent on metaldirected assembly of 12-HSA and dibasic molecules into higher order networks but rather on strong adsorptive interactions between their corresponding salts during co-crystallization in base oil. Thus, it is well-known that the physicochemical properties of diacid complexing agents impact finished grease performance characteristics and manufacturing process parameters (e.g. one-step vs. two-step thickener formation).3,4
Table 1 lists relevant properties such as melting points and water solubilities for a range of α,ωdiacids with increasing chain lengths from adipic (C6) to octadecanedioic (C18) acid.5 As expected, water solubility drops precipitously as chain length increases while melting points plateau between azelaic and sebacic (C10) acids with additional influences from even-odd carbon number effects. For grease, it follows then that shorter chain complexing agents such as adipic acid would be more challenging to process and disperse in nonpolar base oils than longer chain counterparts due to decreased solubility of the corresponding lithium salts and greater susceptibility toward macroscopic phase separation. Thus, mid-chain azelaic acid and to a lesser extent sebacic acid are prevalent in grease owing to more favorable performance properties and manufacturing process parameters despite generally less attractive economics than adipic acid. It is significant to note that longer chain complexing agents dodecanedioic acid (C12) and DM-C18 have been reported to yield acceptable lithium complex greases in mineral oil but widespread adoption of these technologies have most likely been hindered by even less favorable economics relative to mid-chain alternatives. 6,7
For years, the only commercial route to DM-C18 or the corresponding C18 diacid has been through a costly fatty acid fermentation biotechnology process. Metathesis now provides a competitive industrial-scale alternative and this preliminary study was initiated to identify if ERS biorefinery-derived DM-C18 has the potential to expand the slate of economically viable complexing agents available to lithium grease manufacturers.
Experimental
Synfluid® PAO 6 cSt was obtained from Chevron Phillips. Azelaic acid, 12-HSA, and lithium hydroxide monohydrate (LiOH·H2O) were obtained from commercial sources. Gas chromatography was used to confirm DM-C18 in sample compositions. Saponification number (SN) values were determined by ASTM D1387. Three different samples of DM-C18 complexing agents of variable purity were synthesized from palm oil by proprietary olefin metathesis biorefinery processes and hydrogenation: DM-C18-A (SN = 252), DM-C18-B (SN

= 256), and DM-C18-C (SN = 259). The samples were solids with melting points ranging from 56–60°C. Fourier transform infrared (FT-IR) spectroscopy of each sample confirmed methyl ester functional groups at 1739 cm-1.
Four laboratory scale lithium complex base greases were prepared for comparison. An azelaic acid complex grease (1) served as a reference control for experimental greases (2-4) with the three DM-C18 complexing agents (A-C). For each batch, an open kettle two-step reaction scheme was used with total thickener content fixed at 20% w/w. Ratios of 12-HSA to complexing agent were fixed at 2.7:1 (% w/w). Table 2 shows formulation components for each. Grease preparation generally proceeded stepwise as follows:
1) Total batch size (base oil + thickener) approximately 2500 g. 2) A portion of PAO 6 and all 12-HSA were added to the vessel and heated to 80°C (176°F) till a homogeneous melt formed. 3) LiOH·H2O (1 equiv to 12-HSA) was mixed with deionized water (100 mL) and gently heated. 4) The aqueous base was then added to the oil solution under constant mechanical stirring and heated to about 100°C (212°F) for 1 h to complete neutralization (Step 1). 5) The complexing agent was then added to the vessel followed by additional LiOH·H2O necessary to neutralize or saponify this dibasic acid or ester (Step 2). 6) The reaction was gradually heated to 200°C (392°F) to complete lithium soap thickener formation and facilitate dehydration (and evaporation of methanol for DM-C18 samples). 7) Vessel contents were cooled by addition of the remaining PAO 6. 8) Upon cooling, base grease was finished by homogenization at 6000 psi. Chemical and physical properties of each base grease are shown in Table 3.


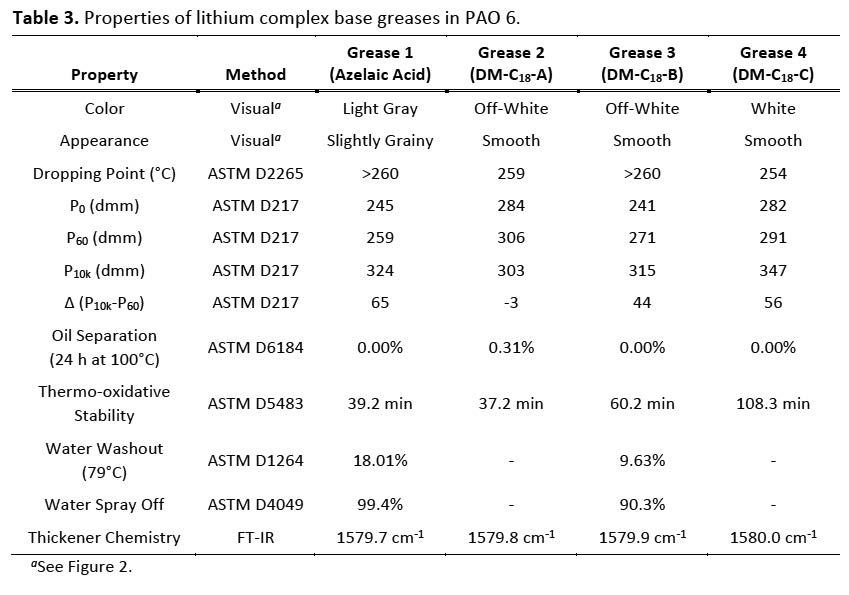
Figure 2. Images of lithium complex base greases.
Results and Discussion
Figure 2 shows a representative picture of each grease prepared in this study. Notably, the smooth textures of greases 2-4 with DM-C18 complex agents were impressive given the highly nonpolar nature of PAO 6. Azelaic acid control grease 1 was slightly grainier in comparison despite high pressure homogenization. These observations suggest the reduced polarity of longer chain C18 complexing agents enabled greater thickener dispersability in PAO which may lead to improved formulation compatibility, manufacturing process parameters (i.e. lower temperatures), and performance properties.
All greases were compared by high temperature performance, unworked and worked penetration, oil separation, and thermo-oxidative stability using ASTM methods (Table 3). FT-IR data for all samples confirmed the formation of lithium carboxylate salt thickeners with characteristic carbonyl stretching frequencies around 1580 cm-1. A dropping point >260°C (500°F) for baseline control grease 1 with azelaic acid confirmed viability of the selected two-step open kettle process for preparing lithium complex greases. Experimental greases 2-4 had dropping points well above simple lithium 12-hydroxy stearate grease with sample 3 performing the best at a value >260°C (500°F) indicating a thermally robust thickener network. Furthermore, oil separation values at elevated temperatures were negligible for all samples. Greases 1 and 2 displayed similar oxidation onset times by PDSC (ASTM D4583 @ 150°C and 500 psi) with values trending higher for 3 and 4, respectively, indicating improved oxidative stability over the baseline.
Consistencies for each grease were determined by worked penetration after 60 strokes (P60) by ASTM D217. Control grease 1 had the highest yield (i.e. the lowest P60) with a penetration value of 259 dmm just between NLGI Grade 2 (265–295 dmm) and Grade 3 (220–250 dmm). Greases 3 and 4 were NLGI Grade 2 with a higher yield for 3. Although grease 2 was not confined to NLGI Grade 2, its mechanical stability was impressive and noteworthy with a slightly firmer consistency from 60 strokes to 10,000 strokes (P10k-P60). Further work is warranted to validate this result from DM-C18-A.
Water resistance properties were compared between base greases 1 and 3 with similar consistencies. The water washout test (ASTM D1264) was used to evaluate the ability of each lubricating grease to adhere to operating bearings while a stream of hot water (79°C) impinges the housing. Grease 3 with long chain complexing agent DM-C18-B displayed a nearly two-fold improved water washout value over azelaic control grease 1. A similarly improved water spray off (ASTM D4049) result was also observed for 3 compared to 1. The relatively poor values for both samples in these tests were most likely due to low base oil viscosity. Nevertheless, these data indicate that the reduced polarity of longer chain complexing agents (i.e. DM-C18) may enable the development of lithium greases with improved water resistance.

Conclusions and Outlook
The results presented herein demonstrate that ERS biorefinery-derived long chain dibasic esters are attractive complexing agent alternatives to azelaic acid for the preparation of lithium complex grease. Notably, in neat PAO at constant soap concentrations and ratios to 12-hydroxystearic acid (12-HSA), base greases with DM-C18 exhibited comparable dropping points with improved water resistance properties and oxidative stability over an azelate control. Furthermore, the long chain complexing agents yielded more uniform and smoother greases than azelaic acid despite high pressure homogenization suggesting improved thickener dispersability which may enable less intensive process parameters (i.e. time and temperature) during grease manufacturing. Future work will be directed toward optimization of thickener component ratios and grease manufacturing processes to improve yields and allow more extensive testing on fully formulated grease.
Acknowledgements
The authors would like to thank Courtnay Shaner and Daniel Mubima of Elevance Renewable Sciences for assistance in purification and characterization of DM-C18 samples.
References
1) NLGI 2012 Annual Production Survey. 2) Lorimor, J. J. “An Investigation into the Use of Boron Esters to Improve the High-Temperature Capability of Lithium 12-Hydroxystearate Soap Thickened Grease,” NLGI Spokesman 2010, 74(4), 27-37. 3) Morgan, D.; Kay, J. S.; Coe, C. R. “Critical Variables in Lithium Complex Grease Manufacturing,” 80th NLGI Annual General Meeting 2013, Tucson, AZ. 4) Tuszynski, W.; Bessette, P. A. “An Evaluation of Sebacic and Azelaic Acid as Thickeners in Lithium Complex Grease,” NLGI Spokesman 2008, 72(4), 30-38. 5) Johnson, R. W.; Pollock, C. M.; Cantrell, R. R. “Dicarboxylic Acids,” Kirk-Othmer Encyclopedia of Chemical Technology 2010, 1-18. 6) Clark, R. D.; Newman, R. H.; Pike, W. C. “Lubricating grease for rock drill bits and bits so lubricated,” Eur. Patent Appl. EP191608 A2, 1986. 7) Mertz, W. J.; Whitson, C. “Investigating the Influence of New Complexing Agents in a Lithium Complex Grease Formulation,” NLGI Spokesman 2002, 66(9), 18-25.






